Note: Descriptions are shown in the official language in which they were submitted.
CA 02236447 2006-05-23
60557-5805
1
AT LEAST PARTLY FUSED PARTICULATES
AND METHODS OF MAKING THEM BY FLAME FUSION
Technical Field
The present invention relates to at least partly fused, particulate products,
including those that are substantially glassy, and to improved flame fusion
methods for making them. Preferred embodiments of the invention include
energy efficient methods for making generally ellipsoidal particulates by at
least
partial direct fusion of feed particles at economically feasible throughput
rates
while controlling unwanted formation of enlarged product particles from
agglomerated feed particles.
CA 02236447 1998-04-30
WO 97/16385 PCTIUS96/16967
2
Background Art Techniques for melting or softening small feed particles under
controlled
conditions to make generally ellipsoidal particulate products are known.
Examples include atomization, fire polishing and direct fusion.
Atomization involves melting myriad feed particles to convert them to bulk
liquid glass. A thin stream of such glass is atomized through contact with a
disruptive air jet. It divides the stream into fme droplets. These are kept
away
from one another and from other objects until they cool and solidify. Then
they
are recovered as substantially discrete, generally ellipsoidal glassy,
amorphous
particles.
In fire-polishing, discrete, irregularly shaped glassy solid feed particles
are
heated to a soft or molten condition while dispersed and suspended in a hot
gaseous medium. Surface tension forms the particles into ellipsoidal shapes.
Kept suspended in cooler gases until reaching their freezing temperatures, the
particles are recovered as solid, generally discrete glassy ellipsoids.
Atomization and fire polishing of glasses may be described as indirect
methods. Their feed materials have been formulated from glass-making raw
materials which were melted and homogenized in the form of bulk liquid prior
to entering the ellipsoid-forming step.
Direct fusion, somewhat similar to fire-polishing, uses feed particles with
irregular shapes that are not glassy, or are at least not fully glassy. Heated
while
in suspension and dispersion in a hot gaseous medium, the feed particles are
softened or melted and formed into molten, generally ellipsoidal shapes,
followed
by cooling, freezing and recovery in an at least partly, but more fully,
glassy
state.
In direct fusion, each ellipsoidal product particle may be formed by fusion
of either a discrete feed particle or by fusion of a group of several mutually
adherent feed particles. Groups of adherent particles are sometimes referred
to
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
3
as clusters or agglomerates, and are described herein as agglomerated. The
product particles resulting from direct fusion respectively and generally
exhibit
the varying chemical compositions of the discrete particles and/or the average
chemical compositions of the groups of agglomerated particles, from which the
ellipsoids are respectively formed, except that there may be losses of
ingredients
through high-temperature volatilization. Thus, direct fusion products do not
necessarily have the more uniformly similar particle-to-particle composition
expected of particles produced by atomization or fire-polishing.
Unlike direct fusion, fire-polishing typically employs solid feed particles
that are in a relatively highly or fully glassy or amorphous state. At some
point
in their history, they have existed in bulk liquid form. In direct fusion,
feed
particles that are not fully glassy or amorphous, and that are often nori-
glassy
minerals, undergo direct conversion to glassy form, or at least to a more
nearly
glassy and amorphous form, in an ellipsoid-forming step, without prior
conversion to bulk liquid form.
Flame fusion, as employed herein, involves formation of at least partly
fused, substantially glassy particulate products by direct fusion or fire-
polishing
of solid feed particles. Such feed particles, as fed to a fusion zone, may
have
physical states ranging from fully crystalline to fully glassy and amorphous.
Various forms of equipment, as well as differing forms of feed handling
and fusion methodology have been employed in known flame fusion processes.
For example, as early as 1935, it was taught in U.S. Patent 1,995,803 to
Gilbert,
at page 1, colunm 1, lines 31-32 and at column 2, lines 33-41, that in order
to
generate well-formed spherulized products, feed particles should be positively
dispersed in the fuel and/or oxygen-containing gas that is fed to a burner
that
heats the fusion zone, and that this can be done upstream of the burner.
Gilbert
also teaches, at page 2, column 1, lines 1-8, that subsequent heating and
expansion of these gases provides an additional dispersive effect. This patent
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
4
does not disclose the geometry of Gilbert's combustion chamber. However, his "
later U.S. Patent 2,044,680, at page 3, column 1, lines 2 and 5, twice
describes
his chamber as having "confining" surfaces.
As a further example, Garnier, in U.S. Patent 4,778,502, at colunm 2,
lines 41-45, discusses production of hollow microspheres from particulate
feeds.
At least 90 percent by weight of the feed particles have particle sizes less
than
20, and preferably less than 10, microns. To combat agglomeration of the feed,
which is recognized as making difficult the production of microspheres of
small
dimensions, the patent proposes pre-treating the feed by distributing over its
particle surfaces a small amount of a "fluidizing agent, " preferably alkanol
amine(s). See colunm 2, lines 46-58. Feed, ball-milled with such agent, can be
dispersed in gases, as taught at colunm 6, lines 19-35 and column 4, lines 50-
55,
and then fused with the aid of either of two burner types. Each of these, as
described at column 4, line 64 through colunul 5, line 43 and in Figures 1 and
2, has a combustion chamber which is of restricted cross-section relative to a
down-stream expansion enclosure. The combustion chamber, which includes fuel
ports 20 and air ports 23,24, has an extension of equally restricted cross-
section
surfaced with refractory 25 (Figure 1) or a liquid-cooled metal wall 27
(Figure
2). In the Figure 1 burner, the feed dispersion is projected into combustion
gases departing the front end or outlet of the combustion chamber through one
or more radially oriented injection ports 30,31. In the burner of Figure 2,
the
feed dispersion is projected into the combustion chamber through an axial pipe
17 in the back end of the burner.
In British Patent No. 2,178,024, at page 5, line 33 through page 6, line
4, Mouligneau et al say that it is most desirable to use feed well dispersed
in the
combustible gases. They teach propelling a stream of gas with entrained feed
through a passageway leading to the combustion chamber and forcing a second
stream of gas transversely into the first stream through an orifice in the
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
= passageway wall, to generate forces said to promote intimate admixture.
Also,
at page 2, lines 6-8, these patentees describe a tendency for feed particles
to
agglomerate and/or stick to the fusion chamber walls. They attributed this
problem to excessive heating of the feed during fusion. As a solution, they
5 proposed, at page 2, lines 15-20, to provide a flowing gaseous sleeve. It
surrounded the stream of flaming combustible gases containing dispersed feed
particles. The sleeve was said to improve yields of high quality beads by
keeping the feed particles wholly enveloped in the flame, encouraging rapid
heating of the feed, adding kinetic energy to the feed and product particles
while
keeping them dispersed and promoting rapid departure of product particles from
the fusion chamber, cooling the fusion chamber walls and thus reducing
agglomeration and sticking tendencies. See page 2, lines 22-31.
Morishita, et al, in Japanese published patent application HEI 2[1990]
59416, published February 28, 1990, discuss direct fusion of silica with
particle
sizes of less than 10 microns. Severe problems of agglomeration of the feed
materials in the flame during fusion and adherence of particles to the furnace
wall are mentioned. They suggest agglomeration may be prevented by working
with plasma induction at temperatures exceeding those of the usual fusion
furnace. However, they explain that this method is not suitable for mass
production and has poor energy efficiency. Morishita, et al proposed to solve
these problems by using feed powder reduced by jet mill to less than 10 micron
particle size, followed by direct fusion in a fusion furnace with an oxygen-
flammable gas (e.g. oxygen-propane) flame. Feed is supplied to a burner having
a powder discharge port at the center, and an opening for the gas flame at the
center axis. The thermal load of the burner and the thermal load per unit
volume
of the furnace were respectively in the ranges of 100,000-200,000 kcal/H and
less than 2,000,000 kcal/m3H. Higher thermal loads were said to lead to
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
6
agglomeration of the feed, and lower burner thermal loads were said to lead to
products of poor quality.
Commenting further on their above-described work, the above inventors
and one other, in Japanese published patent application HEI 2[1990] 199013,
published August 7, 1990, acknowledge that it proved difficult for them to
make
fme spheroidal silica at high yield by direct reduction of fme silica with
control
of thermal load. However, they suggest that this problem may be overcome by
supplying a cooling gas to, and adjustment of, the flame generating area.
Working with a fusion furnace with an oxygen-flammable gas flame again, and
with less than 10 micron feed which is dispersed in carrier gas and fed to the
center of the flame, they blow in cooling gas perpendicular to the flame or
introduce it through a ring. This is done at a selected position downstream of
the burner and is said to effectively eliminate flame generation, i.e. quench
the
flame. By changing the position and other aspects of introduction of the
cooling/quenching gas, it is said that one can adjust the residence time of
the
silica in the flame, prevent growth of the grains by agglomeration in the
flame
and recover high yields of small particles.
In Japanese published patent application No. HEI 4[1992]-147923,
"Manufacturing Method of Spherical Microparticles," by T. Koyama, et al,
published May 21, 1992, the inventors suggest, apparently in the attempt to
recover very small products, grinding the raw material to a particle size in
the
range of 0.1 to 1 micron. However, it appears that the fusion procedure used
suffers from some considerable agglomeration of the molten or soft particles.
Notwithstanding the progress made by prior workers in the art, it appears 25
that there is a need for, and an opportunity to provide, further improvements
in
the yield and energy efficiency of flame fusion processes aimed at producing
very
fme generally ellipsoidal particles. This appears especially true in relation
to
mass production of products, from feeds in the particle size ranges with 50th
CA 02236447 1998-09-23
- 7 -
percentiles (average particle size) of up to about 25, up to
about 20, up to about 15 and up to about 10 microns, or with
90th percentiles of up to about 60, up to about 40, up to
about 30 or up to about 25 microns, by volume. In production
of these products, increasing production rates have tended to
produce agglomeration and ensuing particle size growth during
fusion, while agglomeration has been avoided at the expense of
energy efficiency.
The present invention seeks to fulfill the above-
stated need. This goal has been fulfilled, at least in part,
by development of the products and methods disclosed below.
According to one aspect of the present invention
there is provided a method for the production, in bulk, of
particulate material including solid, generally ellipsoidal
particles, said method comprising: A. bringing into a
dispersed condition feed particles including at least one
material selected from among a) clay, b) talc, c) hydrate of
aluminum oxide, d) water-containing oxide of metal(s) selected
from among iron, zinc, boron or zirconium; and/or e) hydrated
silicate containing 1 to 25o by weight of dissolved or
combined water selected from among asbestos, glauconite,
meershaum, mica, pyrophillite, sepiolite, vermiculite and
zeolite, said feed particles being convertible at least in
part to generally ellipsoidal particles by heating said
particles while they are flowing in suspension in hot
combustion gases; and B. while maintaining said feed
particles in a dispersed condition while in suspension in a
60557-5805
CA 02236447 1998-09-23
- 7a -
mixture of flowing hot combustion gases, heating the feed
particles sufficiently to bring about fusion within at least
the surfaces of the feed particles to produce at least
partially fused bulk particulate product; and C. recovering
bulk product containing generally ellipsoidal, substantially
glassy fused product particles which 1. have a lower specific
gravity, at least 1% lower, than that of product particles
which have been remelted and solidified, and 2. represent
about 15 to 100o by volume of said bulk product.
According to a further aspect of the present
invention there is provided a method for the production, in
bulk, of particulate material including solid, generally
ellipsoidal particles, said method comprising: A. bringing
into a dispersed condition feed particles including at least
one material selected from among a) clay, b) talc, c) hydrate
of aluminum oxide, d) water-containing oxide of metal(s)
selected from among iron, zinc, boron or zirconium; and/or
e) hydrated silicate containing 1 to 25% by weight of
dissolved or combined water selected from among asbestos,
glauconite, meershaum, mica, pyrophillite, sepiolite,
vermiculite and zeolite, said feed particles being convertible
at least in part to generally ellipsoidal particles by heating
said particles while they are flowing in suspension in hot
combustion gases; and B. while maintaining said feed
particles in a dispersed condition while in suspension in a
mixture of flowing hot combustion gases, heating the feed
particles sufficiently to bring about fusion within at least
60557-5805
CA 02236447 1998-09-23
- 7b -
the surfaces of the feed particles to produce at least
partially fused bulk particulate product; and C. recovering
bulk product containing generally ellipsoidal substantially
glassy fused product particles 1. which represent about 15 to
100% by volume of said bulk product, and 2. wherein the
excess, if any, of the indicated 90th percentile particle size
of a sample of said product that has been vigorously agitated
in liquid, minus the 90th percentile particle size of a sample
of the feed particles from which the product particles have
been prepared and which has been vigorously agitated in
liquid, is in the range of up to about 300 of the feed
particle size, on a weight basis or, after suitable
corrections are made for any voids which may be present in the
product particles, on a volume basis.
According to another aspect of the present invention
there is provided a composition of matter comprising solid
particles, A. at least a portion of said solid particles
being substantially glassy, generally ellipsoidal particles;
B. at least a portion of said solid particles respectively
having 1. chemical composition(s) corresponding substantially
with that of at least one of a) clay, b) talc, c) hydrate of
aluminum oxide, d) water-containing oxide of metal(s) selected
from among iron, zinc, boron or zirconium; and/or e) hydrated
silicate containing 1 to 25o by weight of dissolved or
combined water selected from among asbestos, glauconite,
meershaum, mica, pyrophillite, sepiolite, vermiculite and
zeolite, said solid particles, as compared to materials a-e,
60557-5805
CA 02236447 1998-09-23
- 7c -
exhibiting a reduced content of any components of said
materials that are volatile under conditions of fusion of such
particles, and 2. lower specific gravity that is at least lo
lower than that of product particles which have been remelted
and solidified; and C. said composition of matter comprising
about 15 to 1000i by volume of generally ellipsoidal product
particles that have said chemical composition(s) and said
lower specific gravity, based on the total volume of solid
particles present in said composition of matter.
