Note: Descriptions are shown in the official language in which they were submitted.
CA 02237l65 l998-05-08
WO97/17462 rCT~S96/17878
A METHOD OF IDENTIFYING A NONPARAFFINOPHILIC
MICROORGANISM USING VARIOUS A~ILIEUS
AND AN ASSOCIATED APPARArUS
BACKGROUND OF THE INVENTION
This invention relates to a method of identifying a
nonparaffinophilic microorganism using various milieus and an
associated apparatus.
Identification of nonparaffinophilic microorganisms
in a clinical specimen is an important part of medical
treatment of patients. Often times, educated guesses~as to
the nature of the microorganism involved are made. It thus
would be beneficial to improve the process of identifying
these microorganisms with a simple, effective method and
apparatus. ~ ~
United States Patent Nos. 5,153,119 and 5,316,918
disclose methods and apparatus for identifying and testing the
antibiotic sensitivity of Myco~acterium avium-intracellulare
("MAI"), a paraffinophilic microorganism. The inventor named
on those patents is Robert-A. Ollar, one of the co-inventors
of the invention disclosed herein. This method involves
providing a receptacle containing an aqueous solution and
inoculating into the solution a specimen. After this, a
paraffin coated slide is placed into the receptacle. The
slide is then observed for the presence or absence of growth
of MAI .
Despite the efficient, effective and economical
method disclosed in Dr. Ollar's patents, there still remains
a need for a simple and effective method to determine the
presence or absence of a nonparaffinophilic microorganism.
CA 0223716~ 1998-0~-08
WO97/17462 PCT~S96/17878
SUMMARY OF THE INVENTION
The invention has met or surpassed the
above-mentioned need as well as others. The method of
determining the presence of a nonparaffinophilic microorganism
in a specimen taken from a patient includes providing a
receptacle containing an aqueous solution and adjusting the
solution to mimic the in vivo clinical conditions of the
patient. The method further includes inoculating the solution
with the specimen and then placing in the receptacle a slide
coated with a carbon source. The slide is then analyzed after
exposure to the specimen to determine the presence or absence
of the nonparaffinophilic microorganism.
An apparatus to facilitate determination of the
presence of a nonparaffinophilic microorganism in a specimen
taken from a patient is also provided. The apparatus
comprises a receptacle for holding an aqueous solution and a
slide coated with a carbon source adapted to be placed in the
receptacle. The apparatus further comprises means for
adjusting the aqueous solution to mimic the in vivo clinical
conditions of the patient.
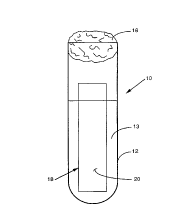
B~F.F DESCRIPTION OF THE DRAWING
A full understanding of the invention can be gained
from the following detailed description of the invention when
read in conjunction with the accompanying lone drawing which
shows a front elevational view of a test tube holding a slide
coated with a carbon source in an aqueous solution inoculated
with a specimen.
DETAILED DESCRIPTION
As used herein, the term "nonparaffinophilic
microorganism" means any microorganism sustained by a carbon
source other than paraffin. Examples of such
,
CA 0223716~ 1998-0~-08
WO97/17462 PCT~S96/17878
nonparaffinophilic microorganisms include, but are not limited
to, the following: Helicobacter pylori; Hemophilus
influenzae; Salmonella typhimurium; Mycobacterium
tuberculosis; Mycobacterium paratuberculosis; Mycobacterium
leprae; Staphylococcus; Streptococcus; ~. Coli; Listeria;
Brucellae; Humemophilusi Treponema; Pneumococcus; Clostridium;
Cryptococcus; Coccidioides; and Histoplasma. Also, as used
herein, the term "patient" refers to a member of the animal
kingdom, including human beings, whose body specimen is being
processed by the method and apparatus of the~inventlon.
The method and apparatus of the invention provide an
efficient, effective and economical way of identifying a
nonparaffinophilic microorganism. Referring now to the lone
Figure, an embodiment of a nonparaffinophilic microorganism
identification apparatus 10 is shown. The apparatus 10
includes a standard test tube 12 which contains an aqueous
solution 13 and a cotton plug 16 to seal the test tube 12.
