Note: Descriptions are shown in the official language in which they were submitted.
CA 02237368 1998-05-12
Ref. 11'484
5'-DEOXY-CYTIDINE DERIVATIVES
The present invention is concerned with novel 5'-deoxy-cytidine derivatives,
pharmaceutical compositions, a kit thereof for assisting a delivery of 5-
fluorouracil
selectively to tumor tissues and process for manufacturing the novel 5'-deoxy-
cytidine derivatives.
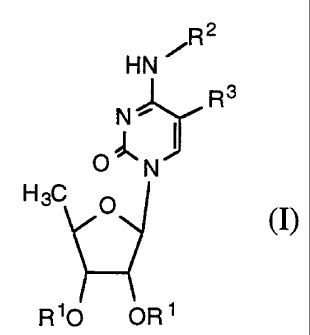
More particularly, the present invention relates to novel 5'-deoxy-cytidine
derivatives represented by the general formula (I),
R2
HN
1R3
N~ I
ON
H 3C O
(I)
R10 OR '
wherein R1 is a hydrogen atom or a group easily hydrolyzable under
physiological conditions; R2 is a hydrogen atom, or -CO-OR4 group
[wherein R4 is a saturated or unsaturated, straight or branched
hydrocarbon group consisting of one to fifteen carbon atoms, or a group
of the formula -(CH2)n-Y (in which Y is cyclohexyl or phenyl; n is an
integer from 0 to 4)]; R3 is a hydrogen atom, bromo, iodo, cyano, a C1-4
alkyl group [which may be substituted with halogen atom(s)], a vinyl or
ethynyl group [which may be substituted with halogen atom(s), C1-4 alkyl,
cycloalkyl, aralkyl, or aromatic ring which may have one or more hetero
atom(s)], or an aralkyl group which may be substituted; with the proviso
that R2 and R3 do not mean a hydrogen atom at the same time.
Although 5-fluorouracil (5-FU) or its derivatives are clinically useful
antitumor agents for the treatment of various solid tumors, in general they
are still
not satisfactory in terms of efficacy and safety. These drawbacks are mainly
due
to rapid inactivation of 5-FU by dihydropyrimidine dehydrogenase (DPD) and/or
the unsatisfactory delivery of 5-FU to tumor tissues with respect to tumor
selectivity. The attempts to enhance the antitumor activity of 5-FU or its
Ar/UI/So 3.4.98
CA 02237368 1998-05-12
-2-
derivatives by inhibition of DPD have already been reported : the co-
administration of 5-FU or its derivative with a DPD inhibitor such as uracil
[USP
4,328,229], 5-ethynyluracil [W092/04901], 5-chloro-2,4-dihydroxypyridine [USP
5,525,603] etc. Such co-administration resulted in enhancement of the
antitumor
activity of 5-FU or its derivatives, but the safety profile was not so
improved due to
insufficient selectivity in delivering the DPD inhibitor to tumor tissues (as
a
consequence, 5-FU level is increased both in tumor and plasma).
In contrast, in accordance with the present invention it has been found that
the co-administration of a novel 5'-deoxy-cytidine derivative represented by
the
general formula (I) with 5-FU or its derivative results in the significantly
improved
delivery of 5-FU selectively to tumor tissues as compared with the combination
of
5-FU or its derivative with a known DPD inhibitor such as 5-ethynyluracil, and
shows significantly improved antitumor activity in human cancer xenograft
models.
The respective groups of the general formula (I) are explained in more
detail as follows;
Explanation of R1:
R1 is a hydrogen atom or a group easily hydrolyzable under physiological
condition.
In the above, the term "a group easily hydrolyzable under physiological
condition" preferably means acetyl, propionyl, benzoyl, toluoyl, glycyl,
alanyl, [i-
alanyl, valyl, lysyl, and the like.
Explanation of R2:
R2 is a hydrogen atom, or -CO-OR4 group [wherein R4 is a saturated or
unsaturated, straight or branched hydrocarbon group consisting of one to
fifteen
carbon atoms, or group of formura -(CH2)n-Y (in which Y is cyclohexyl or
phenyl;
n is an integer of from 0 to 4)].
In the above group R4, the term "a saturated or unsaturated, straight or
branched hydrocarbon group consisting of one to fifteen carbon atoms"
preferably
means methyl, ethyl, n-propyl, 1-isopropyl-2-methylpropyl, 1,1,2-
trimethylpropyl,
n-butyl, isobutyl, 2-ethylbutyl, 3,3-dimethylbutyl, n-pentyl, isopentyl,
neopentyl, 2-
CA 02237368 1998-05-12
-3-
propylpentyl, n-hexyl, 2-ethylhexyl, n-heptyl, n-octyl, allyl, 2-buten-1 -yl,
3-buten-1 -
yl, 3-penten-1 -yl, 4-penten-1-yl, 3-hexen-1 -yl, 4-hexen-1 -yl, 5-hexen-1 -
yl, n-
tridecyl and the like.
The term "a group of the formula -(CH2)n-Y (in which Y is cyclohexyl or
phenyl; n is an integer from 0 to 4)" preferably means cyclohexyl,
cyclohexylmethyl, 2-cyclohexylethyl, 3-cyclohexylpropyl, 4-cyclohexyl-butyl,
phenyl, benzyl, phenethyl, 3-phenylpropyl, 4-phenylbutyl and the like.
In the most preferred embodiment of the compounds in accordance with the
present invention, R4 means n-propyl, n-butyl, n-pentyl, isopentyl, neopentyl,
n-
hexyl, 3,3-dimethylbutyl, 2-ethylbutyl, phenylethyl, and cyclohexylmethyl.
Explanation of R3:
R3 is a hydrogen atom, bromo, iodo, cyano, a C1-4 alkyl group [which may be
substituted with halogen atom(s)], a vinyl or ethynyl group [which may be
substituted with halogen atom(s), C 1-4 alkyl, cycloalkyl, aralkyl, or
aromatic ring
which may have one or more hetero atom(s)], or an aralkyl group which may be
substituted; with the proviso that R2 and R3 do not mean a hydrogen atom at
the
same time.
In the above, the term "a C 1-4 alkyl group which may be substituted with
halogen atom(s)" preferably means methyl, trifluoromethyl, ethyl, propyl and
the
like.
The term "a vinyl or ethynyl group [which may be substituted with halogen
atom(s), C1-4 alkyl, cycloalkyl, aralkyl, or aromatic ring which may have one
or
more hetero atom(s)]" preferably means vinyl, 1 -chlorovinyl, 2-bromovinyl, 2-
bromo-l-chlorovinyl, ethynyl, prop-l-ynyl, but-l-ynyl, pent-l-ynyl, hex-l-
ynyl, 3,3-
dimethyl-but-l-ynyl, cyclopentylethynyl, cyclohexylethynyl, phenylethynyl, 3-
phenylprop-1-ynyl, pyrid-2-ylethynyl, imidazol-2-yiethynyl, and the like. The
most
preferred group is ethynyl and iodo.
The term "an aralkyl group which may be substituted" preferably means 3-
(benzyloxy)benzyl, 3-methoxybenzyl, 3-bromobenzyl, 3-methylbenzyl, 3-
hydroxybenzyl and the like.
Preferred 5'-deoxy-cytidine derivatives of the present invention are:
CA 02237368 1998-05-12
-4-
5'-deoxy-5-ethynylcytidine,
5'-deoxy-5-prop-1 -ynylcytidine,
5-but-1 -ynyl-5'-deoxycytidine,
5'-deoxy-5-pent-1 -ynylcytidine,
5'-deoxy-5-hex-1 -ynylcytidine,
5'-deoxy-5-iodocytidine,
5-bromo-5'-deoxycytidine,
5-(1-chlorovinyl)-5'-deoxycytidine,
5'-deoxy-5-vinylcytidine,
5'-deoxy-5-trifluoromethylcytidine
5-(3-benzyloxybenzyl)-5'-deoxycytidine,
5-cyano-5'-deoxycytidine,
5'-deoxy-N4-(n-pentyloxycarbonyl)cytidine,
5'-deoxy-N4-(n-pentyloxycarbonyl)-5-prop-1 -ynylcytidine,
5-but-1 -ynyl-5'-deoxy-N4-(n-pentyloxycarbonyl)cytidine,
5'-deoxy-5-pent-1 -ynyl-N4-(n-pentyloxycarbonyl)cytidine,
5'-deoxy-5-hex-1 -ynyl-N4-(n-pentyloxycarbonyl)cytidine,
5'-deoxy-5-iodo-N4-(n-pentyloxycarbonyl)cytidine,
5-bromo-5'-deoxy-N4-(n-pentyloxycarbonyl)cytidine,
5-(1-chlorovinyl)-5'-deoxy-N4-(n-pentyloxycarbonyl)cytidine,
N4-(ethoxycarbonyl)-5'-deoxy-5-vinylcytidine,
5'-deoxy-N4-(n-propoxycarbonyl)-5-vinylcytidine,
N4-(n-butoxycarbonyl)-5'-deoxy-5-vinylcytidine,
5'-deoxy-N4-(n-pentyloxycarbonyl)-5-vinylcytidine,
N4-(benzyloxycarbonyl)-5'-deoxy-5-vinylcytidine,
5'-deoxy-N4-(n-pentyloxycarbonyl)-5-trifluoromethylcytidine,
5-(3-benzyloxybenzyl)-5'-deoxy-N4-(n-pentyloxycarbonyl)cytidine,
5-cyano-5'-deoxy-N4-(n-pentyloxycarbonyl)cytidine,
5'-deoxy-5-ethynyl-N4-(methoxycarbonyl)cytidine
5'-deoxy-N4-(ethoxycarbonyl)-5-ethynylcytidine
5'-deoxy-5-ethynyl-N4-(n-propoxycarbonyl)cytidine,
5'-deoxy-5-ethynyl-N4-(isopropoxycarbonyl)cytidine,
N4-(n-butoxycarbonyl)-5'-deoxy-5-ethynylcytidine,
5'-deoxy-5-ethynyl-N4-(isobutoxycarbonyl)cytidine,
5'-deoxy-5-ethynyl-N4-(n-pentyloxycarbonyl)cytidine,
5'-deoxy-5-ethynyl-N4-[(2-propylpentyloxy)carbonyl]cytidine,
5'-deoxy-5-ethynyl-N4-(isopentyloxycarbonyl)cytidine,
CA 02237368 1998-05-12
-5-
5'-deoxy-5-ethynyl-N4-[(2-methylpentyloxy)carbonyl]cytidine,
5'-deoxy-5-ethynyl-N4-[(3-methylpentyloxy)carbonyl]cytidine,
5'-deoxy-5-ethynyl-N4-(n-hexyloxycarbonyl)cytidine,
5'-deoxy-N4-[(2-ethylbutyl)oxycarbonyl]-5-ethynylcytidine,
5'-deoxy- N4-[(2 -ethyl h exyl)oxycarbonyl]-5-ethynylcytid ine,
5'-deoxy-5-ethynyl-N4-[(2-phenylethoxy)carbonyl]cytidine,
N4-(cyclohexyloxycarbonyl)-5'-deoxy-5-ethynylcytidine,
N4-[(cyclohexylmethoxy)carbonyl]-5'-deoxy-5-ethynylcytidine,
5'-deoxy-5-ethynyl-N4-(neopentyloxycarbonyl)cytidine,
5'-deoxy-N4-[(3,3-dimethylbutoxy)carbonyl]-5-ethynylcytidine,
2',3'-di-O-acetyl-5'-deoxy-5-ethynyl-N4-(n-propoxycarbonyl)cytidine
2',3'-di-O-acetyl-5'-deoxy-5-ethynyl-N4-(n-pentyloxycarbonyl)cytidine
2',3'-di-O-acetyl-5'-deoxy-5-vinylcytidine,
2',3'-di-O-acetyl-N4-(ethoxycarbonyl)-5'-deoxy-5-vinylcytidine
2',3'-di-O-acetyl-5'-deoxy-N4-(n-propoxycarbonyl)-5-vinylcytidine,
2',3'-di-O-acetyl-N4-(n-butoxycarbonyl)-5'-deoxy-5-vinylcytidine,
2',3'-di-O-acetyl-5'-deoxy-N4-(n-pentyloxycarbonyl)-5-vinylcytidine,
2',3'-di-O-acetyl-N4-(benzyloxycarbonyl)-5'-deoxy-5-vinylcytidine.