According to a still further aspect of the present
invention there is provided a composition of matter comprising
solid particles, A. at least a portion of said solid
particles being substantially glassy, generally ellipsoidal
particles; B. at least a portion of said solid particles
repspectively having 1. been formed from feed particles of at
least one a) clay, b) talc, c) hydrate of aluminum oxide, d)
water-containing oxide of metal(s) selected from among iron,
zinc, boron or zirconium and/or e) hydrated silicate
containing 1 to 25% by weight of dissolved or combined water
selected from among asbestos, glauconite, meershaum, mica,
pyrophillite, sepiolite, vermiculite and zeolite, and 2.
lower specific gravity, at least lo lower than that of product
particles which have been remelted and solidified; and C.
said composition comprising about 15 to 100% by volume of
substantially glassy, generally ellipsoidal product particles
that are products of at least partial fusion of said feed
particles and have lower specific gravity as aforesaid, said
60557-5805
CA 02236447 2006-05-23
60557-5805
7d
volume being based on the total volume of solid particles
present in said composition of matter.
According to one aspect of the present invention,
there is provided a method for the production, in bulk, of
particulate material comprising solid, generally ellipsoidal
product particles, said method comprising: A. dispersing in
gaseous suspension, in at least one portion of a combustible
gas mixture, solid feed particles that include about 60 to
100% by weight of irregularly shaped particles of at least
one feed material that 1. has an average particle size by
volume of up to about 25 pm (microns), and 2. is convertible
at least in part to generally ellipsoidal particles by
heating the material while it is flowing in suspension in
hot gases generated by combustion of said gas mixture; B.
delivering combustible mixture and suspended feed particles
to a flame front in which the mixture is ignited, the
concentration of feed particles in the mixture being in the
range of about 0.05 to about 2 pounds per pound (Kgrams per
Kgram) of mixture; C. maintaining the flame front and at
least a substantial portion of the resultant flame in a wall
free zone which extends down-stream from the front; D. while
maintaining the suspended particles in a dispersed
condition, heating the particles in said wall free zone with
heat transferred thereto by burning of the combustible
mixture, thereby causing in said zone at least partial
fusion of the irregularly shaped particles within at least
their surfaces; E. expanding the burning gases and causing
fusion of particles in said zone in amounts sufficient to
produce at least partially fused bulk particulate product
wherein about 15 to 100% by volume of the fused bulk
particulate product is generally ellipsoidal discrete
product particles; and F. restricting the total amount of
heat utilized 1. for heating, including pre-heating if any,
CA 02236447 2006-05-23
60557-5805
7e
of feed particles, of any other combustible mixture
components and of the combustible mixture itself, 2. for
fusion of particles, 3. for expansion, and 4. for heat
losses, to an amount in the range of about 500 to about
25,000 B.T.U. per pound (278 to about 13,889 kilocalories
per kilogram) of generally ellipsoidal product particles
produced.
According to another aspect of the present
invention, there is provided a method for the production, in
bulk, of particulate material comprising solid, generally
ellipsoidal product particles, said method comprising: A.
providing solid feed particles that include about 60 to 100%
by weight of irregularly shaped particles of at least one
feed material that has an average particle size by volume of
up to about 28 pm (microns); B. applying to said feed
particles one or both of fluidizing agent and force for
dispersion of the feed particles in gaseous suspension in at
least one portion of a combustible gas mixture; C. while
delivering combustible mixture and feed particles that have
been suspended in at least a portion of said mixture to a
flame front in which the mixture is ignited, inhibiting one
or both of agglomeration and re-agglomeration of suspended
feed particles and distributing particles present in the
suspension across the flame front; D. maintaining the flame
front and at least a substantial portion of the resultant
flame in a wall free zone which extends down-stream from the
front; E. while maintaining the suspended feed particles in
a dispersed condition, heating the dispersed feed particles
with heat transferred thereto by burning of the combustible
mixture, thereby causing at least partial fusion of the
irregularly shaped particles within at least their surfaces;
F. applying sufficient fluidizing agent and/or force in the
dispersion operation of paragraph B above, sufficiently
CA 02236447 2006-05-23
60557-5805
7f
inhibiting one or both of agglomeration and re-agglomeration
during the steps of paragraph C above, sufficiently
expanding the burning gases in the wall free zone of
paragraph D above and establishing a sufficient ratio of
weight of feed particles per unit of heat released in said
zone to produce at least partially fused bulk particulate
product 1. wherein about 15 to 100% by volume of the fused
bulk particulate product is generally ellipsoidal discrete
product particles, and 2. wherein the excess, if any, of the
indicated 90th percentile particle size of said product
relative to the indicated 90th percentile primary particle
size of the feed particles is in the range of up to about
30%, up to about 20% or up to about 10% of the primary
particle size, on a weight basis or, after suitable
corrections are made for any voids which may be present in
the product particles, on a volume basis.
According to still another aspect of the present
invention, there is provided a method for the production, in
bulk, of particulate material comprising solid, generally
ellipsoidal product particles, said method comprising: A.
dispersing in gaseous suspension, in at least one portion of
a combustible gas mixture, solid feed particles that include
about 60 to 100% by weight of irregularly shaped particles
of at least one feed material that 1. has an average
particle size by volume of up to about 25 um (microns), and
2. has a tendency to agglomerate and form clumps when groups
of the particles are subjected to compaction forces when one
or both of at rest and in motion, and 3. is convertible at
least in part to generally ellipsoidal particles by heating
the material while it is flowing in suspension in hot gases
generated by combustion of said gas mixture; B. applying to
said feed particles an amount of one or both of fluidizing
agent and an amount of force sufficient to disperse the feed
CA 02236447 2006-05-23
60557-5805
7g
particles in said gas mixture or a portion thereof so that
the difference in indicated 90th percentile particle size of
said feed particles before and after dispersion is in the
range of up to about 20% or up to about 10% of the feed
particle size before dispersion, on a volume basis; C. after
dispersing the feed particles in the gas mixture or portion
thereof, and while delivering the combustible mixture and
suspended feed particles to a flame front in which the
mixture is ignited, inhibiting one or both of agglomeration
and re-agglomeration of suspended feed particles and
distributing particles present in the suspension
substantially uniformly across the flame front, the
concentration of feed particles in the mixture being in the
range of about 0.05 to about 2 pounds per pound (Kgrams per
Kgram) of mixture; D. maintaining the flame front and at
least a substantial portion of the resultant flame in a wall
free zone which extends down-stream from the front, while
maintaining the suspended feed particles in a dispersed
condition; E. heating the dispersed feed particles with heat
transferred thereto by burning of, and while the particles
are entrained in, the combustible mixture at fusion
temperature being in the range of about 500 to about 2500
degrees centigrade; F. expanding the burning gases and
causing fusion of the entrained feed particles in amounts
sufficient to produce at least partially fused bulk
particulate product wherein about 15 to 100% by volume of
the fused bulk particulate product is generally ellipsoidal
discrete product particles; and G. restricting the total
amount of heat utilized 1. for heating, including pre-
heating if any, of feed particles, of any other combustible
mixture components and of the combustible mixture itself, 2.
for fusion of particles, 3. for expansion, and 4. for heat
losses, to an amount in the range of about 500 to about
25,000 B.T.U.s per pound (278 to about 13,889 kilocalories
CA 02236447 2006-05-23
60557-5805
7h
per kilogram) of generally ellipsoidal product particles
produced.
According to yet another aspect of the present
invention, there is provided a method for the production, in
bulk, of particulate material comprising solid, generally
ellipsoidal particles, said method comprising: A. bringing
into a dispersed condition irregularly shaped feed particles
that 1. are composed substantially of one or more substances
selected from naturally occurring silicas and silicates, 2.
are convertible at least in part to generally ellipsoidal
particles by heating the material while it is flowing in
suspension in hot gases generated by combustion of said gas
mixture, 3. have an average particle size by volume in the
range of up to about 15 pm (microns), and 4. include
sufficient volatile material in the form of combined or
dissolved water to generate voids in at least a portion of
the fused product particles, and B. while maintaining said
feed particles in dispersed condition, heating the feed
particles sufficiently to produce at least partially fused
bulk particulate product 1. having an average particle size
by volume in the range of up to about 15 pm (microns), 2.
containing about 15 to 100% by volume of at least partly
fused, generally ellipsoidal discrete product particles that
are substantially glassy, in that at least the surface
portions of the particles are amorphous, and 3. including
about 1% to about 20% of void volume, based on the volume of
the product particles.
According to a further aspect of the present
invention, there is provided a composition of matter
comprising solid particles wherein: A. at least a portion of
said solid particles are generally ellipsoidal particles
that are substantially glassy in that at least the surface
portions of the particles are amorphous; B. at least a
CA 02236447 2006-05-23
60557-5805
7i
portion of said solid particles respectively constitute
product particles produced by at least partial fusion of
feed particles while they are flowing in suspension in hot
combustion gases, said feed particles containing volatile
material ranging in amount from about 1 to about 25% by
weight based on the weight of the feed particles, are
derived from, and have chemical compositions corresponding
substantially with the chemical composition of, one or more
substances selected from silicas and silicates present in
naturally occurring mineral deposits, except that the amount
of volatile material in the product particles may differ
from the volatile content of the corresponding naturally
occurring mineral, have a Color Quest 457 nanometer
brightness of at least about 60, have an average particle
size by volume in the range of up to about 15 pm (microns),
and include about 1% to about 20% of void volume, based on
the volume of the product particles; and C. said composition
of matter comprises about 15 to 100% by volume of said
generally ellipsoidal particles that have said chemical
compositions, based on the total volume of solid particles
present in said composition of matter.
The invention, depending on which of its various
embodiments is used, is expected to provide one or more of
the advantages set forth in succeeding paragraphs. It
should be understood therefore that the invention includes
embodiments which possess less than all of the advantages
described below.
It is an advantage of the invention that a wide
variety of feed materials can be efficiently melted in an
"open" flame, without special confining furnace walls or
flame quenching processes, to provide generally ellipsoidal
particles which are only a few microns in average particle
size.
CA 02236447 2006-05-23
60557-5805
7j
Particles can be used with an average diameter of
less than 15 microns, such that heat transfer from the
combustion gases to the particles is rapid and the particle
melting or fusing point is reached in the burning zone of
the open flame, without additional confinement by furnace
walls.
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
8
Although it has been taught that dispersion of fme mineral particles in
flames tends to extinguish the flames, due to lack of sufficient heat in the
flames,
the method of the invention can be operated without undue difficulties. As
contrasted with some prior methods which employed temperatures in
excess of 2500 C to produce small unagglomerated feeds, relatively low,
energy
conserving temperatures, for example up to about 2500, more preferably up to
about 2300 and still more preferably up to about 2000 C, can be successfully
used in the methods of the present invention. In general these methods will
employ temperatures of at least about 500, more typically at least about 700
and,
where necessary or desired, at least about 900 C.
Particle compositions can be used with the lowest possible melting point
and preferably with a "fugitive" flux, e.g., bound or dissolved volatile
material
such as water or sulfur oxides. It has been suggested in the prior art that
combustion processes be applied to broad categories of mineral materials,
including some materials containing bound or dissolved volatile materials.
However, these processes generally produce large size ellipsoidal particles
having
a relatively large void space due to the expansion and release of volatile
material
during heating. The present invention teaches the use of particulate feed
material
compositions with bound or dissolved volatile materials and in the size range
of
up to about 25 microns to produce generally ellipsoidal particles of similar
size
distribution, on a weight basis, as the feed materials. Although a portion of
the
product particles may have voids which total, for example, in the range of
about
1 to about 20 volume percent or more, the invention may also be employed to
produce products without voids, including particles with less than theoretical
specific gravity, as will be explained in greater detail below.
When bound or dissolved volatile materials are present in the feed
compositions, they aid in the fusion process. In the presence of volatile
materials, compositions that would otherwise be unaffected can be fused by the
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
9
relatively low flame temperatures obtained through use of stoichiometric
mixtures
of air and natural gas. Apparently the volatile material effectively lowers
the
melting point and viscosity during the fusion process, and then evaporates to
leave solid ellipsoids. No reports of such low temperature energy efficient
means
for producing small-diameter ellipsoids from commonly available powders have
been found. Similar small diameter spheres are reportedly made only by using
high temperature flames generated by combusting propane gas and oxygen.
When the irregularly-shaped particles are carefully dispersed and
homogeneously entrained in the combustion gases prior to ignition, and an open
or unconfined flame is used without "furnace walls," rapid radiation cooling
can
be promoted and can be followed when needed by gradual introduction of cooling
gases (air or water).
Another advantage of the present invention is that it makes possible the
production of generally ellipsoidal particles in abundance while minimizing
unwanted agglomeration. In at least certain of its aspects, the methods of the
present invention avoid slagging, turbulence, collisions of molten particles,
production of fused agglomerates and attendant yield losses.
When the above methods are applied to common and relatively low
melting glasses, very high yields of small diameter ellipsoidal particles are
the
result. In fact, after allowing for the loss of volatile components, yields
can
approach 100 percent, and the size distribution of the products can be
equivalent
to or even less than that of the starting materials, indicating almost no
slagging
or inter-particle collisions in the molten state.
Heretofore, small diameter spheres have been produced expensively as a
by-product of producing large diameter spheres from commercial glasses. This
' has severely restricted commercial availability. Surprisingly, the smaller
diameter
ellipsoids made with the present invention are even more efficient to produce
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
than larger ones, and they can be made from inherently higher melting
compositions. This is contrary to prior art. ,
When common productivity enhancements such as oxygen enrichment and
preheating of combustion gases are used, the small diameter products are
5 produced at the highest microsphere production efficiencies known. One pound
or more of product can be obtained from 2000 B.T.U. of energy. When the
above methods are applied to sphere forming compositions described herein,
unique high-melting ellipsoidal particles can be formed at high efficiency.
It is believed that in one or more of its aspects, the present invention
10 represents the most cost effective means currently known for manufacturing
very
small diameter, substantially non-hollow, generally ellipsoidal particles with
a
high degree of whiteness and transparency.
Moreover, the apparatus and processing requirements for practicing these
methods can be significantly simpler than those described previously by other
workers.
Products can be produced according to the invention for a wide variety of
applications. For example, such products are useful as additives in
thermosetting
and thermoplastic resins such as silicones and fluoropolymers, in engineering
plastics, in lotions and creams, and in composites, paper and other materials
in
any physical form, such as for instance molded products and single or multi-
layer
products including especially webs and laminates. They are also useful as film
antiblocking agents, as anti-caking aids, and as cosmetic powders with unusual
"slip" or lubricity.
