Note: Descriptions are shown in the official language in which they were submitted.
CA 02236851 1998-OS-06
DESCRIPTION
Substituted Vinyl Pyridine Derivative and
Drugs Containing the Same
TECHNICAL FIELD
The present invention relates to a novel substituted
vinylpyridine derivative and salts thereof, which are
endowed with strong and selective phosphodiesterase (PDE) IV
inhibitory action and strong .inhibitory action against
production of the tumor necrotizing factor (TNF-a,), as well
as with high safety. The invention also relates to drugs
containing the derivative or salts and useful for the
prevention and treatment of a broad range of inflammatory
diseases and autoimmune diseases.
BACKGROUND ART
PDE is an enzyme which acts as a catalyst in
hydrolysis of cyclic adenosine 3',5'-phosphate (CAMP) or
cyclic guanosine 3',5'-phosphate (cGMP) into 5'-
monophosphate. cAMP and cGMP are produced from ATP and GTP,,
respectively, following activation of adenylate cyclase or
guanylate cyclase in re:>ponse to a hormone or chemical
transmission substance, and work as intracellular second
messengers. PDE inhibitory agents block the activity of PDE
to increase the amounts of intracellular cAMP and cGMP, to
thereby suppress cellular response. At present, PDE is
known to have type I to type VIII isozymes. These are found
in the central nervous system, circulatory system,
1
CA 02236851 1998-OS-06
respiratory system, digesi~ive system, reproductive system,
and blood cell system. Tree distribution of these isozymes
differs according to the tissue. This suggests that a PDE-
isozyme-specific inhibitor may increase the amount of CAMP
in certain specific tissue.
In recent years, considerable efforts have been
devoted to research and development of highly specific PDE
isozyme inhibitors. For example, attempts have been made to
develop drugs that exhibit organ specificity attributable to
localization of respective isozymes. As a result of such
attempts, PDE IV is considered to be a potential agent
effective for both asthmatic attack and chronic respiratory
tract inflammation, due t:o the facts that PDE IV is present
predominantly in the airway tissue or inflammatory cells,
such as eosinocytes and neutrophilic leukocytes, which are
intimately related to asthmatic symptoms and that drugs that
inhibit the action of PDE IV exhibit bronchodilatation
action as well as inhibitory action against activation of
inflammatory leukocytes. Thus, active studies have been
performed worldwide focusing on development of a selective
inhibitor against PDE IV as a new remedy for bronchial
asthma.
PDE IV, which also exists in the central nervous
system, is expected to improve memory and mitigate anxiety,
based on the considerat~_on that a rolipram, a selective PDE
IV inhibitor, specifica=Lly localizes in the brain tissue to
increase noradrenergic nervous transmission on a synapse or
2
CA 02236851 1998-OS-06
post-synapse level in response to an increased amount of
cAMP, which is a second messenger of noradrenalin.
TNF-a is a cytokine produced by an activated
macrophage. Although TNF-a was first discovered to be a
factor which induces hemorrhagic necrosis in a tumor site,
it is now recognized as a mediator which widely participates
in inflammatory reactions and the immune mechanism.
Excessive production of TNF-a, however, induces disorders
in tissue to cause a variety of pathological conditions.
Rapid release of TNF-a induced by intracellular toxins is
responsible for the lethality.
TNF-a promotes production of platelet-activating
factor (PAF), a variety of inflammatory arachidonic
metabolites, and activated oxygen. Moreover, it induces
production of interleukin(IL)--l, IL-6, and IL-8. As is
understood from this, excessive production of TNF-a
aggravates inflammatory react_Lons and, in the case of
chronic inflammatory diseases such as rheumatism,
osteoporosis, and terminal cancers, results in a persistence~
of complication of diseases, in which the concentrations of
these cytokines are maintained consistently so as exacerbate
the symptoms. Accordingly, ir_ pathological conditions in
which TNF-a is produced excessively, control of its release
is strongly sought by clinicians.
So far, molecular design of a selective PDE IV
inhibitor has not yielded. satisfactory results, and
therefore limitation is imposed on use of the selective PDE
3
CA 02236851 1998-OS-06
IV inhibitor. Theophylline, which is a xanthine-based drug
widely used by clinician~~ as a therapeutic agent for the
treatment of bronchial asthma, exhibits bronchodilating
action stemming from the adenosine antagonizing action and
PDE inhibitory action. However, theophylline sometimes
causes adverse side effects in the circulatory system and
central nervous system, as it inhibits PDE rion-selectively.
Thus, the safety range of theophylline is rather narrow.
Rolipram and Ro20-1724 selectively inhibit PDE IV at a
potency 100 times that at: which they inhibit other PDE
isozymes. However, the inhibitory power itself is not
significant, imposing limitations on applicable diseases.
TNF-a production inhibitors include antiphlogistic
steroids, antihistaminic agents, PAF antagonists, and
active-oxygen quencher. However, these are nonspecific
inhibitors with either weak power or, when their power is
strong, with low tissue specificity, thus limiting their
methods of use. Moreover, protease inhibitors have recently
been reported to be specific 'rNF-a production inhibitors.
The protease inhibitors a.re peptide derivatives and have not
yet been extensively studied with regard to administration
methods, etc.
Accordingly, the present invention i~ directed to the
provision of therapeutics for a variety of diseases based on
the selective PDE IV inhibitory action; the provision of
therapeutics for a variety of diseases based on the TNF-a
production inhibitory action; and the provision of drugs for
4
CA 02236851 1998-OS-06
the prevention and treatment of a wide variety of
inflammatory diseases and autoimmune diseases, which drugs
are designed based on concurrent actions of these two
actions and are endowed with enhanced effects, higher
specificity, and higher safety.
DISCLOSURE OF THE INVENTION
Under the above circumstances, the present inventors
synthesized numerous compounds, and studied their PDE
inhibitory action and inhibitory action against production
of a variety types of cytokines, and as a result found that
the new substituted vinylpyridine derivatives represented by
formula (1) or salts thereof potently and selectively
inhibit PDE IV only, while not acting on other PDE isozymes,
and that production of TNF-a is potently inhibited. As a
result, the below-described substituted vinylpyridine
derivatives have been shown to be effective for the
prevention and treatment of the aforementioned wide ranges
of inflammatory diseases, autoimmune diseases, and other
diseases associated with disturbed matabolism of the - .-
cerebrum. The present invention has been completed based on
these findings.
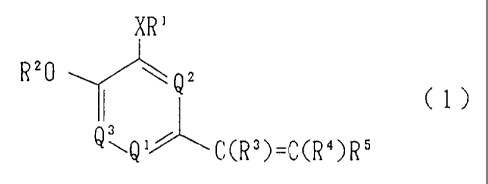
Accordingly, the present invention provides a
substituted vinylpyridine derivative represented by the
following formula (1):
XR'
Rz0 w Q2
~l)
~J
\ 1~ ' 3 _ d S
Q (,CR )-C(R )R
CA 02236851 1998-OS-06
wherein R1 represents a hydrogen atom, an alkyl group, an
alkenyl group, a hydroxya.lkyl group which may have a
substituent, an alkoxyalk:yl group, an alkoxycarbonyl alkyl
group, an alkoxyalkoxyalkyl group, an aminoalkyl group which
may have a substituent, a saturated heterocyclic group which
may have a substituent, an aralkyl group which may have a
substituent, a benzocycloalkyl group which may have a
substituent, or an alkyl group having a heterocyclic group
which may have a substituent; R' represents an alkyl group;
one o.f R3 and R4, which are different from each other,
represents a hydrogen atom and the other represents a
nitrile group, a carboxyl group, or an alkoxycarbonyl group;
R5 represents a monocyclic or ring-condensed aryl group
which may have a substituent or a monocyclic or ring-
condensed heteroaryl group wh-wch may have a substituent; X
represents an oxygen atom or a sulfur atom; and one of Q1,
Q2, and Q3 represents a nitrogen atom and the other two
represent CH; as well as a salt of the derivative, a hydrate
of the derivative, or an N-oxide of the derivative. _ _
The present invention also provides a drug containing
as the active ingredient a substituted vinylpyridine
derivative represented by the above-described formula (1), a
salt thereof, a hydrate thereof, or an N-oxide thereof.
The present invention also provides a pharmaceutical
composition containing a substituted vinylpyridine
derivative represented by the above-described formula (1), a
salt thereof, a hydrate thereof, or an N-oxide thereof; and
6
CA 02236851 1998-OS-06
a pharmacologically acceptable carrier.
The present invention further provides use, as a drug,
of a substituted vinylpyridine derivative represented by the
above-described formula (1), a salt thereof, a hydrate
thereof, or an N-oxide thereof:.
The present invention still further provides a
preventive or therapeutic method for a disease caused by the
production of PDE IV or Tt~F-a., which method comprises the
step of administering to a manunal including a human an
effective amount of a substituted vinylpyridine derivative
represented by the above-described formula (1), a salt
thereof, a hydrate thereof, or an N-oxide thereof.
BEST MODE FOR CARRYING OUT THE; INVENTION
In the substituted vinylpyridine derivative of formula
(1) of the present invention, examples of alkyl groups
represented by R1 include Cl-12 linear, branched, cyclic,
cyclic-linear, or cyclic-:branc:hed alkyl groups: Of these,
linear or branched alkyl groups are preferably Cl-8 alkyl
groups, and examples include methyl, ethyl, n-propyl, i- _ _
propyl, n-butyl, i-butyl, n-pentyl, i-pentyl, n-hexyl, n-
heptyl, and n-octyl. Cyc=_ic alkyl groups are preferably C3-
8 cycloalkyl groups, and examples include cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and
norbornyl. Cyclic-linear or cyclic-branched alkyl groups
are preferably C4-12 alkyl groups, and examples include
cyclopropylmethyl, cyclobutylmethyl, cyclopentylmethyl,
cyclopropylethyl, cyclobutylethyl, and cyclopentylethyl.
7
CA 02236851 1998-OS-06
Examples of alkenyl groups include C2-12 linear,
branched, or cyclic alker,.yl groups. Of these, C5-8 cyclic
alkenyl groups are preferred, and examples include 3-
cyclopentenyl, 2-cyclohexenyl, 3-cyclohexenyl, 4-
cycloheptenyl, and norborneny:L.
Hydroxyalkyl groups may be linear, branched, or cyclic,
and may be substituted with one or more hydroxy groups.
Hydroxyalkyl groups have preferably 2-12 carbon atoms, more
preferably 2-8 carbon atoms. Examples of liner or branched
hydroxyalkyl groups include 2-hydroxyethyl, 3-hydroxypropyl,
4-hydroxybutyl, 5-hydroxypentyl, and 6-hydroxyhexyl. Cyclic
hydroxyalkyl groups are preferably C4-8 hydroxycycloalkyl
groups, and examples include 3-hydroxycyclobutyl, 3-
hydroxycyclopentyl, 3-hydroxycyclohexyl, and 4-
hydroxycyclohexyl. Dihyd:roxyalkyl groups are preferably C3-
7 dihydroxyalkyl groups, and examples include 1,3-dihydroxy-
2-propyl, 1,5-dihydroxypentyl, and 1,7-dihydroxyheptyl. A
hydroxy group of these hydroxyalkyl groups may be
substituted with an alkoxycarbonyl group, an acyl group, or
a TBS (t-butyldimethylsilyl) group.
Alkoxyalkyl groups are preferably those whose total
carbon number is 2-12, and examples include methoxymethyl,
methoxyethyl, and ethoxyethyl.
Alkoxyalkoxyalkyl groups are preferably those whose
total carbon number is 3-12, and examples include
methoxyethoxymethyl.
Alkoxycarbonylalkyl groups are preferably those whose
8
CA 02236851 1998-OS-06
total carbon number is 3--13, and examples include
methoxycarbonylmethyl and ethoxycarbonylethyl.
Examples of aminoalkyl groups which may be substituted
include C2-12 linear or branched aminoalkyl or diaminealkyl
groups. Of these, C2-8 linear or branched aminoalkyl groups
are preferred, and examples include 2-aminoethyl, 3-
aminopropyl, 4-aminobutyl, 5-aminopentyl, and 6-aminohexyl.
Diaminoalkyl groups are preferably those having 3-7 carbon
atoms, and examples include 1,3-diamino-2-propyl, 1,5-
diaminopentyl, and 1,7-diaminoheptyl. An amino group of
these aminoalkyl groups m.ay be substituted with an
alkoxycarbonyl group, an acyl group, etc.
Saturated heterocyclic groups include a 5-6-membered
heterocycle having an oxygen atom, a sulfur atom, or a
nitrogen atom as a hetero atom. Examples of these include
2-tetrahydropyranyl, 3-tetrahydropyranyl, 2-
tetrahydrofuranyl, and 3-tetrahydrofuranyl.
Examples of aralkyl groups which may be substituted
include benzyl, phenethyl, phenylpropyl, and phenylbutyl; _ w
benzyl, phenethyl, and phenylpropyl having one or plurality
o f methoxy group ( s ) , al koxycarbonyl group ( s ) , or
alkylenedioxy groups) at o-, m-, and/or p-position. The
alkoxy groups preferably have 1-6 carbon atom(s), and
examples include methoxy, ethoxy, n-propoxy, and i-propoxy.
Benzocycloalkyl groups which may be substituted have
9-11 carbon atoms, and examples include 1-.indanyl, 2-indanyl,
1,2,3,4-tetrahydro-1-naphthyl and 1,2,3,4-tetrahydro-2-
a
CA 02236851 1998-OS-06
naphthyl.
Examples of alkyl groups which may have a (optionally
substituted) heterocyclic: group include C1-5 linear alkyl
groups substituted with an aromatic heterocycle, a saturated
heterocycle, or an unsaturated heterocycle. Of these,
aromatic heterocycles may be 5- or 6-membered heteroaryl
groups having 1-3 nitrogen atom(s), oxygen atom(s), or
sulfur atom(s), and exam~~les include 2-pyridyl, 3-pyridyl,
4-pyridyl, 2-pyrimidyl, 2-pyrazyl, 2-thiazolyl, 5-thiazolyl,
4-methyl-5-thiazolyl, 1-imidazolyl, 2-imidazolyl, 3-
imidazolyl, 2-oxazolyl, 2-thienyl, 3-thienyl, and 2-furanyl.
Saturated or unsaturated heterocycles may be 5-7-member
groups having 1-3 nitrogen atom(s), oxygen atom(s), or
sulfur atom(s), and examples include 1-pyrrolidyl, 1-
piperidyl, 1-azepanyl, 1-morpholino, pyrrolidin-2-on-1-yl,
and pyridin-2-on-1-yl.
Substituents in benzocyc_Loalkyl groups or a
heterocyclic group of heterocycle-substituted alkyl groups
may be 1-3 groups) selected from hydroxy, halogeno, C1-6
alkyl, C1-6 alkoxy, C1-6 halogenoalkyl, cyano, and nitro.
Alkyl groups represented by Rz preferably have 1-6
carbon atom(s), and examples include methyl, ethyl, n-propyl,
i-propyl, n-butyl, i-butyl, sec-butyl, and tert-butyl.
Alkoxy groups of alkoxycarbonyl groups represented by
R3 and R~ preferably have 1-6 carbon atom(s), and examples
include methoxy, ethoxy, n-propoxy, and i-propoxy.
Examples of monocyclic (optionally substituted) aryl
1. 0
CA 02236851 1998-OS-06
groups represented by RS include a phenyl group which may be
substituted with 1-3 groL.p(s) selected from halogeno, Cl-6
alkyl, Cl-6 alkoxy, C1-6 halogenoalkyl, C1-6 alkoxycarbonyl,
carboxyl, cyano, and nitro. Examples of these include
phenyl, 2-fluorophenyl, 2-chlorophenyl, 2-bromophenyl, 2-
methylphenyl, 2-ethylphenyl, 2-i-propylphenyl, 2-t-
butylphenyl, 2-methoxyphenyl, 2-trifluoromethylphenyl, 2-
cyanophenyl, 2-nitrophenyl, 2-carboxyphenyl, 2-
methoxycarbonylphenyl, 2-ethoxycarbonylphenyl, 3-
carboxyphenyl, 3-methoxycarbonylphenyl, 3-
ethoxycarbonylphenyl, 4-fluorophenyl, 4-chlorophenyl, 4-
bromophenyl, 4-trifluoromethy,-phenyl, 4-cyanophenyl, 4-
nitrophenyl, 4-carboxyphenyl, 4-methoxycarbonylphenyl, 4-
ethoxycarbonylphenyl, 2,6-difluorophenyl, 2,6-dichlorophenyl,
2,6-dibromophenyl, 2,6-dimethylphenyl, 2,6-dimethoxyphenyl,
and 2,6-ditrifluoromethylphenyl.
Examples of condensed-ring (optionally substituted)
aryl groups include a naphthyl group which may be
substituted with 1-3 groups) selected from halogeno, Cl-6_
alkyl, Cl-6 alkoxy, Cl-6 halogenoalkyl, Cl-6 alkoxycarbonyl,
carboxyl, cyano, and nitro. Examples of these include 1-
naphthyl, 2-naphthyl, 2-c:hloro-1-naphthyl, and 2-methoxy-1-
naphthyl.
Examples of monocyclic (optionally substituted)
heteroaryl groups include a 5-6-membered heteroaryl group
(having 1-3 atoms) of nitrogen, oxygen, o~ sulfur) which
may be substituted with 1-3 groups) selected from halogeno,
11
CA 02236851 1998-OS-06
C1-6 alkyl, C1-6 alkoxy, Cl-6 halogenoalkyl, C1-6
alkoxycarbonyl, carboxyl, cyano, and nitro. Examples of
these include 2-pyridyl, 3-pyridyl, 4-pyridyl, 2-carboxy-4-
pyridyl, 2-methoxycarbonyl-4-pyridyl, 2-ethoxycarbonyl-4-
pyridyl, 3-chloro-4-pyriclyl, 3-bromo-4-pyridyl, 3-methoxy-4-
pyridyl, 3,5-dichloro-4-pyridyl, 3,5-dibromo-4-pyridyl, 3,5-
dimethoxy-4-pyridyl, 3-ch.loro-5-methoxy-4-pyridyl, 2-
pyrimidyl, 2-pyrazyl, 2-thienyl, 3-thienyl, and 2-furanyl.
Examples of condensed-ring (optionally substituted)
heteroaryl groups include a condensed-ring heteroaryl group
(containing a nitrogen atom) which may be substituted with
1-3 groups) selected from halogeno, Cl-6 alkyl, C1-6 alkoxy,
Cl-6 halogenoalkyl, C1-6 alkoxycarbonyl, carboxyl, cyano,
and vitro. Examples of these include 2-quinolyl, 4-quinolyl,
and 1-isoquinolyl.
Examples of salts or hydrates of the substituted
vinylpyridine derivative of formula (1) of the present
invention include hydrochlorides, nitrates, hydrobromides,
p-toluenesulfonates, methanesulfonates, fumarates, maleates,_
malonates, succinates, citrates, tartarates, and hydrates
thereof. Examples of N-oxides of the substituted
vinylpyridine derivative incul.de pyridine-N-oxides and N-
oxides of a monocyclic or condensed-ring heteroaryl group
represented by R5.
The substituted vinylpyridine derivative of the
present invention is prepared by, for example, the following
reaction scheme. Briefly, a known compound (2) is easily
12
i i
CA 02236851 2005-05-17
derived from kojic acid (which is inexpensive and available
in large quantities) through two or three reactions (Step
1~; the known compound (2) is processed to yield a key
intermediate (3) or (4) of the synthesis of the present
invention (Step 2 or 3); and the intermediate is condensed
through reaction with commercially available (or separately
synthesized) arylaldehydes (R5-CHO), arylacetonitriles or
arylacetate esters (RS-CFiZR') to thereby obtain a compound
(la) of the present invention. ,
13
CA 02236851 1998-OS-06
Kojic acid
Step 1
OH
Rz0 \
N~ CHZOY
(2)
Step 3 ~ Step 2
OR'° OR~n
2
R 0 \ R20 \
N CHO N ~CH2R6
C4) fig)
R5-CH2R' -H20
-H20 5-CHO
Step 5 \, Step 4
1
R20 \ _ _
~N~ CCRs)=CCR')R5
1 a)
wherein one of R6 and R', which are different from each
other, represents a hydrogen atom and the other represents a
nitrile group or an alkoxycarbonyl group; Y represents a
hydrogen atom or a proteci~ive group (preferably benzyl or
14
CA 02236851 1998-OS-06
tetrahydro-2-pyranyl); R2 and R' have the same meanings as
described above; R'a is identical to R1 except when R' is a
hydrogen atom, with the ~~resence of a protective group being
preferred when Rla is a hvdroxyalkyl group.