5'-deoxy-5-ethynyl-N4-(n-decyloxycarbonyl)cytidine
5'-deoxy-5-ethynyl-N4-[(2,6-dimethylcyclohexyloxy)-carbonyl]cytidine
5'-deoxy-5-ethynyl-N4-(benzyloxycarbonyl)cytidine
5'-deoxy-5-ethynyl-N4-[(1-isopropyl-2-methyl-propoxy)carbonyl]cytidine
5'-deoxy-5-ethynyl-N4-[(3-methoxybenzyloxy)-carbonyl]cytidine.
The novel 5'-deoxy-cytidine derivatives represented by the formula (I) can
be produced according to the following methods. In the following process A-F,
P1 represents a hydroxy protecting group such as acetyl, benzoyl,
trimethylsilyl,
tert-butyidimethylsilyl and the like.
Process A:
Compounds represented by the formula (I) wherein R 1, R2 and R3 are the
same as defined above can be prepared by reacting a compound represented by
the formula (II)
CA 02237368 1998-05-12
-6-
NH2
NR3
OJLNJ
H3 I Oj (II)
-
P1O OP1
[wherein Pl is a hydroxy-protecting group, and R3 is the same as defined
above],
with a compound represented by the general formula (III),
R4OCOX (III)
[wherein R4 is the same as defined above; X is chloro or bromo],
in the presence of acid acceptor, followed, if necessary, by removal of
protecting
group(s),
Process B:
Compounds represented by the formula (I), wherein R 1 and R2 are the
same as defined above and R3 is an ethynyl or vinyl group [which may be
substituted with halogen atom(s), C1_4 alkyl, cycloalkyl, aralkyl, or aromatic
ring
which may have one or more hetero atom(s)], can also be prepared by reacting a
compound represented by the formula (IV)
R2
i
HN
N ~~~
Oj' N (IV)
H3C~0 I
P O OP1
[wherein P1 and R2 are the same as defined above],
with an acetylene or vinyl derivative in the presence of a palladium catalyst,
followed, if necessary, by removal of protecting group(s).
Process C:
Compounds represented by the formula (I), wherein R 1 and R2 are the
same as defined above and R3 is a cyano group, can be prepared by reacting a
compound represented by the formula (IV)
CA 02237368 1998-05-12
-7-
R2
i
HN
N
J.
H3C ~ N (IV)
w
P1 O OP1
[wherein P1 and R2 is the same as defined above],
with alkali metal cyanide, followed, if necessary, by removal of protecting
group(s).
Process D:
Compounds represented by the formula (I), wherein R1 and R3 are the
same as defined above and R2 is a hydrogen atom, can also be prepared by
reacting a compound represented by the formula (V)
0
HN X,,R3
J.J
0
H3 LO (V)
1H
P O OP1
[wherein P1 and R3 are the same as defined above],
with phosphoryl chloride in the presence of an acid acceptor, followed by
treatment with ammonia,
followed, if necessary, by removal of protecting group(s).
Process E:
Compounds represented by the formula (I), wherein R 1, R2 and R3 are the
same as defined above, can also be prepared by coupling a compound
represented by the formula (VI)
R2
HN
R3
N~
~ (VI)
O"H
[wherein R2 and R3 are the same as defined above],
with a compound represented by the formula (VII)
CA 02237368 1998-05-12
-8-
H3C
Vi0~-OP1
1)~ (VII)
P O OP1
[wherein Pl is the same as defined above]
in the presence of Lewis acid catalyst, followed, if necessary, by removal of
protecting group(s).
Process F:
Compounds represented by the formula (I) wherein R3 is a vinyl radical [which
may be substituted with halogen atom(s), C 1 -q alkyl, cycloalkyl, aralkyl, or
aromatic ring which may have one or more hetero atom(s)], R1and R2 are the
same as defined above can be prepared by catalytic hydrogenation of a
compound represented by the formula (VIII)
R2
i
HN
N R3
H O~ N I (VIII)
C
3 O
PlO OP1
[wherein P1 is a hydroxy-protecting radical, R3 is an ethynyl radical (which
may be substituted with halogen atom(s), C 1-4 alkyl, cycloalkyl, aralkyl, or
aromatic ring which may have one or more hetero atom(s)), and R2 is the
same as defined above],
with Lindlar catalyst, followed, if necessary, by removal of protecting
radical(s).
In the following, process for producing novel 5'-deoxy-cytidine derivatives
represented by the formula (I) according to the present invention will be
explained
in more detail.
Process A:
Specific examples of the compounds represented by the general formula
(II) include,
2',3'-di-O-acetyl-5'-deoxy-5-ethynylcytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-ethynylcytidine,
CA 02237368 1998-05-12
-9-
2',3'-di-O-acetyl-5'-deoxy-5-prop-1-ynylcytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-prop-1-ynylcytidine,
2',3'-di-O-acetyl-5-but-1 -ynyl-5'-deoxycytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5-but-1-ynyl-5'-deoxycytidine,
2',3'-di-O-acetyl-5'-deoxy-5-pent-1-ynylcytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-pent-1-ynylcytidine,
2',3'-di-O-acetyl-5'-deoxy-5-hex-1-ynylcytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-hex-1-ynylcytidine,
2',3'-di-O-acetyl-5'-deoxy-5-iodocytidine,
2',3'-bis-O-(tert-butyldimethylsilyi)-5'-deoxy-5-iodocytidine,
2',3'-di-O-acetyl-5-bromo-5'-deoxycytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5-bromo-5'-deoxycytidine,
2',3'-di-O-acetyl-5-(1-chlorovinyl)-5'-deoxycytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5-(1-chlorovinyl)-5'-deoxycytidine,
2',3'-di-O-acetyl-5'-deoxy-5-vinylcytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-vinylcytidine,
2',3'-di-O-acetyl-5'-deoxy-5-trifluoromethylcytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-trifluoromethylcytidine,
2',3'-di-O-acetyl-5-(3-benzyloxybenzyl)-5'-deoxycytidine,
5-(3-benzyloxybenzyl)-2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxycytidine,
2',3'-di-O-acetyl-5-cyano-5'-deoxycytidi ne,
2',3'-bis-O-(tert-butyldimethylsilyl)-5-cyano-5'-deoxycytidine
and the like.
The reaction of the compound of the above general formula (II) with the
compound of the above general formula (III) can be carried out in a solvent
such
as pyridine, dioxane, tetrahydrofuran, acetonitrile, chloroform,
dichloromethane
and the like in the presence of an acid acceptor such as triethylamine,
pyridine,
picoline, 4-(N,N-dimethylamino)pyridine, lutidine and the like. The reaction
can be
carried out at a temperature between 0 and 30 C.
The protecting group(s) may, if necessary, be removed after the reaction by
the
procedures known to those skilled in the art, e.g. by basic or acidic
hydrolysis, or
treatment with fluoride anion.
Process B:
Specific examples of the compound represented by the general formula
(IV) include,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-iodo-N4-
(methoxycarbonyl)cytidine,
CA 02237368 1998-05-12
-10-
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-N4-(ethoxycarbonyl)-5-
iodocytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-iodo-N4-(n-propoxycarbonyl)-
cytidine,
N4-(n-butoxycarbonyl)-2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-
iodocytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-iodo-N4-(n-pentyloxy-
carbonyl)-
cytidine,
2',3'-bis-O-(tert-butyidimethylsilyl)-5'-deoxy-5-iodo-N4-(isopentyloxy-
carbonyl)-
cytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-iodo-N4-(n-hexyloxy-carbonyl)-
cytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-N4-[(2-ethylbutyl)oxycarbonyl]-
5-
iodocytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-iodo-N4-[(2-phenylethoxy)-
carbonyl]cytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-N4-[(cyclohexylmethoxy)carbonyl]-5'-
deoxy-5-
iodocytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-iodo-N4-(neopentyloxy-
carbonyl)cytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-N4-[(3,3-dimethylbutoxy)-
carbonyl]-5-
iodocytidine,
2',3'-di-O-acetyl-5'-deoxy-5-iodo-N4-(ethoxycarbonyl)-cytidine,
2',3'-di-O-acetyl-5'-deoxy-5-iodo-N4-(n-propoxycarbonyl)cytidine
2',3'-di-O-acetyl-N4-(n-butoxycarbonyl)-5'-deoxy-5-iodo-cytidine,
2',3'-di-O-acetyl-5'-deoxy-5-iodo-N4-(n-pentyloxycarbonyl)cytidine
and the like.
Specific examples of the acetylene or vinyl derivatives used for this
coupling reaction are trimethysilyl acetylene, tert-butyidimethysilyl
acetylene, 1-
butyne, 1 -pentyne, 1 -heptyne, 1 -hexyne, 3-methyl-1 -butyne, 3,3-dimethyl-1 -
butyne, cyclohexylacetylene, phenylacetylene, 3-phenyl-l-propyne, tri-n-
butyl(vinyl)stannane and the like.
The coupling reaction of a compound represented by the formula (IV)
with an acetylene derivative can be performed in the presence of a palladium
catalyst such as bis(triphenylphosphine)palladium (II) chloride-copper (I)
iodide,
bis(triphenylphosphine)palladium(II) acetate-copper(l) iodide and the like.