When produced in forms characterized by particular amounts of generally
ellipsoidal particles, e.g. about 30 or more and up to 100% by volume based on
the total volume of the solids contents of the compositions, the products may
be
used, even at relatively high concentrations, to form relatively low viscosity
mixtures in liquids or molten plastics. Products that are abundant in
generally
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
11
ellipsoidal particles can have high levels of hardness coupled with low
abrasiveness.
Highly ellipsoidal products are also characterized by relatively low surface
area and can be made in forms which engage in relatively little surface
interaction with other materials with which they may be formulated in a
variety
of end use applications. However, when the average size distribution of the
generally ellipsoidal particles becomes extremely small, particularly less
than 5
microns, surface interactions of the particles may contribute in an important
way
to the rheology of formulations in which the particles are used.
Products containing some particles having significant surface roughness
may for example be employed to advantage in compositions where some degree
of abrasiveness is desired. Fusion operations conducted according to the
invention can be readily controlled to produce predetermined proportions of
both
substantially glassy and rough, irregular crystalline particles in the
particulate
product, which can thus be used to impart a predetermined degree of
abrasiveness in end use applications. Such products are especially conserving
of
energy since much higher production rates per unit of fuel consumption can be
attained where only partial conversion to ellipsoidal particles is required.
Brief Description of the Drawings
A non-limiting embodiment of the invention, described in text which
follows, is shown in accompanying illustrations, of which:
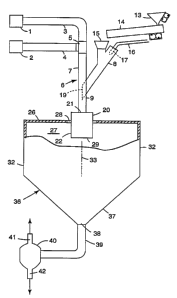
Figure 1 is a schematic, overall diagram of apparatus for converting solid
feed particles of irregular shape to a particulate product characterized by a
substantial proportion of at least partly fused, substantially glassy,
generally
ellipsoidal and discrete particles.
CA 02236447 1998-04-30
WO 97/16385 PCTIUS96/16967
12
Figure 2 is an enlarged portion of the apparatus of Figure 1, disclosing a
mixing device for assisting in dispersion of feed particles into a stream of
combustible gases.
Modes for Carrying Out the Invention
In general, the solid feed particles may include any material which is
fusible to generally ellipsoidal products and which will pass through the
processing equipment, preferably without seriously impairing or frequently
disabling that equipment, and without rendering the ultimate product
unsuitable
for its intended purpose. Thus, these particles may include one or more
materials which are not fusible under the conditions maintained in the
process.
However, according to the invention, the solid feed particles include about 60
to
100% by weight of irregularly shaped particles of at least one feed material
that
is convertible at least in part to generally ellipsoidal particles by heating
the
material while it is flowing in suspension in hot gases generated by
combustion
of a gas mixture in which the gas particles are suspended.
In principle, there is no reason why the full range of materials that are
susceptible to fire polishing and direct fusion methods may not be utilized in
the
method. Some examples of the naturally occurring and at least partly synthetic
materials which may be used include: any of the known species of calcium
silicates, including the wollastonites, these being fibrous structures
attributable
to their containing chains of linked Si04 tetrahedra of the composition
(SiO3),,,
for example wollastonite ("wollastonite per se"), pseudowollastonite and
parawollastonite, and hydrated calcium silicates including xonotlite
(5CaO.5SiO2.H20), foshagite (4CaO.3SiOZ.H20), tobermorite
(4CaO.5SiO2.5H20), girolythe (2CaO.3SiO2.2H20), Flint's hydrate
(CaO.SiO2.H20), chondrodite (5CaO.2SiO2.Ha0), afwillite (3CaO.2SiOa.3H20),
okenite (CaO.2SiO2.2H20) and hillebrandite (2CaO.SiO2.H20); the nephelines,
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
13
referring to any one or combination of the members of the nepheline group,
including nepheline itself (Na3(Na,K)[Al4Si4O16]) and kalsilite (K[A1SiO4]),
in all
of their crystalline structures and solid solutions with each other; alkali
feldspars,
a family of feldspars that includes potassium feldspar (KA1Si3O$) alone or in
combination in varying ratios with sodium feldspar (NaAlSi3O8), and which may
also contain varying but usually small amounts of calcium feldspar
(CaAl2Si2O8);
plagioclase feldspars, a series of materials comprising calcium feldspar
(CaAl2Si2Og) alone or in combination in any ratio with sodium feldspar
(NaA1Si3O8), which may also contain varying amounts, but usually small
amounts, such as about 20% by weight or less, of potassium feldspar
(KA1Si3O8);
volcanic ashes of all types; perlites of all types; garnets of all types;
silicate
glasses of all types; naturally occurring silicas of all types; silica and
silicate
products of all types precipitated from sodium silicate solutions;
precipitates from
silica and silicate sols and gels of all types; clays of all types such as
kaolin
(A12O3SiO2H2O), kaolinite, and halloysite; hydrophilic and hydrophobic talcs
(Mg3Si4H2O1Z); hydrates of aluminum oxides such as gibbsite (A1203=3H20),
boehmite (A12O3=H2O), diaspore (A1203=H20) and bauxite minerals of all types,
as well as aluminum hydroxide (Al(OH)3); and water-containing oxides of other
metal(s) such as iron, zinc, boron, zirconium and/or of any other
intermetallic,
transition metal, metalloid, or non-metallic atom(s). The above enumerated
materials may be used alone or in admixture with other listed and/or unlisted
materials.
Feed materials having combined or dissolved volatile materials are useful
for their property of lowering the respective melting temperatures of the feed
materials. Silicas and silicates are widely prepared by acid precipitation
from
sodium silicate solutions in forms with water as a part of their composition,
either chemically or physically absorbed, dissolved, or as water of hydration
as
CA 02236447 2006-05-23
60557-5805
14
well as with residual sulfates, chlorides or metal ions as part of their
composition.
Examples of feed materials having combined or dissolved volatile
materials which generally lower their respective melting temperatures and can
contribute to the formation of voids include: the hydrated silicates including
calcium silicates, sodium silicates, potassium silicates, and lithium
silicates, e.g.,
perlite; metasilicates; other silicates and silicas having combined or
dissolved
volatile materials; the above mentioned clays; talcs; hydrates of aluminum
oxides
and bauxite minerals; and the above-mentioned metallic, intermetallic,
transition
metal, metalloid, and non-metallic oxides. Examples of volatile materials
which
may be combined with or dissolved in the above feed materials and which
generally lower melting temperature include water, carbon dioxide, nitrogen,
oxides of nitrogen, ammonia, other nitrogen containing volatiles, sulfur
oxide,
sulfur dioxide, sulfur trioxide, other sulfur containing volatiles and various
volcanic components.
For examples of other suitable feed materials containing volatile
components see Industrial Minerals
and Rocks, 5th Edition, Lefond, Stanley J., et al, Society of Mining Engineers
of the American Institute of Mining, Metallurgical, and Petroleum Engineers,
Inc., New York, 1983; Handbook of Glass Properties, Bansal, Narottam P. and
Doremus, R.H., Harcourt Brace Javonovich, 1986; Sol-Gel Science, The Physics
and Chemistry of Sol-Gel Processing, Brinker, C. Jeffrey and Scherer, George
W., Harcourt Brace Javonovich, Boston 1990. For example, one may find in
these works disciosuies of siiicate compositions that can exist in combination
with dissolved or combined water, e.g., 1 to 25% by weight, these being
referred to herein as "hydrated silicates. " These are usually but not
necessarily
crystalline minerals.
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
According to one embodiment of the invention, feed material may be
selected from among oxide(s) of an element, or of any combination of elements,
in the periodic table which: can be obtained in the form of a powdered solid;
has
a fusion temperature of at least about 200 degrees Centigrade; and has/have at
5 least about 0.5 % by weight of associated substances that are volatile under
the
conditions of the fusion step of the method. For purposes of this embodiment,
the term "fusion" and other derivatives of the word "fuse" employed herein
refer
to a high-temperature primarily physical transformation of material from a
less
fused to a more completely fused condition involving vaporization and/or
10 recondensation only to a minor, preferably to a small, and still more
preferably
to no substantial extent, but which may include changes in oxidation state in
the
feed materials and/or in the associated volatiles. Such feed materials as are
above described can be processed in the combustion zone of a flame by means
of a fusion step to provide the generally ellipsoidal particulate products of
this
15 invention and by-products that are liquid or gaseous at room temperature.
Thus,
with feeds having mean melting temperatures of about 200 degrees Centigrade
and higher, for example in the range of about 200 up to about 500 degrees
Centigrade, it is possible to produce generally ellpsoidal particulate
products that
have higher mean melting points than the feeds, for example products that melt
or fuse at about 500 degrees Centigrade and higher.
Materials containing volatile components of one or more kinds that,
together, represent at least about 0.5, at least about 1 or at least about 2
percent
by weight of such materials and up to about 7, up to about 10, up to about 25,
up to about 50 or up to about 80 percent by weight of such materials are
useful
as feed materials in the present invention.
"Perlite" is a hydrated silicate and encompasses both naturally occurring
hydrated volcanic glass and lightweight aggregate that is produced from the
expansion of glass after it has been crushed and sized. Petrologically, it is
CA 02236447 2006-05-23
60557-5805
16
defmed as a glassy rhyolite that has a pearly luster and concentric, onionskin
parting. For a further discussion of the properties and mining of perlite, see
"Perlite" by Frederic L. Kadey Jr. in Industrial Minerals and Rocks, Fifth
Edition, v. 2, p. 997-1010, American Institute of Mining, Metallurgical, and
Petroleum Engineers, Inc., New York, 1983.
In its naturally occurring form, perlite is a rhyolite glass that contains
from about 2 to about 7 weight percent water, which when heated at elevated
temperatures, releases such water to expand the mineral into a relatively
large,
hollow particle of low specific gravity. While perlite also can occur as
andesitic
or dacitic glass, these forms tend to be less important commercially. Typical
naturally occurring perlite compositions include 70-75% SiOZ, 12-14% A1203, 3-
5% Na20, 3-5 % K20, 2-7% H20 and less than 1 % each of Fe203, Ti02, CaO
and MgO.
It is known in the prior art that perlite can be dried to a lower water
content prior to a combustion process to provide smaller, denser and higher
strength ellipsoidal particulates upon combustion. This drying process results
in
higher energy costs per unit of perlite particles. In the present invention,
it has
been found that by simply reducing the particle size of the perlite feed to a
size
which is at or below about 25 microns, preferably about 15 microns, or more
preferably about 10 microns average particle size, generally ellipsoidal
particulates are formed which are stronger, much smaller, and more dense.
A portion of the alkali and plagioclase feldspars are members of the
ternary system NaAlSi3O8--KA1Si3O8--CaAl2SiZO8. Thus, the terms alkali
feldspar and plagioclase feldspar include the full range of solid solutions of
these
three components which can exist in ores that can be mined. Among these are
feldspars containing mostly sodium feldspar in solid solution with equal or
nearly
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
17
equal small quantities of potassium feldspar and calcium feldspar, for
example,
albite and some forms of anorthoclase.
The contents of the relatively pure or concentrated forms of feed materials
that are employed in the present invention need not correspond identically to
their
respective pure compositions or chemical formulas. Some of the factors which
cause such deviation include: slight differences between the ratios of atoms
in the
formulas and the ratios in which those atoms actually combine with one another
when forming mineral material; substitution, a process by which relatively
small
proportions of certain of the atoms predominantly or originally present in the
crystalline lattices have been replaced with or supplanted by small amounts of
other atoms not included in the formulas; the presence of one or more other
minerals in solid solution with a particular mineral; the presence of a small
amount of materials that are given off or lost on strong heating, also called
"ignition;" addition of chemicals to the feed material in small amounts, such
as
to reduce the melting temperature of the feed and promote fusion or otherwise
favorably influence the production process or modify the product.
Thus, when this disclosure refers to feed materials by name or nominal
chemical formula, such reference is intended to include naturally occurring
deviants and man-made modifications which do not render the materials
unsuitable for use in the present invention. From this, it should be apparent
that
where the present disclosure utilizes art-recognized nomenclature of feed
materials, the meaning of that nomenclature is subject to minor adjustments in
meaning as are described herein. Also, chemical formulas are given herein only
for convenience and not to limit the invention.
The identity and classification of feed materials can be determined with
standard petrographic analytical techniques, for example those described in
the
Laboratory Handbook of Petrographic Techniques, by C. S. Hutchison, John
Wiley & Sons, Inc., 1974. With such techniques one can determine the presence
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
18
of designated phases by one or more of the following: X-ray diffraction
patterns;
determinations of chemical composition; microscopic observation; measurements
of refractive index, density and specific gravity; calculations of the Niggli
Molecular Norm (Catanorm); and differential solubility and differential
staining
techniques. See also American Mineralogy, "The Rosiwal method and the modal
determination of rock," by E. S. Larsen and F. S. Miller, Vol. 20, p. 260,
1935.
Many other accepted techniques and refinements are known to those skilled in
the art.
The preparation of feed materials may include all or any portion of the
following steps, and possibly others, depending on the nature of the starting
material used. There may be drying, coarse grinding, magnetic separation,
froth
flotation, final grinding, surface treating and classification.
Some feed materials, even when mined from deposits in which they may
be found at relatively high concentrations, will often require some degree of
refming to produce feed material composed substantially of that mineral. Among
the components which may be removed by such preparatory treatments are
excess accessory minerals and materials which impart color to the ores.
Grinding may be used not only to adjust particle size, but also to liberate
unwanted accessory minerals and/or other ore components which may be present.
Also, grinding may be followed by magnetic separation and/or flotation to
remove the liberated accessory minerals and/or other constituents.
Some feed materials are obtainable in substantially "white, ""colorless"
or "bright" forms convertible to substantially white, colorless or bright
generally
ellipsoidal particles according to the present invention. Brightness of feed
and
product particles in dry, packed powder form may be measured with a
HunterLab Color Quest Spectrocolorimeter System, Model CQS-9400 45/0, or
equivalent means, at 457 nanometers.