In other words, the key intermediate (3) is reacted
with RS-CHO (Step 4) or t:he key intermediate (4) is reacted
with R'-CHAR' (Step 5) to thereby obtain a compound (la) .
These reactions easily proceed in the presence of a base
such as sodium alkoxide, sodium amide, alkali hydroxide,
alkyllithium, or a tertiary alkylamine. These reactions are
preferably conducted in methanol with sodium methoxide or ir~
ethanol with sodium ethoxide in the temperature range from
0°C to room temperature.
The above-described key intermediate (3) may be easily
obtained from the known compound (2) through the following
reaction scheme.
OR'a
~a
2 ) R--~ -~ R ---
Step 2a
Step 2b
N CHzOY -
(5)
OR'°
R20
----~ c 3
N~ CH2C.C Step 2c
C6)
wherein R1a and R', and Y have the same meanings as described
above and Z represents a Leaving group (typically a halogen
CA 02236851 2005-05-17
atom) .
The compound (2) is reacted with halide reagents (Rla_
Z) to obtain a compound (5). If the compound (5) is having a
protective group in Y, then it is de-protected and a compound
(5: Y = a hydrogen atom) is derived therefrom (Step 2a).
Alternatively, the compound (2: Y = a protective group) is
converted to the compound (5: Y = protective group) by
Mitsunobu reaction with primary or secondary alcohols and
the protective group is removed to thereby obtain the
compound (5: Y = a hydrogen atom). Next, the compound (5: Y
- a hydrogen atom) is converted to a chloro compound (6)
(Step 2b). The compound (6) is further~reacted with M-CN to
obtain the key intermediate (3: R6 = a nitrile group), whose
nitrile group undergoes an alcoholysis to obtain the other
key intermediate (3: R6 = an alkoxycarbonyl group) (Step 2c).
Reactions of Step 2a are preferably carried out in a
solvent such as an alcohol, tetrahydrofuran,
dimethylformamide, or dimethyl sulfoxide in the presence of
a base such as potassium carbonate, sodium carbonate, or, in_
some cases, potassium iodide, or sodium iodide in the
temperature range from room temperature to 80°C; or in a
water-alcohol mixed solvent in the presence of sodium
hydroxide or potassium hydroxide as a base in the
temperature range from 0°C to the reflux temperature. Also,
the reaction between the compound (2: Y = a protective
group) and R'°-Z proceeds easily, under conditions other
16
CA 02236851 1998-OS-06
than the above-described reaction conditions, i.e., in a
solvent such as terahydrofuran, 1,2-dimethoxyethane, dioxane,
dimethylformamide, or dirnethyl sulfoxide in the presence of
sodium hydride or potass_Lum hydride as a base in the
temperature range from 0"C to room temperature. The
reaction between the compound (2: Y = a protective group)
and primary or secondary alcohols easily proceeds to yield
the compound (5: Y = a protective group) by the typical
conditions of Mitsunobu reaction, i.e., in the presence of
diethyl azodicarboxylate and triphenylphosphine.
In Step 2a, preferred examples of substituents of Rla
in~~lude alkyl, cycloalkyl, cycloalkylalkyl, hydroxyalkyl,
anc~ (optionally substituted) aralkyl. By Mitsunobu reaction
to obtain the compound (5: Y = a protective group), a
substituent such as cycloalkyl, cycloalkenyl,
helrerocycloalkyl, or benzocycloalkyl is preferred.
When the compound (5: Y = a protective group) is de-
protected, there are employed conditions such as
hydrogenation by use of a catalyst such as palladium or
Raney nickel; reductive removal by use of a compound such as
ammonium formate, cyclopentene, or 1,4-cyclohexadiene (for
benzyl-substituted compound); or hydrolysis in a water-
organic solvent with a mineral acid or an organic acid (for
tetrahydro-2-pyranyl comp~~und).
The reaction between thionyl chloride and the compound
(5) proceeds easily without or within a solvent inert to
thionyl chloride at room temperature to obtain the chloro
17
CA 02236851 1998-OS-06
compound (6) from the cornpound (5: Y = a hydrogen atom)
(Step 2b).
Step 2c, in which the key intermediate (3: R° - a
nitrite group) is obtained from the chloro compound (6), is
preferably carried out in a polar and aprotic solvent such
as dimethyl sulfoxide or dimethylformamide in the presence
of sodium cyanide in the temperature range from room
temperature to 100°C. The also reaction is easily performed
through the cyano-anion-activation method using a phase
transfer catalyst or crown ether.
The key intermediate (3: Rb - an alkoxycarbonyl group)
is obtained from the key intermediate (3: R~ - a nitrite
group) through conversion. of the nitrite group in hydrogen -
ch:Loride-gas-saturated methanol or a lower alcohol in the
temperature range from room temperature to the reflux
temperature.
The above-described key intermediate (4) may be easily
obtained from the known compound (2) through the following
reaction scheme .
(5)
Step 2a Step 3b
C2) C4)
Step 3a OH
RZ() Step 3c
N ~ CHO
(7)
18
CA 02236851 1998-OS-06
wherein R2 has the same meaning as described above.
The formyl compound (4) is obtained through conversion
of the compound (5) obtained in the above-described Step 2a
by use of an oxidant (StE:p 3b); or through oxidation of the
compound (2) to obtain a compound (7) (Step 3a), followed by
reaction with the halide reagent (Rla-Z) (Step 3c).
Step 3a, in which the compound (7) is obtained from
thE: compound (2), is preferably carried out in a solvent
such as tetrahydrofuran, 1,4-dioxane, or dimethylformamide
with an excessive amount of active manganese dioxide or
barium manganate(VI) as an oxidant in the temperature range
from room temperature to 100°C.
Step 3b, in which the formyl compound (4) is obtained
from the compound (5), is easily carried out in a solvent
such as chloroform, dichl~oromethane, or acetone with an
excessive amount of active manganese dioxide or barium
manganate(VI) as an oxidant in the temperature range from
room temperature to the reflux temperature; or through
oxidation by a dimethyl sulfoxide/sulfur trioxide-pyridine- -
complex (Parikh-Doering method) or oxidation by a dimethyl
sulfoxide/oxalyl chloride (Swern method). Also, the formyl
compound (4) may be obtained through oxidation by pyridinium
chlorochromate (PCC) or pyridinium dichromate (PDC).
Step 3c, in which thE: formyl compound (4) is obatined
from the compound (7), is carried out through reaction with
Rla-Z in a solvent such as tetrahydrofuran, 1,2- '
dimE:thoxyethane, dimethylformamide, or dimethyl sulfoxide
19
CA 02236851 1998-OS-06
with a base such as sodium hydride or potassium hydride in
the temperature range from 0°C to room temperature; or in a
solvent such as an alcohol, tetrahydrofuran,
dimethylformamide, or dinethyl sulfoxide with a base such as
potassium carbonate or sodium carbonate, or in some cases,
potassium iodide or sodium iodide in the temperature range
from 0°C to 80°C.
The compound (1b') or (lb") of the present invention
is obtained through removal of an oxyalkyi group from the
compound (1a) of the present invention having a
me~~hoxymethyl or methoxyethoxymethyl group as R1 and a
hydrogen atom as R' (1a') or as R6 (la").
OH
R2~~ \
(la'
N ~ C(Rs)=CHRS
(lb'
OH
R20 \
Cla" ) -~
N CH=CCR')R5
Clb"
wherein R', R5, R6, and R~ have the same meanings as
described above.
De-protection of the compound (la') or (la") of the
present invention is carried out by use of trifluoroacetic
acid or diluted acetic ac=Ld (for R1 - a methoxymethyl group)
or trifluoroacetic acid (for R1 - a methoxyethoxymethyl
CA 02236851 1998-OS-06
group ) .
The compound (lc') o r (lc") of the present invention
is obtained through hydrolysis of the compound (la) of the
present invention having an alkoxycarbonyl group as Rb and a
hydrogen atom as R' (la'''), or a hydrogen atom as R'' and an
alkoxycarbonyl group as F;~ (la"").
OR'
HO- R~0 \
(1a"' )
N ~ CCC02H)=CHRS
(lc' )
OR'
R20 \
Cla~",) H~
N ~ CH=C(COzH)RS
Clc" )
wherein R1, R', and RS have the same meanings as described
above.
The above hydrolysis reaction is carried out under
al~;aline conditions through a widely employed method in
which hydrolysis is allowed to proceed in a lower alcohol,
by use of diluted NaOH aqueous solution or diluted KOH
aqL.eous solution in a temperature range from room
ten.perature to reflux tem~~erature.
Compound (la) may also be obtained by reacting
compound (lb') or (lb ") of the present invention with a
halide reagent (Rla-Z) in the presence of a base, or
alternatively, by Mitsunobu reaction between compound (lb')
21
CA 02236851 1998-OS-06
or (lb" ) and a primary or secondary alcohol (R'a-OH) .
Ills-z ~r R1~
(1 b' ) or (1 b" ) (1 a)
The substituent-introduction reaction by use of a
halide reagent (R1~-Z) is carried out in a solvent such as
alcohol, tetrahydrofuran, dimethylformamide, or dimethyl
su:Lfoxide, in the presence of a base such as potassium
ca..bonate or sodium carbonate, or in some cases potassium
iodide or sodium iodide, in a temperature range from room
temperature to 80°C. Alternatively, this reaction is
carried out in a solvent such as tetrahydrofuran, 1,2-
dirlethoxyethane, dioxane, dimethylformamide, or dimethyl
su7.foxide, by use of sodium hydride or potassium hydride as
a base, in a temperature range from 0°C tc room temperature.
The substituent-introduction reaction through use of a
primary or secondary alcohol (R'3-OH) proceeds easily by the
ty~~ical conditions of Mitsunobu reaction; i.e., in the
presence of diethyl azodicarboxylate and triphenylphosphine.y
Among the formula (1) compounds of the present
invention, those compounds (ld) in which X is a sulfur atom,
are derived using the below-described scheme from the
compound (2').
22
CA 02236851 1998-OS-06
OH
R20 \ R20
POC .~ 3 I \ ~~1-CN
N~ CH20H N~ CH2C.~
c2' ) cs)
C.~ sR~ a
R20 \ R'°SH,K2C0~ R20 \ RS-CHO
--
-HZO
N CHZCN N ~ CHZCN
) (10)
SR'°
RZO \
- N~ CCCM=CHRS
(ld)
wherein Rla, Rz, and R'' hazre the same meanings as described
above, and M represents an alkali metal.
The compound (2') is first transformed to a dichloro
compound (8), then to an acetonitrile compound (9) through_
reaction with M-CN, and after being introduced with an RlaS
group to a key intermediate (10). The thus-obtained
compound (10) is easily derived to compound (ld) of the
present invention through a condensation reaction with RS-
CHC~ .
The reactions for obtaining the compound (9) from the
compound (8) and obtaining the compound (ld) of the present
invention from the key intermediate (10) proceed under
23
CA 02236851 1998-OS-06
conditions same as those described above. The reaction for
obtaining a dichloro compound (8) from the compound (2') is
carried out through reflux with heat in phosphorus
oxychloride. Particularly, the reaction for obtaining the
key intermediate (10) from the compound (9) is preferably
carried out in a solvent such as alcohol, tetrahydrofuran,
dimethylformamide, or dirnethyl sulfoxide, in the presence of
a base such as potassium carbonate or sodium carbonate in a
temperature range from room temperature to 80°C.
When the compound (a?) or (2') is replaced by a 6-
alkoxy-5-hydroxy-3-pyridinemethanol (Q3=N) or 5-alkoxy-6-
hydroxy-2-picoline (Q'=N) , a compound of formula ( 1 ) in
which Qz or Q3 is a nitrogen atom can be prepared.
In the above reactions, separation of compound (1) of
the present invention frc>m reaction mixtures is carried out
using a customary method, e.g., extraction with a solvent,
recrystalization, or column chromatography.
The thus-obtained compound of the present invention
exhibits selective and strong PDE IV inhibitory action and_
inhibitory action against the production of TNF-a..
Therefore, the compound is useful as a PDE IV inhibitor and
a ~CNF-a-production inhibitor, and also as a drug led by one
for the prevention and treatment of diseases involving PDE
IV and/or TNF-a. The drug of the present invention is
useful as a therapeutic agent for the prevention and
treatment of immediate or delayed asthma, allergies such as
airway-hypersensitive allergy and other allergies stemming
24
CA 02236851 1998-OS-06
from the inhibition of acaivation of inflammatory blood
cells such as eosinocyte:>, autoimmune diseases such as atopy
and rheumatism, depression associated with disturbed
metabolism of the cerebrum, cerebral infarction, senile
dementia, and memory disorders associated with Parkinson's
disease, as well as for osteoporosis, type I and type II
diabetes, inflammations, cancers, infections with HIV, AIDS,
an~~ shock caused by intracellular toxins.
The compounds of thE: present invention may be
processed into drugs having a variety of forms, including
tablets, granules, powders, capsules, inhalants, suspensions,
injections, suppositories, and external preparations. When
so:Lid preparations are formed, the compound of the present
in~rention is preferably mixed with a vehicle and if
necessary with a binder, a disintegrant, an extender, a
coating agent, an agent for sugar-coating, etc., and is
subsequently formed into tablets, granules, capsules,
suppositories, etc. When injections are prepared, the
compound of the present invention is dissolved, dispersed,_
or emulsified in an aqueous carrier for injections in
ad~rance, or alternatively the compound is dissolved or
dig>persed, or suspended upon use of the compound. Injection
preparations may be used by way of intravenous
administration, arterial administration, intraperitoneal
administration, subcutaneous administration, or dripping.
When the compounds of the present invention are used
as preventive or therapeutic drugs for the aforementioned
CA 02236851 1998-OS-06
diseases, their doses, which may differ in accordance with
the manner of administrat=ion and the age, body weight, and
conditions of the patient., are preferably 5-100 mg/day in
the case of oral administration to an adult.
EXAMPLES
The present invention will next be described by way of
examples, which should not be construed as limiting the
invention thereto.
Preparation Example 1
Synthesis of 4-subst=ituted-5-alkoxy-2-
pyridinemethanols (5) (Process 1):
A 5-alkoxy-4-hydroxy-2-pyridinemethanol (2) (100 mmol)
was dissolved in dimethylformamide (100 ml). To the
so.Lution were added pota~.sium carbonate (150 mmol) and
potassium iodide (3 mmol). While the mixture was stirred on
a 65°C oil bath, a halide reagent (bromocyclopentane in the
care of preparation of compound (5a)) (130 mmol) was added
dropwise over one hour. 'rhe mixture was stirred for 6-12
additional hours under the same conditions. After being
cooled, the reaction mixture was poured into cold water and
extracted with ethyl acetate. The organic layer was
se<~uentially washed with water and saturated brine, dried,
anc~ then concentrated under reduced pressure. The residue
wa~~ purified by recrystallization or silica gel column
chromatography, to thereby obtain compounds (5a) through
(5cl) shown below.
26
CA 02236851 1998-OS-06
4-Cyclopentyloxy-5-methcxy-2-pyridinemethanol (5a)
'H-NM1R(CDC.~ 3) ~ : 1. 40-2. 20(8H, m), 3. 90(3H, s), =~. 68(2H, s),
4. 70-5. 00(1H, m), 5. 35(1H, s). 6. 82(1H, s), 8. 04(1H, s).
4-Cyclopentyloxy-5-ethoxy-2-pyridinemethanol (5b)
'H-N~9R(CDC.~ a) ~ : 1. 42(~~H, t, J=7. OHz), 1. 50-2. 10(8H, m),
4. 09(~;H, q, J=7. OHz), 4. 65C2H, s), 4. 70-4. 90(1H, m).
6. 73(1H, s), 8. 05(1H, s).
5-Methoxy-4-phenetlzyloxy-2-pyridinemethanol (Sc)
'H-N~9R(CDC.~ 3) ~ : 3. 18(.2H, t, J=7. OHz), 3. 91(3H, s).
4. 26(.2H, t, J=7. OHz), 4. 63(2H, s), 6. 75(1H, s),
7. 29(.SH, s), 8. 05(1H, s).
5-Methoxy-4-(3-phenylpropyloxy)-2-pyridinemethanol
(5d)
'H-NMR(CDC.~ 3) ~ : 2. 00-2. 40(2H, m), 2. 83(2H, t, J=8. OHz),
3. 75(1H, br), 3. 92(3H, s), 4. 05(2H, t, J=8. OHz),
4. 63(2H, s), 6. 70(1H, s), 7. 24(5H, s), 8. 04(1H, s).
4-Butyloxy-5-methoxy-2-pyridinemethanol (5e)
'H-N~~(R(CDC.~ 3) ~ : 0. 98(3H, t. J=7. OHz), 1. 30-2. 00(4H, m), 3. 91(3H,
s),
4. 07(2H, t, J=7. OHz), 4. 66(2H, s), 6. 79(1H, s), _
8. 03(1H, s).
4-(1-Ethylpropyloxy)-5-methoxy-2-pyridinemethanol (5f)
'H-NO~fR(CDC.~ 3) ~ : 0. 96(6I-, t, J=7. OHz), 1. 58-2. 00(4H, m),
3. 90(3H, s), 4. 10-4. 40(1H, m), 4. 65(2H, s), 6. 75(1H, s),
8. 04(1H, s).
5-Methoxy-4-methoxymethyloxy-2-pyridinemethanol (5g)
'H-N~~(RCCDC.~ 3) ~ : 3.51(3H, s), 3. 95(3H, s). 4. 66(2H. s). 5. 31(2H. s).
7. 0=~(1H, s), 8. 11 (1H, s).
27
CA 02236851 1998-OS-06
Preparation Example 2
Synthesis of 4-subst=ituted-5-alkoxy-2-
benzyloxymethylpyridines (5'):
A 5-alkoxy-2-benzyloxymethyl-4-hydroxypyridine (2) (10
mmol), a secondary alcohol (2-indanol in the case of
preparation of compound (5h')) (12.5 mmol), and
triphenylphosphine (15 mniol) were dissolved in
tetrahydrofuran (300 ml). Diethyl azodicarboxylate (15
mm~~l) was added dropwise to the solution under stirring at
ro«m temperature, and the mixture was stirred for one hour.
After addition of water, the reaction mixture was extracted
wivh in chloroform. The organic layer was sequentially
wa:7hed with water and saturated brine, dried, and then
concentrated under reduced pressure. An ether (100 ml) was
added to the residue and insoluble substances were removed
by filtration. After con~~entration of the filtrate, the
residue was purified by recrystallization or silica gel
co__umn chromatography, to thereby obtain compounds (5h')
through (5j') shown below.
2-Benzyloxymethyl-4-(2-indanyloxy)-5-methoxypyridine
( 5h' )
'H-N~~IR(CDC~ 3) ~ : 3. 00-3. 60(4H, m). 3. 83(3H, s). 4. 64(4H, s).
5: 10-5. 40C1H, m), 7. 06(1H, s), 7. 10-r. 50(9H, m),
8. 06(1H, s).
2-Benzyloxymethyl-5-methoxy-4-(exo-2-
norbornyloxy)pyridine (5i')
28
CA 02236851 1998-OS-06
'H-NhIR(CDG.~ 3) ~ : 1. 00-2. 65(IOH, m), 3. 90(3H, s).
4. 10-4. 90(1H, m), 4. 60(4H, s), 6. 95(1H, s),
7. 36(5H, s). 8. 0~(1H, s).
2-Benzyloxymethyl-5--methoxy-4-(tetrahydro-3-
furanyloxy)pyridine (5j')
'H-N6(R(CDC.~ 3) ~ : 2. 05-2. ~0(2H, m), 3. 80-4. 10(4H, m), 3. 91(3H, s),
4. 60(2H. s), 4. 62(2H, s), ~. 90-5. 10(1H, m),
6. 92(1H, s), 7. 36(5H, s), 8. 08(1H, s).
