Some of the information on this Web page has been provided by external sources. The Government of Canada is not responsible for the accuracy, reliability or currency of the information supplied by external sources. Users wishing to rely upon this information should consult directly with the source of the information. Content provided by external sources is not subject to official languages, privacy and accessibility requirements.
Any discrepancies in the text and image of the Claims and Abstract are due to differing posting times. Text of the Claims and Abstract are posted:
| (12) Patent Application: | (11) CA 2236690 |
|---|---|
| (54) English Title: | AQUEOUS ACYCLOVIR FORMULATION AND METHOD FOR STORING SAME |
| (54) French Title: | FORMULATION AQUEUSE D'ACYCLOVIR ET PROCEDE DE STOCKAGE ASSOCIE |
| Status: | Deemed Abandoned and Beyond the Period of Reinstatement - Pending Response to Notice of Disregarded Communication |
| (51) International Patent Classification (IPC): |
|
|---|---|
| (72) Inventors : |
|
| (73) Owners : |
|
| (71) Applicants : |
|
| (74) Agent: | NORTON ROSE FULBRIGHT CANADA LLP/S.E.N.C.R.L., S.R.L. |
| (74) Associate agent: | |
| (45) Issued: | |
| (86) PCT Filing Date: | 1996-11-04 |
| (87) Open to Public Inspection: | 1997-05-22 |
| Availability of licence: | N/A |
| Dedicated to the Public: | N/A |
| (25) Language of filing: | English |
| Patent Cooperation Treaty (PCT): | Yes |
|---|---|
| (86) PCT Filing Number: | PCT/US1996/017615 |
| (87) International Publication Number: | WO 1997017934 |
| (85) National Entry: | 1998-05-04 |
| (30) Application Priority Data: | ||||||
|---|---|---|---|---|---|---|
|
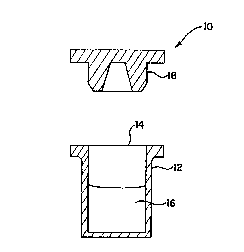
A method for storing a solution containing acyclovir and a pharmaceutical
product containing an acyclovir solution. The method includes the steps of
providing a predetermined volume of an acyclovir solution suitable for
parenteral administration to a patient and providing a container constructed
to contain the predetermined volume. The container is constructed from a
material containing polymethylpentene and defines an opening. The acyclovir
solution is placed into the container. The method further includes the step of
providing a stopper configured to seal fluidly the opening defined by the
container. The container is then sealed using the stopper. The pharmaceutical
product includes an acyclovir solution contained by a container constructed
from a material containing polymethylpentene. The container defines an opening
therethrough. The product further includes a stopper coated with a fluorinated
resin. The stopper fluidly seals the opening defined by the container.
Procédé permettant de stocker une solution contenant de l'acyclovir et produit pharmaceutique contenant une solution d'acyclovir. Le procédé fait appel à un volume prédéterminé d'une solution d'acyclovir convenant pour l'administration parentérale à un patient, et à un récipient conçu pour contenir ledit volume prédéterminé. Le récipient est fabriqué dans un matériau renfermant du polyméthylpentène et définit un orifice. La solution d'acyclovir est placée dans le récipient. Le procédé fait ensuite appel à un bouchon configuré de façon à permettre la fermeture fluidique hermétique de l'orifice défini par le récipient, qui est ensuite fermé à l'aide du bouchon. Le produit pharmaceutique comprend une solution d'acyclovir contenue dans un récipient fabriqué dans un matériau renfermant du polyméthylpentène. Le récipient définit un orifice. Le produit comprend en outre un bouchon, revêtu d'une résine fluorée, qui permet la fermeture fluidique hermétique de l'orifice défini par le récipient.
Note: Claims are shown in the official language in which they were submitted.
Note: Descriptions are shown in the official language in which they were submitted.
2024-08-01:As part of the Next Generation Patents (NGP) transition, the Canadian Patents Database (CPD) now contains a more detailed Event History, which replicates the Event Log of our new back-office solution.
Please note that "Inactive:" events refers to events no longer in use in our new back-office solution.
For a clearer understanding of the status of the application/patent presented on this page, the site Disclaimer , as well as the definitions for Patent , Event History , Maintenance Fee and Payment History should be consulted.
| Description | Date |
|---|---|
| Inactive: IPC expired | 2023-01-01 |
| Time Limit for Reversal Expired | 2002-11-04 |
| Application Not Reinstated by Deadline | 2002-11-04 |
| Inactive: Abandon-RFE+Late fee unpaid-Correspondence sent | 2001-11-05 |
| Deemed Abandoned - Failure to Respond to Maintenance Fee Notice | 2001-11-05 |
| Inactive: Single transfer | 1998-10-20 |
| Classification Modified | 1998-08-11 |
| Inactive: IPC assigned | 1998-08-11 |
| Inactive: First IPC assigned | 1998-08-11 |
| Inactive: Courtesy letter - Evidence | 1998-07-28 |
| Inactive: Notice - National entry - No RFE | 1998-07-22 |
| Application Received - PCT | 1998-07-15 |
| Application Published (Open to Public Inspection) | 1997-05-22 |
| Abandonment Date | Reason | Reinstatement Date |
|---|---|---|
| 2001-11-05 |
The last payment was received on 2000-10-27
Note : If the full payment has not been received on or before the date indicated, a further fee may be required which may be one of the following
Please refer to the CIPO Patent Fees web page to see all current fee amounts.
| Fee Type | Anniversary Year | Due Date | Paid Date |
|---|---|---|---|
| Basic national fee - standard | 1998-05-04 | ||
| Registration of a document | 1998-10-20 | ||
| MF (application, 2nd anniv.) - standard | 02 | 1998-11-04 | 1998-10-23 |
| MF (application, 3rd anniv.) - standard | 03 | 1999-11-04 | 1999-09-29 |
| MF (application, 4th anniv.) - standard | 04 | 2000-11-06 | 2000-10-27 |
Note: Records showing the ownership history in alphabetical order.
| Current Owners on Record |
|---|
| ABBOTT LABORATORIES |
| Past Owners on Record |
|---|
| KATHEE E. KLANDRUD |
| LAWRENCE J. RHODES |