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
19
Feed materials used in the invention may for example have a Color Quest
457 nanometer brightness of at least about 60, more preferably at least about
70
and yet more preferably at least about 80. In general, the preferred mineral
materials, used to produce white and/or transparent products with low color,
contain very small amounts of Fe203 or Fe304, e.g. less than about 0.1 %, and
of FeO, e.g. less than about 1 %. However, use of colored forms of the
designated minerals and production of colored products are also contemplated.
While the feed materials utilized in the present invention do not
necessarily contain minerals or other fusible substance(s)s having an exact or
nominal compositional identity, they may nevertheless be "composed
substantially
of" at least one of these substances. Thus, the feed materials contemplated
for
use in the present invention may contain about 60 to 100%, more specifically
about 75 to 100 % and still more specifically about 90 to 100 % by weight of
one
or more specified substances. These ranges generally embrace those materials
which cause the above-described deviations of contemplated feed substances
from
their nominal chemical formulas. Among these are: excesses of one or more of
the atoms that are included in such formulas; atomic substitutions, i.e. atoms
that
are not included in such formulas and that have been substituted for included
atoms; solid solutions; and such other components of, additions to or
modifications of the feed materials which do not render them unsuitable for
use
in the present invention, including without limitation man-made modifications.
However, loss on ignition materials, although usually present in natural feed
materials or at least in the raw materials from which they are prepared, are
not
to be counted either as part of the feed materials or included in the basis
for
applying the above weight percentage ranges.
The expression "composed substantially of" and the weight ranges just
given are intended to indicate that the feed materials may correspondingly and
respectively contain up to about 40 %, more specifically up to about 25 % and
still
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
more specifically up to about 10% by weight of "remainder" materials.
Remainder materials may for example include accessory minerals, the above
fluidizing agents and any other material or materials which may be present in
the
feed material without making it unfit for making products that contain at
least
5 about 15 % of, and preferably at least about 30% of, at least partially
fused
generally ellipsoidal particles, such as may be useful in one or more of the
end-
use applications disclosed herein or in another end use.
When employing feed particles containing substances that have a specific
composition that is exact or nominal, such as a mineral, it is preferred that
from
10 a major portion up to substantially all of such particles respectively
contain about
60% to 100% by weight of at least one substance(s) of which the particles are
composed. Thus, for example, about 50 to 100%, more preferably about 75 to
100 % and still more preferably about 90 to 100 % by weight of the feed
particles
will respectively contain about 60 to 100% by weight of such substance(s).
15 Thus, it is contemplated that one can formulate feed materials in which
there are
particles that respectively contain above and below 60% by weight of those
substance(s), including for example feed materials in which more than 50% by
weight of the feed particles contain less than 60% by weight of the
substance(s),
but in which the weighted average composition of the feed particles reflects
about
20 60 to 100% by weight of such substance(s). Correspondingly, one can
formulate
feed materials in which the particles respectively contain above and below 40%
by weight of remainder material(s), but in which the weighted average
composition of the feed particles reflects up to about 40% by weight of
remainder material(s).
According to the invention, at least partially fused particulate material is
prepared from feed particles which may be prepared as above described or in
any
other suitable manner. The term particle is used herein in a generic sense
that
CA 02236447 1998-04-30
WO 97/16385 PCTIUS96/16967
21
includes any finely subdivided form of the particular mineral involved, which
may for example include grains, crystals, mixtures of crystals, mixed
crystals,
clusters, agglomerates and fiber fragments.
These materials are supplied to the fusion step of the process in small
particle sizes. The average particle size, on a volume basis, is up to about
25,
up to about 20, up to about 15 or up to about 10 microns, or the 90th
percentile
is up to about 60, up to about 40, up to about 30 or up to about 25 microns,
by
volume. To illustrate the meaning of volume basis, as exemplified by a
preferred particle size for feed material and product of the present
invention, an
average or mean particle size of about 5 microns means that the aggregate
volume of all particles less than 5 microns in size is equal to the aggregate
volume of all particles that are more than 5 microns in size.
Some prior art processes involve grinding the feed material to the 0.1 to
1 micron range. This mode of feed material preparation may be used in
practicing the present invention if desired, although it can be relatively
difficult
and expensive. However, certain of the suitable volatile containing materials
such as talc, aluminum hydroxide, or precipitated silica are soft and easily
ground to small size. For other desired end uses of the products of the
invention, discrete product particles essentially confined to the size range
of 0.1
to 1 microns would be too small, although having some quantities of particles
in
this range will certainly be acceptable if not desirable in many of the end
uses
for the products of the present invention. Thus, in certain preferred
embodiments of the invention, the feed particles have an average particle size
by
volume of at least about 1, at least to about 2 or at least about 3 microns.
Many if not most of the feed materials contemplated will, depending upon
such factors as the chemistry and particle sizes of the ultimate particles,
the
ambient conditions such as temperature and humidity, the manner in which the
materials have been ground, handled and stored, and the manner in which they
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
22
are transported through plant equipment, will have a tendency to agglomerate
and
form clumps composed of multiple particles, especially when groups of the
particles have been subject to compaction forces when at rest and/or in
motion.
The mere act of transporting particles through long conduit systems,
especially
those with coils, elbows and other bends, can tend to concentrate the flow of
particles by centrifugal or gravitational force along the peripheries of
curved
sections, bringing the particles into more intimate contact and encouraging
adhesion. Adhesion can also occur with the particles at rest in bulk. Adhesion
may be promoted by van der Vaals and other inter-atomic and -molecular forces
exerted among adjacent particles.
In view of the variable clumping tendencies encountered among different
feed materials under differing conditions, the particle sizes given herein are
indicated particle sizes determined after agglomerated clumps of ultimate
particles
are broken up insofar as is feasible. Thus, for example, the particle size of
the
feed material may be determined after the sample has been thoroughly agitated,
such as by application of vigorous ultrasonic energy, while in suspension in a
liquid such as water or alcohol with use of dispersants, to separate the
clumps
insofar as feasible in the context of a production control process. The
particle
size determination is then made by any suitable technique, such as laser
diffraction and/or visual analysis of electron microscope photographs so that,
insofar as may be feasible, the particle size measurement is based on the
sizes
of the ultimate particles in the sample. Where the feed material has little if
any
tendency for the ultimate particles to form clumps, as above-described, an
indicated particle size determination may be made without prior agitation in
liquid.
Where the particles in the feed material do have a substantial or unusual
tendency to clump, which will be the case for many if not most feed materials
in the indicated particle size range, it will ordinarily be necessary to take
special
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
23
precautions in order to effectively disperse the feed particles to promote
retention
of particles in discrete form during the fusion operation. The invention
includes
dispersion of the solid particles in a carrier gas. As shown above, prior
workers
in the flame fusion art have called attention to the importance of adequate
dispersion of feed particles. However, it is believed that what previously
passed
for adequate dispersion would prove woefully inadequate for practicing some
aspects or embodiments of the present invention.
Two approaches to dispersion may be employed singly, in combination
with one another and/or in combination with other dispersing techniques. One
such technique is application of a fluidizing agent, with or without
accompanying
grinding. The other involves application of force to clumped and/or unclumped
particles by a gas or solid member to break apart clumps, if such are present,
and to distribute particles uniformly in carrier gas, which is preferably a
combustible gas mixture or at least a component thereof.
For purposes of the invention, a fluidizing agent is any additive which,
when spread over the surfaces of particles of feed material, reduces to a
significant and useful extent whatever clumping tendencies they may have. The
use of certain surfactants as fluidizing agents is known to persons skilled in
the
art through the teachings of U. S. Patent 4,778,502 to Garnier et al. Thus,
Garnier et al described fluidizing agents as materials that have a good
affinity for
glass. Where the feed material employed in the present invention is not a
glass,
such as a crystalline mineral material, the fluidizing agent should have a
good
affulity for that mineral. The fluidizing agents described by Garnier et al
are
composed of substances having molecules with a polar portion, comprising, for
example, hydroxyl or amino radicals. Such compounds also have a non-polar
portion which promotes independence of the particles treated with the
fluidizing
agents. Garnier et al disclosed the use of poly alkanol amines, mono-
propolyeneglycol and similar compounds, which may be used in the, present
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
24
invention. However, in experiments with the present invention it has been
found
that zinc stearate and hexamethyldisilazane are more effective fluidizing
agents.
Triethanolamine may also be used. For additional examples, see Kopatz and
Pruyne in U.S. Patent No. 4,715,878, which describes additional anionic,
cationic and nonionic treatments which can be used in the present invention.
Any other effective surfactant or other fluidizing agent may be used.
Typically, the fluidizing agents employed in the present invention are those
which are effective to substantially inhibit clumping when used in amounts of
up
to about 1%, more preferably up to about 0.5 % and still more preferably up to
about 0.3 % by weight based on the weight of the feed material treated
therewith.
However, smaller feed particles, for example those less than 5 microns, will
have
a much larger aggregate surface than the same weight of larger particles, for
example 15 micron particles. Thus, for the very finest particles and/or for
those
particles which tend to clump stubbornly, larger amounts of fluidizing agent
may
be necessary.
The fluidizing agents may be applied to the feed material particles, and
preferably to the entire mass of solid feed particles in any effective manner,
including the time honored technique of milling the feed material or the
entire
mass of solid feed in contact with the fluidizing agent. It is recommended
that
the fluidizing agent be added to the feed material, and preferably to the
entire
quantity of solid feed particles to be used, during ball mill grinding,
preferably
as several additions during the grinding process. Such additions can be made
as
part of a fmal size reduction step in the preparation of the particles.
Intimate
dispersion over the particle surfaces has for instance been achieved by ball-
milling the particles for about one hour with about 0.5 % by weight of
surfactant,
based on the total weight of particles. However, where the particles are
already
at their desired particle size, the fluidizing agent can be applied by merely
agitating the agent and the particles together in a suitable chamber or zone.
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
Appropriate dispersion forces, which includes where necessary sufficient
force to deagglomerate agglomerated particles, may be applied in any effective
manner. An appropriate example, involving a gas jet and venturi, is described
below. In general, one may employ any form of force, generated in any
5 equipment which is effective to break apart clumps of feed material, other
solid
feed particles or both. Thus, for example, one may employ methods and
apparatus that subject clumped particles to impact with one another, or with
relatively high energy gas streams and/or with solid objects, moving or
stationary.
10 Thus, for example, the particles may be forcibly projected against a
stationary surface such as a wall or target, or may be passed through the
blades
of a fan, including a turbine, to provide collisions and ensuing impact
between
the particles and fan blades. Disc mills, jet mills and hammermills are other
examples of devices that may be suitably adapted to provide sufficient impact
and
15 shear for particle dispersion, including deagglomeration. Impact includes
direct
frontal impacts and skipping contacts, such as those which apply shear and/or
rotational forces to clumps.
In some cases, depending on the properties of the particles and the manner
in which they are handled and transported downstream of the dispersing
20 operation, use of a fluidizing agent alone or use of dispersing force alone
may
be sufficient to adequately disperse the feed. However, when practicing
certain
embod'unents of the invention, the amount of dispersion effort applied in some
prior dispersion operations may be inadequate. Thus, when practicing the
invention with solid feed particles having stubborn clumping tendencies, or
with
25 downstream processing equipment that does not minimize exertion of
compaction
forces on dispersed feed particles, or with high throughput levels described
below, unprecedented levels of dispersion effort may be required. Thus, it may
be necessary to apply fluidizing agent at levels heretofore considered
unnecessary
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
26
or even undesirable and/or to apply dispersion force and energy at levels
beyond
those formerly considered necessary.
In order to determine whether the amount of fluidizing agent and/or force
applied to the feed particles has been sufficient to deagglomerate and
disperse
them, the following tests may be used. The tests involve collecting a first
sample
of feed particles upstream of the dispersion operation and measuring by laser
diffraction methods the particle size of that sample after ultrasonic
agitation in
water or alcohol and a dispersant. Such agitation is sufficient to allow
determination of a deagglomerated size distribution for comparison. This
measurement establishes what is referred to as the "primary" size
distribution.
A second sample dilutely dispersed on a flat surface is collected at any
suitable point in the process downstream of the dispersion operation. For
example, a glass plate may be prepared, having a thin film of "tacky"
substance
on its surface. One may then rapidly pass the glass plate through a stream
diverted from a duct receiving particles from the dispersion operation, such
as
a duct conveying feed particles to a burner. This sample will be measured to
establish a "secondary" size distribution, for example by conventional
microscopy
methods, to determine the presence of agglomerates.
The difference in indicated particle size observed in the primary and
secondary samples is indicative of the extent to which the dispersing
treatment
has been effective. Relatively small differences indicate effective dispersion
treatment, while large differences indicate less effective dispersion
treatment.
This difference at the 90th percentile is preferably in the range of up to
about
20%, and more preferably in the range of up to about 10%, on a weight or
volume basis, based on the primary distribution. That is, for feed with a
primary
distribution of 90% less than 50 microns, the dispersed sample will have 90
percent less than 60 microns or preferably less than 55 microns.
CA 02236447 1998-04-30
WO 97/16385 PCTIUS96/16967
27
An alternative procedure may be used. Samples of solid particles may be
collected upstream of the dispersion step and measured while dispersed in
water
or alcohol as before. Downstream of the dispersion step a sample may be
diverted and air conveyed through a laser detection device and the dispersed
size
distribution compared as in the previous paragraph. The laser diffraction
techniques have the advantage that they may be applied on a "real-time" basis
to
side streams diverted from the main stream of dispersed and undispersed feed
particles upstream and downstream of the dispersing operation.
As still another alternative, the effectiveness of the dispersing step can be
determined by comparing the primary size distribution of the feed particles
when
dispersed in water or alcohol with the size distribution of the flame fused
generally ellipsoidal particles, also when dispersed in water or alcohol.
Suitable
corrections must be made for the presence of voids when present in the product
particle. If both fluidizing agent and force are applied to disperse the
particles, it may prove most convenient to apply the fluidizing agent first
and
then follow with application of force. However, operation in the reverse order
is also possible. Nevertheless, since the ultimate goal is to disperse the
feed
particles in a carrier gas, starting with application of fluidizing agent is
preferred,
since this operation may then be immediately followed by application of force
to
the feed particles in the presence of the desired carrier gas.