Preparation Example 3
Synthesis of 4-subst=ituted-5-alkoxy-2-
py:ridinemethanols (5) (Process 2):
A 4-substituted-5-a~_koxy-2-benzyloxymethylpyridine
(5') (10 mmol) was dissolved in acetic acid (50 ml). Pd-
black (2 g) was added to the solution, which was
hydrogenated at room tem~~erature for 2-6 hours. After
removal of the catalyst, the filtrate was concentrated under
reduced pressure. The residue was fractionated by a
chloroform-aqueous saturated sodium hydrogencarbonate
so=Lution. The organic layer was dried and concentrated _ w
under reduced pressure. The obtained crystalline residue
was optionally recrystallized, to thereby obtain compounds
(5h) through (5j) shown below.
4-(2-Indanyloxy)-5-methoxy-2-pyridinemethanol (5h)
'H-NMRCCDC.~ 3) ~ : 3. 00-3. 55(4H, m), 3. 84(3H, s), 4. 69(2H, s),
5. 10-5. ~0(1H, m), 6. 85(1H, s), 7. 10-7. 40(4H, m).
8. 04(1H~ s).
5-Methoxy-4-(exo-2-n.orbornyloxy)-2-pyridinemethanol
29
CA 02236851 1998-OS-06
(5i)
1 H-N~IR CCDC .~ 3 ) ~ : 1. 00-2. 00 (8H, m) . 2. 30-2. 60 C2H, m) , 3. 90
(3H, s) ,
4. 10-4. 40(1H, m), 4. 65(2H, s), 6. 69(1H, s),
8. 03(1H, s).
5-Methoxy-4-(tetrahydro-3-furanyloxy)-2-
pyridinemethanol (5j)
H-NMR (CDC .~ 3 ) ~ : 2. 10-2., 40 (2H, m) , 3. 80-4. 20 C4H, m) , 3. 91 C3H,
s) ,
4. 66(2H, s), 4. 90-5. 10(1H, m). 6. 71 (1H, s). 8. 07(1H, s).
Preparation Example 4
Synthesis of 4-substituted-5-alkoxy-2-
chloromethylpyridines (6);
A 4-substituted-5-al:koxy-2-pyridinemethanol (5) (0.2
mol) was dissolved in dichloromethane (200 ml). Thionyl
chloride (0.3 mol) was added dropwise to the solution under
stirring at 5°C. The mixture was allowed to react for 30
minutes under the same conditions. The resultant solution
was concentrated under reduced pressure and the residue was
fractionated by a chloroform-aqueous saturated sodium _
hydrogencarbonate solution. The organic layer was dried and
concentrated under reduced pressure. Obtained crystalline
or oily compounds (6a) through (6j) were used for the
subsequent reaction without additional purification.
2-Chloromethyl-4-cyc:Lopentyloxy-5-methoxypyridine (6a)
'H-NN1R(CDC.~ 3) ~ : 1. 40-2. 10C8H, m), 3. 90C3H, s). 4. 60C2H, s).
4. 75-4. !37(1H, m), 6. 96(1H, s). 8. 07(1H, s).
CA 02236851 1998-OS-06
2-Chloromethyl-4-cyclopentyloxy-5-ethoxypyridine (6b)
'H-N~~(RCCDC.~ 3) ~ : 1. 40(3H, t, J=7. OHz), 1. 60-2. 10(8H, m).
4. 11 (2H, q, J=7. OHz), 4. 59(2H, s), 4. 70-4. 90(1H, m),
6. 95(1H, s). 8. 05(1H, s).
2-Chloromethyl-5-met::~oxy-4-phenethyloxypyridine (6c)
'H-Nh~IRCCDC,~ 3) S : 3. 18C2H, t, J=7.OHz), 3. 92(3H, s),
4. 27(2H, t, J=7. OHz), 4. 57(2H, s). 6. 94C1H. s),
7. 30(5H, s), 8. 06(1H, s).
2-Chloromethyl-5-met:hoxy-4-(3-phenylpropyloxy)pyridine
(6d)
' H-NMR (CDC .~ 3 ) ~ : 2. 00-2. 40 C2H, m) , 2. 83 C2H, t , J=8. OHz) ,
3. 93(3H, s), 4. 07(2H, t. J=8. OHz). 4. 57(2H, s).
6. 88C1H, s). 7. 24C5H, s), 8. 06(1H, s).
4-Butyloxy-2-chloromethyl-5-methoxypyridine (6e)
'H-Nh(RCCDC.~ 3) s : 0. 99C3H, t, J=7. OHz), 1. 30-2. 00C4H, m),
3. 93(3H, s), 4. 09C2H, t, J=7. OHz), 4. 60(2H, s),
6. 97C1H, s). 8. 06(1H, s).
2-Chloromethyl-4-(1-ethylpropyloxy)-5-methoxypyridine
(6f)
'H-NM1R~CDC.~ 3) cS : 0. 98C6~1, t, J=7. OHz), 1. 58-2. 00(4H, m), 3. 92(3H,
s).
4. 10-4. 40(1H, m), 4. 60(2H, s), 6. 94(1H, s), 8. 07(1H. s).
2-Chloromethyl-5-met:hoxy-4-methoxymethyloxypyridine
(6g)
'H-N~-fR(CDC~ 3) o : 3.52C3H, s). 3.96(3H, s), 4.59(2H. s), 5. 32(2H. s).
7. 23(1H, s), 8. 13(1H, s).
2-Chloromethyl-4-(2-indanyloxy)-5-methoxypyridine (6h)
31
CA 02236851 1998-OS-06
' H-NhIR (CDC .~ 3 ) a : 3. 20 (2H. dd, J=4. 0, 17. OHz) ,
3. 50(2H, dd, J=6. 0. 17. OHz). 3. 87(3H, s), 4. 63C2H, s),
5. 15-5. 40(1H, m). 7. 0~(1H, s), 7. 23C=1H, s), 8. 06(1H, s).
2-Chloromethyl-5-metlzoxy-4-(exo-2-
norbornyloxy)pyridine (6i;~
' H-N~~IR (CDC .~ 3 ) ~ : 1. 00-2. 10 (8H, m) , 2. 25-2. 60 (2H, m) , 3. 91
(3H. s ) .
4. 15-4. 40C1H, m), 4. 60(2H, s), 6. 90(1H, s). 8. 04C1H, s).
2-Chloromethyl-5-metlzoxy-4-(tetrahydro-3-
furanyloxy)pyridine (6j)
'H-N~~IR(CDC.~ 3) o : 2. 10-2. 40(2H, m), 3. 80-4. 20(4H, m). 3. 92(3H, s),
4. 61 C2H, s), 4. 90-5. 10(1H, m), 6. 90(1H. s). 8. 08(1H. s).
Preparation Example 5
Synthesis of 4-substituted-5-alkoxy-2-
pyridineacetonitriles (3):
A 4-substituted-5-al:koxy-2-chloromethylpyridine (6)
(0.20 mol) was dissolved in dimethyl sulfoxide (200 ml).
Sodium cyanide (0.24 mol) was added to the solution and the
mixture was allowed to react at room temperature for 12 _
hours or at 100°C for one hour, depending on the reaction
rate of the substrate. Th.e reaction mixture was poured into
water (500 ml), extracted with ethyl acetate, sequentially
washed with water and saturated brine, dried, and then
concentrated under reduced pressure. The residue was
purified by recrystallizai=ion or silica gel column
chromatography, to thereby obtain compounds (3a) through
(3j) shown below.
32
CA 02236851 1998-OS-06
4-Cyclopentyloxy-5-methoxy-2-pyridineacetonitrile (3a)
'H-NMR(CDC.~ 3) o : 1. 46-2. 20(8H, m), 3. 84(2H, s). 3. 90(3H, s).
4. 72-5. 00(1H, m), 6. 90(1H, s), 8. 06(1H, s).
4-Cyclopentyloxy-5-ethoxy-2-pyridineacetonitrile (3b)
'H-N~~(RCCDC.~ 3) ~ : 1. 42(3H, t, J=7. OHz), 1. 60-2. 20C8H, m), 3. 84(2H,
s),
4. 11 (2H, q, J=7. OHz), 4. 75-5. OOC1H, m), 6. 88C1H, s),
8. 04(1H, s).
5-Methoxy-4-phenethy:Loxy-2-pyridineacetonitrile (3c)
'H-N~~(R(CDC.~ 3) ~ : 3. 18C2H, t, J=7.OHz), 3. 82C2H, s), 3. 92(3H, s),
4. 27(2H, t. J=7. OHz), 6. 86(1H. s). 7. 30(5H. s),
8. 05C1H, s).
5-Methoxy-4-(3-pheny:Lpropyloxy)-2-pyridineacetonitrile
(3d)
'H-N~dR(CDC.~ 3) S : 2. 00-2. ~O(2H, m). 2. 84(2H, t. J=8. OHz), 3. 82(2H, s),
3. 94(3H, s), 4. 08C2H. t, J=8. OHz), 6. 81C1H. s),
7. 25(5H, s), 8. 06(1H, s).
4-Butyloxy-5-methoxy--2-pyridineacetonitrile (3e)
'H-Nh~(RCCDC.~ 3) ~ : 0. 99~3H, ~;, J=7. OHz), 1. 35-2. 10(4H, m), 3. 86C2H,
s),-
3. 93C3H, ;>), 4. 09(2H, t. J=7. OHz), 6. 90(1H, s).
8. 05C1H, s).
4-(1-Ethylpropyloxy)--5-methoxy-2-pyridineacetonitrile
(3f)
'H-N~~(R(CDC.~ 3) o : 0. 98(6H, t, J=7. OHz), 1. 60-2. 00(4H, m), 3. 85(2H,
s),
3. 92(3H, s), 4. 10-4. 40(1N. m). 6. 88C1H, s),
8. 06C1H, s).
33
CA 02236851 1998-OS-06
5-Methoxy-4-methoxymethyloxy-2-pyridineacetonitrile
(3c.)
'H-NMR(CDC.~ 3) ~ : 3. 52(3H, ~), 3. 84C2H, s), 3. 96(3H, s), 5. 32(2H, s),
7. 14C1H, sO, 8. 13(1H, s).
4-(2-Indanyloxy)-S-methoxy-2-pyridineacetonitrile (3h)
'H-NII-1R(CDC.~ a) ~ : 3. 22C2H, cid. J=4. 0, 17. OHz),
3. 51 C2H, cld, J=6. 0, 17. OHz), 3. 86(3H, s), 3. 88C2H, s),
5. 10-5. 40(1H, m), 6. 97(1H. s), 7. 23(4H, s). 8. 05(1H, s).
5-Methoxy-4-(exo-2-norbornyloxy)-2-
pyridineacetonitrile (3i)
' H-N~iR CCDC .~ 3 ) c~ : 1. 10-2. 00 (8H, m) , 2. 30-2. 70 C2H, m) , 3. 85
C2H, s) ,
3. 90(3H, s), 4. 15-4. 40(1H, m). 6. 84(1H, s).
8. 03(1H, s).
5-Methoxy-4-(tetrahyc~ro-3-furanyloxy)-2-
pyridineacetonitrile (3j)
' 1~-NMR CCDC .~ 3 ) ~ : 2. 10-2. 50 C2H, m), 3. 80-4. 20 (4H, m) , 3. 86 C2H,
s ) ,
3. 92(3H, s), 4. 90-5. 20(1H, m), 7. 28(1H, s).
8. 08(1H, s).
Preparation Example 6
Synthesis of methyl ~1-cyclopentyloxy-5-methoxy-2-
pyridineacetate (3k):
A HC1-saturated methanol solution (30 ml) was added
to 4-cylopentyloxy-5-methc>xy-2-pyridineacetonitrile (3a)
(2.32 g, 10 mmol) and the solution was refluxed for 30
minutes. The reaction mixture was evaporated to dryness.
The residue was dissolved in chloroform and the solution was
34
CA 02236851 1998-OS-06
sequentially washed with ~iqueous saturated sodium
hydrogencarbonate solution and saturated brine, then dried,
and then concentrated undE:r reduced pressure, to thereby
obtain the title compound (2.36 g, yield 89~).
'H-~~lfR(CDC.~ 3) ~ : 1. 60-2. 10(81, m). 3.72C3H, s). 3. 76C2H. s).
3. 89C3H. s). ~. 70-=>~. 90C1H, m). 6. 81C1H, s).
8. 0~(1H, s~.
Preparation Example 7
Synthesis of 3,5-dichloro-4-pyridinecarbaldehyde:
Under an argon atmosphere, to a solution of
diisopropylamine (33.6 ml,. 0.24 mol) in tetrahydrofuran (400
ml) at -65°C was added a _L.6 M solution of n-butyl lithium
in hexanes (156 ml). After 20 minutes later, a solution of
3,5-dichloropyridine (29.6 g, 0.20 mol) in tetrahydrofuran
(150 ml) was added dropwise, and the mixture was stirred for
30 minutes. Subsequently, the mixture was treated with
dimethylformamide (23.2 m=L, 0.30 mol) in tetrahydrofuran (50
ml), and then stirred for one hour under the same conditions
The reaction mixture was poured into a 5% aqueous ammonium
chloride solution (1,000 rnl) and extracted with ethyl
acetate. The organic layer was sequentially washed with
water and saturated brine,. dried, and then concentrated
under reduced pressure. The residue was chromatographed on
silica gel, to thereby obt=ain the title compound (27.2 g,
yield 770).
CA 02236851 1998-OS-06
'H-N~~fR(CDC.~ 3) ~ : 8. 6a3(2H, s), 10.44(1H, s).
Preparation Example 8
Synthesis of 3,5-dim~~thoxy-4-pyridinecarbaldehyde:
In a similar manner to that in Preparation Example 7,
the title compound was prf~pared.
'H-N~~IR(CDC.~ 3) ~ : 4.02(6H, s). 8. 17(2H, s). 10. 50(1H, s).
Preparation Example 9
Synthesis of 5-alkox:y-4-chloro-2-chloromethylpyridines
(8)
Phosphorus oxychloric~e (10 ml) was added to a 5-
alkoxy-4-hydroxy-2-pyridinemethanol (10 mmol) and the
solution was refluxed for three hours. The reaction mixture
was concentrated under reduced pressure and the residue was
fractionated with a chloroform-aqueous saturated sodium
hydrogencarbonate solution. The obtained organic layer was
dried and then concentrated under reduced pressure. An
obtained crystalline compound (8a) described below was used._
for the subsequent reaction without additional purification.
4-Chloro-2-chloromethyl-5-methoxypyridine (8a)
'H-NMR(CDC.~ 3) ~ : 4. 00(3H, s). 4. 60(2H. s), 7. 50(1H, s), 8. 25(1H, s).
Preparation Example 10
Synthesis of 5-alkoxy-4-chloro-2-pyridineacetonitrile
(9) :
The procedure of Preparation Example 5 was repeated
36
CA 02236851 1998-OS-06
through use of 5-alkoxy-4--chloro-2-chloromethylpyridine (8)
and sodium cyanide, to thereby obtain a compound (9a) shown
below.
4-chloro-5-methoxy-2--pyridineacetonitrile (9a)
'H-NMR(CDC.~ 3) ~ : 3. 87C2H, ~;), 4. 01C3H, s), 7.44(1H, s), 8.23C1H, s).
Preparation Example 11
Synthesis of 4-substituted-thio-5-alkoxy-2-
pyridineacetonitriles (10):
A 5-alkoxy-4-chloro-'<?-pyridineacetonitrile (9) (4.5
mmol) was dissolved in dimethylformamide (9 ml). To the
solution were added potas:~ium carbonate (5.4 mmol) and R1'SH
(cyclopentanethiol in the case of preparation of compound
(l0a)) (5.2 mmol) and the mixture was stirred on a 60°C oil
bath for 3-12 hours. After being cooled, the reaction
mixture was poured into cold water and extracted with ethyl
acetate. The organic layer was sequentially washed with
water and saturated brine, dried, and then concentrated
under reduced pressure. The residue was purified by _. _
recrystallization or silica gel column chromatography, to
thereby obtain a compound (l0a) shown below.
4-Cyclopentylthio-5-rlethoxy-2-pyridineacetonitrile
(103)
'f(-N~IRCCDC.~ ~) o : 1. 55-2. ~0(.BH, m). 3. 55-3. 80C1H, m;. 3. 87C2H. s).
3. 97C3H. s). 7. 20C1H, s). 7. 99C1H. s).
Preparation Example 12
37
CA 02236851 1998-OS-06
Synthesis of 3-fluor~~-4-pyridinecarbaldehyde:
The procedure of Preparation Example 7 was repeated to
thereby obtain the title compound.
'H-Nl(R(CDC.~ a) ~ : 7. 71(1H, t, J=5. OHz), 8. 65(1H, d. J=5. OHz).
8. 73(1H, d, J=1. OHz), 10. ~5(1H, s).
Preparation Example 13
Synthesis of 3,5-diclzloro-4-pyridinecarbaldehyde N-
oxide:
To a benzene solution (100 ml) of 3,5-dichloro-4-
pyridinecarbaldehyde (Preparation Example 7) (5.00 g, 28.4
mmol) were added ethylene glycol (10 ml) and p-
toluenesulfonic acid hydrate (0.19 g, 1.0 mmol). The
mixture, placed in a react=ion vessel equipped with a Dean-
Stark water separator, wa:~ refluxed for eight hours. After
being cooled, the reaction mixture was washed with water,
dried, and then concentrated under reduced pressure, to
thereby obtain the corresponding acetal compound (6.19 g,
yield 99%) . - -
' H-NbIR(CDC.~ ~) ~ : 3. 95-4. 22(=~H. m). 6. 38(1H. s). 8. =~9(2H. s).
The above-described acetal compound (5.50 g, 25 mmol)
was dissolved in dichloromethane (50 ml). To the solution
was added 85o m-chloroperbenzoic acid (6.10 g, 30 mmol), and
the mixture was stirred at: room temperature for 15 hours.
The reaction mixture was crashed with an aqueous saturated
sodium hydrogencarbonate aolution, dried, and then
con~~entrated under reduced pressure. The residue was
38
CA 02236851 1998-OS-06
purified by silica gel column chromatography, to thereby
obtain the corresponding lV-oxide compound (5.24 g, yield
89° ) .
'H-~iVR(CDC .~ ~) o : 3. 95-~. ;?2(=1H. m). 6. 28(1H, s). 8. 15C2~-~. s).
The above-described a~-oxide compound (4.72 g, 20 mmol)
was dissolved in acetone (40 ml)-H~O (10 ml) solution. To
the solution was added p-~~oluenesulfonic acid hydrate (3.80
g, 20 mmol). The mixture was refluxed for two hours. The
reaction mixture was evaporated to dryness. An aqueous
saturated sodium hydrogencarbonate solution was added to the
residue, which was then e:~tracted with chloroform. The
organic layer was washed, dried, and then concentrated under
reduced pressure. The residue was purified by silica gel
column chromatography, to thereby obtain 3,5-dichloro-4-
pyridinecarbaldehyde N-ox=_de (3.78 g, yield 98~).
'H-f~fR(CDC.~ 3) o : d. 19~;2H. s). 10.35C1H. s).
Preparation Example 14
Synthesis of 4- (2-inc~anyloxy) -5-methoxy-2- - -
pyridinecarbaldehyde:
To a dimethyl sulfoxide solution (20 ml) of 4-(2-
indanyloxy)-5-methoxy-2-p5~ridinemethanol (5h) (2.71 g, 10
mmo 1 ) and triethylamine ( ~~ . 03 g, 30 mmol ) was added sulfur
tri~~xide-pyridine complex (4.77 g, 30 mmol), and the mixture
was stirred at room temperature for 7 hours. The reaction
mixture was poured into cold water to cause precipitation of
crude crystals. The crude crystals were collected by
39
CA 02236851 1998-OS-06
filtration, dried, and purified by silica gel column
chromatography, to thereb~~ obtain the title compound (1.95 g,
yield 720).
