Note: Descriptions are shown in the official language in which they were submitted.
CA 02236~2 1998-0~-04
WO97/17424 PCT~S96115317
DETERM]:NIN6 ANTIMICROBIAL AGENT SENSITIVITY FOR PARAFFINOPHILIC
MICROORGANISMS
BACKGROUND OF THF. INVF~TION
This invention relates to a method for determining
the antimicrobial agent sensitivity of a paraffinophilic
microorganism using various milieus and an associated
apparatus, and more particularly, to a method involving the
use of a receptacle containing an aqueous solution that mimics
the ~n vivo conditions of a patient. A paraffin coated slide
is pLaced into the receptacle. The paraffinophilic microor-
ganism to be tested for antimicrobial agent sensitivity is
baited by the paraffin.
United States Patent Nos. 5,153,119 and 5,316,918,
the disclosures of whic:h are incorporated by reference herein,
disc:Lose methods and apparatus for identifying and testing the
antibiotic sensitivity of Mycobacterium a~ium-intracel 7 u~are
("MAI"). One of the co-inventors herein, Robert-A. Ollar, was
the named inventor on these patents. The method of identi-
fying MAI includes placing a paraffin coated slide in a
receptacle containing a sterile aqueous solution inoculated
with a specimen from a patient and analyzing the slide after
exposure to the specimen to determine the presence or absence
of M~I. The analysis step involves performing a number of
speciation assays, such as a tellurite reduction test. The
method for testing t:he sensitivity of MAI to different
antimicrobial agents amd dosages thereof includes providing a
plurality of test tubes adapted to contain an amount of an
antimicrobial aqent to be tested and MAI to be assayed and a
separate paraffin coated slide adapted for placement in each
of t~le test tubes. Observing the growth of MAI on the slide
CA 02236~2 1998-0~-04
WO97/17424 PCT~S96/15317
can be used to determine the concentration of the antimicro-
bial aqent necessary to resist MAI growth on the slide.
The inventions provide effective, efficient and
economical methods for identifying MAI and testing MAI for
~i antimicrobial agent sensitivity. These methods avoid the use
of expensive, complicated equipment, and thus can be used in
places such as field hospitals and third world locations where
the more expensive and hard to use equipment is not available.
Identifying and treating opportunistic infections
very often involves educated guesses as to the nature of the
microorganism involved and, once identified, the quantity of
antimic:robial agent needed to effectively treat the
microorganism. Some antimicrobial agents are extremely
expensive, so it would be beneficial to use only that amount
lS necessary to treat the infection. Furthermore, and more
importantly, antimicrobial agents can have undesired side
effects, so it is prudent to use only that amount needed to
effectively treat the infection. Vnfortunately, however,
there is presently no method by which a physician may rapidly
ascertain which antimicrobial agent will work best in order to
assure effective inhibition of the growth of the
microorganism. This has the consequence of greater expense,
less efficacy and the potential for more damaging side
effect,. This state of affairs exists because medical care
givers frequently do not have the type of information
regarding antimicrobial agent sensitivity that would make a
more exact selection of an antimicrobial agent possible and,
once an appropriate antimicrobial agent is selected,
facilit;ate a more precise concentration for use. In addition,
certain antimicrobial agents react differently under different
conditions. For example, one type of antimicrobial agent may
not be effective in a low pH environment. It would thus be
desirable to mimic the in vivo clinical condition of a patient
in vitro in any type of antimicrobial agent sensitivity
CA 02236~2 1998-0~-04
WO97/17424 PCT~S96/15317
testing so that antimicrobial agent efficacy against the
microoryanism can be maximized.
Thus, there remlains a need for a method of testing
the antimicrobial agen,t sensitivity of one or more
paraffinophilic microorganisms in a way that maximizes the
efficacy of the antimicrobial agent used to inhibit growth of
the one or more paraffinophilic microorganisms that may be
present in a patient.