The carrier gas may or may not be a combustion supporting gas. It may
in fact be an inert gas, but in that case the amount used must be regulated
carefully. It is particularly preferred that the carrier gas be a combustion
supporting gas, which includes one or both of the components required for
combustion, including fuel and/or oxygen containing gas.
Among the appropriate fuel gases are hydrogen, methane, ethane,
propane, butane, and other gases, including vapors of heavier hydrocarbon
fuels
and/or carbonaceous gases such as carbon monoxide. The heavier hydrocarbon
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
28
fuels include those that are liquids or semi-solids at ambient conditions (20
C.
and atmospheric pressure) but that can exist substantially in vapor form at
the
conditions under which they are mixed with feed particles. Preferably, the
hydrocarbon fuels are those that are gases at ambient conditions, including
for
example acetylene and particularly those hydrocarbon fuels in which the
hydrogen to carbon mole ratio is about 2.5 or more. This includes for example
butane, propane, ethane and methane, e.g. in the form of natural gas.
As oxygen-containing gas one may use substantially pure oxygen, oxygen
enriched air or unenriched air as drawn from the atmosphere, it being an
advantage of the invention that suitable oxygen-containing gases may be used
that
have nitrogen contents in the range of about 50 to about 80 mole percent, the
balance being primarily oxygen.
The combustion supporting gases are preferably substantially free of
sources of cinders, including ash and carbon particles. However, the presence
of very fme, clean burning, particles of carbon and solid carbonaceous fuels
is
acceptable.
Preheating of the fuel, air, oxygen enriched air and feed particles
generally increases productivity and decreases the time of contact between the
feed particles and the combustion gases required to at least partially fuse
the
particles. Preheating of the feed particles can also assist in "conditioning"
the
materials by removing surface moisture or electrostatic charges and thereby
provide improved dispersion into the combustion gases.
Either the fuel or the oxygen containing gas may be described as "at least
one portion of a combustible gas mixture." It should be understood that the
foregoing expression includes either of these components, the ultimate
combustion gas mixture that will be burned later in the process and/or any
other
gas that may acceptably included in the gas mixture fed to the burner which
generates heat in the combustion zone. Thus, the combustible mixture may be
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
29
formed prior to or after dispersion of the feed particles, and dispersion of
feed
particles in a portion of the gas mixture is intended to generically include
dispersion in the entire mixture to be fed to the burner or in any portion
thereof,
provided dispersion can be maintained if such portion is subsequently mixed
with
the remaining components of the ultimate combustible mixture.
In certain instances, the feed particles will be dispersed in only a portion
of the combustible mixture and transported with that portion to the burner,
where
mixing with the remaining parts of the combustible mixture will take place. In
other circumstances, the entire gaseous (including vaporous) components of the
combustible mixture may be formed upstream of the dispersion location, in
which
event the dispersion operation will disperse the feed particles in the entire
combustible mixture. A variety of intermediate options are possible in which
the
feed particles are first dispersed with portions of any part of the
combustible
mixture, followed by admixture with the remaining part or parts of the
combustible mixture at or prior to the flame front in the burner. However, in
carrying out the processes of the invention it is definitely preferred that
the entire
combustible mixture be formed, and that the entire amount of suspended feed
particles be fully dispersed in that mixture, upstream of, and less preferably
as
it enters, the flame front.
The flame front is an imaginary "surface" or "surfaces" in which the
mixture is ignited. The shape and number of the surface(s) will be dependent
upon the shape and design of the burner that is utilized.
The possibility of performing all or at least the last stages of dispersion
and the burning of the mixture in a burner, i.e. in one and the same
apparatus,
is contemplated. However, in most applications, it is anticipated that the
dispersion apparatus and the flame front in the burner will be separated from
one
another by some distance, and that the suspended feed particles must therefore
be transported between these two locations. Where it is necessary to transport
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
dispersed feed particles from a dispersion location to the flame front,
considerable care must be exercised.
Feed particles that have been well-dispersed in the dispersion operation
can be re-agglomerated during transport. Transport through long ducts, or
ducts
5 with sufficient aggregate curvature in the form of elbows, coils and the
like, can
produce concentration of particles in a portion of the cross-section of the
conduit
system, for example, through centrifugal or gravitational forces. These forces
and the ensuing concentration of particles represent compaction forces which
can
agglomerate or re-agglomerate dispersed particles. Thus, the best practice
will
10 be to restrict the overall distance between the dispersion location and the
flame
front and to maintain flow velocities of at least about 5, and preferably at
least
about 20, meters per second.
Where the suspension must traverse ducts or conduits from a dispersion
location to the flame front, the conduit system should be designed to minimize
15 compaction forces. This goal is normally best attained by arrangements
which
minimize exertion of centrifugal and/or gravitational forces of the kind that
would tend to concentrate the flow of particles in a portion of the cross-
section
of the conduits. Thus, for best results it is believed that one should tend to
avoid
conduit arrangements with short radius curves or sharp corners and to avoid
long
20 horizontal runs of piping. Rather, relatively straight runs of vertical
piping
are preferred, and if bends are necessary, it is preferred to employ long
smooth
curves, especially those with radii equalling 5 or more and preferably 10 or
more
times the cross-sectional or diametral dimension of the conduit or duct. A
particularly preferred arrangement is illustrated in the drawings and
following
25 text of this application. In that arrangement, the suspension is fed to a
burner
from above, through quite short transport tubes, there being acute angles
between
any merging tubes, and the transport tubes and burner are oriented vertically,
or
at least substantially vertically (within about 20 of vertical). The burner
has a
CA 02236447 2006-05-23
60557-5805
31
generally axial flow pattern with an at least substantially vertically
oriented throat
and substantially horizontal outlet. Thus, the suspension can pass essentially
straight through the burner without any major change of direction and
preferably
with minimal or no change in direction except for such directional changes as
may be attendant to lateral spreading of the gas stream as it moves from the
vertical supply pipe to the burner outlet with components of motion that are
primarily axial and radial.
A specific embodiment of an appropriate burner is described below and
in the accompanying drawings. However, a variety of burners can be used to
ignite the combustible gas mixture containing entrained feed particles.
Examples
may be found in North American Combustion Handbook, edited by Richard J.
Reed, 2d Ed., North American Manufacturing Company, Cleveland, Ohio,
U.S.A., 1978.
See also Soviet Union Patents Nos. 1,654,272 and 1,654,273 to Nosach, et al,
both assigned to As UKR Thermo-Phys. Stekloplastik Prodn. Assoc. Persons
skilled in the art, with the benefit of the present disclosure, will select or
adapt
such burners as necessary to facilitate their acceptance and transmission of
combustible gas mixtures containing entrained feed particles, adjusting the
sizes
of passages and orifices as required to keep such particles in a dispersed
condition and avoid clogging of the burner.
Still other forms of burners may be employed. However, the preferred
burners are those which do not subject particles to compaction forces and tend
to re-agglomerate them. Moreover, the preferred burners are consistent with
the
suspension of feed particles in the combustible mixture being formed upstream
of the flame front and with delivery of the particles to the flame front with
the
dispersion distributed uniformly across and passing through the flame front in
a
very uniform manner, rather than being projected into a zone downstream of the
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
32
flame front or into the side or the center of the flame, as has been done in a
number of prior art processes.
Observance of these precautions is particularly important in practicing
some aspects of the invention. which involve unusually high concentrations of
feed particles in the combustible mixture fed to the flame front. More
particularly, the concentration of feed particles in the combustible gas
mixture
may for example be at least about 0.05 (0.05) or preferably at least about 0.1
(0.1) and still more preferably at least about 0.2 (.2) lbs. (kilograms) per
lb. (per
kilogram) of gases in the mixture. Concentrations of up to about 1 or up to
about 1.5 or up to about 2 (2) lbs. (kilograms) per lb. (per kilogram) of
gases in
the mixture are contemplated.
Given the small particle size of the feed particles employed in the
invention, such concentrations were high enough to create an expectation that
the
aggregate surface area of such small particles employed in such large
concentrations would quench the flame emanating from the burner. Such
concerns were particularly great in relation to operating at a restricted
level of
heat utilization, in accordance with one aspect of the invention described
below.
Contrary to expectations, it is possible to operate successfully at these high
concentrations, without quenching of flame and without unwanted agglomeration
of the particles with each other and with the chamber walls, as the particles
soften or partially melt and reshape themselves in a chamber downstream of the
burner.
In accordance with at least one aspect of the invention, the flame front and
at least a substantial portion of the resultant flame are maintained in a wall
free
zone which extends downstream of the front, while maintaining the suspended
feed particles in a dispersed condition in said zone. The nature and
significance
of a wall free zone and a schematic specific example thereof are described in
the
following text and the accompanying drawings. The wall free zone shape and
CA 02236447 1998-04-30
WO 97/16385 PCTIUS96/16967
33
dimensions are such as to substantially inhibit molten particle contact with
surfaces of the burner, zone and conveying devices, preferably limiting such
contact to negligible amounts.
Several features of the present method are performed in the wall free
zone. The dispersed feed particles are heated with heat transferred thereto by
burning of the combustible mixture. There is at least partial fusion of the
irregularly shaped particles within at least their surfaces. The burning gases
are
caused to expand and the feed particles are caused to fuse to produce at least
partially fused bulk particulate product containing generally ellipsoidal
discrete
product particles.
Preferably, there is expansion of the burning gases and fusion of the feed
sufficient so that there will be at least about 15 %, or preferably at least
about
30% by volume of discrete, generally elliptical particles produced. Preferred
embodiments produce up to about 90% and more preferably up to about 99% of
discrete, generally elliptical particles. Expansion of the combusting gas
stream
tends to keep the particles separated from one another while they are in a
softened or semi-molten or fully molten condition, reducing opportunities for
particle collision and agglomeration. Agglomeration reduces the population of
discrete particles and causes the resultant agglomerated particles to have a
larger
particle size than the feed particles.
Sufficient amounts of expansion and a sufficient ratio of feed particles to
heat released can be established and monitored using the following test. The
excess, if any, of indicated particle size of the product particles, relative
to the
indicated particle size of the feed particles, is determined. This
determination
is based, in the case of the feed particles, on the condition of those
particles prior
to application of the fluidizing agent and/or dispersing force. Each of the
CA 02236447 1998-04-30
WO 97/16385 PCTIUS96/16967
34
particle size indications is based on a sample subjected to agitation, as
above-
described, in water, alcohol or other suitable liquid.
The indications of particle size may be obtained in any appropriate
manner, such as by laser diffraction techniques or by analyzing photographs of
scanning electron microscope images of the samples. Preferably, the excess, if
any, of the indicated 90th percentile particle size of a sample of the
product,
minus the 90th percentile primary particle size of a sample of the feed from
which the product has been prepared, is in the range of up to about 30%, more
preferably up to about 20% and still more preferably up to about 10% of the
primary particle size, on a weight basis or, after suitable corrections are
made
for any voids which may be present in the product particles, on a volume
basis.
The correction for voids is made because voids make the product appear
larger than it would in the absence of voids and thus misleadingly suggest
there
has been agglomeration which did not in fact occur. This correction can be
made by use of particle specific gravity measurements and optical methods.
According to one aspect of the invention, the total amount of heat utilized
is in the range of about 500 (278) to about 25,000 (13,889) B.T.U.s
(kilocalories) per pound (kilogram) of generally ellipsoidal particles
produced.
This total amount of heat includes heat utilized for heating, and for
preheating
if any, of the particles, of other combustible mixture components and of the
combustible mixture itself. The total also includes heat utilized in expansion
and
for heat losses, including such heat losses as may be attendant to the use of
cooling gas, where applicable. When controlling operation of the methods of
the
invention generally, and particularly when practicing those embodiments in
which
the feed material involves larger amounts of volatile material, for example
more
than about 7, more than about 10 or more than about 25 percent by weight, it
is
preferred to exclude from the calculation of the above heat ratio that heat
which
is consumed in removing the volatiles from the feed. For example, when
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
operating with aluminum hydroxide, about 540 (300) or more B.T.U.s
(kilocalories) per pound (kilogram) of feed are excluded from the calculation
of
the ratio, this being the amount of heat required to remove and volatilize the
combined water of hydration.
5 Heat utilization is lower for easily fused particles such as perlite, and
higher for difficultly fused particles such as silica. Feed materials can be
rated
by Penfield's "Material Fusibility Index" from 1 to 7. Using the present
invention, and using this index as a discriminator for materials of differing
resistance to fusion, it will be found that the claimed methods can provide
10 products with a heat consumption, in B.T.U.s per pound of product, of less
than
about 7,000 +(3000 X (Material Fusibility Index) / 7) or preferably less than
about 5,000 +(3000 X (Material Fusibility Index) /7), which is equivalent, in
Kcal/Kgram, to 3,889 + (1667 X (Material Fusibility Index) / 7) and preferably
2,778 + (1191 X (Material Fusibility Index) / 7). Note: BTU/lb x .55556 =
15 Kcal/Kgram. When practicing the invention, and particularly when practicing
those embodiments in which the feed material involves larger amounts of
volatile
material, for example more than about 7, more than about 10 or more than about
25 percent by weight, the preferred operating conditions are those which
provide
the above-described heat consumption when that heat which is consumed in
20 removing the volatiles from the feed is added to the estimated heat
consumption
per pound (kilogram) of product.
Differences between the melting or softening temperatures of different feed
materials and the extent of conversion of feed to generally ellipsoidal
particles
will require suitable adjustment of feed rate and/or heat input. An
appropriate
25 balance between feed particle size, melting or softening point and feed
rate on
the one hand and combustible gas composition and flow rate on the other, will
be readily established by persons skilled in the art with the aid of this
disclosure
and without undue experimentation.
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
36
It is preferred that the particles be cooled rapidly after fusion has
progressed to the desired extent. For example, a cooling rate in excess of
about
1000, more preferably in excess of about 200 or still more preferably in
excess
of about 300 per second is preferred. Radiant and convective cooling of the
particles is preferably assisted by cooling air brought into contact with the
fused
particles with a minimum of turbulence. This minimizes the potential for
accretions resulting from collisions of still-molten or still-soft particles
with one
another or with surfaces of the production apparatus.