'H-N~~iRCCDC.~ 3) ~ : 3. 10-3. '70(4H, m), 3. 96(3H, s), 5. 20-5. 42(1H, m),
7. 23(4H, s), 7. 55C1H, s), 8. 27(1H, s), 9. 95C1H, s).
Preparation Example 15
Synthesis of N-hydroayethyl-2-pyridone:
2-Pyridone (4.76 g, .'~0 mmol) and ethyl bromoacetate
(10.02 g, 60 mmol) were dussolved in acetone (100 ml) .
Potassium carbonate (8.28 g, 60 mmol) was added to the
solution and the mixture was refluxed for two hours with
stirring. After being cooled, insoluble substances were
removed by filtration and the filtrate was evaporated to
dryness. The residue was purified by silica gel column
chromatography, to thereb~~ obtain N-ethoxycarbonylmethyl-2-
pyridone (7.60 g, yield 8~~° ) .
'H-NMR(CDC.~ 3) o : 1. 29(3H, t, J=7.OHz), 4.2=1(2H, q, J=7. OHz).
4. 64(2H, s), 6. 10-6. 30(1H, m), 6. 60(1H, d, J=9. OHz)~ _
7. 15-7. ~2(2H, m).
In 100 ml of dioxan.e, was dissolved N-
ethoxycarbonylmethyl-2-pyridone (3.62 g, 20 mmol). To the
solution was added 90 o LiF3H~ ( 0 . 96 g, 40 mmol ) and the
mixture was refluxed for ~?0 minutes. Subsequently, ethyl
acetate (20 ml) was added to the mixture and the refluxing
was carried out for five minutes. The reaction mixture was
evaporated to dryness and water was added to the residue.
CA 02236851 1998-OS-06
The aqueous solution was extracted with chloroform (three
tin.es), dried, and then c~~ncentrated under reduced pressure.
The residue was purified by silica gel column chromatography,
to thereby obtain the title compound (1.15 g, yield 41°).
'H-I~N1R(CDC.~ 3) ~ : 3. 80-=1. 30(5H, m), 6. 10-6. 62(2H, m),
7. 20-7. 50 (2H, m) .
Preparation Example 16
Synthesis of 5-cyclopentyloxy-6-methoxy-3-pyridine
acetonitrile:
The procedure of Pre~~aration Example 1 was repeated
through use of 5-hydroxy-6-methoxy-3-pyridinemethanol and
bromocyclopentane, to thereby obtain 5-cyclopentyloxy-6-
methoxy-3-pyridinemethanol.
'H-N~~iR(CDC.~ 3) b : 1. 50-2. 20(8H, m). 3. 98(3H, s). 4. 60(2H, s).
4. 73C1H, m). 7. 10(1H, d. J=2. OHz). 7. 63(1H, d. J=2. OHz).
The procedure of Preparation Example 4 was repeated
through use of 5-cyclopent:yloxy-6-methoxy-3-pyridinemethanol
and thionyl chloride, to thereby obtain 3-chloromethyl-5- _ _
cyclopentyloxy-6-methoxypyridine.
'H-N~-~R(CDC.~ 3) ~ : 1. 50-2. 10C8H, m), 3. 93(3H, s). 4. 54C2H, s),
4. 70(lH, m). 7. 06C1H, d. J=2. OHz). 7. 67(1H, d, J=2. OHz).
The procedure of Preparation Example 5 was repeated
through use of 3-chloromet:hyl-5-cyclopentyloxy-6-
methoxypyridine and sodium cyanide, to thereby obtain 5-
cyc:Lopentyloxy-6-methoxy-~~-pyridineacetonitrile.
41
CA 02236851 1998-OS-06
'H-NMRCCDC.~ 3) ~ : 1. 50-2. 20C8H, m). 3. 67C2H, s), 3. 98C3H. s).
4. 75(1H, m), 6. 99C1H, d. J=2. OHz), 7. 61C1H, d, J=2. OHz).
Preparation Example 17
Synthesis of 6-cyclopentyloxy-5-methoxy-2-
pyridineacetonitrile:
The procedure of Pr~epa~ation Example 1 was repeated
through use of 6-hydroxy-!~-methoxy-2-picoline and
bromocyclopentane, to thereby obtain 6-cyclopentyloxy-5-
methoxy-2-picoline.
'E':-N~~iR(CDC.~ 3) ~ : 1. 58-2. 03C8H, m), 2. 36C3H, s), 3. 80(3H, s),
5. 44(1H, m), 6. 59(1H, d, J=8. OHz), 6. 92C1H, d, J=8. OHz).
6-Cyclopentyloxy-5-mf=thoxy-2-picoline (0.83 g, 4.0
mmol) and N-bromosuccinimide (0.80 g, 4.4 mmol) were
dissolved in carbon tetrachloride (10 ml). The solution in
which benzoyl peroxide was added in a catalytic amount was
refluxed for two hours. P.fter being cooled, water was added
to the reaction mixture, which was extracted with ethyl
acetate. The organic layer was washed with water, dried,
and then concentrated under reduced pressure, to thereby
obtain a residue which was used for the subsequent reaction.
The procedure of Preparation Example 5 was repeated
through use of the above-described residue and sodium
cyanide, to thereby obtain 6-cyclopentyloxy-5-methoxy-2-
pyridineacetonitrile (0.7a? g, yield 77=).
'H--Nl4iRCCDC.~ 3) ~ : 1. 50-2. 40(8H, m). 3. 73(2H, s). 3. 84(3H, s).
5. 40(1H, m), 6. 80(1H, d. J=8. OHz). 7. 00(1H, d. J=8. OHz).
42
CA 02236851 1998-OS-06
Preparation Example 18
Synthesis of 5-methoxy-4-[2-(4-methyl-5-
thiazolyl)ethyloxy]-2-pyr:idineacetonitrile:
The procedure of Preparation Example 1 was repeated
through use of 4-hydroxy-:~-methoxy-2-pyridinemethanol and 5-
(2-chloroethyl)-4-methyltlziazole, to thereby obtain 5-
methoxy-4-[2-(4-methyl-5-'~hiazolyl)ethyloxy]-2-
pyridinemethanol.
'H-N~~fRCCDC.~ 3) ~ : 2. 46(3H, s), 3. 33(2H, t. J=6. 5Hz), 3. 92(3H. s),
4. 22(2H, t, J=6. 5Hz), 4. 65C2H, s), 6. 75C1H, s),
8. 07(1H, s), 8. 61 (1H, s).
The procedure of Preparation Example 4 was repeated
through use of 5-methoxy-~~-[2-(4-methyl-5-
thiazolyl)ethyloxy]-2-pyr_Ldinemethanol and thionyl chloride,
to thereby obtain 2-chloromethyl-5-methoxy-4-[2-(4-methyl-5-
thiazolyl)ethyloxy]pyridine.
'H-Nb~(RCCDC.~ 3) o : 2. =17C3H, ~~), 3. 3=(C2H, t, J=6. 5Hz), 3. 94C3H, s).
4. 25C2H. t. J=6. 5Hz), 4. 59(2H, s), 6. 94(1H. s),
8. 08(1H, s), 8. 61(1H, s). -
The procedure of Preparation Example 5 was repeated
through use of 2-chloromet;hyl-5-methoxy-4-[2-(4-methyl-5-
thiazolyl)ethyloxy]pyridine and sodium cyanide, to thereby
obtain 5-methoxy-4-[2-(4-methyl-5-thiazolyl)ethyloxy]-2-
pyridineacetonitrile.
'H-Nh(RCCDC~ 3) ~ : 2. 47(3H, s;~, 3. 35(2H, t, J=6. SHz~. 3. 85(2H, s),
3. 93(3H, s; , 4. 24(2H, t, J=6. SHz~. 6. 87(1H, s).
8. 07(1H, s;~, 8. 62C1H, s).
43
CA 02236851 1998-OS-06
Example 1
Synthesis of (Z)-2-(4-cyclopentyloxy-5-methoxy-2-
pyridyl)-3-(3,5-dichloro-4-pyridyl)propenenitrile (formula
( 1 ) , wherein R1 - cyclopentyl, Rz - CH3, R3 - CN, R~ - H, R' -
3, ~~-dichloro-4-pyridyl, a:nd X = O)
4-Cyclopentyloxy-5-methoxy-2-pyridineacetonitrile (3a)
(16.24 g, 70 mmol) and 3,5-dichloro-4-pyridinecarbaldehyde
(Preparation Example 7) (12.94 g, 73.5 mmol) were dissolved
in methanol (180 ml). While the solution was stirred at 5°C,
a CH30Na-CH30H solution (1 M, 77 ml) was added dropwise. The
mixture was stirred for 3~ additional minutes under the same
conditions. Precipitated crystals were collected by
filtration and recrystallized from ethanol, to thereby
obtain the title compound (24.83 g, yield 91°).
Melting point: 126-126.5°C:
'H-N~1R(CDC.~ 3) ~ : 1. 60-2. 15(8H, m~. 3. 98C3H, s). ~. 92-4. 98(1H, m).
7.26(1H, s). 8. 19(1H, s). 8.20C1H, s). 8. 61C2H, s).
Example 2 _
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-methox:~-2-pyridineacetonitrile (3a) and
2,6-dichlorobenzaldehyde, to thereby obtain the compound
shown below.
(Z)-2-(4-Cyclopentyl~~xy-5-methoxy-2-pyridyl)-3-(2,6-
dichlorophenyl)propenenitrile (formula (1), wherein R1 -
cyclopentyl, R' - CH3, R3 -- CN, Rq - H, R~ - 2, 6-
dichlorophenyl, and X = Oj
44
CA 02236851 1998-OS-06
Melting point: 129-130°C
'H-N~dRCCDC.~ 3) d : 1. 60-2. 20(8H, m). 3. 97C3H, s), 4. 92-4. 98C1H, m).
7. 25(1H, s). 7. 26-7. 31 (1H, m). 7. =12C2H, d, J=7. OHz),
8. 20C1H, s). 8. 23C1H, s).
Example 3
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-methox~~-2-pyridineacetonitrile (3a) and
3-pyridinecarbaldehyde, to thereby obtain the compound shown
below.
( Z ) -2- ( 4-Cyclopentyloxy-5-methoxy-2-pyridyl ) -3- ( 3-
pyridyl)propenenitrile (formula (1), where,~n R1 -
cyclopentyl, Rz - CH3, R3 -- CN, R9 - H, R' - 3-pyridyl, and X
- O)
Melting point : 122 . 5-123 . !~°C
'I-:-NI4fR(CDC.~ 3) ~ : 1. 50-2. 20(:BH, m). 3. 98(3H. s). 4. 84-5. 06C1H, m).
7. 30(1H, s;. 7. 48(1H, dd. J=5. 0. 8. OHz), 8. 20(1H, s).
8. 38C1H, s;~. 8. 55(1H, dt, J=2. 0, 8. OHz),
8. 70C1H, dd. J=2. 0. 5. OHz), 8. 98C1H, d, J=2. OHz).
Example 4
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-methoxy-2-pyridineacetonitrile (3a) and
4-pyridinecarbaldehyde, to thereby obtain the compound shown
below.
(Z)-2-(4-Cyclopentyloxy-5-methoxy-2-pyridyl)-3-(4-
pyridyl)propenenitrile (formula (1), wherein R1 -
CA 02236851 1998-OS-06
cyclopentyl, R' - CH3, R3 -- CN, Ra - H, RJ - 4-pyridyl, and X
- O)
Melting point: 142-142.5°C
' H--Nh~R (CDC .~ 3 ) ~ : 1. 64-2. 14 CBH, m) , 3. 97 C3H, s) . 4. 92-4. 98 C
1 H, m) .
7. 28(1H, s), 7. 74C2H, dd, J=2. 0. 6. OHz). 8. 16(1H, s),
8. 26(1H, s). 8. 74(2H, dd. J=2. 0. 6. OHz).
Example 5
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-methox~l-2-pyridineacetonitrile (3a) and
2-methoxy-1-naphthaldehyde, to thereby obtain the compound
shown below.
(z)-2-(4-Cyclopentylc~xy-5-methoxy-2-pyridyl)-3-(2-
methoxy-1-naphthyl)propenenitrile (formula (1), wherein R
cyclopentyl, Rz = CH3, R3 -- CN, Rq - H, RS - 2-methoxy-1-
naphthyl, and X = 0)
Melting point: 168-169°C
'H-N~~(R(CDC.~ ~) ~ : 1. 60-2. 15(8H, m), 3. 97(3H, s), 4. 04(3H, s).
=~. 80-5. 00 C 1. H. m) . 7 . 29 C 1 H, s ) , 7. 3=~-7. =~2 C2H, m) .
7. 48-7. 53C1.H, m). 7. 80-7. 93(3H, m). 8. 21C1H, s).
8. 69(1H, s).
Example 6
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-methox~,~-2-pyridineacetonitrile (3a) and
2-chlorobenzaldehyde, to i~hereby obtain the compound shown
below.
46
CA 02236851 1998-OS-06
(Z)-3-(2-chlorophenyl)-2-(4-cyclopentyloxy-5-methoxy-
2-pyridyl)propenenitrile (formula (1), wherein R1 -
cyclopentyl, Rz = CH3, R3 -- CN, R~ - H, R7 = 2-chlorophenyl,
and X = 0)
Melting point: 122-123°C
'H--N~IRCCDC.~ 3) o : 1. 60-2. 15(8H, m), 3. 96(3H, s), ~. 92-~. 97(1H, m).
7. 26(1H, s), 7. 35-7. 41(2H, m). 7. 46-7. 50(1H. m).
8. 10-8. 18( H, m), 8. 19(1H, s), 8. 63(1H, s).
Example 7
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-methoxy-2-pyridineacetonitrile (3a) and
4-cyanobenzaldehyde, to thereby obtain the compound shown
below.
(Z)-3-(4-cyanophenyl)-2-(4-cyclopentyloxy-5-methoxy-2-
pyridyl)propenenitrile (formula (1), wherein R1 -
cyclopentyl, RZ - CH3, R3 -- CN, Ra - H, R' - 4-cyanophenyl,
and X = 0 )
Melting point: 149-150°C
'H-N~4R(CDC.~ 3) ~ : 1. 55-2. 20(8H, m). 3. 97C3H. s), 4. 80-5. lOCIH, m),
7. 28(1H, s), 7. 75(2H. d. J=9. OHz), 8. 03C2H, d. J=9. OHz).
8. 15C1H, s), 8. 33(1H, s).
Example 8
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-methox:~-2-pyridineacetonitrile (3a) and
4-trifluoromethylbenzaldehyde, to thereby obtain the
compound shown below.
( Z ) -2- ( 4-Cyclopentyloxy-5-methoxy-2-pyridyl ) -3- ( 4-
47
CA 02236851 2005-05-17
trifluoromethylphenyl)propenenitrile (formula (1), wherein R1
- cyclopentyl, RZ = CH3, R3 = CN, R4 = H, RS = 4-
trifluoromethylphenyl, and X = 0)
Melting point: 116-117°C
'H-(~11(R(CDC:~ ~) ~ : 1. 55-2. 20(8H, m). 3. 97(3H, s). 4. 80-5. lOCIH, m),
7. 27(1H, s). 7. 72C2H, d. J=9. OHz).
8. 04C2H. d. J=9. OHz). 8.15C1H, s). 8. 35(1H. s).
Example 9
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-methoxy-2-pyridineacetonitrile (3a) and
2,6-dimethoxybenzaldehyde, to thereby obtain the compound
shown below.
(Z)-2-(4-Cyclopentyloxy-5-me~hoxy-2-pyridyl)-3-(2,6-
dimethoxyphenyl)propenenitrile (formula (1), wherein R1
cyclopentyl, R2 = CH3, R3 = CN, R' = H, RS = 2, 6-
dimethoxyphenyl, and X = O)
Melting point: 168-170°C
'H-NhiR(CDC ~C s) ~ : 1. 55-2.10(8H, m). 3. 89(6H. S). 3. 94(3H. S).
4. 80-5. lOClH. m). 6. 60(2H. d. J=8. OHz). 7. 24(1H. s).
7. 42(1H, t, J=8. OHz). 8.16(1H. s). ~ 8. 27(IH. s).
Example 10
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-methoxy-2-pyridineacetonitrile (3a) and
4-quinolinecarbaldehyde, to thereby obtain the compound
shown below.
(Z)-2-(4-Cyclopentyloxy-5-methoxy-2-pyridyl)-3-(4-
48
CA 02236851 1998-OS-06
quinolyl)propenenitrile (~=ormula (1), wherein R1 -
cyclopentyl, R' - CH3, R3 -- CN, R~ - H, RS = 4-quinolyl, and
X = 0)
Melting point: 160-161°C
'H-Niv(RCCDC.~ 3) ~ : 1. 55-2. 10(8H, m). ~. 00(3H, s), ~. 80-5. lOCIH, m),
7. 32(1H. s). 7. 45-8. 30(5H, m), 8. 21(1H. s),
9. 02(1H, s), 9. 04(1H, d, J=4. OHz).
Example 11
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-methoxy-2-pyridineacetonitrile (3a) and
3,5-dimethoxy-4-pyridinecarbaldehyde (Preparation Example 8),
to thereby obtain the compound shown. below.
(Z)-2-(4-Cyclopentyl~~xy-5-methoxy-2-pyridyl)-3-(3,5-
dimethoxy-4-pyridyl)propenenitrile (formula (1), wherein Ri
- cyclopentyl, R2 - CH3, R3 - CN, R4 - H, R~' - 3, 5-dimethoxy-
4-pyridyl, and X = O)
Melting point: 143-144°C
'H-~N~~fR(CDC.~ ~) ~ : 1. 55-2. 10(8H. m), 3. 96(3H, s), 4. 00C6H, s),
4. 80-5. 10~;1H, m), 7. 25C1H, s). 8. 11 (2H, s),
8. 17(2H, s).
Example 12
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-methox:y-2-pyridineacetonitrile (3a) and
benzaldehyde, to thereby ~~btain the compound shown below.
(Z)-2-(4-Cyclopentyloxy-5-methoxy-2-pyridyl)-3-
phenylpropenenitrile (formula (1), wherein R1 - cyclopentyl,
49
CA 02236851 1998-OS-06
RZ -- CH3, R3 - CN, R4 = H, RS - phenyl, and X = O)
Melting point: 107-108°C
'H-NhfR(CDC.~ 3) ~ : 1. :~0-2. 30(~~H, m). 3. 96(3H, s),
~. 80-5. 10(lH, m), 7. 26(1H, s),
7. 35-7. 60 (~>H, m) , 7. 80-8. 10 (2H, m) , 8. 15 C 1 H, s) ,
8. 32(1H, s).
Example 13
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-methoxl~-2-pyridineacetonitrile (3a) and
2-t:hiophenecarbaldehyde, t:o thereby obtain the compound
shown below.
(Z)-2-(4-Cyclopentyloxy-5-methoxy-2-pyridyl)-3-(2-
thienyl)propenenitrile (formula (1), wherein R1 -
cyclopentyl, Rz = CH3, R' -~ CN, Rq - H, R5 - 2-thienyl, and X
- O)
Melting point: 89-90°C
' H-Nl4iR (CDC .~ 3 ) ~ : 1. 60-2. 20 (~~H, m) , 3. 95 ( 3H, s ) , ~. 80-5. 00
( 1 H, m) ,
7. 05-7. 25(lH, m). 7. 19(1H, s), 7. 57(1H, d, J=5. OHz),
7. 72(1H, d, :f=~. OHz), 8. 12(1H, s), 8. ~~(1H, s).
Example 14
The procedure of Example 1 was repeated through use of
5-m~thoxy-4-phenethyloxy-~'.-pyridineacetonitrile (3c) and
3,5-dichloro-4-pyridinecarbaldehyde (Preparation Example 7),
to thereby obtain the compound shown below.
( Z ) -3- ( 3, 5-Dichloro-~l-pyridyl ) -2- ( 5-methoxy-4-
CA 02236851 1998-OS-06
phenethyloxy-2-pyridyl)propenenitrile (formula (1), wherein
R1 -- phenethyl, R' - CH3, R3 - CN, R~ - H, R~' - 3, 5-dichloro-
4-pyridyl, and X = O)
Melting point: 141.5-142.5°C
'li-NIt-IR(CDC.~ 3) o : 3. 21(2H, t> .1=8. OHz). 4. 00(3H. sO.