According to the invention, a specimen to be tested for the
presence or absence of a nonparaffinophilic microorganism is
inoculated into the aqueous solution 13. A slide 18 having a
coating comprising or containing a carbon source 20 is then
placed into the test tube 12. The carbon source 20 is a
growth media for growing the nonparaffinophilic microorganism.
It will be appreciated that the aqueous solution 13 should not
contain any carbon source, as it is desired to provide a sole
carbon source 20 on the slide 18 in order to effectively grow
the nonparaffinophilic microorganism to be identified on the
slide 18 and not in the aqueous solution 13. Growth on the
slide 18, which can either be seen or unseen by the unaided
human eye, can be analyzed to determine the presence or
absence of a nonparaffinophilic microorganism. Preferably, a
minimum of twenty-four (24) hours incubation time is necessary
for growth to occur. In order to analyze the slide 18 after
the incubation period, the slide 18 can be scraped using a
flame sterilized spatula and subcultured on an agar-like
CA 02237l6~ l998-0~-08
WO 97/l7462 PCT/US96/17878
tryptic soy agar (TSA). If the scrapings include growth, the
growth on the TSA can be analyzed using classical
microbiological procedures or can be analyzed using a
DNA extraction process involving either organic solvent
5 extraction or column chromatographic extraction.
The specimen to be inoculated into the test tube 12
can be a blood sample; any biopsy or tissue specimen; stomach
fluid; urine; cerebral spinal fluid; nasopharyngeal mucosa or
saliva. These specimens can be obtained from the patient in
10 the doctor's office or in the emergency room of a hospital,
for example, by known techniques in known standard ways.
The carbon source 20 on the slide 18 can include a
gelatinous matrix containing a carbon source. A carbon source
can be one or more of those selected from the group consisting
15 of glucose, fructose, glycenol, mannitol, asparagine and
casein, among others. Another embodiment can include
providing a slide and coating the slide with an adhesive and
securing a plurality of gel beads to the adhesive. The carbon
source can then be either ionically or affinity bound to the
20 gel beads.
The slide 18 with the gelatinous matrix containing
a carbon source can be prepared by the following method. A
receptacle, such as a laboratory beaker, is first filled with
lOO ml of distilled water. Into the beaker is placed
25 two (2) grams of agar (the gelatinous matrix) and
three (3) grams of a carbon source (such as glucose) . This
mixture is then boiled and steam sterilized and the molten
gelatinous matrix with a carbon source is poured into a
petri dish, which is sitting on a hot plate. In this way the
30 gelatinous matrix/carbon source remains molten. After this,
a sterile slide 18 is dropped into the molten gelatinous
matrix/carbon source and becomes coated therewith. The now
coated slide is removed from the petri dish and allowed to
stand for a minute or two in order to solidify the coating 20
35 thereon. The slide with the coating of a gelatinous matrix
CA 02237165 1998-05-08
WO97/17462 ~ P-CT~596/17878
containing a carbon source is then ready~to~b~e~placed in the
test tube 12 containing the aqueous solution~l3 and the
specimen.
An alternative method of preparing the slide
involves first coating the slide with an adhesive, such as
collodion and then applying a plurality of gel beads
(commercially avallable from Pharmacia ~of~==P=arsippany,
New ~ersey) to the adhesive. The gel beads are approximately
one micron in diameter. The slide containing the coating of
gel beads is now immersed in a buffering a~ent containing the
carbon source (such as glucose) to attach the~carbon source t=o
the gel beads either ionically or affinity-wise
Nonparaffinophilic microorganisms that can be
identified using the method of the invention include any
microorganism sustained by a carbon_ source other than
paraffin. Nonparaffinophilic microorganisms include, but are
not limited to, Helicobacter pylori; Hemophilus influenzae;
Salmonella typhimurium; Mycobacterium tuberculosis;
Mycobacterium paratuberculosis; Mycobacterium leprae;
Staphylococcus; Streptococcus; E. Coli; Listeria; Brucellae;
Numemophilus; Treponema; Pneumococcus; Clostridium,
Cryptococcus; Coccidioides; and Histoplasma.