The entire fusion operation may be performed in one step, with at least
partial conversion of irregularly shaped crystalline feed particles to
generally
ellipsoidal form. Thus, for example, about 15 to 100%, more preferably about
50 to 100 % and still more preferably about 75 to 100 % by volume of the
solids
content of the compositions of the invention will be in the form of generally
ellipsoidal particles. For certain applications in which it is important to
minimize
the quantity of irregularly shaped particles found in the product, the
percentage
of generally ellipsoidal particles may be in the range of about 90 to 100%
based
on the solids content of the compositions.
When solid feed particles that have been previously melted are used, for
example ground glass of any type, the present methods partake to that limited
extent of prior flame polishing technology. However, the preferred mode for
using the invention is as a direct fusion method, in which the feed material,
or
at least a substantial portion thereof, has not previously existed as a bulk
melt.
Thus, the term direct fusion is used to refer to methods by which irregularly
shaped solid feed particles composed substantially of one or more specified
materials may be dispersed, heated and melted or softened sufficiently to
convert
them, while maintaining them dispersed and suspended in hot gases and under
the influence of surface tension, to generally ellipsoidal particles. This
method
of formation makes powders in which the constituent particles may have
particle-
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
37
to-particle variations in chemical composition, and in some cases residual
crystallinity, of a kind not found in particles made by indirect methods.
While the particles may be pre-heated in any suitable manner, in the
fusion step, heat is transferred to the feed particles through contact with
flaming
combustion gases in which the particles are dispersed. More particularly, the
present method involves premixing and entraining feed particles in flowing
combustible gases and heating them to fusion temperature by igniting the gases
in the presence of the particles and maintaining the particles in a dispersed
state
in the flaming gases and possibly also for some distance downstream of the
flame.
During their residence in the flame, and possibly during continued contact
with the hot combustion gases outside the flame, the particles are maintained
for
a time at a temperature sufficient to soften or melt them to the extent that
surface
tension within the resultant fused or partially fused particles or droplets is
sufficient to convert appreciable amounts of the feed particles to generally
ellipsoidal form. The flow of particles as they progress from their original
un-
fused state to an at least partially fused state may be in any appropriate
direction
or directions, including for example horizontal and/or vertical, with vertical
down-flow being preferred.
When operating in the above manner, it is possible to obtain partially
fused bulk particulate products in which the average particle size, on a
volume
basis, is up to about 25, up to about 20, up to about 15 or up to about 10
microns, or the 90th percentile is up to about 60, up to about 40, up to about
30
or up to about 25 microns, also on a volume basis. Obtaining products of still
smaller particle size can be very valuable. The invention makes it possible to
obtain glassy rhyolite, silica based and silicate based products with average
particle sizes, on a volume basis, of up to about 8, or preferably, up to
about 6
microns.
CA 02236447 1998-04-30
WO 97/16385 PCTIUS96/16967
38
Possibly depending at least in part on whether the feed particles contain
sufficient volatile material, operation with high concentration of feed in the
combustible gas mixture and with efficient control of heat utilization, both
of
which have been described above, tend to produce one or more useful effects on
the products. These may include development of void volume and/or
compositional phases of low specific gravity within the product particles
and/or
the presence of some irregular feed particles among the product particles, or
a
combination of two or more of these effects. In these embodiments of the
invention, the specific gravity of the products is typically lower than the
specific
gravity of the corresponding composition as reported in the technical
literature
such as Lanize's Handbook of ChemistrY.
The reductions in specific gravity may partly be the result of the presence
of some hollow particles with trapped voids in the products or may be the
result
of a phenomenon related to the loss of crystallinity and conversion to a lower
density "glassy" phase. Whatever their explanation, these reductions provide
advantages in manufacturing and in application of the resultant powders. The
increased volume and lower densities are generally preferred characteristics
of
the products, and even small volume increases can, over large production runs,
provide equivalent volumes of saleable products with considerable fuel
savings.
Thus, the specific gravities of the generally ellipsoidal products of the
invention
may be lower, in the range of about 1 to 15 %, and, more beneficially, about 1
to about 10% lower, than the specific gravities of the feed materials.
In the alternative, and possibly preferably, the reduction in specific gravity
may be measured by a "before and after" test that is performed on a product
sample. This test compares the specific gravity of the solid products, as
recovered from the production process, to the specific gravity exhibited by
those
products after further, post-production, melting and solidification. After
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
39
measuring the specific gravity of a sample of the recovered product, the
sample
is re-heated, for example in a crucible, for a time and at a temperature
sufficient
for causing the products to melt, lose void content (if present), develop
crystallinity (if possible), or otherwise convert to a dense phase and thereby
provide a composition that, on cooling and solidification, has an increased
specific gravity, which is measured. Any observed change in specific gravity
is
subject to adjustment to offset any portion of the observed change that is
attributable to differences, if any, in the temperature(s) of the test sample
at the
time of the "before and after" specific gravity measurements. The specific
gravity of the re-heated portion of the sample will generally be in agreement
with
the values reported in the technical literature for corresponding substances
of the
same composition. In general, the specific gravities of the as-recovered
generally
ellipsoidal products of the invention may be lower, for example at least about
1%, or at least about 5%, or at least about 10 % or at least about 15 % lower,
than the specific gravity exhibited by the products after further melting and
solidification. Moreover, the lower specific gravities of the as-recovered
products may for example range up to about 10% lower, or preferably up to
about 15 % lower, or more preferably up to about 25% lower, or still more
preferably up to about 50% lower, than the increased specific gravity
exhibited
by the products after melting and solidification.
Controlling the amount of heat energy released in the flame by adjusting
or maintaining fuel, air mixture and feed material quantity or other process
materials or conditions in such a way as to maintain some detectable voids in
the
product over and above those that are inevitably produced, such as in amounts
as low as about 1 to about 3 percent, or about 1 to about 2 percent, leads to,
and
can be used as an indicator of, efficient use of combustion energy. Thus, it
is
preferred that process conditions be controlled to produce in the partially
fused
bulk particulate product at least about 1 % of void volume, based on the
volume
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
of the product. However, the quantity of void volume in the product may be at
least about 3 or at least about 5 percent. On the other hand the void volume
may
be up to about 12 or up to about 15 or up to about 20 percent. For example,
void volumes in the range of about 1 to about 15 percent or about 1 to about
10
5 percent by weight are contemplated.
The above-indicated high feed concentration and controlled heat utilization
may cause some irregular feed particles to be present in the products, whether
significant quantities of voids are generated or not. This mode of operation
is
also advantageous from the standpoint of energy utilization, and may for
example
10 involve production of products containing up to about 99%, more preferably
up
to about 95% and even more preferably up to about 90% by volume of fused
generally ellipsoidal discrete particles that are substantially glassy, with
corresponding amounts of irregular product particles being present.
It is preferred that, in the compositions of matter according to the
15 invention, the carbon content of the solid particles should be restricted.
Other
than carbon present in the form of organic material applied to the surfaces of
the
solid particles, it is preferred that the carbon content be limited to up to
about
0.2%, more preferably up to about 0.15% or still more preferably up to about
0.1 % by weight, based on the total weight of the solid particles.
20 Preferred products according to the invention have little or essentially no
hematite, emery, magnetite, or other highly colored iron-containing minerals.
They may for example contain up to about 0.2, more preferably up to about 0.1
and still more preferably up to about 0.05% by weight of Fe203 and/or Fe3 4.
Similar limits apply to Manganese, e.g. MnO, and to those other metals whose
25 oxides or other compounds tend to color the products. In the case of
ferrous iron
oxide, FeO, which is not so strongly colored, the preferred products may
contain
up to about 5 %, more preferably up to about 2 % and still more preferably up
to
about 1 % by weight.
CA 02236447 1998-04-30
WO 97/16385 PCTIUS96/16967
41
When practicing the invention with exercise of control over the kinds and
amounts of carbon in the fuels and the kinds and amounts of carbon and other
colorants in the feed materials, one can produce solid particle products
having
brightness levels that make the products particularly suitable for various end
uses, certain of which are described below. For example, products with
brightness levels of at least about 60 and preferably at least about 80 are
contemplated.
Products of the invention may be characterized by having chemical
compositions corresponding substantially with that of one or more feed
materials,
including mixtures thereof. The terminology "corresponding substantially with"
is intended to embrace chemical compositions similar to those which would
result
from at least partial fusion of feed material composed substantially of at
least one
of the materials. However, the words corresponding substantially with have
been
chosen to embrace the possibilities that different production techniques can
be
employed and that there can be differences between the chemical compositions
of the feed materials and those of the resultant products. For example,
differences between feed material and product chemical compositions can result
from departure of the loss on ignition materials and of varying amounts of
other
portions of the minerals as a result of high temperature volatilization, such
other
portions usually being in the range of up to about 5 % by weight of the feed
material.
When the feed materials or other portions of the solid feed particles
include crystalline matter, the process of at least partial fusion destroys at
least
a portion of their crystalline character. The mechanism by which this occurs
has
not been proven, but it is theorized that at least portions of the respective
particles are raised to temperatures above the dissolution temperature of the
crystalline material. At least a portion and usually the major portion of the
crystalline structure in the respective particles will be destroyed.
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
42
It should be understood that the resultant particles, although having
reduced crystallinity, may not in every instance be properly described as
fully
amorphous. For this reason, the particulate product is referred to herein as
"substantially glassy. " This terminology is intended to include the
possibility that
generally ellipsoidal product particles may contain some but not all of their
original crystallinity, while having been converted to a form with a generally
ellipsoidal surface that resembles glass in terms of its smoothness, at least
the
surface portions of the product particles being amorphous in nature.
There is however no reason in principle why the crystal content of the
generally ellipsoidal particles produced from the crystalline feed materials
should
not be reduced to a major extent. Thus, in these particles, it is contemplated
and
possibly also even preferred, that most if not all of the crystalline
structure
originally present in these particles should be destroyed during the fusion
operation.
It is of course also contemplated that products according to the invention,
containing generally ellipsoidal substantially glassy particles respectively
including particles of particular chemical compositions may also contain
particles
of the same or other compositions that are or are not of a substantially
glassy
nature. Such particles that are not of a substantially glassy nature, having
passed
through a fusion zone, may or may not have undergone fusion, and in the latter
case may retain most if not all of any original crystallinity and/or surface
roughness which they may have originally possessed. Those fusion products that
contain both significant amounts of crystallinity and of substantially glassy
particles may be referred to as "crysto-morphic. "
The crystallinity of products produced according to the invention may be
tested "in gross," meaning that X-ray diffraction can be used to measure the
crystallinity of samples containing both fused and essentially un-fused
particles
without measuring the quantum of crystallinity present in the two different
kinds
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
43
of products. Crystallinity that is so measured may be expressed in terms of a
weight percentage, based on the total weight of the sample. Based on this mode
of measuring, products containing up to about 90%, more preferably about 0.1
to about 75 % and still more preferably about 5 to about 60 % of crystallinity
are
contemplated. In some circumstances, nearly complete conversion to generally
ellipsoidal products may occur in combination with surprisingly high residual
levels, e.g. 20%, of crystallinity. Understandably, products produced from non-
crystalline feed materials remain essentially amorphous or glassy.
A preferred form of apparatus which has been employed to produce the
products of the present invention using the method of the present invention,
and
which has also been used to conduct the examples set forth below, will now be
described with the aid of the drawings. It should be understood however that
such apparatus disclosure is illustrative only, and that the invention is not
intended to be limited by or to the particular apparatus described.
The illustrative equipment shown in Figures 1 and 2 includes separate
sources 1 and 2 for oxygen-containing gas and fuel, which may or may not
include facilities for pre-heating of the oxygen-containing gas and/or fuel.
Thus,
for example, filtered oxygen-containing gas is conducted from its source 1
through a suitable compressor or blower (not shown), valving (not shown) and
flow measuring equipment (not shown) into oxygen-containing gas pipe 3 to
provide an adjustable, stable flow of such oxygen-containing gas. Fuel gas,
after
passing from its source 2 through its own independent valving (not shown),
flow
measuring device (not shown) and delivery pipe 4 is adjustably drawn by
aspiration and at a stable rate of flow into pipe 3 at junction 5. There, if
needed
or desired, a flow-control orifice is provided to properly match the volume of
the
fuel to the usually larger volume of oxygen-containing gas. For example where
the oxygen-containing gas is air and the fuel is natural gas, a volume ratio
of
about 10:1 may be employed.
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
44
Pre-mixing of the resulting combustion-supporting gas mixture with feed
material prior to igniting the fuel may be performed in a Y 6, a generally "Y"-
shaped mixing connection having upper intersecting gas and feed entry legs 7
and
8 which join and feed together into a lower exit leg 9. Gas entry leg 7 is a
vertically oriented extension of oxygen-containing gas pipe 3. Feed entry leg
8
also extends upwardly but is inclined from the vertical, intersecting at an
acute
angle, for example about 10-45 , with gas entry leg 7.
A uniform rate of flow of feed into feed entry leg 8 is effected by feeding
the feed under moderate humidity and temperature, e.g. at room temperature,
from a vibrating discharge funnel 13 onto a vibratory conveyor 14 and from
that
conveyor into inlet 15 of the feed entry leg. Loss-in-weight screw feeders
with
mechanically stirred hoppers and vibration assisted discharge are useful for
feeding very fine powders. Supply pipe 16 provides a supply of dispersion gas
such as air, which may thus represent a small portion of the combustion-
supporting gas to be burned. As shown in greater detail in Figure 2, which is
an enlarged, partial cross section of Figure 1, dispersion gas discharged from
supply pipe 16 passes through jet nozzle 17 into feed entry leg 8 to aspirate
feed
from inlet 15 into leg 8 and through venturi 18 to assist in dispersion of the
feed
particles. Particles of feed, pre-dispersed in dispersion gas, are delivered
through chamfered end 19 of feed entry leg 8 into the intersection of Y 6,
where
they are then mixed with and further dispersed in the combustion gases passing
downward mechanically through gas entry leg 7.
Dispersal of the feed in the combustion gases can be achieved and
enhanced by selection of the ratio of gas to feed mixed in the Y and the
volume
rate of gas flow per unit of cross-section of the gas tube provided by the
continuation of gas entry leg 7 into exit leg 9 of Y 6. In experiments
conducted
in the apparatus described herein, ratios in the range of about 0.9 to about 9
pounds of feed per 1000 ft.3 (cubic feet at 15 C) of fuel-air mixture were
used.