The coupling reaction of a compound represented by the formula (IV) with a
vinyl
derivative can be performed in the presence of palladium catalyst such as
CA 02237368 1998-05-12
-11-
tris(dibenzylideneacetone)dipalladium, tetrakis(triphenylphosphine)palladium,
bis(acetonitrile)palladium (II) chloride in the presence of tri-2-
furylphosphine,
triphenylphosphine and the like.
These reaction can be carried out in a solvent such as chloroform,
dichloromethane, tetrahydrofurane, N-methylpyrrolidone, N,N-dimethyformamide
and the like. The reaction can be carried out at a temperature between 0 and
800 C, preferably between 10 and 60 C.
Process C:
The reaction of the compound of the above general formula (IV) with alkali
metal cyanide such as sodium cyanide, potassium cyanide etc. can be carried
out
in a solvent such as N,N-dimethylformamide, dimethylsulfoxide, acetonitrile
and
the like. The reaction can be carried out at a temperature between 0 and 100
C,
preferably between 10 and 30 C.
Process D:
Specific examples of the compounds represented by the general formula
(V) include,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-ethynyluridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-prop-1-ynyluridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5-but-1-ynyl-5'-deoxyuridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-pent-1-ynyluridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-hex-1-ynyluridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-iodouridine,
5-bromo-2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxyuridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5-(1-chlorovinyl)-5'-deoxyuridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-vinyluridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-trifluoromethyluridine,
5-(3-benzyloxybenzyl)-2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxyuridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5-cyano-5'-deoxyuridine
and the like.
The starting materials listed above can be prepared from the known 5-
substituted uracil derivatives by the method similar to the process E wherein
5-
substituted uracil derivative is used instead of 5-substituted cytosine
derivative.
CA 02237368 1998-05-12
-12-
The reaction of the compound of the above general formula (V) with
phosphoryl chloride can be carried out in a solvent such as pyridine, dioxane,
tetrahydrofuran, acetonitrile, chloroform, dichloromethane and the like in the
presence of acid acceptor such as triethylamine, pyridine, picoline, 4-(N,N-
dimethylamino)pyridine, lutidine, imidazole, N-methylimidazole, triazole and
the
like at a temparature between 0 and 30 C, followed by treatment with aqueous
ammonia or ammonia gas in a solvent such as methanol, ethanol, acetonitrile,
N,N-dimethylformamide and the like at a temperature between 0 and 30 C.
Process E:
Specific examples of the compounds represented by the general formula
(VI) include 5-ethynylcytosine, 5-prop-1 -ynylcytosine, 5-prop-1 -
ynylcytosine, 5-but-
1-ynyl-5'-deoxycytosine, 5-pent-1-ynylcytosine, 5-hex-1-ynylcytosine, 5-
iodocytosine, 5-bromocytosine, 5-(1 -chlorovinyl)-cytosine, 5-vinylcytosine, 5-
trifluoromethylcytosine, 5-(3-benzyloxy-benzyl)cytosine, 5-cyanocytosine, 5-
ethynyl-N4-(n-pentyloxycarbonyl)cytosine and the like.
Specific examples of the compound represented by the general formula
(VII) include known 5-deoxy-1,2,3-O-triacetyl-D-ribofuranoside, 5-deoxy-1,2,3-
0-
tribenzoyl-D-ribofuranoside and the like.
A compound of the formula (VI) may be first converted to the trimethylsilyl
derivative with silylation reagent such as hexamethyldisilazane, followed by
the
coupling reaction with a compound represented by the formula (VII) in the
presence of Lewis acid catalyst such as tin-(IV)-chloride, titanium-(IV)-
chloride
and the like. This coupling reaction proceeds in a solvent such as
acetonitrile,
dichloromethane, chloroform, 1,2-dichloroethane, nitromethane, toluene and the
like, at a temperature between 0 and 30 C, preferably between 0 and 10 C.
Process F:
Specific examples of the compounds represented by the general formula (VIII)
include
5'-deoxy-5-ethynylcytidine,
5'-deoxy-N4-(ethoxycarbonyl)-5-ethynylcytidine
5'-deoxy-5-ethynyl-N4-(n-propoxycarbonyl)cytidine,
N4-(n-butoxycarbonyl)-5'-deoxy-5-ethynylcytidine,
CA 02237368 1998-05-12
-13-
5'-deoxy-5-ethynyl-N4-(n-pentyloxycarbonyl)cytidine,
N4-(benzyloxycarbonyl)-5'-deoxy-5-ethynylcytidine,
2',3'-di-O-acetyl-5'-deoxy-5-ethynylcytidine
2',3'-di-O-acetyl-5'-deoxy-5-ethynyl-N4-(ethoxycarbonyl)cytidine,
2',3'-di-O-acetyl-5'-deoxy-5-ethynyl-N4-(n-propoxycarbonyl)cytidine,
2',3'-di-O-acetyl-5'-deoxy-5-ethynyl-N4-(n-pentyloxycarbonyl)cytidine and the
like.
The catalytic hydrogenation of the ethynyl group of the compound of formula
(VIII)
can be performed using Lindlar catalyst according to the method known to those
skilled in the art [ cf. Synthetic Method, 1952, vol. 7, P38 ( Interscience
Publishers,
Inc., New York)].
The novel 5'-deoxy-cytidine derivatives of the present invention can be
used as antitumor agent together with known physiologically acceptable
pharmaceutical carriers.
The present invention also provides a pharmaceutical composition
comprising a 5'-deoxy-cytidine derivative represented by the general formula
(I)
and 5-fluorouracil (5-FU) or a derivative thereof. With this composition, the
5'-
deoxy-cytidine derivative potentiates the antitumor effect of 5-fluorouracil
or its
derivative by delivering a significantly higher amount of 5-FU selectively to
tumor
tissues without significant increase of 5-FU concentration in plasma.
For the combination of a 5'-deoxy-cytidine derivative represented by the
general formula (I) with 5-FU or a derivative thereof for the treatment of
cancer
with an improved efficacy and safety profile, the 5-FU derivative is
preferably
selected from the group consisting of:
5-fluoro-1 -(2-tetrahydrofuryl)uracil,
1 -(n-hexyloxycarbonyl)-5-fluorouracil,
5'-deoxy-5-fluorouridine,
5'-deoxy-5-fluoro-N4-(n-propoxycarbonyl)cytidine,
N4-(n-butoxycarbonyl)-5'-deoxy-5-fluorocytidine,
5'-deoxy-5-fluoro-N4-(n-pentyloxycarbonyl)cytidine,
5'-deoxy-5-fluoro-N4-(isopentyloxycarbonyl)cytidine,
5'-deoxy-5-fluoro-N4-(n-hexyloxycarbonyl)cytidine,
CA 02237368 1998-05-12
- 14-
5'-deoxy-N4-[(2-ethylbutyl)oxycarbonyl]-5-fluorocytidine,
5'-deoxy-5-fluoro-N4-[(2-phenylethoxy)carbonyl]cytidine,
N4-[(cyclohexylmethoxy)carbonyl]-5'-deoxy-5-fluorocytidine,
5'-deoxy-5-fluoro-N4-(neopentyloxycarbonyl)-cytidine,
5'-deoxy-N4-[(3,3-dimethylbutoxy)carbonyl]-5-fluorocytidine,
5'-deoxy-5-fluoro-N4-(3,5-dimethylbenzoyl)cytidine,
5'-deoxy-5-fluoro-N4-(3,5-dichlorobenzoyl)cytidine,
2',3'-di-O-acetyl-5'-deoxy-5-fluoro-N4-(n-pentyloxycarbonyl)cytidine and the
like.
A compound of the formula (I) can be administered either alone or
simultaneously with 5-FU or a derivative thereof.
Accordingly, the pharmaceutical composition of the present invention can
be obtained by formulating a compound of the formula (I) and 5-FU or a
derivative
thereof into a single preparation or may be provided in the form of two
separate
individual preparations.
A pharmaceutical composition of the formula (I) can be administered before
or simultaneously with the administration of 5-FU or a derivative thereof;
preferably, within 3 hour before or simultaneously with the administration of
5-FU
or a derivative thereof.
In the pharmaceutical composition of the present invention comprising 5-
FU or a derivative thereof and a 5'-deoxy-cytidine derivative represented by
the
general formula (I), the suitable molar ratio of two components is about 0.001-
10
moles, preferably 0.002-0.5 mole of a compound of the formula (I) per mole of
5-
FU or its derivative.
The present invention also provides a kit comprising a pharmaceutical
composition (component A) containing a compound of the formula (I) and a
pharmaceutical composition (component B) containing 5-FU or a derivative
thereof.
Thus, the present invention is also concerned with pharmaceutical
compositions of a compound of formula (I) and, optionally, 5-FU or a
derivative
thereof and a kit thereof for the treatment of colorectal cancer, breast
cancer,
stomach cancer, lung cancer, cervical cancer, bladder cancer and other
malignant
diseases and the like.
CA 02237368 1998-05-12
- 15-
The pharmaceutical compositions and the components A and B of the kit of
the present invention can be administered in any form, for example, tablets,
pills,
suppositories, capsules, granules, powders, or emulsions etc. Pharmaceutically
acceptable carriers and excipients useful in formulating the pharmaceutical
composition of the present invention are those commonly used. Pharmaceutically
acceptable materials can be an organic or inorganic inert carrier material
suitable
for enteral, percutaneous or parenteral administration such as water,
gelatine,
gum arabic, lactose, starch, magnesium stearate, talc, vegetable oils,
polyalkylene glycols and petroleum jelly. The pharmaceutical composition
provided by the present invention can be administered orally, e.g., in form of
tablets, capsules, pills, powders, granules, solutions, syrups, suspensions or
elixirs. The administration can also be carried out parenterally, e.g. in form
of
sterile solutions, suspensions or emulsions; or locally, e.g., in form of
solutions,
suspensions, salves, powders or aerosols. The pharmaceutical composition can
be sterilized and/or can contain further adjuvants such as preserving,
stabilizing
setting, emulsifying agents, flavor-improving agents , salts for variation of
the
osmotic pressure or substances acting as buffers.
The pharmaceutical composition can be prepared in a conventional
manner.
Dosage ranges for the pharmaceutical composition of the present invention
depend on the route of administration, the age, weight and condition of the
patient
and the particular disease to be treated. In the case of oral, rectal or
parenteral
administration for adults, an approximate daily dosage is in the range of from
about 1 mg to about 2,000 mg of a compound of formula (I) and from about 10 mg
to about 4,000 mg of 5-FU or its derivative, depending on the kind of 5-FU
derivative used. Oral administration is a preferred route of administration of
the
pharmaceutical composition according to the present invention.