SUMMARY OF THF INVF.~TION
The invention has met or surpassed the
above-mentioned need as well as others. The method of
determining the sensitivity of at least one paraffinophilic
microorganism from a specimen obtained from a patient to
different antimicrobial agents and predetermined guantities
thereof includes providing at least one receptacle containing
an aqueous solution and i~djusting the solution to mimic the
in vivo clinical conditions of the patient. The method then
includes inoculating the solution with the specimen and then
placing into the receptacle ~i) a paraffin coated slide to
bait the at least one paraffinophilic microorganism and (ii) a
predetermined quantity of an antimicrobial agent to be tested.
The slide is then observed for paraffinophilic microorganism
growth or lack thereof to determine whether the predetermined
quantity of the antimicrobial agent is effective in inhibiting
growth of the paraffinophilic microorganisms on the slide.
An associated apparatus is also provided that
includes a receptacle adapted to contain an aqueous solution,
an amow~t of antimicrobial agent to be tested and the patient
specimen and means for adjusting the aqueous solution to mimic
the in vivo clinical conditions of the patient. The apparatus
further includes a paraffin coated slide adapted to being
placed in said receptacle. In this way, observation of the
growth of the paraffinophilic microorganisms from the specimen
on the slide can be used to determine the concentration of the
CA 02236~2 1998-0~-04
W097/17424 PCT~S96/15317
antimic:robial a~ent necessary to resist paraffinophilic
microorganism growth on the slide.
BP~F.F DF.SCR~PTION OF THF. DRAW~l~G
A full understanding of the invention can be gained
from the following detailed description of the invention when
read in conjunction with the accompanying lone drawing which
shows one embodiment of the antimicrobial agent sensitivity
apparatus.
D~TArT.F.n DFSCRIPTION
lO As used herein, the term "patient" refers to a
member of the animal kingdom, including human beings, whose
body sp,ecimen is being processed by the method and apparatus
of the invention.
As used herein, the term "paraffinophilic" means an
organism that can employ paraffin wax as a source of carbon in
a basal salt media, devoid of other forms of carbon. The
organism can be bacteria:L or funqal in nature.
The method and,apparatus of the invention provide an
efficient, effective and economical way of determining the
sensitivity of at least one paraffinophilic microorganism to
different antimicrobial agents and predetermined quantities
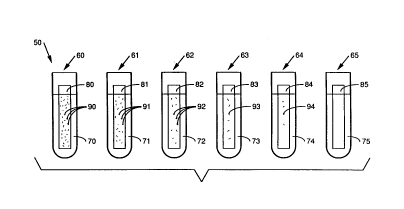
thereof. Referring now to the lone Figure, the antimicrobial
agent sensitivity method will be explained with reference to
one embodiment of the antimicrobial agent sensitivity
apparatus 50. The apparatus 50 consists of six receptacles in
the form of test tubes 60, 61, 62, 63, 64, 65 each containing
an amount of an aqueous solution, such as distilled water 70,
71, 72, 73, 74, 75. It will be appreciated that the aqueous
solution should not contain any carbon source, as it is
desired to provide a sole carbon source on the slide in order
to effe!ctively grow the microorganism to be tested on the
slide and not in the aqueous solution. The aqueous solutions
CA 02236~2 1998-0~-04
WO97117424 PCT~S96/l5317
contain uniform intervalc; of increasing concentrations of an
antimicrobial agent to be tested. For example, the
microorganism whose antimicrobial agent sensitivity is to be
tested can be MAI, with the antimicrobial agent ciproflaxacin
being placed in test tubes 61, 62, 63, 64 and 65 along with
aqueous solution 71, 72, 73, 74 and 75. The ciproflaxacin can
be placed into each test tube 61, 62, 63, 64 and 65 in
increasing concentrations. Test tube 60 is used as a control
tube that does not contain any antimicrobial agent.
The specimen from the patient is then inoculated
into each of the test tubes 60-65. The specimen can be a
blood sample; any biopsy or tissue specimen; stomach fluid;
urine; c:erebral spinal fluid; nasopharyngeal mucosa or saliva.
These s,pecimens can be obtained from the patient in the
doctor':3 office or in the emergency room of a hospital, for
example, by known techniques.