4. 34C2H, t, J=8. OHz). 7. 20-7. ~OC6H, m).
8. 16C1H, s). 8. 22C1H, s). 8. 60(2H, s).
Example 15
The procedure of Example 1 was repeated through use of
5-methoxy-4-(3-phenylpropyloxy)-2-pyridineacetonitrile (3d)
and 3,5-dichloro-4-pyridinecarbaldehyde (Preparation Example
7), to thereby obtain the compound shown below.
( Z ) -3- ( 3, 5-Dichloro-4-pyridyl ) -2- [ 5-methoxy-4- ( 3-
phenylpropyloxy)-2-pyridy=L]propenenitrile (formula (1),
wherein R1 - 3-phenylpropyl, Rz = CH=, R3 - CN, Rq - H, R5 -
3,5-dichloro-4-pyridyl, and X = 0)
Melting point: 101-102°C
'H-N~~(RCCDC.~ 3) ~ : 2. 00-2. 40(2H, m). 2. 86(2H, t. J=8. OHz). 4. 02(3H,
s).
4. 15(2H, t, a=8. OHz). 7. 10-7. 40(6H, m). 8. 16(1H, s).
8. 22(1H, s). 8. 60(2H, s).
Example 16
The procedure of Example 1 was repeated through use of
4-butyloxy-5-methoxy-2-py~~idineacetonitrile (3e) and 3,5-
dichloro-4-pyridinecarbalc~ehyde (Preparation Example 7), to
thereby obtain the compound shown below.
( Z ) -2- ( 4-Butyloxy-5-methoxy-2-pyridyl ) -3- ( 3, 5-
51
CA 02236851 1998-OS-06
dichloro-4-pyridyl)propenenitrile (formula (1), wherein R1 -
butyl, R'' - CH3, R3 = CN, Ra - H, R5 - 3, 5-dichloro-4-pyridyl,
and X = 0 )
Melting point: 108-109°C
'H-NhiRCCDC.~ 3) o : 1. 00(3H, t, J=7. OHz), 1. 30-2. 10(4H, m), ~. 00(3H, s),
4. 16(2H, t, J=7. OHz), 7. 27(1H, s), 8. 19(1H, s),
8. 21 (1H, s), 8. 61 (2H, s).
Example 17
The procedure of Example 1 was repeated through use of
4-(1-ethylpropyloxy)-5-mei=boxy-2-pyridineacetonitrile (3f)
and 3,5-dichloro-4-pyridinecarbaldehyde (Preparation Example
7), to thereby obtain the compound shown below.
( Z ) -3- ( 3, 5-Dichloro-~~-pyridyl ) -2- [ 4- ( 1-
ethylpropyloxy)-5-methoxy--2-pyridyl]propenenitrile (formula
( 1 ) , wherein R- - 1-ethyl~~ropyl, RZ = CH3, R3 - CN, Rq - H, R'
- 3,5-dichloro-4-pyridyl, and X = 0)
Melting point: 127-127.5°C:
'H-N~~1R(CDC.~ 3) o : 1. 00(6H, t, J=7. OHz), 1. 60-2. 00(4H, m), =1. 00C3H,
s),
~. 10-~!. 40(1H, m), r. 25(1H, s). 8. 19C1H, s), 8. 22(1H, s).
8. 61 (2H, s).
Example 18
The procedure of Example 1 was repeated through use of
4-cyclopentyloxy-5-ethoxy--2-pyridineacetonitrile (3d) and
3,5-dichloro-4-pyridineca:~baldehyde (Preparation Example 7),
to thereby obtain the com~~ound shown below.
(Z)-2-(4-Cyclopentyl~~xy-5-ethoxy-2-pyridyl)-3-(3,5-
52
CA 02236851 1998-OS-06
dichloro-4-pyridyl)propenenitrile (formula (1), wherein R~ -
cyclopentyl, R' - C2H5, R3 = CN, Rq - H, RS - 3, 5-dichloro-4-
pyridyl, and X = 0)
Melting point: 90-91°C
'H-:V~IR(CDC.~ 3) ~ : 1. 46(3H, t. J=7. OHz). 1. 60-2. 20(8H, m).
4. 19(2H, q. J=7. OHz). 4. 80-5. 00(1H, m). 7. 26(1H, s).
8. 18(1H, s). 8. 19(1H, s). 8. 61 C2H. s).
Example 19
The procedure of Example 1 was repeated through use of
4-(2-indanyloxy)-5-methoxy-2-pyridineacetonitrile (3h) and
3,5-dichloro-4-pyridinecarbaldehyde (Preparation Example 7),
to thereby obtain the compound shown below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[4-(2-indanyloxy)-5-
methoxy-2-pyridyl]propenenitrile (formula (1), wherein R1 -
2-indanyl, RZ - CH3, R3 - <:N, Rq - H, RS - 3, 5-dichloro-4-
pyridyl, and X = 0)
Melting point: 185-185.5°C:
H-N~~(R (CDC .~ ~ ) d : 3. 28 (2H. ~Jd. J=3. 5. 17. OHz) .
3. 51 C2H. ~~d, J=6. 0. 17. OHz). 3. 92C3H, s).
5. 32-5. 3~3 C 1 H, m) . 7. 17-7. 28 CSH, m) . 8. 21 ( 1 H, s ) .
8. 22(1H. :>). 8. 61 (2H, s).
Example 20
The procedure of Example 1 was repeated through use of
5-methoxy-4-(tetrahydro-3--furanyloxy)-2-pyridineacetonitrile
(3j) and 3,5-dichloro-4-p~~ridinecarbaldehyde (Preparation
53
CA 02236851 1998-OS-06
Example 7), to thereby obtain the compound shown below.
(Z)-3-(3,5-Dichloro-~~-pyridyl)-2-[5-methoxy-4-
(tetrahydro-3-furanyloxy)--2-pyridyl]propenenitrile (formula
( 1 ) , wherein R1 - tetrahydro-3-furanyl, R' - CH3, R3 - CN, Ra
- H, RS - 3, 5-dichloro-4-pyridyl, and X = 0)
Melting point: 138-140°C
'H-~J~-(RCCDC.~ 3) ~ : 2. 10-2. 50C2I~, m), 3. 80-4. 20(4H, mi. 3. 99C3H, s),
5. 00-5. 20(11, m). 7. 20C1H, s), 8. 21 C1H, s~, 8. 24C1H, s),
8. 61C2H, s).
Example 21
The procedure of Exarlple 1 was repeated through use of
5-methoxy-4-(exo-2-norbornyloxy)-2-pyridineacetonitrile (3i)
and 3,5-dichloro-4-pyridinecarbaldehyde (Preparation Example
7), to thereby obtain the compound shown below.
( Z ) -3- ( 3, 5-Dichloro-~l-pyridyl ) -2- [ 5-methoxy-4- ( exo-2-
norhornyloxy)-2-pyridyl]propenenitrile (formula (1), wherein
Rl -- exo-2-norbornyl, R' - CH3, R3 - CN, R4 - H, R~ - 3, 5-
dic:zloro-4-pyridyl, and X = 0)
Melting point: 145.5-146. 'i°C
'H-N6fR(CDC.~ 3) o : 1. 10-2. 00(8H, m), 2. 30-2. 70(2H, m), 3. 98C3H, sO
4. 30-4. 50(l~f, m). 7.21C1H, s), 8. 19(2H, s), 8. 61C2H, s).
Example 22
Synthesis of methyl (E)-2-(4-cyclopentyloxy-5-
metlzoxy-2-pyridyl) -3- ( 3, 5-~dichloro-4-pyridyl) propenoate
( fo=rmula ( 1 ) , wherein R~ - cyclopentyl, RZ - CH3, R3 - COzCH3,
54
CA 02236851 1998-OS-06
Rq -- H, R5 - 3, 5-dichloro-4-pyridyl, and X = O)
Under the same condi~~ions in Example 1 and through use
of methyl 4-cyclopentylox~~-5-methoxy-2-pyridineacetate (3k)
and 3,5-dichloro-4-pyridinecarbaldehyde (Preparation Example
7) in methanol containing a CH30Na-CH30H solution (1 M), the
title compound was prepared.
Melting point: 119-120°C
'H-N~~1RCCDC.~ 3) ~ : 1. 55-1. 90C8H, m>, 3. 86C3H, s), 3. 91C3H, s),
4. 56-4. 60(1H, m), 6. 70(1H, s), 7. 57(1N, s),
7. 94(1H, s), 8. 40C2H, s).
Example 23
Synthesis of (Z)-3-(4-cyclopentyloxy-5-methoxy-2-
pyridyl)-2-(3-pyridyl)propenenitrile (formula (1), wherein
R1 -- cyclopentyl, Rz - CH3, R3 - H, Rq - CN, R' - 3-pyridyl,
and X = 0 )
4-Cyclopentyloxy-5-mE=_thoxy-2-pyridinecarbaldehyde
(4.42 g, 20 mmol) and 3-p~lridineacetonitrile (2.36 g, 20
mmol) were dissolved in methanol (60 ml). While the
solution was stirred at 5"C, a CH30Na-CH30H solution (1 M, 23
ml) was added dropwise. The mixture was stirred for 30
additional minutes under l.he same condition. The reaction
mixture was poured into cold water and extracted with
chloroform. The organic layer was dried and then
concentrated under reduce<~ pressure. The residue was
chromatographed on silica gel, to thereby obtain the title
compound (4.54 g, yield 7_L°) from a lg (v/v) methanol-
CA 02236851 1998-OS-06
chloroform-eluted fraction.
Melting point: 119-119.5°C'.
H-NIdR CCDC .~ a ) o : 1. 60-2. 20 CBH, m) . 4. 00 (3H, s ) . 4. 92-'' . 98 C
1 H, m) ,
7..-"tlClH. dd~ J=5. 0, 7. OHZ), 7. 6=~C1H, s). 7. r~(1_ia. s).
7. 98-8. 03(1H, m). 8. 26C1H. s).
8. 6~C1H, dd, J=1. 5, S. OHz). 8. 97(1H, d. J=2. 0Hz).
Example 24
The procedure of Example 23 was repeated through use
of 4-cyclopentyloxy-5-methoxy-2-pyridinecarbaldehyde and 2-
thiopheneacetonitrile, to thereby obtain the compound shown
below.
(Z)-3-(4-Cyclopentyloxy-5-methoxy-2-pyridyl)-2-(2-
thienyl)propenenitrile (formula (1), wherein Rl -
cyclopentyl, Rz - CHI, R3 -- H, R~ - CN, RS - 2-thienyl, and X
- O) .
Melting point: 101-102°C
'H-Nh(RCGDC.~ 3) o : 1. 60-2. 15C8fi. m). 3. 98C3H, s); 4. 92-4. 98C1N, m).
7. 08C1H, dd, .1=a. 0. 5. OHz), 7. 33CiH. dd, .~=1. 0, 5. OHz),
7. ~3C1H, s). 7. =!='.C1H, dd, J=1. 0. 4. OHz).
7. 62(1H. s). 8. 22(1H, s).
Exa:~ple 25
The procedure of Example 23 was repeated through use
of 4-cyclopentyloxy-5-methoxy-2-pyridinecarbaldehyde and
phenylacetonitrile, to thereby obtain the compound shown
belsw.
56
CA 02236851 1998-OS-06
(Z)-3-(4-Cyclopentyloxy-5-methoxy-2-pyridyl)-2-
phenylpropenenitrile (formula (1), wherein R1 - cyclopentyl,
R'' -- CH3, R3 - H, Rq - CN, R' - phenyl, and X = 0) .
Melting point: 91-91.5°C
'H-N~dR(CDC~ 3) ~ : 1. 60-2. 20(8H, m), 3. 99(3H. s). 4. 92-~. 98(1H, m).
7. 38- 7. =~9 C3N, m) , 7. 62 C 1 H, s) , 7. 70-7. 75 C2H, m) .
7. 77(1H, s). 8. 23(1H, s).
Example 26
The procedure of Example 1 was repeated through use
of 5-methoxy-4-methoxymethyloxy-2-pyridineacetonitrile (3g)
and 3,5-dichloro-4-pyridinecarbaldehyde (Preparation Example
7), to thereby obtain the compound shown below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-(5-methoxy-4-
methoxymethyloxy-2-pyridy-L)propenenitrile (formula (1),
wherein R1 - CHZOCH3, R' - CH3, R3 - CN, R~ - H, RS - 3, 5-
dichloro-4-pyridyl, and X = 0).
Melting point: 140-141°C
'H-NI~fR(CDC.~ ~) ~ : 3. 5.~C3H. s). ~. 03C3H, s). 5. 38C2H, s). 7. 54C1H, s),
8. 14(1H, s), 8. 28(1H, s). 8. 61(2H, s).
Example 27
Synthesis of (Z) -3-~ (3, 5-dichloro-4-pyridyl) -2- (4-
hydroxy-5-methoxy-2-pyridyl)propenenitrile (formula (1),
wherein R1 - H, RZ - CH3, R3 = CN, R~ - H, R' - 3, 5-dichloro-
4-pyridyl, and X = 0):
In 8 ml of dichloromethane, was dissolved (Z)-3-(3,5-
57
CA 02236851 2005-05-17
dichloro-4-pyridyl)-2-(5-methoxy-4-methoxymethyloxy-2-
pyridyl ) propenenitrile ( formula ( 1 ) , wherein R' = CH20CH3, RZ
- CH3, R3 = CN, R4 = H, RS = 3, 5-dichloro-4-pyridyl, and X =
0) (0.73 g, 2 mmol). While the solution was stirred at 0°C,
trifluoroacetic acid (2 ml) was added. The mixture was
stirred for additional two hours. The reaction mixture was
evaporated to dryness. The residue was dissolved through
addition of water, and the pH of the solution was adjusted
to about 6 through addition of an aqueous saturated sodium
hydrogencarbonate solution. Precipitated crystals were
collected by filtration, washed with water, and
recrystallized from ethanol, to thereby obtain the title
compound (0.56 g, yield 88~).
Melting point: 218-219.5°C
'H-~lIfRCD~~SO-dB) o : 3. 94C3H. s). r. 33C1H. s). 8. 19C1H. s). o. 29C1H. s).
8. 81 C2H. s).
Example 28
Synthesis of (Z) -3- (3, 5-dichloro-4-pyridyl) -2- [4- (3-
hydroxypropyloxy)-5-methoxy-2-pyridyl]propenenitrile
(formula (1), wherein R1 = (CHZ)30H, RZ = CH3, R3 = CN, R4 = H,
RS = 3, 5-dichloro-4-pyridyl, and X = 0)
In 4 ml of dimethylformamide, was dissolved (Z)-3-
(3,5-dichloro-4-pyridyl)'-2-(4-hydroxy-5-methoxy-2-
pyridyl) propenenitrile ( formula ( 1 ) , wherein R1 = H, RZ = CH3,
R3 = CN, R" - H, R5 = 3, 5-dichloro-4-pyridyl, and X = 0) (400
mg, 1.24 mmol). To the solution were added 3-bromopropanol
58
CA 02236851 1998-OS-06
(0.132 ml, 1.48 mmol) and potassium carbonate (204 mg, 1.48
mmol). The mixture was stirred on a 60°C oil bath for three
hours. After being cooled., the reaction mixture was poured
into cold water and extracted with in ethyl acetate. The
organic layer was sequentially washed with water and
saturated brine, dried, and then concentrated under reduced
pressure. The residue was purified by silica gel column
chromatography. Crystals obtained from the lv hexane-ethyl
acetate (1 . 1)-eluted friction were recrystallized from
hexane-diethyl ether, to thereby obtain the title compound
(360 mg, yield 76%).
Melting point: i11-113°C
' H-od~fRCCDC .~ 3) o : 1. 91C1H. t. J=5. OHz). 2. 12-2. 20(2H. m).
3. 87-3. °2C2H. m), =~. OOC3H, s). s. 33C2H, t, J=6. C~~z).
7. 30C1H. s). 8. 18C1H. s). 8. 22(1H. s). 3. 61C2H. s).
Example 29
The procedure of Example 1 was repeated through use
of 4-cyclopentylthio-5-met:hoxy-2-pyridineacetonitrile (l0a)
and 3,5-dichloro-4-pyridinecarbaldehyde (Preparation Example
7), to thereby obtain the compound shown below.
(Z)-2-(4-Cyclopentylthio-5-methoxy-2-pyridyl)-3-(3,5-
dic:zloro-4-pyridyl)propenenitrile (formula (1), wherein R1 -
cyc Lopentyl, R' - CH3, R3 - CN, R4 - H, RS - 3, 5-dichloro-4-
pyridyl, and X = S).
Melting point: 147-148°C
59
CA 02236851 1998-OS-06
'H-NI~IRCCDC.~ 3) d : 1. 50-2. ~0C8H. m), 3. 60-3. 95C1H. m), 4. 04C3H. s).
7. 59(1H, s), 8. 14(1H, s), 8. 18C1H, s), 8. 61C2H, s).
Example 30
The procedure of Example 23 was repeated through use
of 4-cyclopentyloxy-5-met:hoxy-2-pyridinecarbaldehyde and
3,5-dichloro-4-pyridineacetonitrile, to thereby obtain the
compound shown below.
(Z)-3-(4-Cyclopentyloxy-5-methoxy-2-pyridyl)-2-(3,5-
dichloro-4-pyridyl ) propenenitrile ( formula ( 1 ) , wherein R' -
cyclopentyl, R' - CH3, R3 -- H, Ra - CN, RS - 3, 5-dichloro-4-
pyridyl, and X = 0) .
Melting point: 140.5-142°<:
lIi-N~4RCCDC.~ 3) ~ : 1. 50-2. 00(8H, m), 3. 88C3H, s). 4. 50-4. 70(1H, m),
6. 68C1H, s), 7. 51C1H, s). 7. 89C1H, s), 8.57C2H, s).
Example 31
Synthesis of (E)-2--(4-cyclopentyloxy-5-methoxy-2-
pyridyl)-3-(3,5-dichloro-4-pyridyl)propenoic acid (formula
( 1 ) , wherein R1 = cyclopentyl, R' - CH3, R3 - CO~H, R~ - H, R5
- 3,5-dichloro-4-pyridyl, and X = O):
Methyl (E)-2-(4-cyclopentyloxy-5-methoxy-2-pyridyl)-3-
(3,5-dichloro-4-pyridyl)propenoate (formula (1), wherein R1
- cyclopentyl, R' - CH3, R3 - CO~CH;, Ra - H, RS - 3, 5-
dichloro-4-pyridyl, and X = 0) (423 mg, 1 mmol) was
dissolved in methanol (4 nl). A 1N aqueous NaOH solution (2
ml) was added to the solution. The mixture was stirred for
CA 02236851 1998-OS-06
twc hours at room temperature and the reaction mixture was
poured into a 5% aqueous ammonium chloride solution.
Precipitated crystals were collected by filtration, washed,
and recrystallized from ethanol, to thereby obtain the title
compound (240 mg, yield 59° ) .
Melting point: 178-180°C ;decomposition)
'H-I~~~9RCCDC.~ 3) ~ : 1. 40-2. 00(8H, m), 3. 77C3H, s). 4. 58-4. 80(1H, m),
6. 78C1H, s). 7. 50(1H, s). 7. 94C1H, s). 8. 56C2H. s).
Example 32
The procedure of Example 1 was repeated through use
of 4-cyclopentyloxy-5-met:hoxy-2-pyridineacetonitrile (3a)
and 3-nitrobenzaldehyde, to thereby obtain the compound
shown below.
( Z ) -2- ( 4-Cyclopentyloxy-5-methoxy-2-pyridyl ) -3- ( 3-
nitrophenyl)propenenitrile (formula (1), wherein R1 -
cyclopentyl, Rz - CH3, R~ -- CN, R~ - H, R' - 3-nitrophenyl,
and X = 0 ) .
Melting point: 126-127°C
'H-N~~1RCCDC.~ 3) ~ : 1. 50-2. 20(81-1, m), 3. 98C3H, s), 4. 82-5. 10(1H, m),
7. 28C1H, s), 7. 67C1H, t. J=8. OHz~, 8. 16(1H, s),
8. 20-8. 40 C3 H, m) , 8. 60-8. 70 ( 1 H, m) .