The tumor selective delivery of 5-FU through the tumor DPD selective
inhibition by a compound of formula (I) is evident from the test described
hereafter.
1. Tumor DPD Selective Inhibition by Compound A of Example 6
The activity of compound A of Example 6 to inhibit DPD activity was
CA 02237368 1998-05-12
-16-
compared with that of the known DPD inhibitor 5-ethynyluracil (5-EU) in BALB/c
nude mice bearing the human prostate cancer xenograft PC-3. The liver and
tumor tissues were resected out from each group of three mice at 2 and 8 hours
after the administration of the Compound A (0.5 mmol/kg) and 5-EU (0.05
mol/kg). DPD activity in these tissues was then measured as described
elsewhere (Naguib et al., Cancer Research 45, 5405-5412, 1985). 5-EU inhibited
the DPD activity both in the liver and tumor tissue, whereas compound A
strongly
inhibited the activity only in tumor tissue (Table 1). These results suggest
that
compound A of Example 6 inhibits DPD activity selectively in tumor tissue.
Table 1. Inhibition of DPD activity by Compound A of Example 6
DPD activities (pmol/mg protein/min)
Tissues Control 5-EU Compound A
2 hr 8 hr 2 hr 8 hr 2 hr 8 hr
Liver 288 162 46 83 177 326
Tumor 31 29 17 13 9 9
2. Selective increase of 5-FU levels in tumors by compound A of Examp le 6 in
mice treated with fluoropyrimidines
The experiment shown in Table 2 demonstrates that compound A of
Example 6 increases 5-FU AUC (Area under curve) selectively in tumors in mice
treated with fluoropyrimidines. In this study, fluoropyrimidines, such as 5-
FU,
doxifluridine [5'-deoxy-5-fluorouridine] and capecitabine [5'-deoxy-5-fluoro-
N4-(n-
pentyloxycarbonyl)cytidine], were given to BALB/c nude mice bearing the human
gastric cancer xenograft MKN28 in combination with either compound A or 5-EU.
Then, 5-FU levels in the plasma and tumor tissues were measured at 0.25, 0.5,
2,
4 and 7 hours after each fluoropyrimidine administration (n = 3 mice), and 5-
FU
AUC was calculated. The known DPD inhibitor 5-EU greatly increased the 5-FU
AUCs both in the plasma and tumor tissues in mice treated with either 5-FU,
capecitabine or doxifluridine . Since the increase of 5-FU levels in the
plasma
results in the systemic toxicity of 5-FU, 5-EU should enhance both the
efficacy
and toxicity of the fluoropyrimidines.
In contrast, compound A greatly increases the 5-FU AUCs only in tumor tissues
probably as a result of compound A's tumor selective inhibition of DPD
activity
that catabolizes 5-FU. Compound A of Example 6 therefore enhances the
efficacy of fluoropyrimidines with little increasing their toxicity.
CA 02237368 1998-05-12
-17-
Table 2. 5-FU AUC in the plasma and tumors in mice treated with
fluoropyrimidines
Test compounds Fluoropyrimidines 5-FU AUC (nmolohr/mL)
( mol/kg) (mmol/kg) Plasma Tumor
Exp. 1
- 5-FU (0.3) 9.3 1.3
Compound A (2) 5-FU (0.3) 9.5 6.0
5-EU (1) 5-FU (0.3) 75 48
- Capecitabine (1.5) 1.3 30
Compound A (2) Capecitabine (1.5) 3.1 67
5-EU (1) Capecitabine (1.5) 53 120
Exp. 2
- Doxifluridine (0.75) 2.6 8.0
Compound A (2) Doxifluridine (0.75) 11 30
5-EU (1) Doxifluridine (0.75) 86 73
- Capecitabine (1.5) 1.5 30
Compound A (2) Capecitabine (1.5) 3.8 76
5-EU (1) Capecitabine (1.5) 54 120
3. Enhancement of antitumor activily of Capecitabine by Compound A of
Example 6
Compound A of Example 6 was examined for its activity to enhance the
efficacy of capecitabine in BALB/c nude mice bearing the human prostate cancer
xenograft PC-3. Compound A and capecitabine were orally given simultaneously
or sequentially 5 successive days per week for 3 weeks starting on day 53
after
the tumor inoculation when the tumor becomes palpable. On day 75, tumor
volume gain and the percentage of the tumor growth inhibition were calculated.
As
Table 3 shows, capecitabine inhibited tumor growth to a greater extent when
compound A was given in combination either simultaneously or sequentially.
Since compound A itself is not cytotoxic (data not shown), it enhances the
efficacy
of capecitabine by inhibiting DPD activity.
CA 02237368 1998-05-12
- 18-
Table 3. Enhancement of Capecitabine Efficacy by compound A of Example 6
Tumor volume Tumor growth Body weight Survivors
Capecitabine Compound A change (mm3) inhibition (%) change (g) on Day 75
(mmol/kg/d) ( mol/kg/d) Day 53-75 Day 75 Day 75
- - 981 - -3.6 5/5
1.0 - 757 23 -3.4 5/5
1.0 1.0 323* 67 -1.8 5/5
1.0 1.0# 201* 80 -0.3 4/5
* p < 0.05 as compared with the control group
# Compound A was given one hour prior to the capecitabine administration.
The following Examples are intended to illustrate the present invention in
more detail, but are not intended to limit its scope in any manner.
Reference example 1:
a) Preparation of 2'.3'-di-O-acetyl-5'-deox~r-5-ethynyluridine
5-Ethynyluracil (12g, 88.2 mmol) was suspended in a solution of
ammonium sulfate (570mg, 4.3 mmol) in hexamethyldisilazane (240ml). The
suspension was refluxed for 6hr. After concentrating the reaction mixture
under
reduced pressure, a solution of 5-deoxy-1,2,3-tri-O-acetyl-D-ribofranoside
(27.5g,
105.8 mmol) in acetonitrile (300m1) was added to the residue. Then, a solution
of
anhydrous stannic tetrachloride (27.6g, 105.8 mmol) in nitromethane (60m1) was
added dropwise to the mixture with keeping the temperature below 0 C. After
stirring the mixture at 0 C for additional 4hr, sodium bicarbonate was added
and
followed by dropwise addition of water. After the mixture was stirred for 2hr,
the
reaction mixture was filtered to remove insoluble material, which was washed
with
ethyl acetate.The filtrate and washing were combined and dried over MgSO4 and
filtered. The filtrate was evaporated under reduced pressure.
Purification of the residue by silica gel chromatography ( using n-hexane :
ethyl
acetate= 1: 2 as an eluent) gave 2',3'-di-O-acetyl-5'-deoxy-5-ethynyluridine
(1 3.7g,
48%yield).
CA 02237368 1998-05-12
-19-
MALDI-MS : (m/z) 359[M+Na]+, 375[M+K]+
1 H-NMR :(270MHz;CDCI3) : S 1.47 (3H, d, J = 6.6), 2.10 (3H, s), 2.12 (3H,
s), 3.23 (1 H, s), 4.19-4.28 (1 H, m), 5.01-5.05 (1 H, m), 5.30-5.34 (1 H, m),
5.90 (1 H, d, J = 4.95), 7.57 (1 H, s), 8.34 (1 H, br.s)
b) Preparation of 2',3'-bis-O-(tert-butyidimethylsilyl)-5'-deoxy-5-
ethynyluridine
To a solution of 2',3'-di-O-acetyl-5'-deoxy-5-ethynyluridine (13.7g, 40.7
mmol) was dissolved in methanol (100ml) was added dropwise a solution of
sodium hydroxide (3.3g, 81.4 mmol) in water (10mI) with stirring at 0 C. After
stirring at 0 C for additional 30min, the reaction mixture was adjusted to pH7
with
aqueous 1 N-hydrochloric acid. Then the mixture was evaporated under reduced
pressure.
The residue was dissolved in DMF (250ml), and imidazole (41.6g, 610 mM) and
tert-butyldimethylchlorosilane (30.7g, 203 mmol) was added to the solution
with
stirring. The mixture was continued to stir for 23hr. The reaction mixture was
partitioned between ethyl acetate and water. The aqueous layer was back-
extracted with ethyl acetate. The combined organic layers were washed with
brine, dried over Na2SO4, filtered and evaporated under reduced pressure.
Purification of the residue by silica gel chromatography ( using n-hexane :
ethyl
acetate = 3:1 as an eluent) gave 2',3'-bis-O-(tert-butyldimethylsilyl)-5'-
deoxy-5-
ethynyluridine (14.9g, 76%yield).
FAB-MS : (m/z) 481 [M+H]+
1 H-NMR :(270MHz;CDCI3) : S 0.10-0.13 (12H, m), 0.91 (18H, m), 1.40 (3H, d, J
= 6.6), 3.21 (1 H, s), 3.58 (1 H, dd, J = 4.29, 6.6), 4.08-4.17 (2H, m),
5.62(1 H, d, J
2.64), 7.68(1 H, s), 8.24(1 H, br.s)
The following compounds were prepared in the same manner as described
above using the corresponding known 5-substituted uracil derivatives:
2' 3'-bis-O-(tert-butyidimethylsilyl)-5'-deoxy-5-iodouridine,
FAB-MS : (m/z) 583[M+H]+, 605[M+Na]+
1 H-NMR :(270MHz;DMSO-d6) : 5 -0.09 (3H, s), -0.03 (3H, s), 0.00 (3H, s),
0.02 (3H, s), 0.75 (9H,s), 0.81 (9H,s), 1.24 (3H, d, J = 6.6), 3.75 (1 H,dd, J
=
4.6, 4.0), 3.86 (1 H, m), 4.36 (1 H, dd, J = 5.3, 5.0), 5.59 (1 H, d, J =
5.6), 7.91
(1 H, s), 11.69 (1 H, br.s)
CA 02237368 1998-05-12
-20-
2',3'-bis-O-(tert-butyidimethylsilyl)-5'-deoxy-5-trifluoromethyluridine,
FAB-MS : (m/z) 525 [M+H]+
1 H-NMR :(400MHz;CDCI3) : S 0.00 (6H, s), 0.02 (3H, s), 0.06 (3H, s), 0.83
(9H, s), 0.83 (9H, s), 1.32 (3H, d, J = 5.9), 3.47 (1 H, m), 4.05 (1 H, m),
4.16
(1 H, m), 5.54 (1 H, d, J = 2.2), 7.84 (1 H, s), 8.43 (1 H, br.s)
2',3'-bis-O-(tert-butyldimethylsilyl)-5-(3-benzyloxybenzyl)-5'-deoxy-uridine,
FAB-MS : (m/z) 653 [M+H]+
1 H-NMR :(270MHz;CDCI3) : 8 -0.09-0.01 (12H, m), 0.77-0.82 (18H, m),
0.90 (3H, d, J = 6.3), 3.27 (1 H, m), 3.31 (1 H, d, J = 16.5), 3.61 (1 H, d, J
=
16.5), 3.86 (1 H, m), 3.95 (1 H, m), 4.94 (2H, s), 5.50 (1 H, d, J = 2.0),
6.68-
6.78 (4H, m), 7.12-7.34 (6H, m), 8.54 (1 H, br.s)
The following compounds can be prepared in the same manner as
described above using the corresponding known 5-substituted uracil
derivatives:
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-prop-1-ynyluridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5-but-1-ynyl-5'-deoxyuridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-pent-1-ynyluridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-hex-1-ynyluridine,
5-bromo-2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxyuridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5-(1-chlorovinyl)-5'-deoxyuridine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-vinyluridine,
Example 1:
Preparation of 2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-
ethynylcytidine
To a solution of dimethylaminopyridine (19.0g, 155.5 mmol) in acetonitrile
(120 ml) and pyridine (12.6m1, 155.5 mmol) phosphoryl chloride (14.4g, 93.8mM)
was added dropwise in an ice bath under Ar atmosphere. After stirring the
mixture for 1 h at room temperature, a solution of 2',3'-bis-O-(tert-
butyldimethyl-
silyl)-5'-deoxy-5-ethynyluridine (14.9g, 31.1 mmol) in acetonitrile (80 ml)
was
added at 50C with cooling in an ice bath. The mixture was stirred at room
temperature for 2h. Then, 25% aq. ammonia solution (10 ml) was added in one
portion to the reaction mixture while keeping the temperature below 100C. A
second portion of 25 % aq. ammonia solution (65m1) was added to the reaction
mixture while keeping the temperature below 100C. The mixture was stirred at
room temperature for 45 min. Then the reaction mixture was diluted with water
CA 02237368 1998-05-12
-21-
(200m1) at room temperature and extracted three times with ethyl acetate. The
combined organic layers were washed successively with aq. 1 N-hydrochloric
acid
solution, aq. saturated sodium bicarbonate and brine. The organic layer was
dried over MgSO4, filtrered and evaporated under reduced pressure.