Slides 80, 81, 82, 83, 84 and 85 coated with
paraffin are then placed into respective test tubes 60, 61,
62, 63, 64 and 65. The slides are incubated for a period of
eight days. By observing MAI growth 90, 91, 92, 93, 94 on the
slides 80-85, the minimum inhibitory concentration ("MIC") of
the antimicrobial agent necess~ry to prevent paraffinophilic
microorganism growth can be determined. In this case,
slide 85 has no MAI growth, thus the MIC concentration is
found in test tube 75.
It will be appreciated that although apparatus 50 is
shown with multiple receptacles and multiple slides 80-85,
that the invention is not limited to multiple receptacles and
multiple slides, but covers also a single receptacle and a
single slide.
The method of the invention can be used to determine
the antibiotic sensitivity of at least one of the
paraffinophilic microorganisms selected from the group
consist:ing of Micrococcus Paraffinae; Corynebacterium Simplex;
Ahnl; M~cococcus fRhodococcus) CinnabareUsi Ahnl. Mycococcus
CA 02236~2 1998-0~-04
WO97/17424 PCT~S96/15317
~Rhodoc~ Rhodochrous; M~cobact. Perrugosum Var. Athanicum;
Mycobact. ~ubrum Var. P;ropanicum; Mycobacterium Hyalinum;
Mycobaciterium Lacticola; Mycobacterium Album, M. Luteum;
Mycobacterium Microti; Mycobacterium Rubrum,
Mycob~cterium Phlei.; Mycobacterium Phlei, M. Smegmatis;
Mycobacterium Testudo; Mycobacterium-Avium-Intracellu~are;
Nocardia Spp.; Actinomyc-es; Candida Lipolytica; Candida
Tropica.lis, Torulopsis Colliculosa; Monila Sp., ~n-e6nl~7a Sp.,
Torula rossa; Penicillium Sp.; I~NL. Aspergillus Flavus;
10 Asperg~llus sp., Penicillium Sp.; Citromyces Sp.,
Scopulariopsis Sp.; Pseudomonas Fluorescens Liquef~ciens;
Ahnl, P~m. Fluorescens Denitrificans; Pseudomo~as Aeruginosa.
It will be appreciated that in clinical medical
practice, there are situations both where a patient has a
single paraffinophilic mi.croorganism (such as MAI) or where
another patient may have multiple paraffinophilic
microorganisms (such as MAI and Mycobacterium Xansasii). It
is imperative that all paraffinophilic microorganisms be
treated as each one is probably causing pathogenicity in the
patient Thus, if an immunocompromised patient has a lung,
liver or kidney Ah-C~SS, the physician is interested in the
antimicrobial agent that will inhibit all bacterial growth on
the slide, whether or not that bacterial growth involves one
or mult:iple paraffinophilic microorganisms.
In accordance with the invention, the aqueous
solutions 70-75 in each of the test tubes 60-65 can be
adjusted to mimic the in vivo "clinical conditions" of the
patient By "clinical conditions" it is meant at least one of
the follLowing: the pH oI the in vivo milieu of the patient
where the paraffinophilic microorganism can be found and the
electro:Lyte levels of a patient's blood where paraffinophilic
microorc~anism can be found. Adjusting the aqueous solution
can be effected by numerous different methods. Adjusting the
pH of t:he aqueous solution can be accomplished by adding
hydroch:Loric acid (HCl) to obtain a more acidic solution or by
CA 02236~2 1998-0~-04
WO97/17424~ PCT~S96115317
- 7 -
adding sodium hydroxide (NaOH) or potassium hydroxide (KOH) in
order to obtain a more b~sic solution. Electrolytes such as
one or more selected from the group consisting of sodium,
potassium, chloride, magnesium, phosphate and calcium, can be
added to the solution in desired quantities in order to mimic
the electrolytes in the blood of a patient from which a blood
sample which may contain the microorganism is obtained.
EXAMPT F. 1
An AIDS patient comes to an emergency room at a
hospital complaining of severe abdominal pain probably
suffering from an MAI infection and possibly a Mycobacterla
Ransasii infection. A gastroenterologist uses a
gastrointestinal scope to obtain a specimen of the patient's
stomach fluid. The scope indicates that the pH in the
patient's stomach is l.5 In the meantime, a lab technician
using 1:he apparatus of the Figure adjusts the pH of the
aqueous solutions ?0-75 by adding HCl thereto so that the
aqueous solutions 70-75 have a pH of l.5. Thus, the pH in the
patient's stomach is mimicked by the pH of the aqueous
solution in the apparatus shown in the Figure.