CA 02236447 1998-04-30
WO 97/16385 PCTIUS96/16967
The combustible gas through-put was for example 400 ft.3/hour through a gas
tube having an area of about one square inch. Persons skilled in the art will
appreciate that the ranges of ratios and velocities that will work in other
types of
equipment, and the ranges that will work to best advantage in such other
5 equipment, may vary from the values just given and can be found through
tests
which such persons can readily conduct with the aid of this disclosure and
without undue experimentation. It is particularly important to maximize the
amount of combustion-supporting gases injected into the feed particles by high-
energy jets to accelerate the feed particles and break up agglomerates by
shear
10 and impact. For adequate dispersal of fine powders at high rates, the
particles
and combustion air can be dispersed by passing them through a hammermill, disc
mill or other deagglomerating device, functioning as the sole dispersion means
or as an upstream pre-treatment for feed particles that will also be dispersed
with
the aid of fluidizing agent and/or the disclosed air jet.
15 In the present preferred embodiment, as may be seen in Figure 1, the
burner 20 is a downwardly discharging "stick-tight" gas burner having a 1.75
inch diameter flame-retaining nozzle 22 adapted so that the internal pilot is
fed
a separate air and gas stream free of feed particles. Such a burner is
described
at page 431 of the above-mentioned Reed work. In the present embodiment, this
20 burner has at its top a common inlet 21 for the particle and combustion-
supporting gas mixture, received from exit leg 9 of Y 6.
Nozzle 22 of burner 20 penetrates the upper, horizontal wall 26 of a
combustion chamber 27. An annular opening in wall 26 surrounding the outer,
= peripheral surface of nozzle 22 represents an inlet port 28 for cooling air.
A
25 short distance below this port, at the bottom of nozzle 22, is a generally
horizontal burner mouth 29 for the discharge of combustible gas and entrained
feed into combustion chamber 27. Combustion occurs as the particle-combustible
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
46
gas mixture exits burner mouth 29 and continues downward in combustion
chamber 27.
While it is possible to widely vary the internal cross-sections of the
above-mentioned gas channel in the Y and of the burner, a certain balance
between these dimensions should be maintained. The objective to be satisfied
in
selection of these dimensions is keeping feed particles dispersed in the
resulting
flame, while keeping sufficient velocity of flow through burner mouth 29,
given
the available volume rate of the gas and feed, to discourage or effectively
bar
"back-fire," retreat of the flame into the interior of burner 20. As those
skilled
in the art will appreciate, a variety of other burner designs are available
which
can accomplish these objectives.
It is believed beneficial to generate the flame from the burner in a "wall-
free" environment. By this it is meant that the side walls 32 of combustion
chamber 27 are positioned at a predetermined distance laterally or
transversely
from the path of the flame emanating from burner mouth 29. There should be
a sufficient distance laterally or transversely from the perimeter of the
flame to
the walls 32 to afford the flame a substantial amount of freedom to expand in
the
lateral or transverse direction. Alternatively, this distance should be
sufficient
to substantially inhibit or substantially prevent molten or soft and still un-
solidified particles that have been at least partially fused in the flame from
contacting the side walls 32 and adhering thereto. Preferably, the distance
should be sufficient both to afford the freedom to expand and to inhibit the
adherence of particles, as above described.
In the present burner embodiment, burner mouth 29 is located on the
extended axis 33 of the burner and projects a flame along that axis, generally
in
the direction in which the axis extends. Thus, in this case, the side walls 32
are
positioned at a predetermined lateral or transverse distance from that axis,
to
provide the freedom and/or inhibition described above. The side walls 32 may
CA 02236447 1998-04-30
WO 97/16385 PCTIUS96/16967
47
be of any suitable configuration, but are cylindrical in the present
embodiment,
as viewed in a plane perpendicular to axis 33, and have a diameter of about 3
feet.
Prior art suggests introducing cooling gas to the combustion area,
perpendicular to the path of the flame and presumably a short distance
downstream from the burner. According to those teachings, the flame disappears
where it contacts the cooling gas, and the technique could thus be used to
control
the amount of time during which feed particles are held at fusion temperature.
That system may optionally be used with the present invention. However, the
present invention also provides and preferably employs a different and
advantageous cooling technique, as described below.
In connection with the present invention it has been found that assistance
in isolating molten or soft particles from the combustion chamber side walls
32,
and in some cases from the upper wall 26, can be obtained from a current of
cooling gas, such as air introduced through the above-mentioned port 28. This
current may for example, and preferably is, caused to pass gently in co-
current
flow along the side of the flame between the flame and one or more of such
walls. The term gently, as used herein, signifies that the direction and/or
rate
of flow of the cooling gas is co-current with the flame and allows lateral
expansion of the combustion gases. This co-current flow occurs at least along
an appreciable portion of the length of the zone in which flame is present in
the
hot combustion gases, and possibly also for an appreciable distance downstream
of that zone.
It is recommended that the cooling gas direction be established or
controlled in a way such that the hot combustion gases can continue to expand
laterally and the cooling gas can flow co-currently downstream for an
appreciable
distance with such gases, during which the combustion gases may continue to
expand laterally. In aid of this goal it is recommended that the cooling gas
linear
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
48
flow rate be controlled or sufficiently limited to substantially inhibit or
substantially prevent the cooling gas flow from generating turbulent flow at
the
central axis, or in the core, of the adjacent hot combustion gases.
It should be understood however that the mere presence of cooling gas
adjacent the hot combustion gases, especially when it is substantially cooler
and/or substantially slower-moving than the combustion gases, will encourage
formation of some eddy currents in the outer or peripheral portion of the
combustion gases. Thus, the goal of the foregoing limits or control that are
impressed upon the cooling gas is the substantial inhibition or substantial
prevention of any tendency for the cooling gas to bring about an immediate
overall disruption of the flame, and preferably also of the flow of combustion
gases that continues downstream from the zone in which flame is present. In
the
present embodiment, in which the air inlet port 28 that surrounds burner
nozzle
22 in combustion chamber upper wall 26 is substantially annular, cooling air
is
admitted to the chamber in the form of a moving curtain, induced by the draft
produced by the burner and downstream collection equipment, that substantially
entirely surrounds the flame while performing the particle dispersion,
agglomeration inhibition and other cooling gas functions described above.
Optionally, additional air, water, or other suitable dilution gas can be
admitted
to the combustion chamber downstream of the burner. In any case, enough
cooling is preferably introduced to bring the hot gases to below about 800 to
about 1200 C before entering ducts for conveyance to collection devices.
Any suitable means and measures may be used to collect the at least
partially fused particulate product. Persons skilled in the art are well aware
of
suitable systems. In the present embodiment combustion chamber 27 has an
integral hopper section 36 with a conical or upright funnel-like bottom
section 37
into which product falls by gravity and/or is drawn by the draft provided by
downstream collection equipment. An outlet 38 at the bottom of hopper 36 is
CA 02236447 1998-04-30
WO 97/16385 PCTIUS96/16967
49
connected through conduit 39 with collection equipment, such as a gas-solids
separator 40, which may be of the cyclone type having top and bottom outlets
41
and 42 for gases and particulate products respectively. Outlet 41 may be
connected to a bag filter (not shown), if desired, and to a blower (not shown)
to
provide a draft through the collection equipment.
In the fusion of feed particles by the above described method, sufficient
heat is transmitted to the particles, while dispersed, to cause enough
softening or
melting in the respective particles so that surface tension is able to convert
an
appreciable portion of them from their original irregular form to a
substantially
more regular shape, while providing them with smooth surfaces. Then the
particles are kept out of contact with one another and with other surfaces
until
they have been cooled to a non-tacky state.
If it were possible for each individual particle to undergo fusion and
experience the effects of surface tension with no interference by air
currents, by
other particles or by fusion apparatus components, with no particle
composition
inhomogeneities, with sufficient time at a suitable viscosity, and with
uniformly
rapid cooling, the resultant product particles would be perfectly spherical.
However, in practice, a certain amount of interference, inhomogeneities and
variations in residence time and viscosity will occur. Thus, to some extent,
there
will be product particles that are less than perfectly spherical.
Some of these less than perfectly spherical particles may be quite irregular
in shape, and in some instances a substantial percentage of irregular
particles will
be retained intentionally in the resultant products. Yet, the objects of the
invention are attained when a substantial portion of the irregular feed
particles
are converted to a form that appears at least generally ellipsoidal when
viewed
under magnification as described below and when the resultant product, as
originally produced, or as packaged, or as combined with other materials for
any
suitable end use, contains about 15 to about 99%, or about 50 to about 99%, or
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
about 75 to about 99 % or about 90 to about 99 % by volume of generally
ellipsoidal particles.
According to a particularly preferred embodiment of the invention, the
products contain substantially spherical particles in amounts within at least
one
5 of these volume percentage ranges. More particularly, for those end uses in
which discreetness of the product particles is deemed important, it is
preferred
that, in the compositions of matter according to the invention, the above
identified portion of the resultant product that represents about 15 to 100%
by
volume of generally ellipsoidal particles should itself contain about 50 to
about
10 99 %, more preferably about 70 to about 99 % and still more preferably
about 90
to about 99% by volume of substantially discrete particles.
"Generally ellipsoidal" particles are those whose magnified two-
dimensional images appear generally rounded and free of sharp corners or
edges,
whether or not they appear to have truly or substantially circular,
elliptical,
15 globular or any other rounded shape. Thus, in addition to the truly
circular and
elliptical shapes, other shapes with curved but not circular or elliptical
outlines
are included.
"Substantially spherical" particles are those whose magnified two-
dimensional images appear at least substantially circular. A particle will be
20 considered substantially spherical if its outline fits within the
intervening space
between two concentric, truly circular outlines differing in diameter from one
another by up to about 10% of the diameter of the larger of these outlines.
In general, a given particle will be considered "substantially discrete" if
the outline of its image does not touch or overlap that of any other particles
25 visible in a magnified view of the given particle and of such other
particles.
However, a given particle will still be considered substantially discrete if
its
image touches or overlaps the outline of one or any number of other particles,
CA 02236447 1998-04-30
WO 97/16385 PCTIUS96/16967
51
if the largest visible dimensions of all si.tch other particles are
respectively in the
range of up to about 10% of the largest visible dimension of the given
particle.
Shape, discreetness and particle size of feed material and product particles
may in general be judged by viewing their two-dimensional photographic images
at a magnification of X1000, as in Figures 3 and 4 herein. Such images may be
provided by an optical or scanning electron microscope or by a suitable
alternative magnifying device at the same or equivalent magnification. Only
particles entirely visible within the image under review are considered in
applying the above defmitions and in determining quantities of particles
present.
Samples used for such analyses should, unlike Figures 3 and 4, be prepared in
a manner that sufficiently scatters the particles in the magnified views in
order
to minimize particle-to-particle overlap of discrete particles. The number of
particles counted for determining the volume percentage of particles of a
particular type in a sample should be sufficient to provide an acceptable
level of
confidence, such as about 95 %.
The definitions of generally ellipsoidal, substantially spherical and
substantially discrete given above are applied on the basis of the above-
described
images as viewed at the indicated magnification, even if the particles in
question
would not conform to these definitions if viewed at higher levels of
magnification. Thus, for example, particles whose outlines appear rounded and
whose surfaces appear mostly or substantially entirely smooth at this level of
magnification should be considered generally ellipsoidal even if they may
appear
less rounded and/or less smooth at higher levels of magnification.
Determinations of particle size, discreetness and volume percent for
particles of different sizes and shapes, whether generally ellipsoidal,
substantially
spherical or irregular, may be based on procedures described in Handbook of
Mineral Dressing, by A. F. Taggart, John Wiley & Sons, Inc., New York, 1945,
chapter 19, pages 118-120. Many refinements of this basic method are known
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
52
to those skilled in the art. For instance, one may analyze the magnified two-
dimensional images of suitably prepared samples using a Leica Q570 image
analysis system in conjunction with a Leitz Ortholux microscope or a source
that
inputs data from scanned SEM (scanning electron microscope) micrographs.
Such automated image analysis systems can make direct measurements of
particle area, perimeter and aspect ratio to determine equivalent circular
diameter
values for the two-dimensional images of all observed particles, regardless of
shape. These substantially correspond to the actual values for all observed
particles. Such systems readily determine equivalent circular diameter values
for
particles in selected particle size categories.
When supplied by the operator with a suitably defined "discriminating
factor, " such systems can distinguish particles that are substantially
ellipsoidal or
substantially spherical from those that are not and can determine area values
that
substantially correspond with the aggregate areas of the particles within and
without these categories. A discriminating factor that has been used with
apparently acceptable results for distinguishing generally ellipsoidal
particles
from those that are not, and which may or may not be subject to further
refinement, is as follows:
CSF = AR > 0.55, wherein
CSF = circular shape factor (4-ir X area of particle = particle
perimeter2) as derived by the system and
AR = aspect ratio (largest particle dimension or diameter =
smallest particle dimension or diameter) as derived by the system.
The respective aggregate image areas for particles whose images are and are
not
within the generally ellipsoidal or substantially spherical category may then
be
converted to volume percentages by formulas familiar to persons skilled in the
art.
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
53
Automated image analysis systems of the above type are available with
displays on which an operator may view particles under analysis. Such displays
permit the operator to visually discriminate between particles that are and
are not
in a selected category, for example generally ellipsoidal, substantially
spherical
or substantially discrete, as above defmed. Particles so identified may be
selected for inclusion in groups of particles whose aggregate areas may then
be
determined automatically, followed by conversion of these areas to volume
percentages as above described.
The following examples, conducted in apparatus as depicted in Figures 1
and 2, offered as illustrations, are not intended to limit the scope
invention.
Bxanaple 1
800 grams of Kansas volcanic ash (72. 8% Si0a; 14.6 % A 1203; 5.8 % K20;
3.9% Na2O; 0.75% Fe203; 0.28% CaO; 2% H20) is placed in ajar mill with 20
grams of hexamethyldisilazane and 1500 grams of 1/4" alumina balls. After
tumbling for ten hours the ash is recovered as a free-flowing powder with 90
percent of the somewhat platy, irregularly shaped, particles having a diameter
of
less than 10 microns and a density of 2.5 g/cc.