Purification
of the residue by silica gel chromatography (using n-hexane : ethyl acetate =
2: 1
as an eluent ) gave 2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-
ethynylcytidine
(14.8g, 99% yield).
MALDI-MS : (m/z) 502[M+Na]+, 518[M+K]+
1 H-NMR :(400MHz;CDCI3) : S 0.05 (3H, s), 0.06 (3H, s), 0.12 (3H, s), 0.24
(3H,
s), 0.89 (9H, s), 0.92 (9H, s), 1.41 (3H, d, J = 6.35), 3.36 (1 H, s), 3.46 (1
H, dd, J
3.91,7.81), 4.19-4.26 (2H, m), 5.57 (1 H, s), 5.79 (1 H, br.s), 7.57 (1 H,
br.s),
7.80(1 H, s)
The following compounds were obtained in a manner analogous to that of
Example 1.
Example 2: 2',3'-bis-O-(tert-butyldimethylsilyl -5'-deoxy-5-iodocytidine,
FAB-MS : (m/z) 582[M+H]+
1 H-NMR :(270MHz;DMSO-d6) S 0.00 (3H, s), 0.02 (3H, s), 0.06 (3H, s),
0.08 (3H, s), 0.82 (9H, s), 0.88 (9H, s), 1.30 (3H, d, J = 6.6), 3.78 (1 H,
dd, J
4.6, 4.3), 3.93 (1 H, m), 4.33 (1 H, dd, J = 4.9, 4.6), 5.67 (1 H, d, J =
5.0),
6.67(1 H, br.s), 7.87(2H, br.s)
Example 3: 2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-trifluoro-
methylcytidine,
FAB-MS : (m/z) 524 [M+H]+
1 H-NMR :(400MHz;CDCI3) : 5 0.00 (6H, s), 0.08 (3H, s), 0.19 (3H, s), 0.84
(9H, s), 0.87 (9H, s), 1.35 (3H, d, J = 6.6), 3.38 (1 H, m), 4.15 (1 H, m),
4.21
(1 H, m), 5.51 (1 H, s), 7.97 (1 H, s)
Example 4: 5-(3-benzyloxybenzyl)-2',3'-bis-O-(tert-butyldimethylsilyl -5'-
deoxycytidine,
FAB-MS : (m/z) 652 [M+H]+
1 H-NMR :(270MHz;CDCI3) : 8 -0.01 (3H, s), 0.00 (3H, s), 0.09 (3H, s), 0.22
(3H, s), 0.86 (9H, s), 0.90 (9H, s), 1.10 (3H, d, J = 6.6), 3.37 (1 H, m),
3.57
(2H, s), 4.08-4.18 (2H, m), 5.03 (2H, s), 5.59 (1 H, s), 6.75-6.90 (3H, m),
7.11
(1 H, s), 7.26 (1 H, m), 7.31-7.44 (5H, m)
CA 02237368 1998-05-12
-22-
Example 5: 2',3'-bis-O-(tert-butyldimethylsilyl -5-cyano-5'-deoxycytidine
FAB-MS : (m/z) 481 [M+H]+
1 H-NMR :(270MHz;DMSO-d6) : 5 -0.04 (3H, s), 0.00 (3H, s), 0.02 (3H,s),
0.76 (9H, s), 0.82 (9H, s), 1.21 (3H, d, J 6.3), 3.81 (1 H, m), 4.05 (1 H, t,
J
5.0), 4.71 (1 H, t , J = 5.0), 5.65 (1 H, d, J 5.3), 6.41 (1 H, s), 7.69 (1 H,
br.s),
7.85 (1 H, br.s)
The following compounds can be obtained according to a manner
analogous to that of Example 5.
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-prop-1-ynylcytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5-but-1-ynyl-5'-deoxycytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-pent-1-ynylcytidine,
2',3'-bis-O-(tert-butyidimethylsilyl)-5'-deoxy-5-hex-1-ynylcytidine,
5-bromo-2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxycytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5-(1-chlorovinyl)-5'-deoxycytidine,
2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-vinylcytidine,
Example 6:
Preparation of 5'-deoxy-5-ethynyl-N4-(n-pentyloxYcarbonyl)cytidine,
a) 2',3'-Bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-ethynylcytidine (45 mg,
0.09
mmol) was dissolved in dichloromethane (1 ml) and pyridine (33 I,0.42mM). To
the mixture, n-pentyl chloroformate (42mg, 0.28 mmol) was added dropwise in an
ice bath under Ar. The reaction mixture was stirred at room temperature for
2h.
Water was aded and the reaction mixture stirred for 30 mins. The reaction
mixture
was partitioned between dichloromethane and water. The aqueous layer was
extracted with dichloromethane. The combined organic layers were dried over
Na2SO4 and filtered. The filtrate was evaporated under reduced pressure.
Purification of the residue by silica gel chromatography (using n-hexane :
ethyl acetate = 4: 1 as an eluent ) gave 2',3'-bis-O-(tert-butyldimethylsilyl)-
5'-
deoxy-5-ethynyl-N4-(n-pentyloxycarbonyl)cytidine (40 mg, 72% yield).
FAB-MS : (m/z) 594[M+H]+
1 H-NMR :(270MHz;CDCI3) : S 0.12-0.27 (12H, m), 0.90-0.92 (21 H, m), 1.26-1.42
(7H, m), 1.64-1.74 (2H, m), 3.25-3.51 (2H, m), 4.15-4.23 (4H, m), 5.55-5.60 (1
H,
m), 7.62 (0.5H, br.s), 7.73 (0.5H, br.s), 8.00 (0.5H, br.s), 12.3 (0.5H, br.s)
CA 02237368 2007-02-14
-23-
b) To a solution of 2',3'-bis-O (tert-butyldimethylsilyl)-5'-deoxy-5-ethynyl-
N4-(n-
pentyloxycarbonyl)cytidine (19mg, 0.03 mmol) in tetrahydrofuran (500 I) was
added dropwise tetrabutylammonium fluoride (93 1, 0.09 mmol) [1.OM
tetrahydrofuran solution] at room temperature under Ar atmosphere. After the
mixture was stirred at room temperature for 2h., the reaction mixture was
evaporated under reduced pressure. The residue was partitioned between
dichloromethane and water. The aqueous layer was back-extracted with
dichioromethane. The combined organic layers were dried over Na2SO4, filtered
and evaporated under reduced pressure. Purification of the residue by silica
gel
chromatography (using dichloromethane : methanol = 20 : 1 as an eluent) gave
5'-
deoxy-5-ethynyl-N4-(n-pentyloxycarbonyl)cytidine (compound A) (9 mg, 81 %
yield).
FAB-MS : (m/z) 366[M+H]+
1 H-NMR :(400MHz;DMSO-d6) : S 0.88 (3H, t, J=6.84), 1.30-1.32 (7H, m), 1.59-
1.63 (2H, m), 3.67-3.71 (1 H, m), 3.90-4.46 (5H, m), 5.07 (1 H, m), 5.42 (1 H,
m),
5.66 (1 H, m), 7.89 (0.5H, br.s), 8.14 (0.5H, br.s), 9.53 (0.5H, br.s), 11.7
(0.5H,
br.s)
The following compounds (examples 7-41) were obtained in a manner
analogous to that of Example 6.