Example 33
The procedure of E~>ample 1 was repeated through use
of 4-cyclopentyloxy-5-met:hoxy-2-pyridineacetonitrile (3a)
and 3-fluoro-4-pyridinecarbaldehyde (Preparation Example 12),
61
CA 02236851 1998-OS-06
to thereby obtain the compound shown below.
(Z)-2-(4-Cyclopentyloxy-5-methoxy-2-pyridyl)-3-(3-
fluoro-4-pyridyl)propenen.itrile (formula (1), wherein Ri -
cyclopentyl, RZ - CH3, R3 -- CN, RQ - H, R' - 3-fluoro-4-
pyridyl, and X = 0).
Melting point: 120-121°C
'H-N~dR(CDC.~ 3) o : 1. 60-2. 15(8H, m). 3. 98C3H, s), 4. 92-4. 97(1H. m).
7. 28(1H, s), 8. 10(1H, t, J=6. OHz), 8. 18(1N, s),
8. 47C1H, s), 8. 56C1H, d, J=5Hz), 8. 60C1H, d, J=2Hz).
Example 34
The procedure of E~;ample 1 was repeated through use
of 4-(2-indanyloxy)-5-metlzoxy-2-pyridineacetonitrile (3h)
and methyl 4-formyl-2-picolinate, to thereby obtain the
compound shown below.
( Z ) -2- [ 4- ( 2-Indanyl_oxy-5-methoxy-2-pyridyl ] -3- ( 2-
methoxycarbonyl-4-pyridyl)propenenitrile (formula (1),
wherein R1 - 2-indanyl, R~ - CH3, R3 - CN, Rq - H, RS - 2-
methoxycarbonyl-4-pyridyl, and X = O).
Melting point: 177-179°C
'H-N~dRCCDC.~ 3) ~ : 3. 14-3. 85C4H, m), 3. 93(3H, s), 4. 05C3H, s),
5. 29-5. 36(:lH, m), 7. 24-7. 37(5H, m), 8. Ol-8. 09(1H, m).
8. 18(1~~, s), 8. 35~1H, s), 8. 47(1H, s),
8. 89(1H, d, J=5. OHz).
Example 35
The procedure of E~;ample 1 was repeated through use
62
CA 02236851 1998-OS-06
of 4-(2-indanyloxy)-5-methoxy-2-pyridineacetonitrile (3h)
and 4-pyridinecarbaldehyde, to thereby obtain the compound
shown below.
( Z ) -2- [ 4- ( 2-Indany:Loxy) -5-methoxy-2-pyridyl ] -3- ( 4-
pyridyl)propenenitrile (formula (1), wherein Rl - 2-indanyl,
RZ - CH3, R3 - CN, R4 - H, R5 - 4-pyr idyl, and X = 0) .
Melting point: 205-206°C
' H-N~iR (CDC .~ 3 ) d : 3. 15-3. 75 C 4H, m) , 3. 92 C3H, s ) . 5. 20-5. 50 C
1 H, m) .
7. 2~(4H, s). 7. 36C1H, s). 7. 75C2H. d, J=7. OHz).
8. 17(1H, s). 8. 28C1H, s). 8. 78C2H, d. J=7. OHz).
Example 36
The procedure of Example 1 was repeated through use
of 4-(2-indanyloxy)-5-methoxy-2-pyridineacetonitrile (3h)
and 4-pyridinecarbaldehyde N-oxide, to thereby obtain the
compound shown below.
4- [ ( Z ) -2-Cyano-2- ( ~~- ( 2-indanyloxy) -5-methoxy-2-
pyridyl)-1-ethenyl]pyridine N-oxide (formula (1), wherein R'
- 2-indanyl, Rz = CH3, R~ ~- CN, Ra - H, RS - 1-oxo-4-pyridyl,
and X = 0 ) .
Melting point: 231-232°C
' H-NMR CCDC .~ s ) ~ : 3. 15-3. 72 (4H, m) . 3. 92 (3H, s ) , 5. 20-5. 45 ( 1
H, m) ,
7. 2=~C4H, s), 7. 33C1H. s), 7. 85(2H, d, J=7. OHz),
8. 10-8. 30 ( ~H, m) .
Example 37
The procedure of Example 1 was repeated through use
63
CA 02236851 1998-OS-06
of 4-(2-indanyloxy)-5-methoxy-2-pyridineacetonitrile (3h)
and 3,5-dichloro-4-pyridinecarbaldehyde N-oxide (Preparation
Example 13), to thereby obtain the compound shown below.
4-[(Z)-2-Cyano-2-(4-(2-indanyloxy)-5-methoxy-2-
pyridyl)-1-ethenyl]-3,5-dichloropyridine N-oxide (formula
( 1 ) , wherein R1 = 2-indanyl, R2 - CH,3, R3 - CN, R~ - H, RS -
3,5-dichloro-1-oxo-4-pyridyl, and X = 0).
Melting point: 229-230°C
' H-N~~fRCCDC .C 3) o : 3. 10-3. 72(.4H, m). 3. 93C3H, s), 5. 20-5. 43C1H. m).
7. 23C5H. s;, 8. 13(1H, s). 8. 20(1H, s), 8. 26(2H, s).
Example 38
Synthesis of (Z)-3--(3-carboxyphenyl)-2-(4-
cyclopentyloxy-5-methoxy-2-pyridyl)propenenitrile (formula
(1) , wherein R1 - cyclopentyl, R' - CH3, R3 - CN, R~ - H, R5 -
3-carboxyphenyl, and X = O):
4-Cyclopentyloxy-5--methoxy-2-pyridineacetonitrile
(3a) (4.64 g, 20 mmol) and 3-formylbenzoic acid (3.00 g, 20
mmol) were dissolved in methanol (60 ml). While the
solution was stirred at 5°C, 1 M aqueous CH30Na-CH30H
solution (46 ml) was added dropwise. The reaction mixture
was stirred for 30 minute: under the same conditions and was
poured into a 5% aqueous ammonium chloride solution.
Precipitated crystals were collected by filtration, washed,
and recrystallized from ei=hanol, to thereby obtain the title
compound (4.00 g, yield 550).
Melting point: 253-254°C
64
CA 02236851 1998-OS-06
' H-N~1R (Dh~(SO-d s ) ~ : 1. 50-2. 20 (8H, m) . 3. 90 (3H, s ) , 4. 98-5. 20
( 1 H, m) ,
7. 49(1H, s). 7. 67(1H, t, J=7. 5Hz), 8. 00-8. 30(2H, m),
8. 25(1H, s). 8. 35(1H, s). 8. 56(1H, s).
Example 39
The procedure of Example 23 was repeated through use
of 4-cyclopentyloxy-5-met:hoxy-2-pyridinecarbaldehyde and
methyl 3-cyanomethylbenzoate, to thereby obtain the compound
shown below.
(Z)-3-(4-Cyclopentyloxy-5-methoxy-2-pyridyl)-2-(3-
methoxycarbonylphenyl)propenenitrile (formula (1), wherein
R1 - cyclopentyl, RZ - CH3,. R3 - H, R~ - CN, RS - 3-
methoxycarbonylphenyl, and X =
Melting point: 116-117°C
'H-N6-1R(CDC.~ 3) d : 1. 55-2. 30(8H, m), 3. 96(3H, s). 4. 00C3H, s),
4. 80-5. 10(:[H, m), 7. 42-8. 50(7H, m).
Example 40
The procedure of Example 23 was repeated through use
of 4-(2-indanyloxy)-5-methoxy-2-pyridinecarbaldehyde
(Preparation Example 14) and methyl 3-cyanomethylbenzoate,
to thereby obtain the compound shown below.
( Z ) -3- [ 4- ( 2-Indanyloxy) -5-methoxy-2-pyridyl ] -2- ( 3-
methoxycarbonylphenyl)propenenitrile (formula (1), wherein
R1 - 2-indanyl, R' - CH3, F;j - H, R~ - CN, R' - 3-
methoxycarbonylphenyl, and X = 0).
Melting point: 183-184°C
CA 02236851 1998-OS-06
'H-N~-[RCDM1S0-ds) ~ : 3. 10-3. 70C4H, m). 3. 94C3H, s), 3. 96(3H, s).
5. 20-5. 40C1H, m). 7. 23C4H, s), 7. 40-8. 40C7H, m)
Example 41
The procedure of Example 23 was repeated through use
of 4-(2-indanyloxy)-5-metaoxy-2-pyridinecarbaldehyde
(Preparation Example 14) and 4-pyridineacetonitrile, to
thereby obtain the compound shown below.
(Z)-3-[4-(2-Indanyloxy)-5-methoxy-2-pyridyl]-2-(4-
pyridyl)propenenitrile (formula (1), wherein R1 - 2-indanyl,
R2 - CH3, R3 - H, R9 - CN, RS - 4-pyridyl, and X = O) .
Melting point: 195-197°C
'H-N~1R(CDC.~ 3) ~ : 3. 10-3. 75(4H. m), 3. 96(3H, s). 5. 20-5. 45(1H. m).
7. 23C4H, s). 7. 62(2H, d, J=6. OHz), 7. 82(1H, s).
7. 87(1H, s). 8. 29(1H, s), 8. 73(2H, d. J=6. OHz).
Example 42
The procedure of Example 28 was repeated through use
of (Z)-3-(3,5-dichloro-4-pyridyl)-2-(4-hydroxy-5-methoxy-2-
pyridyl ) propenenitrile ( formula ( 1 ) , wherein R1 - H, R' - CH3,
R3 - CN, Rq - H, RS - 3, 5-dichloro-4-pyridyl, and X = O) and
methyl bromoacetate, to thereby obtain the compound shown
below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-(5-methoxy-4-
methoxycarbonylmethyloxy-2.-pyridyl)propenenitrile (formula
( 1 ) , wherein R1 - CH~COZCH3, R- - CH;, R' - CN, Ra - H, RS -
66
CA 02236851 1998-OS-06
3,5-dichloro-4-pyridyl., and X = 0).
Melting point: 131-132°C
'H-N~~R(CDC,~ ~) o : 3. 85(3H, s). 4. 04(3H, s); 4. 84(2H, s), 7. 14(1H, s),
8. 19(1H, s). 8. 27(1H, s), 8. 61 (2H, s).
Example 43
The procedure of Example 28 was repeated through use
of (Z)-3-(3,5-dichloro-4-:pyridyl)-2-(4-hydroxy-5-methoxy-2-
pyridyl ) propenenitrile ( formula ( 1 ) , wherein R1 - H, R' = CH3,
R3 - CN, R9 - H, RS - 3, 5-dichloro-4-pyridyl, and X = 0) and
2-bromoethanol, to thereby obtain the compound shown below.
( Z ) -3- ( 3, 5-Dichloro-4-pyridyl ) -2- [ 4- ( 2-
hydroxyethyloxy)-5-methoxy-2-pyridyl)propenenitrile (formula
( 1 ) , wherein R1 - (CHI ) 20H,, R' - CH3, R3 - CN, R~ - H, R5 -
3, 5-dichloro-4-pyridyl, a:nd X = 0) .
Melting point: 159-160°C
'N-N~IR(CDC.~ 3) ~ : 2. 24(1H, t. J°=6. OHz). 4. O1(3H, s). 4. 04-4.
08(2H, m).
4. 27(2H, t, J~=4. OHz), 7. 29(1H, s>> 8. 19(1H, s),
8. 24(1H, s), 8. 61 (2H, s).
Example 44
The procedure of E~:ample 28 was repeated through use
of ( Z ) -3- ( 3, 5-dichloro-4-pyridyl ) -2- ( 4-hydroxy-5-methoxy-2-
pyridyl ) propenenitrile ( formula ( 1 ) , wherein R1 - H, Rz - CH ~,
R3 - CN, Ra - H, R5 - 3, 5-dichloro-4-pyridyl, and X = 0) and
4-bromobutanol, to thereb:~ obtain the compound shown below.
( Z ) -3- ( 3, 5-Dich:loro-4-pyridyl ) -2- [ 4- ( 4-
67
CA 02236851 1998-OS-06
hydroxybutyloxy)-5-methoxy-2-pyridyl)propenenitrile (formula
( 1 ) , wherein R1 = ( CHI ) OOH, R' - CH3, R3 - CN, R~ - H, RS -
3,5-dichloro-4-pyridyl, and X = 0).
Melting point: 120.5-122°C:
'N-N~4RCCDC.~ 3) ~ : 1. 74-1. 82<3H, m), 1. 99-2. 05(2H, m),
3. 75(2H, m?, 4. 00(3H, s). 4. 21 (2H. t, J=6. OHZ).
7.27(1H, s;~, 8. 19C1H, s), 8.21(1H, s), 8. 61C2H, s).
Example 45
The procedure of Example 28 was repeated through use
of (Z)-3-(3,5-dichloro-4-pyridyl)-2-(4-hydroxy-5-methoxy-2-
pyridyl ) propenenitrile ( formula ( 1 ) , wherein R1 - H, R' - CH,;,
R3 - CN, R4 - H, Ry - 3, 5-~~ichloro-4-pyridyl, and X = 0) and
5-bromopentanol, to there:oy obtain the compound shown below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[4-(5-
hydroxypentyloxy)-5-metho:xy-2-pyridyl)propenenitrile
( formula ( 1 ) , wherein R1 -- (CHI ) SOH, RZ = CHz, R3 - CN, R4 - H,
RS - 3, 5-dichloro-4-pyridyl, and X = O) .
Melting point: 86-87°C
'H-NM(R(CDC.~ 3) o : 1. 35(1H, m>, 1. 58-1. 70(4H, m). 1. 93-1. 97C2H, m).
3. 71 C2H, m;>, 4. 00(3H, s), 4. 16C2H, t. J=6. OHZ).
7.26(1H, s), 8. 19(1H, s). 8. 21C1H, s). 8. 61(2H, s).
Example 46
Synthesis of (Z)-3-(3,5-dichloro-4-pyridyl)-2-[5-
metboxy-4-(3,4-methylenedioxyphenylmethyloxy)-2-
pyridyl]propenenitrile (formula (1), wherein Rl - 3,4-
68
CA 02236851 1998-OS-06
met:hylenedioxyphenylmethyl, R' - CH_3, R~' - CN, Ra - H, RS -
3,5-dichloro-4-pyridyl, and X = 0):
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-(4-hydroxy-5-methoxy-
2-pyridyl ) propenenitrile ( formula ( 1 ) , wherein R1 - H, R' -
CH3, R3 - CN, Ra - H, R5 - 3, 5-dichloro-4-pyridyl, and X = 0)
(0.97 g, 3.0 mmol), 3,4-methylenedioxyphenylmethanol (0.50 g,
3.3 mmol), and triphenylphosphine (1.18 g, 4.5 mmol) were
dissolved in tetrahydrofuran (100 ml). While the solution
was stirred at room temperature, diethyl azodicarboxylate
(0.78 g, 4.5 mmol) was added dropwise. The reaction mixture
was stirred for four hours at room temperature and then
water was added thereto. The mixture was extracted with
ethyl acetate. The organic layer was sequentially washed
with water and saturated brine, dried, and then concentrated
under reduced pressure. (;rystals precipitated from the
residue were recrystallized from an isopropyl ether-ethanol,
to thereby obtain the title compound (1.01 g, yield 74~).
Melting point: 182-183°C
'H-N~~(RCCDC.~ 3) ~ : 4. 00C3H, s;, 5. 15C2H, s), 5. 98(2H, s),
6. 80-7. OOc;3H, m), 7. 36(1H, s), 8. 16(1H, s),
8.23(1H, s;~, 8. 61(2H, s).
Example 47
The procedure of Example 46 was repeated through use
of (Z)-3-(3,5-dichloro-4-pyridyl)-2-(4-hydroxy-5-methoxy-2-
pyridyl) propenenitrile ( f srmula ( 1 ) , wherein R1 - H, R- - CH;,
R3 -- CN, Rq - H, RS - 3, 5-dichloro-4-pyridyl, and X = Oj and
69
CA 02236851 1998-OS-06
4,5-dimethoxy-2-pyridinemethanol, to thereby obtain the
compound shown below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[4-(4,5-dimethoxy-2-
pyridylmethyloxy)-5-metho:xy-2-pyridyl]propenenitrile
( formula ( 1 ) , wherein R1 -- 4, 5-dimethoxy-2-pyridylmethyl, R'
- CH3, R~ - CN, R~ - H, R~ - 3, 5-dichloro-4-pyridyl, and X =
0) .
Melting point: 146.5-147..5°C
'H-N~~fRCCDC.~ 3) ~ : 3. 94(31f, s), 3. 96C3H, s), 4. 03C3H, s). 5.31C2H, s).
7. 07(1H, s), 7. 52(1H, s). 8. 09(1H, s). 8. 14C1H. s).
8. 24(111, s). 8. 60(2H, s).
Example 48
The procedure of Example 46 was repeated through use
of ( Z ) -3- ( 3, 5-dichloro-4-pyridyl ) -2- ( 4-hydroxy-5-methoxy-2-
pyridyl ) propenenitrile ( formula ( 1 ) , wherein R1 - H, R' - CH;,
R3 -- CN, R9 - H, R5 - 3, 5-dichloro-4-pyridyl, and X = 0) and
cyclopropanemethanol, to i~hereby obtain the compound shown
below.
(Z)-2-(4-Cyclopropylmethyloxy-5-methoxy-2-pyridyl)-3-
(3,5-dichloro-4-pyridyl)propenenitrile (formula (1), wherein
Rl -- cyclopropylmethyl, R' - CHI, R3 - CN, R~ - H, RJ - 3, 5-
dichloro-4-pyridyl, and X = 0).
Melting point: 118-119°C
'H-N~1RCCDC.~ 3) ~ : 0. 44C21~, m), 0. 72(2H, m), 1. 36(1H. m).
3. 99C2H, d, .I=7. 5Hz), 4. 02(3H, s), 7. 23C1H, s).
8. 19(1H, s). 8.23(1H, s). 8. 61C2H, s).
CA 02236851 1998-OS-06
Example 49
The procedure of Example 46 was repeated through use
of (Z)-3-(3,5-dichloro-4-pyridyl)-2-(4-hydroxy-5-methoxy-2-
pyridyl)propenenitrile (formula (1), wherein R1 - H, R' - CH3,
R3 - CN, R~ - H, RS - 3, 5-dichloro-4-pyridyl, and X = 0) and
2-pyridineethanol, to thereby obtain the compound shown
below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-methoxy-4-(2-(2-
pyridyl)ethyloxy)-2-pyridyl]propenenitrile (formula (1),
wherein R1 = 2- (2-pyridyl ) ethyl, R~ - CH3, R3 - CN, R~ - H, R
- 3,5-dichloro-4-pyridyl, and X = O).
Melting point: 134-135°C
'H-N~~1RCCDC.~ 3) ~ : 3. 38C2H. t, J=~. OHz). 3. 96(3H, s),
4. 58(2H, t, J=7. OHz), 7. 00-7. 30C2H, m), 7. 32C1H, s),
7. 65(1H, m), 8. 15(1H, s), 8. 21(1H, s), 8. 55C1H, m),
8. 61 C2H, s) .
Example 50
The procedure of Example 46 was repeated through use
of (Z)-3-(3,5-dichloro-4-pyridyl)-2-(4-hydroxy-5-methoxy-2-
pyridyl ) propenenitrile ( formula ( 1 ) , wherein R1 - H, R' - CH3,
R3 - CN, R4 - H, RS - 3, 5-dichloro-4-pyridyl, and X = 0) and
4-methyl-5-thiazoleethanol, to thereby obtain the compound
shown below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-methoxy-4-(2-(4-
methyl-5-thiazolyl)ethyloxy)-2-pyridyl]propenenitrile
71
CA 02236851 1998-OS-06
( formula ( 1 ) , wherein R' -- 2- ( 4-methyl-5-thiazolyl ) ethyl, R-
- CH3, R3 - CN, Ra - H, RS - 3, 5-dichloro-4-pyridyl, and X =
0) .
Melting point: 136-137°C
'H-N~~R(CDC.~ 3) o : 2. 48C3H, s), 3. 36C2H, t, J=7. OHz), ~. OOC3H, s),
4. 31 C2H. t, J=7. OHz). 7. 22(1H, s), 8. 18(1H, s).