Into the apparatus of Figures 1 and 2, air is metered to the oxygen-
containing gas pipe 3 at about 270 ft.3/hr (cubic feet per hour at 20 C).
Natural
gas, with a heating value of 1,000 B.T.U./ft.3 is separately metered and
aspirated
into pipe 3 from fuel delivery pipe 4 at junction 5 at about 35 ft.3/hr. An
additiona180 ft.3/hr. of air is injected from supply pipe 16 and nozzle 17
through
venturi 18 into the feed entry leg 8 of Y 6.
Over a period of about 6.6 minutes, one hundred grams of the ash,
aspirated and entrained with a stoichiometric mixture of air and natural gas
as
above described, is supplied to a downward directed flame of 50,000 btu per
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
54
hour in the apparatus of Figures 1 and 2. The mixture of hot gases and
entrained
ellipsoidal particles is cooled by mixing with room temperature air.
Using a cyclone, the solid particles are separated from the gases. The
powdered product has a density of 2.1 g/cc and an average particle size of 4.5
microns. Greater than 90 percent of the ash ellipsoidal particles contain a
void,
visible on microscopic observation, and these "bubbles" account for the
reduction
in average particle density compared with the starting volcanic ash.
Example 2
Synthetic precipitated silicas products: "FK320"; "FK16"; "SIPERNAT
22"; "SIPERNAT D17"; and "EXTRUSIL", and a synthetic alumino silicate,
"SIPERNAT 44", are obtained from the Degussa Corporation. Each of these
powders, which contain from 3 to 22 percent water, is dispersed into a
stoichiometric flame of air and natural gas in the above described manner to
produce powders with an abundance of spherical particles with average particle
diameters of a few microns. Once again voids are evident in some of the
otherwise spherical particles.
Example 3
Into the apparatus of Figures 1 and 2, air was metered to the oxygen-
containing gas pipe 3 at about 420 ft.3/hr. (cubic feet per hour at 20 C) .
Natural
gas, with a heating value of 1,000 B.T.U./ft.3 was separately metered and
aspirated into pipe 3 from fuel delivery pipe 4 at junction 5 at about 64
ft.3hr.
An additional 80 ft.3/hr. of air was injected from supply pipe 16 and nozzle
17
through venturi 18 into the feed entry leg 8 of Y 6. About 28 ft.3/hr. of
oxygen
gas was metered to the combustion air supply line. The material used for this
example was ALCOA OC-1000 aluminum hydroxide having a composition of
65 % A1Z03; 0.2 % Na20; 34.7 %o HZO. After the aluminum hydroxide was treated
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
with 0.25% hexamethyldisilazane and ground in a ball mill for nine hours, the
size distribution was 90%, 50%, and 10% less than 14.91, 6.99, and 3.03
microns respectively. The free-flowing powder had a B.E.T. surface area of
18.8 m2 per gram and a specific gravity of 2.42 grams per cubic centimeter.
5 Seven hundred fifty-five grams of sample was aspirated through inlet 15 into
venturi 18, at a rate of 1.8 lb. per hr., and dispersed into the ignited
burner 29.
After entraimmmet and dilution with additional air drawn into the combustion
chamber 27 through port 28, the particles were then exhausted from the hopper
36 at about 130 C. The free flowing white powder product, slippery to the
10 touch, was collected using a Premier reverse pulse FILTER RECEIVER "bag
house. "
By microscopic observation, 90% of the particles in the product were
generally ellipsoidal. The aluminum oxide product has: a B.E.T. surface area
of 2.21 m2/g; a size distribution wherein 90%, 50% and 10% of the particles
15 have diameters less than 13.09, 4.94, and 2.52 microns respectively; and a
specific gravity of 2.95 g/cc;. For comparison aluminum oxide as reported in
Lange's Handbook of Chemistry has a specific gravity of 4.00 g/cc.
Industrial Applicability
It is expected that products according to the invention will be supplied to
20 industry as compositions of matter that are composed substantially of the
solid
particles, including generally ellipsoidal particles with or without particles
of
other shapes. However, due to the diverse practical uses of the particulate
products, it is expected that compositions of matter of the present invention,
referred to in the accompanying claims, will take many different and varied
25 forms. Some illustrations are given below.
Compositions of matter comprising the solid particles disclosed herein may
take the form of mixtures of such solid particles, including the generally
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
56
ellipsoidal particles, with polymeric materials of all types, for instance
thermoplastic and thermosetting resins, elastomers and other forms, including
for
example all materials popularly known as plastics. In such mixtures, the
volume
of solid particles, based on the total volume of such particles and polymeric
material, can vary through out the range of about .05% (e.g., when small
amounts of particles are present in films as anti-blocking agents) to about
99.9%
(e.g. when small amounts of polymer are present as a surface treatment on the
particles).
Katz and Milewski, supra, at pages 311 to 315, discuss uses of glass beads
in polymeric materials. The products of the invention will be useful in many
of
these applications, especially since the invention provides an economical
source
of generally ellipsoidal particles in the range of up to about 15 microns in
average diameter. Similarly, with only minor formulation adjustments, the
generally ellipsoidal particles will be useful for most if not all of the
applications
described in the literature for fused silica, spherical alumina, silica,
feldspar,
calcium carbonate, nepheline syenite, alumina trihydrate and other
particulates
used as additives or neat powders. Products of this invention can replace at
least
partly and in many cases fully the volume of particulate additives used or
contained in a given application or formulation. Only minor additional
adjustments to attain the desired viscosity, texture or other properties of
importance will be required.
Particles in the size range with an average diameter of about 15 microns
or less are important for producing composites, including molded products and
laminates, with smooth surfaces that have high resistance to abrasion and
staining. Consequently, these particles will be especially useful in amino
polymer plastics, polyesters, phenolics, epoxies and other resins used for
preparing a wide variety of molding compounds and molded members for the
electrical transportation industry and other industries, as well as for
preparing
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
57
laminating mixes, laminates and other items for counter tops, vanities and
other
applications for the building and construction industries. For these purposes,
the
solid particles of the present invention, in their various mixtures with
polymeric
material, are preferably present in amounts of about 5 to about 65 % by
volume,
based on the volume of the entire composition.
Another valuable end-use is in polymeric films of any kind that contain
said solid particles. For example, when incorporated in polymeric films in a
sufficient amount, the particulate products impart anti-blocking properties to
said
films. To illustrate, homogeneously blending about .05 to about .5 % by volume
of these products into polyethylene and/or other films enables those films to
be
stored in layered (including wound) form under typical warehouse conditions,
e.g. at film temperatures up to about 45 C, without "blocking" or fusing of
the
film layers to one another. In preferred products for these anti-blocking
applications, 90 to 100% by volume of the particles have diameters of up to
about 25 microns and about 80 to 100% by volume of the particles are generally
ellipsoidal.
The products of this invention are valuable as additives for adjusting the
viscosity, thixotropy, or other rheological properties of formulations for
paints,
coatings of all types, caulks, sealants, plastic materials of all types,
cosmetics,
inks, etc. For these applications generally ellipsoidal spheres with
relatively
small diameter and high surface area will be most suitable. Preferred products
will have average diameters of up to about 2 microns and preferably less, and
may advantageously contain 20 to 30 percent or more of irregular, non-
ellipsoidal particles.
Extenders for paint represent another valuable application. Economical
availability of products with low color in small sizes that are abundant in
rounded
particles makes it possible to add these products to liquid coating
compositions
as fillers at loadings in the range of about 5 to about 50% of the total
volumes
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
58
of said compositions. With particulate products having very small particle
sizes
and an abundance of substantially spherical particles, only relatively modest
viscosity increases, e.g. less than half the viscosity increase that would be
expected when using fillers in the form of typical irregularly shaped
particles, are
experienced. Preferred examples of particulate products useful for such
applications are those having Color Quest 457 nanometer brightness of at least
about 60, more preferably at least about 70 and still more preferably at least
about 80, with about 90 to 100% by volume of the particles having diameters in
the range of up to about 25 microns and with about 75 to 100% by volume of the
particles being generally ellipsoidal or substantially spherical.
Also, the compositions of the present invention include liquid coating
compositions that are curable to solid decorative or protective coatings,
including
architectural paints, industrial coatings, wood stains and other coatings. In
these
compositions, the particulate materials may be used if desired to displace
other
ingredients that are expensive or environmentally troublesome, such as
solvents.
Also, products composed to a large extent of rounded particles, for example
those that contain about 70 to about 100% by volume of generally ellipsoidal
particles, can be incorporated in coatings to provide improved durability.
The products of the invention can also be used in coatings in sufficient
amounts to impart controlled surface texture to them and thereby to provide
gloss
reduction and "flatting" effects in combination with improved stain and scrub
resistance. Products in which about 90 to 100% by volume of the particles have
diameters of up to about 25 microns and which contain about 60 to 100% of
generally ellipsoidal particles are preferred for these applications.
The solid particles of the present invention, which can readily be made
with melting points higher than those of glass beads, are potentially useful
in
shaped metallic members of the kind that include a matrix of metallic material
in which said solid particles are dispersed, for example as an additive to
improve
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
59
durability or hardness. Such metallic materials may for example be selected
from among zinc, aluminum and alloys containing at least one of said metallic
materials. In such compositions, the products of the invention offer potential
savings in both weight and cost.
Inert, non-abrasive generally ellipsoidal fillers are useful in soap and
cosmetic formulations, because of the smooth texture they impart to such
formulations. Thus, it is possible to provide compositions in the form of
smooth-
textured fluent or spreadable material comprising the solid particles of the
present
invention dispersed in a pharmacologically acceptable vehicle for application
to
the skin or other body parts of humans or animals. Freedom of the particulate
products from heavy metals and other noxious materials will be required in
many
if not all of these applications. In the products preferred for these
applications,
about 90 to 100% by volume of the solid particles will have diameters in the
range of up to 10 microns and about 90 to 100% by volume of the particles will
be generally ellipsoidal or substantially spherical.
The paper industry has large requirements for specialty fillers of all types,
and the invention offers the opportunity of formulating papers with a high
degree
of surface smoothness and durability. Thus, the invention makes possible
compositions of matter in the form of smooth-surfaced webs comprising woven
or non-woven fibers as the principal structural elements of the webs, with the
solid particles of the invention being present in said webs as an additive,
whether
or not such webs include polymeric material. For these applications, products
with average particle sizes in the range of up to about 10 microns are
preferred.
Solid particles in accordance with the invention are useful for preparing
many caulks, organic and inorganic cements, and other compositions. Among
these are compositions of matter in the form of smooth-textured fluent or
spreadable adhesives comprising said solid particles dispersed therein. It is
anticipated that products of this invention that are abundant in rounded
particles,
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
preferably those containing about 50 to 100% by volume of generally
ellipsoidal
or substantially spherical particles and having an average particle size in
the
range of up to about 10 microns, will be useful as additives for modifying the
properties of adhesives, providing combinations of tack, elasticity,
elongation and
5 possibly other properties that were not previously available. Other useful
compositions include powders comprising at least an inorganic cement-forming
component in admixture with said solid particles. White grades of the products
of the invention are useful in these compositions where appearance is an
important feature. For example transparent products having a Color Quest 457
10 nanometer brightness of at least about 80 and average particle diameters in
the
range of up to about 10 microns are preferred for use in dental compositions.
Katz and Milewski, supra, in chapter 4, describe using mixtures of
particles with large and small diameters to provide combinations with high
"packing" factors or high bulk density. Such combinations are important for
the
15 formulation of composites in which generally ellipsoidal particles
represent a
very high volume percentage of the solid particles therein, and consequently
contain a minimum of other ingredients. Composites giving high performance
at elevated temperatures, such as may be used in aerospace and other
applications, are made possible by such formulating techniques. The invention
20 makes readily available products that are abundant in particles within the
small
size ranges needed for these mixtures.
The generally ellipsoidal particles of this invention, either by themselves
or in combination with other materials, including for instance other kinds of
solid
or cellular particulates, can be used to form non-flowable porous structures.
The
25 particles of such structures may be rendered temporarily or permanently
adherent
to one another by high-temperature sintering or by bonding the particles
together
in bulk, such as with small additions of adhesives or cements. These products
are useful in block, slab, or other shaped forms to act as lightweight
structural
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
61
materials. By suitable selection of particle size and level of bonding agents,
the
porosity of these materials can be controlled to provide utility as filters,
such as
for gases and/or liquids. Particles in accordance with the invention are
useful
in curable liquid and solid polymeric compositions generally. At least some of
them are however particularly useful in UV-curable compositions due to their
relatively high UV transparency, as compared with other fillers.
Neat or powdered forms of the products of this invention, because of the
rounded particle shapes, have an unusual degree of lubricity or slipperiness
to the
touch. This property causes those embodiments of the invention which are
abundant in free flowing generally ellipsoidal particles to be useful in a
wide
range of applications, such as lubricants for a variety of friction control
applications, powders for skin protection, slip agents between film and paper
layers and agents for controlling the tackiness or stickiness of surfaces in
general.
Any form of surface treatment with silane coupling agents, organic
titanates, surfactants, dispersants, wetting agents, etchants (acidic or
basic), or
other agents, and any other method of surface modification, may be used to
enhance the performance of the generally ellipsoidal particles in any
application.
See Silane CouplingA ents, Plueddemann, E. P., 2d Ed., Plenum Press, 1991.
For additional information regarding organic titanate and silane coupling
agents,
to improve bonding with polymeric materials, see also U.S. Patents 3,834,924
to Grillo, 3,290,165 and 3,567,680 to lannicelli, and 4,268,320 and 4,294,750
to Klingaman and Ehrenreich.
The end-uses of the products of the present invention that are described
above are those which presently appear most attractive. The foregoing
disclosures of embodiments of the invention and end-uses therefor have been
given merely for purposes of illustration and not to limit the invention.
Thus,
CA 02236447 1998-04-30
WO 97/16385 PCT/US96/16967
62
the invention should be considered to include all embodiments falling within
the
scope of the following claims and equivalents thereof.