Example 7: 5'-deoxy-5-ethyl-N4-(n-gentyloxycarbonyl)cytidine
FAB-MS : (m/z) 370[M+H]+
1 H-NMR :(270MHz;CDCI3) : 5 0.91(3H, t, J = 6.93), 1.16 (3H, t, J= 7.5),
1.36 (4H, m), 1.41 (3H, d, J = 6.6), 1.72 (2H, m), 2.47 (2H, q, J = 7.5), 3.22
(1 H, br.s), 3.93 (1 H, m), 4.16 (2H, t, J = 6.93), 4.28 (2H, m), 4.49 (1 H,
br.s),
5.66 (1 H, d, J = 3.63), 7.37 (1 H, br.s), 12.46(1 H, br.s)
Example 8: 5'-deoxy-5-iodo-N4-(n-pentyloMcarbonyl)cytidine
FAB-MS : (m/z) 468 [M+H]+, 490[M+Na]+
1 H-NMR : (270 MHz; DMSO-d6) : S 1.36 (3H, t, J = 7.0), 1.76-1.78 (7H, m),
2.09 (2H, m), 4.18 (1 H, m), 4.36 (1 H, m), 4.54 (2H, t, J = 5.9), 5.54 (1 H,
br.d,
J= 5.0), 5.84 (1 H, br.d, J = 5.0), 6.09 (1 H, d, J = 4.3), 8.47 (1 H, s),
12.24
(1 H, br.s)
Example 9: 5'-deoxy-N4-(n-pentyloxycarbonyl)-5-trifluoromethylcytidine
FAB-MS : (m/z) 410[M+H]+
CA 02237368 1998-05-12
-24-
1 H-NMR :(270MHz;CDC13) : S 0.88-0.94 (3H, m), 1.32-1.39 (4H, m), 1.42
(3H, d, J = 6.6), 1.68-1.75 (2H, m), 3.09-3.30 (1 H, m), 3.92 (1 H, m), 4.15-
4.27 (5H, m), 5.67 (1 H, d, J = 3.3), 8.05-8.31 (1 H, m), 12.6 (1 H, br.s)
Example 10: 5-(3-benzyloxybenzyl -5'-deoxy-N4-(n-pentyloxy-carbonyl)cytidine
FAB-MS : (m/z) 538 [M+H]+
1 H-NMR :(270MHz;CDCI3) : S 0.90 (3H, t, J= 6.9), 1.04 (3H, d, J = 6.6),
1.26-1.39 (4H, m), 1.72 (2H, m), 3.16 (1 H, br.s), 3.67 (1 H, d, J = 16.5),
3.71
(1 H, m),3.75 (1 H, d, J = 16.5), 4.10 (2H, m), 4.16 (2H, t, J = 6.9), 4.40 (1
H,
br.s), 5.04 (2H, s), 5.62 (1 H, d, J = 3.3), 6.79 (1 H, d, J = 7.6), 6.84-6.89
(2H,
m), 6.97 (1 H, br.s), 7.22-7.43 (6H, m), 12.41 (1 H, br.s)
Example 11: 5-cyano-5'-deoxy-N4-(n-pentyloxycarbonyl)cytidine
FAB-MS : (m/z) 367[M+H]+
1 H-NMR :(270MHz;DMSO-d6) : S 0.88 (3H, t, J = 6.9), 1.30 (4H, s),
1.31(3H, d, J = 6.3), 1.62 (2H, m), 3.81 (1 H, quin., J = 6.3), 3.91 (1 H,
quin., J
=6.3),4.13(2H,t,J=6.6),4.39(1H, m), 5.09 (1 H, d, J = 6.3), 5.31 (1 H, d,
J = 5.3), 5.83( 1 H, d, J = 4.0), 7.57 (1 H, s), 11.23 (1 H, br.s)
Example 12: 5'-deoxv-5-ethynyl-N4-(n-propoxycarbonyl)cytidine
FAB-MS : (m/z) 338[M+H]+, 360[M+Na]+
1 H-NMR :(270MHz;DMSO-d6) : S 0.91 (3H, t, J = 7.3), 1.31 (3H, d, J = 6.3),
1.63 (2H, sextet, J = 7.3), 3.69 (1 H, dt, J = 5.9,5.3), 3.91 (1 H, quin., J =
5.9),
4.03 (2H, t, J = 6.6), 4.13 (1 H, dt, J = 5.0,4.3), 4.35 (1 H, br.s), 5.05 (1
H, d, J
= 5.9), 5.41 (1 H, d, J = 5.3), 5.66 (1 H, d, J = 4.0), 8.01 (1 H, br.s)
Example 13: 5'-deoxy-5-ethynyl-N4-(isopropoxycarbonyl)cytidine
FAB-MS : (m/z) 338[M+H]+
1 H-NMR : (270MHz;DMSO-d6): S 1.24 (6H, d, J = 5.9), 1.31 (3H, d, J = 6.6),
3.68 (1 H, dt, J = 5.9,5.6), 3.90 (1 H, quin., J = 5.9), 4.12 (1 H, m), 4.30
(1 H, s),
4.85 (1 H, m), 5.05 (1 H, d, J = 5.9), 5.40 (1 H, d, J = 5.3), 5.66 (1 H, d, J
=
3.6), 8.02 (1 H, br.s)
Example 14: N4-(isobutoxycarbonyl)-5'-deoxy-5-ethynylcytidine
FAB-MS : (m/z) 352[M+H]+
1 H-NMR : (270MHz;DMSO-d6): fi 0.91 (6H, d, J = 6.6), 1.30 (3H, d, J = 6.3),
1.91 (2H, m), 3.68 (1 H, dt, J = 5.9,5.3), 3.84 (2H, d, J = 6.6), 3.89 (1 H,
quin.,
6.3), 4.11 (1 H, m), 4.30 (1 H, s), 5.03 (1 H, d, J = 5.9), 5.38 (1 H, d, J =
5.3),
CA 02237368 1998-05-12
-25-
5.66 (1 H, d, J = 4.0), 7.96(1 H, s)
Example 15: 5'-deoxy-5-ethynyl-N4-[(2-methylpentyloxy)carbonYl-cytidine
FAB-MS : (m/z) 380[M+H]+,402[M+Na]+
1 H-NMR : (270M Hz; DMSO-d6): S 0.85-0.93 (7H, m), 1.31 (3H, d, J = 6.3),
1.28-1.37 (3H, m), 1.77 (1 H, m), 3.69 (1 H, dt, J = 5.9, 5.6), 3.88(2H, m),
3.92(1 H, m), 4.13 (1 H, dt, J = 4.9, 4.6), 4.37(1 H, br.s), 5.06 (1 H, d, J =
5.9),
5.41 (1 H, d, J = 5.3), 5.66 (1 H,d,J = 4.0), 8.02 (1 H, br.s),
Example 16: 5'-deoxy-5-ethynyl-N4-j(3-methylpentyloxy)carbonyll-cytidine
FAB-MS : (m/z) 380[M+H]+
1 H-NMR :(270MHz;CDCI3) : 8 0.86-0.98 (6H, m), 1.15-1.80 (8H, m), 3.25-
3.26 (1 H, m), 3.53 (1 H, brs), 3.90-3.95 (1 H, m), 4.25-4.37 (4H, m), 5.33 (1
H,
brs), 5.71 (1 H, d, J = 4.28), 7.69 (1 H, br.s), 8.13 (1 H, br.s),
Example 17: 5'-deoxy-5-ethynyl-N4-[(2-propylpentyloxy)carbonyll-c)tidine
MALDI-MS : (m/z) 408.5[M+H]+, 430.5[M+Na]+, 446[M+K]+
1 H-NMR : (270MHz;DMSO-d6): 5 0.87 (6H, br.m), 1.29 (11 H, br.m),1.66
(1 H, br.m), 3.69 (1 H, br.m), 3.94-4.5 (5H, br.m), 5.06 (1 H, br.m), 5.42 (1
H,
br.m), 5.66 (1 H, br.m), 7.90 (0.5H, br.s), 8.14 (0.5H, br.s), 9 .53 (0.5H,
br.s)
Example 18: 5'-deoxy-5-ethynyl-N4-(n-octyloxycarbonyl)c)tidine
FAB-MS : (m/z) 408[M+H]+, 430[M+Na]+
1 H-NMR :(270MHz;DMSO-d6) : S 0.86 (3H, t, J = 5.0), 1.26 (10H, m), 1.31
(3H, d, J = 6.0), 1.60 (2H, m), 3.69 (1 H, dt, J = 5.9,5.6), 3.90 (1 H, quin.,
J =
6.3), 4.06 (2H, t, J = 6.3), 4.13 (1 H, m), 4.35 (1 H, br.s), 5.05 (1 H, d, J
= 5.9),
5.41 (1 H, d, J = 5.3), 5.66 (1 H, d, J = 4.0), 8.02 (1 H, br.s)
Example 19: 5'-deoxy-N4-[(2-ethylhexY~xycarbonyll-5-ethynLrl-cytidine
FAB-MS : (m/z) 408[M+H]+
1 H-NMR :(270MHz;CDCI3) : S 0.88-0.94 (6H, m), 1.30-1.41 (12H, m), 3.25
(1 H, d, J = 3.63), 3.53 (1 H, m), 3.92-3.94 (1 H, m), 4.15-4.37 (4H, m), 5.32
(1 H, m), 5.70 (1 H, dt, J = 4.61), 7.86 (1 H, br.s), 8.14 (1 H, br.s)
Example 20: 5'-deoxy-5-ethynyl-N4-((2-phenylethoxy)carbonyll-cytidine
FAB-MS : (m/z) 400[M+H]+
1 H-NMR : (270MHz;DMSO-d6): 8 1.31 (3H, d, J = 6.3), 2.94 (2H, t, J = 6.9),
3.69 (1 H, dt, J = 5.9,5.6), 3.90 (1 H, quin., J = 6.3), 4.14 (1 H, m), 4.28
(2H, t,
CA 02237368 1998-05-12
-26-
J= 6.9), 4.31 (1 H, br.s), 5.05 (1 H, d, J = 5.9), 5.41 (1 H, d, J = 4.9),
5.66 (1 H,
d, J = 4.0), 7.27 (5H, m), 8.01 (1 H, br.s),
Example 21: N4-(cyclohexyloxycarbonyl)-5'-deoxy-5-ethynylcytidine
FAB-MS : (m/z) 378[M+H]+
1 H-NMR :(270MHz;DMSO-d6) : b 1.06-1.48 (9H, m), 1.69 (2H, m), 1.86 (2H,
m), 3.65-3.72 (1 H, m), 3.88-3.93 (1 H,m), 4.13-4.61 (3H, m), 5.06 (1 H, d, J
6.27), 5.42 (1 H, d, J = 4.95), 5.66 (1 H, d, J = 3.63), 7.9-8.1 (1 H, m), 9.4
(0.5H, br.s), 11.8(0.5H, br.s)
Example 22: N4-f(cyclohexylmethoxy)carbonyll-5'-deoxy-5-ethynylcytidine
FAB-MS : (m/z) 392[M+H]+, 414[M+Na]+
1 H-NMR : (270MHz;DMSO-d6): S 0.86-1.25 (5H, m), 1.31 (3H, d, J = 6.3),
1.61-1.72 (6H, m), 3.69 (1 H, dt, J = 5.9,5.6), 3.89 (2H, d, J = 6.3), 3.90 (1
H,
m), 4.14 (1 H, m), 4.36 (1 H, br.s), 5.05 (1 H, d, J = 5.9), 5.41 (1 H, d, J =
5.3),
5.66 (1 H, d, J = 4.0), 8.02 (1 H, br.s).