In accordance with the invention, the aqueous
solution 13 can be adjusted to mimic the in vivo "clinical
conditions" of the patient before placing the slide 18 in the
receptacle 12 and before inoculating the specimen into the
aqueous solution 13. By "clinical conditions" it is meant at
least one of the following: (i) the pH of the in vivo milieu
of the patient where the nonparaffinophilic microorganism can
be found and (ii) the electrolyte levels of a patient's blood
where nonparaffinophilic microorganisms can be found.
Adjusting the aqueous solution can be~effected by numerous
different methods. Adjusting the pH of the aqueous solution
can be accomplished by adding hydrochloric acid (HCl) to
obtain a more acidic solution or by adding sodium hydroxide
,
CA 0223716~ 1998-0~-08
WO97/17462 PCT~S96tl7878
- 6 -
(NaOH) or potassium hydroxide (KOH) in order to obtain a more
basic solution. Electrolytes such as one or more selected
from the group consisting of sodium, potassium, chloride,
maynesium, phosphate and calcium, can be added to the solution
in desired quantities in order to mimic the electrolytes in
the blood of a patient from which a blood sample which may
contain the nonparaffinophilic microorganism is obtained.
EXAMPLE I
A patient comes to an emergency room at a hospital
complaining of severe abdominal pain. A gastroenterologist
uses a gastrointestinal scope to obtain a specimen of the
patient's stomach fluid. The scope indicates that the pH in
the patient's stomach is 3.5. In the meantime, a lab
technician using the apparatus of the Figure adjusts the pH of
the aqueous solution 13 by adding HCl thereto so that the
aqueous solution 13 has a pH of 3.5. Thus, the pH in the
patient's stomach is mimicked by the pH of the aqueous
solution in the apparatus shown in the Figure. After this,
the specimen of stomach fluid taken by the gastroenterologist
from the patient is inoculated into the receptacle 12 holding
a slide coated with a carbon source 18. After about
eight days a growth appears on the slide 18. The growth is
then analyzed by conventional methods to determine the
presence or absence of a nonparaffinophilic microorganism,
such as for example Helicobacter pylori.
EXAMPLE 2
A patient comes to an emergency room complaining of
high fever and apparently has pneumonia. As is standard in
almost every emergency room, a chemical screen ("CSS") is
performed on a blood specimen obtained from the patient. The
CSS lists the electrolyte content of the patient's blood. The
electrolyte content is communicated to a lab technician who in
CA 0223716~ 1998-0~-08
WO97/17462 PCT~S96/17878
turn adjusts the aqueous solution 13 in the receptacle ~2
holding the slide coated with a carbon~ source 18. For
example, the CSS reveals that the patient has a sodium level
of 120. The lab technician adjusts the sodium level of the
aqueous solution ( f or exampl e, distil l ed water) by adding
sodium thereto in order to mimic the 120 level of sodium found
in the patient's blood. The blood specimen is then inoculated
into the adjusted aqueous solution. After about two days a
growth appears. The growth is analyzed and is found to be a
nonparaffinophilic microorganism.
It will be appreciated that a method for identifying
a nonparaffinophilic microorganism has been disclosed in which
the aqueous solution in which the paraffin coated slide and
the nonparaffinophilic microorganism are placed is adjusted to
mimic the in vivo clinical conditions of a patient from whom
the specimen containing the nonparaffinophilic microorganism
to be identified is obtained. The method is effective and
efficient and does not involve the use of expensive and
complicated equipment. An associated apparatus is also
disclosed.
While specific embodiments of the invention have
been disclosed, it will be appreciated by those skilled in the
art that various modifications and alterations to those
details could be developed in light of the overall teachings
of the disclosure. Accordingly, the particular arrangements
disclosed are meant to be illustrative only and not limiting
as to the scope of the invention which is to be given the full
breadth of the appended claims and any and all equivalents
thereof.