8.23(1H, si. 8. 61C2H, s). 8. 61(1H, s).
Example 51
The procedure of Example 46 was repeated through use
of (Z)-3-(3,5-dichloro-4-pyridyl)-2-(4-hydroxy-5-methoxy-2-
pyridyl ) propenenitrile ( formula ( 1 ) , wherein R1 - H, R' - CH;,
R3 - CN, R9 - H, R5 - 3, 5-dichloro-4-pyridyl, and X = 0) and
N-hydroxyethyl-2-pyridone (Preparation Example 15), to
thereby obtain the compound shown below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-methoxy-4-(2-(2-
pyridon-1-yl)ethyloxy)-2-;oyridyl]propenenitrile (formula (1),
wherein R' - 2- (2-pyridon--1-yl) ethyl, R' - CH,, R3 - CN, Ra -
H, RS - 3, 5-dichloro-4-pyridyl, and X = 0) .
Melting point: 142-143°C
' H-N~dR(CDC.~ 3) o : 3. 97(3H, s), 4. 41 C2H, t, J=5. OHz),
4. 48C2H, t, J=5. OHz), 6. 17-6. 22C1H, m),
6. 58(1H, d, J=10. OHz), 7. 26C1H, s), 7. 32-7. ~OC1H, m),
7. 45(1H, dd, J=2. 0, 7. OHz), 8. 10<,1H, s), 8. 22(1H, s).
8. 60C2H, s).
Example 52
72
CA 02236851 1998-OS-06
The procedure of Example 46 was repeated through use
of (Z)-3-(3,5-dichloro-4-pyridyl)-2-(4-hydroxy-5-methoxy-2-
pyridyl ) propenenitrile ( f~~rmula ( 1 ) , wherein R1 - H, R' - CH3,
R3 = CN, R4 - H, R5 - 3, 5-c~ichloro-4-pyridyl, and X = 0) and
3-~~yridinepropanol, to thereby obtain the compound shown
below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-methoxy-4-(3-(3-
pyridyl)propyloxy)-2-pyridyl]propenenitrile (formula (1),
whe rein R1 - 3- ( 3-pyridyl ) propyl, R' - CH3, R3 - CN, R4 - H,
R5 - 3, 5-dichloro-4-pyrid~~l, and X = 0) .
Melting point: 91-93°C
'H-Nh~R(D~~fSO-ds) o : 2. 12(2H, m), 2. 79C2H, t, J=6. SHz~. 3. 95(3H, s).
4. 21 (2H, t, J=6. 5Hz), 7. 30(1H, m), 7. 56(1H, s),
7. 67(1H, m~, 8. 28C1H, s), 8. 33(1H, s), 8. ~7C2H, m).
8. 82(2H, s).
Example 53
The procedure of Example 45 was repeated through use
of (Z)-3-(3,5-dichloro-4-hyridyl)-2-(4-hydroxy-5-methoxy-2-
pyridyl) propenenitrile ( formula ( 1 ) , wherein R1 - H, Rz - CH3,
R3 -- CN, Ra - H, RS - 3, 5-<~ichloro-4-pyridyl, and X = 0) and
4-pyridinepropanol, to thereby obtain the compound shown
below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-methoxy-4-(3-(4-
pyridyl)propyloxy)-2-pyridyl]propenenitrile (formula (1),
wherein R1 - 3- ( 4-pyridyl ) propyl, R' - CH3, R3 - CN, Ra - H,
R5 -- 3, 5-dichloro-4-pyridy 1, and X = 0) .
73
CA 02236851 1998-OS-06
Melting point: 124-125°C
' H-N~dR(CDC .~ 3) ~ : 2. 25C2H. m;~, 2. 87(2H, m), 4. Ol (3H, s),
4. 16(2H, t, J=6. OHz), 7. 17(2H, d, J=6. OHz), 7. 22C1H, s).
8. 19(1H, s;~, 8. 23(1H, s), 8. 50(2H, d, J=6. OHz),
8. 61 (2H. s;'.
Example 54
The procedure of Example 46 was repeated through use
of (Z)-3-(3,5-dichloro-4-oyridyl)-2-(4-hydroxy-5-methoxy-2-
pyridyl ) propenenitrile ( f~~rmula ( 1 ) , wherein R1 - H, R' - CH_j,
R' - CN, Ra - H, Ry - 3, 5-c~ichloro-4-pyridyl, and X = 0) and
N-r.ydroxyethylmorpholine, to thereby obtain the compound
shown below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-methoxy-4-(2-(N-
morpholino)ethyloxy)-2-pyridyl]propenenitrile (formula (1),
whE:rein R1 - 2- (N-morpholino) ethyl, R' - CH3, R3 - CN, R~ - H,
R5 - 3, 5-dichloro-4-pyrid5~l, and X = 0) .
Melting point: 130-131°C
'H--N~1R(CDC.~ 3) d : 2. 50-2. 70(~H, m). 2. 89(2H, t. J=6. OHz),
3. 60-3. 82C4H, m). 4. 00(3H, s), ~. 28C2H, t, J=6. OHz),
7. 29(1H, s), 8. 19(1H, s). 8. 22(1H, s). 8. 61 C2H, s).
Example 55
The procedure of Example 46 was repeated through use
of (Z)-3-(3,5-dichloro-4-pyridyl)-2-(4-hydroxy-5-methoxy-2-
pyridyl ) propenenitrile ( formula ( 1 ) , wherein R~ - H, R'' - CH,"
R3 - CN, R~ - H, R5 - 3, 5-c3ichloro-4-pyridyl, and X = O) and
74
CA 02236851 1998-OS-06
1,~'~-bis(BOC-amino)-2-propanol, to thereby obtain the
compound shown below.
(Z)-2-[4-(1,3-bis(BOC-amino)-2-propyloxy)-5-methoxy-2-
pyridyl]-3-(3,5-dichloro-4-pyridyl)propenenitrile (formula
( 1 ) , wherein R1 - 1, 3-bis (BOC-amino ) -2-propyl, R~ - CH3, R3 -
CN, Ra - H, RS - 3, 5-dichloro-4-pyridyl, and X = O) .
Melting point: 181-181.5°C:
':-I-N~iR(CDC~ 3) o : 1. 43C18H, s), 3. 23(2H, m), 3. 66C2H, m), 3. 99C3H, s),
4. 54(1H, m), 5. 21 C2H, m), 7. 93C1H, s), 8. 16C1H, s),
8. 25(1H, s), 8. 60C2H, s).
Example 56
The procedure of Example 46 was repeated through use
of ( Z ) -3- ( 3, 5-dichloro-4-pyridyl ) -2- ( 4-hydroxy-5-methoxy-2-
pyridyl ) propenenitrile ( formula ( 1 ) , wherein R1 - H, Rz - CH3,
R3 -- CN, R4 - H, R5 - 3, 5-dichloro-4-pyridyl, and X = O) and
cis-1,3-cyclopentanediol monoTBS-ether, to thereby obtain
the compound shown below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-methoxy-4-(cis-3-
(TBS-oxy)cyclopentyloxy-2--pyridyl]propenenitrile (formula
(1) , wherein R1 - cis-3- (TBS-oxy) cyclopentyl, R- - CH3, R-3 -
CN, R'' - H, RS - 3, 5-dichloro-4-pyridyl, and X = O) .
'H-f~MIRCCDC.~ 3) b : 0. 05(6H, s), 0. 88(9H, s), 1. 60-2. 62(6H, m).
3. 98(3H, s), 4. 20-4. 40(1H, m), 4. 75-5. 00(1H, m~,
7. 19C1H, s), 8. 19(2H, s), 8. 61 (2H, s).
Example 57
CA 02236851 1998-OS-06
The procedure of Example 46 was repeated through use
of (Z)-3-(3,5-dichloro-4-pyridyl)-2-(4-hydroxy-5-methoxy-2-
pyridyl)propenenitrile (formula (1), wherein R1 - H, R' - CHI,
R3 -- CN, Rq - H, R5 - 3, 5-<~ichloro-4-pyridyl, and X = 0) and
trans-1,3-cyclopentanedio:l monoTBS-ether, to thereby obtain
the compound shown below.
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-methoxy-4-(trans-
3-(TBS-oxy)cyclopentyloxy-2-pyridyl]propenenitrile (formula
( 1 ) , wherein R1 = trans-3-~ (TBS-oxy) cyclopentyl, R'' - CH3, R3
- CN, R~ - H, Ry' - 3, 5-dichloro-4-pyridyl, and X = 0) .
'H-N~dRCCDC.~ 3) o : 0. 06(6H, s). 0. 89(9H, s). 1. 50-2. 50C6H, m).
3. 98(3H, s). 4. 30-4. 60C1H, m). 4. 95-5. 22C1H, m~.
7. 23~1H, s). 8. 17(1H, s). 8. 20C1H. s). 8. 61 C2H, s).
Example 58
Synthesis of ( Z ) -2- ( 3-carboxyphenyl ) -3- [ 4- ( 2-
indanyloxy)-5-methoxy-2-p:yridyl]propenenitrile (formula (1),
wherein R1 - 2-indanyl, RZ = CH3, R' - H, Ry - CN, R5 - 3-
carboxyphenyl, and X = 0):
In 4 ml of dioxane, was dissolved (Z)-3-[4-(2-
indanyloxy)-5-methoxy-2-p:yridyl]-2-(3-
methoxycarbonylphenyl)propenenitrile (formula (1), wherein
R1 - 2-indanyl, R' - CH3, R3 - H, Rq - CN, R' - 3-
methoxycarbonylphenyl, and X = 0) (426 mg, 1 mmol). To the
solution was added 1N aqueous NaOH solution (1.2 ml). The
mixture was stirred for 0.5 hours at room Temperature. The
reaction mixture was poured into 5~ aqueous ammonium
76
CA 02236851 1998-OS-06
chloirde solution. Precipitated crystals were collected by
filtration, washed with w<~ter, and recrystallized from
ethanol, to thereby obtain the title compound (353 mg, yield
86~) .
Melting point: 281.5-282..'°C
' H-~l~~iR (D~~1S0-d 6 ) ~ : 2. 90-3. 65 (4H, m) . 3. 89 (3H, s ) , 5. 20-5.
42 ( 1 H, m) .
7. 05-7. 40 (4H, m) , 7. 50-7. 75 (2H, m) , 7. 90-8. 10 (3H, m) ,
8. 37(2H, s).
Example 59
The procedure of Example 58 was repeated through use
of (Z)-2-[4-(2-indanyloxy;-5-methoxy-2-pyridyl]-3-(2-
methoxycarbonyl-4-pyridyl;propenenitrile (formula (1),
wherein R1 - 2-indanyl, R' - CH;, R3 - CN, R.~ - H, R5 - 2-
methoxycarbonyl-4-pyridyl, and X = 0), to thereby obtain the
compound shown below.
(Z) -3- (2-Carboxy-4-p~~ridyl) -2- [4- (2-indanyloxy) -5-
methoxy-2-pyridyl]propenenitrile (formula !1), wherein R'
2-indanyl, R'' - CH3, R3 - C:N, R~ - H, R~' - 2-carboxy-4-
pyridyl, and X = O).
Melting point: 224-225°C
'~-,-NMRCD~~SO-ds) b : 3. 08-3. 5fiC4H, m~. 3. 85C3H, s). 5. 50C1H, br).
7. 18-7. 30C4H, m). 7. 66(1H, s). 8. 09-8. lOClN. mJ.
8. 29C1H, s). 8. 39(1H, s). 8. 53(1H, s).
8. 89C1H, ci, J=5. 3Hz).
Example 60
77
CA 02236851 1998-OS-06
The procedure of Exa::nple 58 was repeated through use
of (Z)-3-(4-cyclopentylox:y-5-methoxy-2-pyridyl)-2-(3-
methoxycarbonylphenyl)propenenitrile (formula (1), wherein
R1 - cyclopentyl, R' - CH;,, R' - H, R~ - CN, RJ - 3-
methoxycarbonylphenyl, an~~ X = 0), to thereby obtain the
con.pound shown below.
(Z)-2-(3-Carboxyphenyl)-3-(4-cyclopentyloxy-5-
methoxy-2-pyridyl)propenenitrile (formula (1), wherein R1 -
cyclopentyl, Rz - CH3, R3 -- H, Ra - CN, R5 - 3-carboxyphenyl,
anc. X = 0 ) .
Melting point: 264-266°C
H-N~~R (Dh~SO-d s ) ~ : 1. 58-1. f>2 C6H, m) , 1. 97-2. 10 (2H, m) , 3. 94
C3H, s ) .
4. 88-4. ~~4C1H, m). 7. 54(1H, s), 7. 63(1H, t, J=8. OHz).
7. 96-8. 03(3H, m). 8. 33(1H, s), 8. 35C1H, s).
Example 61
Synthesis of (Z)-2-[4-(1,3-diamino-2-propyloxy)-5-
methoxy-2-pyridyl]-3-(3,5-dichloro-4-pyridyl)propenenitrile
trihydrochloride (formula (1), wherein R'' -- 1,3-diamino-2-
prcpyl, R' - CH3, R3 - CN, Ra - H, RS - 3, 5-dichloro-4-
pyridyl, and X = 0)
In 4 ml of ether, was dissolved (Z)-2-[4-(1,3-bis(BOC-
amino)-2-propyloxy)-5-met:Boxy-2-pyridyl]-3-(3,5-dichloro-4-
pyridyl)propenenitrile (formula (1), wherein R1 - 1,3-
bis (BOC-amino) -2-propyl, R' - CH3, R3 - CN, R9 = H, R5 - 3, 5-
dic.hloro-4-pyridyl, and X = 0) (594 mg, 1 mmol). To the
solution was added 4N HCl-dioxane solution (2 ml). The
78
CA 02236851 1998-OS-06
mi~s:ture was stirred for three hours at room temperature.
Precipitated crystals were collected by filtration, washed
with ether, and recrystallized from ethanol, to thereby
obtain the title compound (450 mg, yield 89=).
Melting point: 174-177°C
' H-N~~iR CD~~4S0-d 6 ) o : 3. 23 (2H, m) . 3. 40 C2H, m) , 3. 97 (3H, s) , 5.
32 ( 1 H, m) .
8. 22(1H. ~;), 8. 41(1H, s). 8. 72(1H, s). 8. 83C2H, s).
Example 62
Synthesis of (Z)-3-(3,5-dichloro-4-pyridyl)-2-[4-(cis-
(3-hydroxy)cyclopentyloxy)-5-methoxy-2-
pyridyl]propenenitrile (formula (1), wherein R1 - cis-(3-
hyc.roxy) cyclopentyl, Rz - CH3, R3 - CN, Ra - H, R' - 3, 5-
dichloro-4-pyridyl, and X = 0):
In 2 ml of THF, was dissolved (Z)-3-(3,5-dichloro-4-
pyridyl)-2-[5-methoxy-4-(cis-3-(TBS-oxy)cyclopentyloxy)-2-
pyridyl]propenenitrile (formula (1), wherein R1 - cis-3-
(TES-oxy) cyclopentyl, R2 -- CH3, R' - CN, R9 - H, R5 - 3, 5-
dichloro-4-pyridyl, and X = 0) (520 mg, 1 mmol). To the
solution was added 1M tetrabutylammnoium fluoride-THF
solution (2.2 ml), and the mixture was stirred for five
hours at room temperature. The reaction mixture was
evaporated to dryness and then water was added to the
residue. The mixture was extracted with ethyl acetate. The
organic layer was washed with saturated brine, dried, and
then concentrated under reduced pressure. The residue was
purified by silica gel column chromatography and
79
CA 02236851 1998-OS-06
rec:rystallized from a benzene-hexane, to t:zereby obtain the
title compound (240 mg, yield 59~).
Melting point: 134-135°C
'11-N~dR(CDC.~ 3) d : 1. 90-2. 24~;6H, m), 2. 39C1H, d. J=8. OHz),
3. 98C3H, s;>, ~. 38-4. 50C1H, m). 5. 04-5. 08(1H, m),
7.26(1H, s), 8.20C1H. s). 8.22(1N. s~. 8. 61C2H, s).
Example 63
The procedure of Example 62 was repeated through use
of (Z)-3-(3,5-dichloro-4-:pyridyl)-2-[5-methoxy-4-(trans-3-
(TE,S-oxy)cyclopentyloxy)-2-pyridyl]propenenitrile (formula
(1) , wherein R1 - trans-3-- (TBS-oxy) cyclopentyl, R' - CH3, R'
- C'N, Rq - H, RS - 3, 5-dichloro-4-pyridyl, and X = 0) , to
thereby obtain the compound shown below.
(Z)-3-(3,5-Dichloro-4-pyridyl}-2-[4-(trans-(3-
hyc.roxy)cyclopentyloxy}-5-methoxy-2-
pyridyl]propenenitrile(formula (1), wherein Ri - trans-(3-
hydroxy) cyclopentyl, R' - CHI, R~ - CN, R' - H, R' - 3, 5-
dichloro-4-pyridyl, and X = 0).
Melting point: 136-137°C
'H-N~~(R(CDC.~ 3) ~ : 1. 58(1H, ~~r), 1. 70-1. 80C1H, m), 1. 90-2. 00(1H, m),
2. 07-2. 28(3H, m). 2. 35-2. 46(1H, m). 3. 98C3H. s),
4. 58-4. 62(1H, m), 5. 04-5. 07(1H, m), 7. 24(1H, s),
8. 17(1H, s), 8.20(1H, s~, 8. 61(2H, s).
Example 64
The procedure of Example 1 was repeated through use of
CA 02236851 1998-OS-06
5-c:yclopentyloxy-6-methoxy-3-pyridineacetonitrile
(Preparation Example 16) and 3,5-dichloro-4-
pyridinecarbaldehyde (Pre.oaration Example '7), to thereby
obtain the compound shown below.
(Z)-2-(5-Cyclopentyloxy-6-methoxy-3-pyridyl)-3-(3,5-
di c~hloro-4 -pyridyl ) propeneni tri 1 a .
Melting point: 143-144°C
'H-T~~dR(CDC.~ 3) ~ : 1. 60-2. 05(8H. m), 4. 05(8H, s). 4. 86(1H, m),
7. 25(1H, d, J=2. OHz). 7. 27(1H, s), 8. 11 (1H, d, J=2. OHz).
8. 62(2H, s).
Example 65
The procedure of Example 1 was repeated through use of
6-cyclopentyloxy-5-methox_~-2-pyridineacetonitrile
(Preparation Example 17) and 3,5-dichloro-4-
pyridinecarbaldehyde (Preparation Example 7), to thereby
obtain the compound shown below.
(Z)-2-(6-Cyclopentyloxy-5-methoxy-2-pyridyl)-3-(3,5-
dichloro-4-pyridyl)propenenitrile.
Melting point: 137-138°C
'H--NhiRCCDC.~ ~) o : 1. 61-2. 12(8H, m), 3. 91(3H, s), 5. 50(1H, m),
7. 06C1H, d. J=8. OHz), 7. 31 (1H, d. J=8. OHz), 8. 07C1H, s),
8. 60C2H, s).
Example 66
The procedure of Example 1 was repeated through use of
5-methoxy-4-[2-(4-methyl-:~-thiazolyl)ethyloxy]-2-
81
CA 02236851 1998-OS-06
pyridineacetonitrile (Preparation Example 18) and methyl 9-
formyl-2-picolinate, to thereby obtain the compound shown
below.
(Z)-2-[5-methoxy-4-(2-(4-methyl-5-thiazolyl)ethyloxy)-
2-pyridyl]-3-(2-methoxycarbonyl-4-pyridyl)propenenitrile
( formula ( 1 ) , wherein R1 -- 2- ( 4-methyl-5-thiazolyl ) ethyl, R'
- CH3, R3 - CN, R~ - H, R5 - 2-methoxycarbonyl-4-pyridyl, and
X = 0) .
Melting point: 161-162°C
'N--N6fR(CDC.~ 3) o : 2. 49(3H, s), 3. 37(2H, t. J=6. 5Hz), 4.01(3H, s),
4. 05(3H, s), 4. 32(2H, t, J=6. 5Hz). 7. 25(1H, s),
8. 04C1H, dd, J=1. 0, 5. 5Hz). 8. 20(1H, s), 8. 34(1H, s),
8. 47(1H, d. .J=1. OHz). 8. 63C1H, s), 8. 89(1H, d, J=5. 5Hz).