After this, the lab technician is instructed by the
physician to add specified amounts of an antimicrobial agent
to each receptacle 61-65. Receptacle 60 is a control test
tube alld thus does not contain any antimicrobial agent.
Receptacle 65 is to contain the highest concentration of
antimicrobial agent, with each successive receptacle 64-61
receiving half as much as the adjacent receptacle. In this
example, the physician wants to test the sensitivity of the
paraffinophilic microorganisms to the antimicrobial agent
risabutin. The technician is instructed to add the following
amounts to each receptac].e (each receptacle contains 5 cc of
aqueous solution)
CA 02236~2 1998-0~-04
WO97tl7424 PCT~S96/15317
R~-~.AC$E AMOUNT
0
61 0.003125 mg
62 0.00625 mg
63 0.0125 mg
64 0.025 mg
0.05 mg
After this, portions of the specimen of stomach
fluid t:aken by the gast:roenterologist from the patient are
inocula.ted into each of the receptacles 60-65 holding a
respective paraffin coated slide 80-85. After about
eight d.ays the growth or lack thereof is observed to indicate
which c:oncentration of antimicrobial agent is effective to
inhibit paraffinophilic microorganism growth on the slide.
EXAMP~ F 2
An AIDS patient comes to an emergency room
complaining of high fever and apparently has pneumonia. The
physician suspects that there is an infection caused by
Nocardia bactereremia. As is standard in almost every
emergency room, a chemical screen ~"CSS") is performed on a
blood specimen obtained :Erom the patient. The CSS lists the
electrolyte content of the patient's blood. The electrolyte
content is communicated to a lab technician who in turn
adjusts the aqueous solutions 70-75 in the receptacles 60-65
each holding a respective paraffin coated slide 80-85. For
example, the CSS reveals that the patient has a sodium level
of 120. The lab technician adjusts the sodium level of the
aqueous solution (for e.xample, distilled water) by adding
sodium thereto in order to mimic the 120 level of sodium found
in the patient's blood.
CA 02236~2 1998-0~-04
WO 97/17424 PCT/US96/15317
After this, the lab technician is instructed by the
physician to add specified amounts of an antimicrobial agent
to each receptacle 61-65. Receptacle 60 is a control test
tube and thus does not contain any antimicrobial agent.
5 Receptarle 65 is to contain the highest concentration of
antimic:robial agent, with each successive receptacle 64-61
receiving half as much as the adjacent receptacle. In this
example, the physician wants to test the sensitivity of the
paraffinophilic microorganisms to the antimicrobial agent
lO clarithromycin. The technician is instructed to add the
following amounts to each receptacle (each receptacle contalns
cc of a~ueous solution)
a~ .AcL~ AMo~
0
61 0.00625 mg
62 0.0125 mg
63 0.025 mg
64 0.05 mg
O.l mg
After this, a portion of the blood specimen taken
from th,e patient are inoculated into each receptacle 60-65
holding a respective paraffin coated slide 80-85. After about
eight days the growth or lac~c thereof is observed to indicate
25 which concentration of antimicrobial agent is effective to
inhibit paraffinophilic microorganism growth on the slide.
It will be appreciated that a method for determining
the antimicrobial agent sensitivity of a microorganism is
providecl. The method includes adjusting the aqueous solutions
30 used in the testing appar-atus to mimic the in vivo clinical
conditions of a patient from whom the specimen containing the
microorqanism to be identified and tested is obtained. The
method ia effective and efficient and do not involve the use
CA 02236~2 1998-0~-04
WO97/1742'1 PCT~S96/15317
-- 10 --
of expensive and comp:Licated equipment. An associated
apparat:us is also disclosed.
While specific embodiments of the invention have
been disclosed, it will be appreciated by those skilled in the
art that various modifications and alterations to those
details could be developed in light of the overall teachings
of the disclosure. Accordingly, the particular arrangements
disclosed are meant to be illustrative only and not limiting
as to the scope of the invention which is to be given the full
breadth of the appended claims and any and all equivalents
thereof.