Example 23: 5'-deoxy-5-ethynyl-N4-(neopentyloxycarbonyl)-cytidine
FAB-MS : (m/z) 366[M+H]+, 388[M+Na]+
1 H-NMR : (270MHz;DMSO-d6): 8 0.93 (9H, s), 1.30 (3H, br.d), 3.67-4.27
(5.5H, br.m), 4.47 (0.5H, br.s), 5.06 (1 H, br.m), 5.39 (1 H, br.m), 5.43 (1
H,
br.m), 7.88 (0.5H, br.s), 8.16 (0.5H, br.s), 9.56 (0.5H, br.s), 11.69 (0.5H,
br.s)
Example 24: 5'-deoxy-N4-f(3,3-dimethylbutoxy)carbonyll-5-ethynylcytidine
FAB-MS : (m/z) 380[M+H]+
1 H-NMR : (270MHz;DMSO-d6): S 1.01 (9H, s), 1.39 (3H, br.d), 1.63 (2H,
br.t), 3.77 (1 H, br.m), 3.98-4.32 (4.5H, br.m), 4.56 (0.5H, br.s), 5.13 (1 H,
br.m), 5.45-5.51 (1 H, br.m), 5.73-5.75 (1 H, br.m), 7.96 (0.5H, br.s), 8.23
(0.5H, br.s), 9.57 (0.5H, br.s), 11.76 (0.5H, br.s)
Example 25: 5'-deoxy-5-ethynyl-N4-(tridecyloxycarbonyl)cytidine
MALDI-MS : (m/z) 478[M+H]+, 516[M+K]+
1 H-NMR :(270MHz;DMSO-d6) : S 0.85(3H, d, J = 4.6), 1.24(20H, m), 1.30
(3H, d, J = 6.3), 1.60 (2H, m), 3.68 (1 H, dt, J = 5.9,5.6), 3.90 (1 H, quin.,
J
6.3), 4.05 (2H, t, J = 6.6), 4.13 (1 H, dt, J = 5.0,4.3), 4.34 (1 H, br.s),
5.05
(1 H, d, J = 5.9), 5.40 (1 H, d, J = 5.3), 5.65 (1 H, d, J = 3.6), 8.00 (1 H,
br.s)
CA 02237368 1998-05-12
-27-
Example 26: N4-(n-butoxycarbonyl)-5'-deoxy-5-ethynylcytidine
FAB-MS : (m/z) 352 [M+H]+, 374 [M+Na]+
1 H-NMR :(270 MHz; DMSO-d6) : S 0.89 (3H, t, J = 7.2), 1.28 - 1.41 (5H, m),
1.53 - 1.64 (2H, m), 3.64 -3.71 (1 H, m), 3.85 - 3.92 (1 H, m), 4.03 - 4.15
(3H,
m),4.34(1H,s),5.04(1H,d,J=5.9),5.39(1H,d,J=5.3),5.64(1H,d,J=
3.6), 8.06 (1 H, br.s)
Example 27: 5'-deoxy-5-ethynyl-N4-(n-hexyloxycarbonyl)cytidine
FAB-MS : (m/z) 380 [M+H]+, 402 [M+Na]+
1 H-NMR :(270 MHz; DMSO-d6) : S 0.95 (3H, t, J = 6.6), 1.38 - 1.40 (9H, m),
1.63-1.71 (2H, m), 3.74 -3.80 (1 H, m), 3.94 - 4.03 (1 H, m), 4.14 (2H, t, J =
6.6), 4.19 - 4.24 (1 H, m), 4.43 (1 H, s), 5.13 (1 H, d, J = 5.9), 5.49 (1 H,
d, J =
5.3), 5.74 (1 H, d, J = 4.0), 8.09 (1 H, br.s)
Example 28: 5'-deoxy-5-ethynyl-N4-(n-decyloxycarbonyl)cytidine
MS:FAB-MS: (m/z) 436 [M+H]+
1 H-NMR :(270MHz; DMSO-d6) : d 0.85 (3H, t, J= 6.4), 1.15 -
1.42 (17H, m), 1.60 (2H, m), 3.69 (1 H, m), 3.90 (1 H, m), 4.05 (2H,
t, J = 6.6), 4.13 (1 H, m), 4.34 (1 H, br.s), 5.04 (1 H, d, J = 5.6), 5.40
(1 H, d, J = 4.9), 5.66 (1 H, d, J = 3.6), 8.01 (1 H, br.s)
Example 29: 5'-deoxy-5-ethynyl-N4-[(2,6-dimethylcylohexyloxy)-
carbonyllc)tidine
MS:FAB-MS: (m/z) 406 [M+H]+
1 H-NMR :(270MHz; DMSO-d6) : d 0.83 (36H, d, J = 6.3), 1.20 -
1.50 (9H, m), 1.55 - 1.75 (2H, m), 3.68 (1 H, m), 3.93 (1 H, m) 4.12
- 4.20 (2H, m), 4.45 (0.7H, s), 4.86 (0.3H, s), 5.04 (1 H, d, J= 5.6),
5.43 (1 H, br.s), 5.67 (1 H, br.s), 7.96 (0.3H, br.s), 8.14 (0.7H, br.s),
9.50 (0.7H, br.s) 12.00 (0.3H, br.s)
Example 30: 5'-deoxy-5-ethynyl-N4-(benzyloxycarbonyl cytidine
MS:FAB-MS: (m/z) 386 [M+H]+
1 H-NMR :(270MHz; DMSO-d6) : d 1.30 (3H, d, J = 6.3), 3.69 (1 H,
m), 3.89 (1 H, m), 4.13 (1 H, m), 4.35 (1 H, br.s), 5.05 (1 H, d, J =
5.9), 5.14 (2H, s), 5,41 (1 H, d, J = 5.3), 5.66 (1 H, d, J = 3.6), 7.31
- 7.45 (5H, m), 8.01 (1 H, br.s)
CA 02237368 1998-05-12
-28-
Example 31: 5'-deoxy-5-ethynyl-N4-[(1-isopropyl-2-methypropoxy)-
carbonyllcytidine
MS:FAB-MS: (m/z) 394 [M+H]+
1 H-NMR :(270MHz; DMSO-d6) : d 0.93 (12H, d, J = 6.6), 1.40
(3H, d, J 6.6), 1.97 (2H, m), 3.33 (1 H, d, J = 3.6), 3.55 (1 H, s),
3.91 (1 H, m), 4.30 (1 H, m), 4.36 (1 H, m), 4.62 (1 H, m), 5.40 (1 H, s),
5.72 (1 H, d, J = 4.3), 7.69 (1 H, s), 8.11 (1 H,s)
Example 32: 5'-deoxy-5-ethynyl-N4-f(3-methylbenzyloxy)-carbonyllcytidine
MS:FAB-MS: (m/z) 416 [M+H]+
1 H-NMR : (270MHz; DMSO-d6) : d 1.31 (3H, d, J = 6.0), 3.70 (1 H,
m), 3.76 (3H, s), 3.90 (1 H, m) 4.14 (1 H, m), 4.26 (0.5H, br.s),
4.44 (0.5H, br.s), 5.06 (2H, s), 5.16 (1 H, br.s), 5.41 (1 H, br.s),
5.66 (1 H, m), 6.91 (1 H, d, J = 7.9), 7.00 (2H, m), 7.30 (1 H, dd, J
7.9, 7.9), 7.89 (0.5H, br.s), 8.14 (0.5H, br.s), 9.72 (0.5H, br.s),
11.7 (0.5H, br.s)
Example 33: 5'-deoxv-5-ethynyl-N4-(methoxycarbonYl)cytidine
MS:FAB-MS: (m/z) 310 [M+H]+
1 H-NMR :(270MHz; DMSO-d6) : d 1.30 (3H, d, J = 6.3), 3.66 (3H,
s), 3.70 (1 H, m), 3.90 (1 H, quin., J = 6.3), 4.13 (1 H, m), 4.34 (1 H,
s), 5.05 (1 H, d, J = 5.9), 5.40 (1 H, d, J = 5.3) 5.66 (1 H, d, J= 4.0),
8.00 (1 H, br.s)
Example 34: 5'-deoxy-5-ethynyl-N4-(ethYlo_xycarbony)cytidine
MS:FAB-MS: (m/z) 324 [M+H]+
1 H-NMR : (270MHz; DMSO-d6) : d 1.23 (3H, t, J = 6.93), 1.31 (3H,
d, J = 6.27), 3.69 (1 H, m), 3.90 (1 H, m), 4.08 - 4.14 (3H, m), 4.35
(1 H, br.s), 5.05 (1 H, d, J = 5.94), 5.40 (1 H, d, J = 5.27), 5.66 (1 H,
d, J = 3.63), 8.02 (1 H, br.s)
Example 35: 5'-deoxy-N4-(n-pentyloxycarbonyl)cytidine
FAB-MS : (m/z) 342[M+H]+,
1 H-NMR : (270MHz;DMSO-d6): 8 0.88 (3H, t, J=6.9), 1.31(4H, m), 1.32 (3H,
CA 02237368 1998-05-12
-29-
d, J=6.3), 1.55-1.63 (2H, m), 3.63 (1 H, dt, J=5.6, 5.6), 3.93 (1 H, quin.,
J=6.3),
3.98 (1 H, m), 4.01 (2H, t, J=6.9), 5.04 (1 H, d, J=5.9), 5.42 (1 H, d,
J=4.6),
5.73 (1 H, d, J=3.0), 7.07 (1 H, d, J=7.6), 7.97 (1 H, d, J=7.6), 10.66 (1 H,
br. s)
Example 36: 5'-deoxy-N4-(n-pentyloxycarbonyl)-5-vinylc)tidine
MS:LC-MS: (m/z) 368 [M+H]+
' H-NMR :(270MHz; DMSO-d6) : S 0.88 (3H, t, J = 7.1), 1.31 (7H, m), 1.61 (2H,
m), 3.74 (1 H, m), 3.91 (1 H, m), 4.06 (2H, t, J = 6.4), 4.22 (1 H, m), 5.08
(1 H, d, J
= 5.3), 5.20 (1 H, d, J = 11.3), 5.40 (1 H, d, J = 4.9), 5.69 (1 H, d, J =
4.0), 5.88
(1 H, d, J = 17.9), 6.57 (1 H, dd, J = 11.3, 17.9), 7.78 (1 H, s), 11.88 (1 H,
s)
Example 37: 5'-deoxy-N4-(benzyloxycarbonyl)-5-vinylc)tidine
MS:FAB-MS: (m/z) 388 [M+H]+, 410 [M+Na]+,
~ H-NMR : (270 MHz; DMSO-d6) : S 1.30 (3H, d, J = 6.3), 3.73 (1 H, m), 3.92
(1 H, m), 4.23 (1 H, m), 5.13 (2H, s), 5.04-5.22 (2H, m), 5.42 (1 H, d, J =
5.3),
5.69 (1 H, d, J = 4.3), 5.69 (1 H, dd, J = 15.8, 2.0), 6.55 (1 H, dd, J=11.2,
15.8),
7.36-7.42 (5H, m), 7.78 (1 H, s), 11.87 (1 H, s).