Similarly, the folloiaing compounds can also be
prepared.
( Z ) -2- ( 6-Cyclopentyloxy-5--methoxy-2-pyridyl ) -3- ( 2, 6-
dic:~lorophenyl)propenenitrile
(Z)-2-(6-Cyclopentyloxy-5-~methoxy-2-pyridyl)-3-(3-
pyridyl)propenenitrile
(Z)~-2-(6-Cyclopentyloxy-5-methoxy-2-pyridyl)-3-(4-
pyr:idyl)propenenitrile
(Z)--3-(3,5-Dichloro-4-pyridyl)-2-(5-methoxy-6-phenethyloxy-
2-p~lridyl)propenenitrile
(Z)--3-(3,5-Dichloro-4-pyridyl)-2-(5-methoxy-6-(3-
phenylpropyloxy)-2-pyridyl)propenenitrile
(Z)--2-(6-Butyloxy-5-methoxy-2-pyridyl)-3-(5,5-dichloro-4-
82
CA 02236851 1998-OS-06
pyridyl)propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[6-(1-ethylpropyloxy)-5-
met:boxy-2-pyridyl]propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyridyl ) -2- [ 6- ( 2-indanyloxy) -5-
met:boxy-2-pyridyl]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-methoxy-6-(tetrahydro-3-
furanyloxy)-2-pyridyl]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-methoxy-6-(exo-2-
norbornyloxy)-2-pyridyl]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-(5-methoxy-6-
methoxymethyloxy-2-pyridy.l)propenenitrile
(Z)-3-(3,5-Dichloro-4-pyr:idyl)-2-(6-hydroxy-5-
methoxypyridyl)propenenit:rile
(Z)-3-(3,5-Dichloro-4-pyr:idyl)-2-[6-(3-hydroxypropyloxy)-5-
methoxy-2-pyridyl]propenenitrile
( Z ) -3- ( 6-Cyclopentyloxy-5--methoxy-2-pyridyl ) -2- ( 3, 5-
dichloro-4-pyridyl)propenenitrile
(Z)-2-(6-Cyclopentyloxy-5--methoxy-2-pyridyl)-3-(3-fluoro-4-
pyridyl)propenenitrile
( Z ) -2- [ 6- ( 2-Indanyloxy) -5--methoxy-2-pyridyl_ ] -3- ( 2-
methoxycarbonyl-4-pyridyl)propenenitrile
( Z ) -2- [ 6- ( 2-Indanyloxy) -5--methoxy-2-pyridyi ] -3- ( 4-
pyridyl)propenenitrile
4-[(Z)-2-Cyano-2-(6-(2-indanyloxy)-5-methoxy-2-pyridyl)-1-
eth~~nyl]pyridine N-oxide
4- [ ( Z ) -2-Cyano-2- ( 6- ( 2-inclanyloxy) -5-methoxy-2-pyridyl ) -1-
ethenyl]-3,5-dichloropyricline N-oxide
83
CA 02236851 1998-OS-06
(Z)-3-(3-Carboxyphenyl)-2-(6-cyclopentyloxy-5-methoxy-2-
pyridyl)propenenitrile
(Z)-3-(6-Cyclopentyloxy-5-methoxy-2-pyridy:L)-2-(3-
methoxycarbonylphenyl)propenenitrile
(Z)-3-[6-(2-Indanyloxy)-5-methoxy-2-pyridyl]-2-(3-
methoxycarbonylphenyl)pro~?enenitrile
(Z)-3-[6-(2-Indanyloxy)-5-methoxy-2-pyridyl]-2-(4-
pyridyl)propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyr:idyl ) -2- [ 6- ( 2-hydroxyethyloxy) -5-
methoxy-2-pyridyl]propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyr:idyl ) -2- [ 6- ( 4-hydr_oxybutyloxy) -5-
methoxy-2-pyridyl]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyr:Ldyl)-2-[6-(5-hydroxypentyloxy)-5-
methoxy-2-pyridyl]propenenitrile
(Z)-2-(6-Cyclopropylmethy:Loxy-5-methoxy-2-pyridyl)-3-(3,5-
dichloro-4-pyridyl)propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyr=Ldyl ) -2- [ 5-methoxy-6- ( 2- ( 2-
pyridyl ) ethyloxy) -2-pyrid~ll ] propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-methoxy-6-(2-(4-methyl-
5-thiazolyl)ethyloxy)-2-p~~ridyl]propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyr:_dyl ) -2- [ 5-methoxy-6- ( 2- ( 2-
pyridone-1-yl)ethyloxy)-2--pyridyl]propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyridyl ) -2- [ 5-methoxy- 6- ( 3- ( 3-
pyridyl)propyloxy)-2-pyridyl]propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyridyl ) -2- [ 5-methoxy-6- ( 3- ( 4-
pyridyl)propyloxy)-2-pyric~yl]propenenitrile
(Z) -3- (3, 5-Dichloro-4-pyridyl) -2- [5-methoxy-6- (2- (N-
84
CA 02236851 1998-OS-06
morpholino)ethyloxy)-2-pyridyl]propenenitrile
(Z)-2-(3-Carboxyphenyl)-3--[6-(2-indanyloxy;-5-methoxy-2-
pyridyl]propenenitrile
(Z)-3-(2-Carboxy-4-pyridy:L)-2-[6-(2-indanyloxy)-5-methoxy-2-
pyridyl]propenenitrile
(Z)-2-(3-Carboxyphenyl)-3--(6-cyclopentyloxy-5-methoxy-2-
pyridyl)propenenitrile
(Z)-2-[5-Methoxy-6-(2-(4-methyl-5-thiazolyl_)ethyloxy)-2-
pyridyl]-3-(2-methoxycarbonyl-4-pyridyl)propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-methoxy-6-(cis-3-(TBS-
oxy)cyclopentyloxy)-2-pyridyl]propenenitrile
(Z) -3- (3, 5-Dichloro-4-pyr=idyl) -2- [ 5-methoxy-6- (trans-3- (TBS-
oxy)cyclopentyloxy)-2-pyr__dyl]propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyr__dyl ) -2- [ 6- ( cis- ( 5-hydroxy)
cyclopentyloxy)-5-methoxy--2-pyridyl]propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyridyl ) -2- [ 6- ( trans- ( 3-hydroxy)
cyclopentyloxy)-5-methoxy--2-pyridyl]propenenitrile
(Z)-2-(5-Cyclopentyloxy-6--methoxy-3-pyridyl)-3-(2,6-
dichlorophenyl)propenenitrile
(Z)-2-(5-Cyclopentyloxy-6--methoxy-3-pyridyl)-3-(3-
pyridyl)propenenitrile
( Z ) -2- ( 5-Cyclopentyloxy-6--methoxy-3-pyridyl ) -3- ( 4-
pyridyl)propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-(6-methoxy-5-phenethyloxy-
3-pyridyl)propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[6-methoxy-5-(3-
phenylpropyloxy)-3-pyridy7-]propenenitrile
CA 02236851 1998-OS-06
(Z)-2-(5-Butyloxy-6-methoxy-3-pyridyl)-3-(3,5-dichloro-4-
pyridyl)propenenitrile
(Z) -3- (3, 5-Dichloro-4-pyridyl ) -2- [ 5- ( 1-ethylpropyloxy) -6-
met:boxy-3-pyridyl]propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyridyl ) -2- [ 5- ( 2-indanyloxy) -6-
met:boxy-3-pyridyl]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[6-methoxy-5-(tetrahydro-3-
furanyloxy)-3-pyridyl]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[6-methox:y-5-(exo-2-
norbornyloxy)-3-pyridyl]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-(6-methoxy-5-
met:hoxymethyloxy-3-pyridyl)propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-(5-hydroxy-6-methoxy-3-
pyridyl)propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-(3-hydroxypropyloxy)-6-
methoxy-3-pyridyl]propene:nitrile
(Z)-3-(5-Cyclopentyloxy-6-methoxy-3-pyridy:l)-2-(3,5-
dic:hloro-4-pyridyl)propenenitrile
(Z)-2-(5-Cyclopentyloxy-6-methoxy-3-pyridyl)-3-(3-fluoro-4-
pyridyl)propenenitrile
(Z)-2-[5-(2-Indanyloxy)-6-methoxy-3-pyridyl]-3-(2-
methoxycarbonyl-4-pyridyl)propenenitrile
(Z)-2-[5-(2-Indanyloxy)-6-methoxy-3-pyridyl]-3-(4-
pyridyl)propenenitrile
4- [ ( Z ) -2-Cyano-2- ( 5- ( 2-inc~anyloxy) -6-methoxy-3-pyridyl j -1-
ethenyl]pyridine N-oxide
4-[(Z)-2-Cyano-2-(5-(2-inc3anyloxy)-6-methoxy-3-pyridyl)-1-
86
CA 02236851 1998-OS-06
et~-~enyl]-3,5-dichloropyridine N-oxide
(Z)-3-(3-Carboxyphenyl)-2-[5-cyclopentyloxy-6-methoxy-3-
pyridyl]propenenitrile
(Z)-3-(5-Cyclopentyloxy-6-methoxy-3-pyridyl)-2-(3-
methoxycarbonylphenyl)pro:oenenitrile
( Z ) -3- [ 5- ( 2-Indanyloxy) -6-methoxy-3-pyridy:L ] -2- ( 3-
methoxycarbonylphenyl)propenenitrile
(Z)-3-[5-(2-Indanyloxy)-6-methoxy-3-pyridyl]-2-(4-
pyridyl)propenenitrile
(Z)-3-(3,5-Dichloro-4-pyr.idyl)-2-[5-(2-hydroxyethyloxy)-6-
methoxy-3-pyridyl]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyr.idyl)-2-[5-(4-hydroxybutyloxy)-6-
methoxy-3-pyridyl]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyr:idyl)-2-[5-(5-hydroxypentyloxy)-6-
methoxy-3-pyridyl]propenenitrile
(Z)-2-(5-Cyclopropylmethy:Loxy-6-methoxy-3-pyridyl)-3-(3,5-
dichloro-4-pyridyl)propenE:nitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[6-methoxy-5-(2-(2-
pyridyl)ethyloxy)-3-pyrid~~l]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[6-methoxy-5-(2-(4-methyl-
5-thiazolyl)ethyloxy)-3-p~lridyl]propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyridyl ) -2- [ 6-methoxy-5- ( 2- ( 2-
pyridone-1-yl)ethyloxy)-3--pyridyl]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[6-methoxy-5-(3-(3-
pyridyl)propyloxy)-3-pyric~yl]propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyr:_dyl ) -2- [ 6-methoxy-5- ( 3- ( 4-
pyridyl)propyloxy)-3-pyric~yl]propenenitrile
87
CA 02236851 1998-OS-06
( Z ) -3- ( 3, 5-Dichloro-4-pyr idyl ) -2- [ 6-methoxy-5- ( 2- (N-
morpholino)ethyloxy)-3-py:ridyl]propenenitrile
( Z ) -2- ( 3-Carboxyphenyl ) -3- [ 5- ( 2-indanyloxy) -6-methoxy-3-
pyridyl]propenenitrile
(Zj -3- (2-Carboxy-4-pyridy.L) -2- [5- (2-indany-Loxy) -6-methoxy-3-
pyridyl]propenenitrile
(Z)-2-(3-Carboxyphenyl)-3--(5-cyclopentyloxy-6-methoxy-3-
pyridyl)propenenitrile
(Z)-2-[6-Methoxy-5-(2-(4-methyl-5-thiazolyl)ethyloxy)-3-
pyridyl]-3-(2-methoxycarbonyl-4-pyridyl)propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[6-methoxy-5-(cis-3-(TBS-
oxy)cyclopentyloxy)-3-pyr_Ldyl]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[6-methoxy-5-(trans-3-(TBS-
oxy)cyclopentyloxy)-3-pyridyl]propenenitrile
(Z)-3-(3,5-Dichloro-4-pyridyl)-2-[5-(cis-(3-
hydroxy)cyclopentyloxy)-6--methoxy-3-pyridyl]propenenitrile
( Z ) -3- ( 3, 5-Dichloro-4-pyr=_dyl ) -2- [ 5- ( trans- ( 3-
hydroxy)cyclopentyloxy)-6--methoxy-3-pyridyl]propenenitrile
Test Example 1 PDE Inhibitory Activity Test
A variety of PDE isoymes shown below were isolated
fro::n human tissue and purified in accordance with the method
described in literature.
PDE III Human platelet
H.. Hidaka, et al., Bioph. Bioch. Acta, (1976), X29, p485
P. Grant, et al., Biochemistry, (1984), ~3, p1801
PDE IV Human histocytic~ lymphoma (U-937)
88
CA 02236851 1998-OS-06
T . Torphy, et al . , J. Pharm. Exp. Ther. , ( 1992 ) , X63,
p1195
M. DiSanto, et al., BBRC;, (1993) , 197, pi.126
PDE V Human platelet
H. Hidaka, et a1. , Bioph. Bioch. Acta, (1976) , ~9, p485
P. Grant, et al., Biochemistry, (1984), c3, p1801
C.D. Nicholson, et al., Trends Pharmacol. Sci., (1991), 1~,
p19.
The PDE activity was determined through use of a
modified two-step assay method described by Hidaka et al.
(Bioph. Bioch. Acta., (19'76), ,~9_, p485) .
Briefly, [3H]CAMP and [3H]cGMP are hydrolyzed by their
respective PDE isozymes to form [3H] 5'-AMP and [~H] 5'-GMP,
respectively. Subsequently, [3H] 51-AMP and [3H] 51-GMP are
transformed into [3H]adencsine and [3H]guanosine,
respectively, due to the action of nucleotidases. Unreacted
[3HicAMP and [3H]cGMP are :removed, causing them to bond to
ion exchange resin, and the quantity of eluted [3H]adenosine
or [3H]guanosine was counted in a liquid scintillation
counter.
The diluted enzyme solution contains 50 mM Tris-HC1
(pH 8. 0) , 5 mM MgCl2, and .'~0 ~,g of bovine serum albumin,
wherein the concentration~> represent final concentrations.
The substrate concentratic>n is 1 ~.M. The concentration of
eac;z test compound varies between 0.1 nM and 100 N.M. Each
test sample is incubated for 20 minutes at 30°C, and the PDE
rea~~tion is terminated by boiling for 2 minutes. The
89
CA 02236851 1998-OS-06
nucleotidase reaction was induced by adding snake venom
nucleotidase to the above--described reaction mixture and
incubating the resultant rnixture for 20 minutes at 30°C.
The ICso values of the test compounds were obtained
from concentration-reaction curves within the concentration
range of 0.1 nM to 100 ~.M..
Table 1
PDE inhibitory Activity
PDE III: PDE IV PDE V
Compound ICSO (~) IC~o (~.M) IC~o, (~.M)
Example 1 10 0.0026 >100
Example 14 >100 0.0059 5.6
Example 15 91 0.0048 56
Example 16 15 0.015 31
Example 17 >100 0.036 >100
Example 18 18 0.036 12
Example 19 >100 0.00065 >100
Example 20 56 0.011 >100
Rolipram >100 5.0 >100
Test Example 2 TNF-a Production Inhibition Test
Human promonocytic leukemia cells (U 937 cells, 1 x 105
cells/400 ~,1) were inocul~~ted on a 24-well culture plate.
By use of an RPMI 1640 cu7_ture liquid (supplemented with 10°
FCS) containing 50 nM PMA, the cells were incubated for 72
hours, to thereby induce differentiation into
monacyte/macrophage (see t~arkiz Daniel-Issakani, Allem M.
Spiegel and Berta Strulovici (1989), J. Biol. Chem. ?~,
p20240-20247). Subsequently, the culture supernatant was
discarded, and an RPMI 1640 culture liquid (supplemented
wit:z 10% FCS) containing 1.0 ng/ml of LPS
(li~opolysaccharide; E. cc>li, 0111: B4) was added. Each
CA 02236851 1998-OS-06
compound was added one hour before the LPS treatment, so as
to achieve concentrations of 100 ~.M, 10 ~.M, 1 ~.M, and 0.1 ~.M.
Six: hours after addition of LPS, the quantity of produced
TNF'-a in the supernatant was measured by use of a human
TNF'-a ELISA kit (Amersham, code RPN 2758). For respective
doses of each compound, ~~~ontrol was calculated, wherein the
quantity of TNF-a produce~~ when LPS treatment was performed
anc. the compound was not ;added was considered 1000. Further,
based on primary regression curves, there were calculated
ICS; values in terms of TNF-a production inhibitory activity
for respective compounds.
Table 2
TNF'-a Production Inhibition
Compound ICja (N,M)
Example 1 10.2
Example 4 7.6
Example 10 10
Example 11 5.5
Example 16 13.1
Example 19 2.9
Example 20 7.1
Example 21 13.3
Rolipram 100
Preparation Example 1 Tablet
Compound of Example 1 50 mg
Crystalline cellulose 50 mg
91
CA 02236851 1998-OS-06
Lactose 50 mg
Hydroxypropylcellulose 18 mg
Magnesium stearate 2 mg
Total 170 mg
Tablets each having the above composition were
prepared through a customary method. If necessary, these
tablets may be processed into sugar-coated tablets or film-
coated tablets.
Preparation Example 2 Capsule
Compound of Example 1 50 mg
Light silicic acid anhydride ?5 mg
Lactose 100 mg
Starch 50 mg
Talc 25 mg
Total 250 mg
The above ingredients were filled in a No.l capsule,
to thereby prepare capsule preparations.
Preparation Example 3 Granules
Compound of Example L 50 mg
Lactose 600 mg
Cornstarch 200 mg
Carboxymethylcellulo;~e-Na 20 mg
Hydroxypropylcellulo;~e 130 mg
92
CA 02236851 1998-OS-06
Total 1000 mg
Granules of the above composition were prepared using
a customary method.
Preparation Example 4 Powder
Compound of Example 1 50 mg
Light silicic acid anhydride 20 mg
Precipitated calcium carbonate 10 mg
Lactose 250 mg
Starch 70 mg
Total 400 mg
A powder product having the above composition was
prepared using a customar:~ method.
Preparation Example 5 Injection
Compound of Example 19 5 mg
Hydrogenated castor oil 85 mg
Propylene glycol 60 mg
Glucose 50 mg
Distilled water for injection Suitable amount
Total 1 ml in total
An injection having the above composi.'_ion was prepared
through a customary method.
Preparation Example 6 Intravenous drip infusion
93
CA 02236851 1998-OS-06
Compound of Example 19 50 mg
Glucose 5 g
Na~HP04 anhydrate 10 mg
Citric acid 14.5 mg
Distilled water for injection Suitable amount
Total 100 ml in total
An intravenous drip infusion was prepared through a
customary method.
INDUSTRIAL APPLICABILITY
The 2-substituted vinylpyridiene derivative of the
present invention is endowed with strong and selective PDE
IV inhibitory activity, and strong TNF-a production
inhibitory activity. Thu~~, drugs designed on the basis of
the selective PDE IV inhibitory activity are useful for the
prevention and treatment of various diseases, including
immediate or delayed asthma; allergies such as airway-
hypersensitive allergy anc3 other allergies stemming from the
inhibition of activation of inflammatory blood cells such as
eosinocytes; autoimmune diseases such as atopy and
rheumatism; depression as:~ociated with disturbed metabolism
of the cerebrum; cerebral infarction; seni,~e dementia; and
memory disorders associated with Parkinson's disease. Also,
drugs designed on the basis of the TNF-a, production
inhibitory activity are useful for the prevention and
treatment of various diseases, including rheumatism,
94
CA 02236851 1998-OS-06
osteoporosis, type I and type II diabetes, cancers,
inj=ections with HIV, AIDS, and shock caused by intracellular
to~:ins. Moreover, since 'she compounds of the present
in~rention have both selective PDE IV inhibitory action and
TNF-a production inhibitory action, they are useful for the
prevention and treatment of a wide variety of inflammatory
di;~eases and autoimmune diseases .