Une partie des informations de ce site Web a été fournie par des sources externes. Le gouvernement du Canada n'assume aucune responsabilité concernant la précision, l'actualité ou la fiabilité des informations fournies par les sources externes. Les utilisateurs qui désirent employer cette information devraient consulter directement la source des informations. Le contenu fourni par les sources externes n'est pas assujetti aux exigences sur les langues officielles, la protection des renseignements personnels et l'accessibilité.
L'apparition de différences dans le texte et l'image des Revendications et de l'Abrégé dépend du moment auquel le document est publié. Les textes des Revendications et de l'Abrégé sont affichés :
| (12) Demande de brevet: | (11) CA 2236690 |
|---|---|
| (54) Titre français: | FORMULATION AQUEUSE D'ACYCLOVIR ET PROCEDE DE STOCKAGE ASSOCIE |
| (54) Titre anglais: | AQUEOUS ACYCLOVIR FORMULATION AND METHOD FOR STORING SAME |
| Statut: | Réputée abandonnée et au-delà du délai pour le rétablissement - en attente de la réponse à l’avis de communication rejetée |
| (51) Classification internationale des brevets (CIB): |
|
|---|---|
| (72) Inventeurs : |
|
| (73) Titulaires : |
|
| (71) Demandeurs : |
|
| (74) Agent: | NORTON ROSE FULBRIGHT CANADA LLP/S.E.N.C.R.L., S.R.L. |
| (74) Co-agent: | |
| (45) Délivré: | |
| (86) Date de dépôt PCT: | 1996-11-04 |
| (87) Mise à la disponibilité du public: | 1997-05-22 |
| Licence disponible: | S.O. |
| Cédé au domaine public: | S.O. |
| (25) Langue des documents déposés: | Anglais |
| Traité de coopération en matière de brevets (PCT): | Oui |
|---|---|
| (86) Numéro de la demande PCT: | PCT/US1996/017615 |
| (87) Numéro de publication internationale PCT: | WO 1997017934 |
| (85) Entrée nationale: | 1998-05-04 |
| (30) Données de priorité de la demande: | ||||||
|---|---|---|---|---|---|---|
|
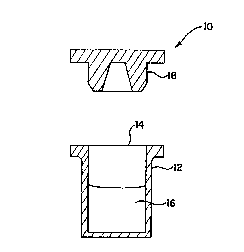
Procédé permettant de stocker une solution contenant de l'acyclovir et produit pharmaceutique contenant une solution d'acyclovir. Le procédé fait appel à un volume prédéterminé d'une solution d'acyclovir convenant pour l'administration parentérale à un patient, et à un récipient conçu pour contenir ledit volume prédéterminé. Le récipient est fabriqué dans un matériau renfermant du polyméthylpentène et définit un orifice. La solution d'acyclovir est placée dans le récipient. Le procédé fait ensuite appel à un bouchon configuré de façon à permettre la fermeture fluidique hermétique de l'orifice défini par le récipient, qui est ensuite fermé à l'aide du bouchon. Le produit pharmaceutique comprend une solution d'acyclovir contenue dans un récipient fabriqué dans un matériau renfermant du polyméthylpentène. Le récipient définit un orifice. Le produit comprend en outre un bouchon, revêtu d'une résine fluorée, qui permet la fermeture fluidique hermétique de l'orifice défini par le récipient.
A method for storing a solution containing acyclovir and a pharmaceutical
product containing an acyclovir solution. The method includes the steps of
providing a predetermined volume of an acyclovir solution suitable for
parenteral administration to a patient and providing a container constructed
to contain the predetermined volume. The container is constructed from a
material containing polymethylpentene and defines an opening. The acyclovir
solution is placed into the container. The method further includes the step of
providing a stopper configured to seal fluidly the opening defined by the
container. The container is then sealed using the stopper. The pharmaceutical
product includes an acyclovir solution contained by a container constructed
from a material containing polymethylpentene. The container defines an opening
therethrough. The product further includes a stopper coated with a fluorinated
resin. The stopper fluidly seals the opening defined by the container.
Note : Les revendications sont présentées dans la langue officielle dans laquelle elles ont été soumises.
Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2024-08-01 : Dans le cadre de la transition vers les Brevets de nouvelle génération (BNG), la base de données sur les brevets canadiens (BDBC) contient désormais un Historique d'événement plus détaillé, qui reproduit le Journal des événements de notre nouvelle solution interne.
Veuillez noter que les événements débutant par « Inactive : » se réfèrent à des événements qui ne sont plus utilisés dans notre nouvelle solution interne.
Pour une meilleure compréhension de l'état de la demande ou brevet qui figure sur cette page, la rubrique Mise en garde , et les descriptions de Brevet , Historique d'événement , Taxes périodiques et Historique des paiements devraient être consultées.
| Description | Date |
|---|---|
| Inactive : CIB expirée | 2023-01-01 |
| Le délai pour l'annulation est expiré | 2002-11-04 |
| Demande non rétablie avant l'échéance | 2002-11-04 |
| Inactive : Abandon.-RE+surtaxe impayées-Corr envoyée | 2001-11-05 |
| Réputée abandonnée - omission de répondre à un avis sur les taxes pour le maintien en état | 2001-11-05 |
| Inactive : Transfert individuel | 1998-10-20 |
| Symbole de classement modifié | 1998-08-11 |
| Inactive : CIB attribuée | 1998-08-11 |
| Inactive : CIB en 1re position | 1998-08-11 |
| Inactive : Lettre de courtoisie - Preuve | 1998-07-28 |
| Inactive : Notice - Entrée phase nat. - Pas de RE | 1998-07-22 |
| Demande reçue - PCT | 1998-07-15 |
| Demande publiée (accessible au public) | 1997-05-22 |
| Date d'abandonnement | Raison | Date de rétablissement |
|---|---|---|
| 2001-11-05 |
Le dernier paiement a été reçu le 2000-10-27
Avis : Si le paiement en totalité n'a pas été reçu au plus tard à la date indiquée, une taxe supplémentaire peut être imposée, soit une des taxes suivantes :
Veuillez vous référer à la page web des taxes sur les brevets de l'OPIC pour voir tous les montants actuels des taxes.
| Type de taxes | Anniversaire | Échéance | Date payée |
|---|---|---|---|
| Taxe nationale de base - générale | 1998-05-04 | ||
| Enregistrement d'un document | 1998-10-20 | ||
| TM (demande, 2e anniv.) - générale | 02 | 1998-11-04 | 1998-10-23 |
| TM (demande, 3e anniv.) - générale | 03 | 1999-11-04 | 1999-09-29 |
| TM (demande, 4e anniv.) - générale | 04 | 2000-11-06 | 2000-10-27 |
Les titulaires actuels et antérieures au dossier sont affichés en ordre alphabétique.
| Titulaires actuels au dossier |
|---|
| ABBOTT LABORATORIES |
| Titulaires antérieures au dossier |
|---|
| KATHEE E. KLANDRUD |
| LAWRENCE J. RHODES |