Example 38: N4-(ethoxycarbonyl)-5'-deoxy-5-vinylcytidine
MS:FAB-MS: (M/Z) 326 [M+H]+, 348 [M+NA]+
1H-NMR :(270 MHZ; DMSO-Dg) : 0 1.23 (3H, T, J = 7.26), 1.32 (3H, D, J
6.27), 3.70-3.76 (1 H, M), 3.89-3.94 (1 H, M), 4.11 (2H, Q, J = 7.26), 4.22 (1
H,
M), 5.09 (1 H, D, J = 5.61), 5.18-5.22 (1 H, M), 5.42 (1 H, D, J = 5.61), 5.69
(1 H,
D, J= 3.96), 5.85-5.92 (1 H, M), 6.57 (1 H, DD, J=11.88, 17.82), 7.79 (1 H,
S),
11.88 (1 H, BR.S)
Example 39: 5'-deoxy-5-iodo-N4 j(2-phenylethoxy)carbonyl]cytidine
MS:FAB-MS: (m/z) 502 [M+H]+
'H-NMR :(270MHz; DMSO-d6) : S 1.30 (3H, d, J= 6.3), 2.96 (2H, t, J = 7.1),
3.69 (1 H, m), 3.88 (1 H, m), 4.17 (1 H, m), 4.29 (2H, t, J = 7.1), 5.07 (1 H,
d, J
5.9), 5.38 (1 H, d, J = 5.3), 5.62 (1 H, d, J = 4.6), 7.19 - 7.35 (5H, m),
8.01 (1 H,
s), 11.70 (1 H, br.s)
CA 02237368 1998-05-12
-30-
Example 40: 5'-deoxy-5-iodo-N4_(isopropoxycarbonyl)cytidine
MS:MALDI-TOF: (m/z) 462.5 [M+Na]+
'H-NMR : (270MHz; DMSO-d6) : S 1.24 (6H, d, J = 6.3), 1.30 (3H, d, J = 6.3),
3.69 (1 H, m), 3.88 (1 H, m), 4.17 (1 H, m), 4.87 (1 H, m), 5.07 (1 H, d, J =
5.6),
5.38 (1 H, d, J = 5.3), 5.62 (1 H, d, J = 4.3), 8.02 (1 H, s), 11.77 (1 H,
br.s)
Example 41: N4-(cyclohexyloxycarbonvll-5'-deoxy-5-iodoc)tidine
MS:LC-MS: (m/z) 479.9 [M+H]+
'H-NMR :(270MHz, DMSO-d6) : 8 1.23-1.42 (6H, m), 1.29 (3H, d, J = 6.3),
1.70 (2H, m), 1.89 (2H, m), 3.69 (1 H, m), 3.88 (1 H, m), 4.16 (1 H, m), 4.60
(1 H,
m), 5.05 (1 H, d, J = 5.9), 5.37 (1 H, d, J = 5.3), 5.62 (1 H, d, J = 4.3),
8.00 (1 H,
s)
The following compounds can also be obtained in a manner analogous to
that of Example 6:
5'-deoxy-N4-(n-pentyloxycarbonyl)-5-prop-1 -ynylcytidine,
5-but-1 -ynyl-5'-deoxy-N4-(n-pentyloxycarbonyl)cytidine,
5'-deoxy-5-pent-1 -ynyl-N4-(n-pentyloxycarbonyl)cytidine,
5'-deoxy-5-hex-1 -ynyl-N4-(n-pentyloxycarbonyl)cytidine,
5'-deoxy-5-bromo-N4-(n-pentyloxycarbonyl)cytidine,
5'-deoxy-5-(1-chlorovinyl)-N4-(n-pentyloxycarbonyl)cytidine,
5'-deoxy-N4-(n-pentyloxycarbonyl)-5-vinylcytidine,
5'-deoxy-5-ethynyl-N4-(isopentyloxycarbonyl)cytidine, and
5'-deoxy-N4-[(2-ethylbutyl)oxycarbonyl]-5-ethynylcytidine.
Example 42:
Preparation of 2',3'-di-O-acetyl-5'-deoxy-5-iodocytidine
5-lodocytosine (1.0 g; 4.22 mmol) and a catalytic amount of (NH4)2SO4
were suspended in a solution of toluene (10 ml) and hexamethyidisilazane (20
ml). The suspension was heated at 110 C for 18 hours to become a clear
solution. After concentrating the reaction solution under reduced pressure,
acetonitrile (25 ml) and 5-deoxy-1,2,3-tri-O-acetyl-D-ribofuranoside (1.32 g;
5.06
CA 02237368 1998-05-12
-31-
mmol) were added to residue. Then, anhydrous stannic chloride(0.58 ml; 5.06
mmol) in nitromethane (5 ml) was added dropwise to the mixture over 5 minutes.
During the addtion, the mixture was kept below 0 C by ice cooling. After
stirring
the mixture at 0 - 5 C for 2 hours, 2 g of sodium bicarbonate was added,
follwed
by dropwise addition of water (0.7 ml). After the addtion, the mixture was
stirred
vigorously at room temperature for 30 minutes. The reaction mixture was
filtered
to remove insoluble material, which was washed with CH2CI2. The filtrate and
washing were combined, and washed with water and sat.aq. sodium bicarbonate,
and then dried over Na2SO4 and filtered. The filtrate was evaporated under
reduced pressure. The crude product was purified by flash chromatography on
Si02 (eluent : 5% MeOH / CH2CI2 ) to give 5'-deoxy-2',3'-di-O-acetyl-5-
iodocytidine as a colorless solid. (1.22 g, 66% yield)
FAB-MS : (m/z) 438[M+H]+, 460[M+Na]+
1 H-NMR :(270MHz=,DMSO-d6) : S 1.32(3H, d, J = 6.3), 2.04(3H, s), 2.06(3H,
s), 4.02(1 H, quin., J = 6.3), 5.14(1 H, t, J = 6.6), 5.48(1 H, dd, J = 6.6,
4.3),
5.69(1 H, d, J = 4.0), 6.78(1 H, br.s), 8.01(1 H, br.s), 8.11(1 H, s)
Example 43:
Preparation of 2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-iodo-N4-(n-
pentyloxycarbonyl)cytidine
a) 5'-deoxy-2',3'-di-O-acetyl-5-iodocytidine (200 mg; 0.46mmol) was dissolved
in
methanol (5 mi). To this solution 1 mol/I sodium hydoxide solution was added
dropwise at 0 C. After stirring for 10 minutes, the reaction mixture was
adjusted to
pH 7 with 1 N-hydrochloric acid solution. The reaction mixture was evaporated
under reduced pressure.
A mixture of imidazole(467 mg; 6.9 mmol) in DMF(5 ml) was added to the
residue.
Then tert-butyldimethylchlorosilane(345 mg; 2.29 mmol) was added to the
mixture. The reaction mixture was stirred at 50 C for 1 hour. The mixture was
extracted with dichloromethane, washed with water and then dried over Na2SO4
and filtered. The filtrate was evaporated under reduced pressure. The crude
product was purified by flash chromatography on Si02 (eluent :70% EtOAc / n-
hexane to 100% EtOAc) to give 5'-deoxy-2',3'-di-O-tert-butyldimethysilyl-5-
iodocytidine as a colorless solid. (176.5 mg, 66 % yield)
FAB-MS : (m/z) 582[M+H]+
1 H-NMR :(270MHz;DMSO-d6) S 0.00 (3H, s), 0.02 (3H, s), 0.06 (3H, s), 0.08
(3H,
s), 0.82 (9H, s), 0.88 (9H, s), 1.30 (3H, d, J = 6.6), 3.78 (1 H, dd, J = 4.6,
4.3), 3.93
CA 02237368 1998-05-12
-32-
(1 H, m), 4.33 (1 H, dd, J = 4.9, 4.6), 5.67 (1 H, d, J = 5.0), 6.67(1 H,
br.s), 7.87(2H,
br.s)
b) To a stirred solution of 5'-deoxy-2',3'-bis-O-(tert-butyldimethylsilyl)-5-
iodocytidine (116 mg, 0.200 mmol) in CH2CI2 (2 ml) pyridine (84 l, 1.00
mmol),
N,N-dimethylamino-pyridine (6 mg, 0.05 mmol), and n-pentyl chloroformate (95
l,
0.600 mmol) was added at room temperature under Ar. After stirring for 30
min.,
the reaction mixture was partitioned with dichloromethane and water and the
organic phase was separated and the water phase was extracted with CH2CI2 (15
ml x 4). The combined organic phase was washed with water and brine, dried
over Na2SO4 and filtered. The filtrate was evaporated under reduced pressure.
The crude product was purified by flash chromatography on Si02 (eluent: 20%
EtOAc/n-hexane) to give 2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-iodo-
N4-(n-
pentyloxycarbonyl)cytidine as a colorless amorphous solid. (132.4 mg, 91 %
yield)
FAB-MS : (m/z) 696 [M+H]+
1 H-NMR :(270MHz; DMSO-d6) : 5 0.00 (3H, s), 0.03 (3H, s), 0.05 (3H, s),
0.07 (3H, s), 0.77 (9H, s), 0.81 (9H, s), 1.20-1.27 (10H, m), 1.46-1.55 (2H,
m), 3.74 (1 H, dd, J = 4.6, 4.6), 3.89-4.01 (3H, m), 4.37 (1 H, dd, J = 4.5,
4.6),
5.55 (1 H, d, J = 4.6), 7.92 (1 H, s), 11.70 (1 H, br.s)
Example 44:
Preparation of 2'.3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-
[(trimethylsilyl)-
ethynyl]-N4-(n-pentyloxycarbonyl)cytidine
To a solution of 2',3'-bis-O-(tert-butyldimethylsilyl)-5'-deoxy-5-iodo-N4-(n-
pentyloxycarbonyl)cytidine (130mg, 0.18mmol) in CH2CI2(2ml) and Et3N(2mI) Cul
(1 0.7mg, 0.1056mmol), Pd(PPh3)2CI2 (2.6mg, 0.0036mmol), and
trimethylsilylacetylene (58.6 1, 0.40mmol) were added and stirred for 2 h at
room
temperature under Ar in the dark. The reaction mixture was concentrated under
reduced pressure and the residue was dissolved in EtOAc(25 mlx3), washed with
2% aq. EDTAo2Na(10mlx2), water and brine, dried over Na2SO4 and filtered. The
filtrate was evaporated under reduced pressure. The crude product was purified
by flash chromatography on Si02 (eluent : 10%EtOAc/n-hexane) to give 2',3'-bis-
O-(tert-butyldimethylsilyl)-5'-deoxy-5-[(trimethylsilyl)ethynyl]-N4-(n-
pentyloxycarbonyl)cytidine as a colorless amorphous solid. (30.2 mg, 26%
yield)
FAB-MS : (m/z) 666[M+H]+, 688[M+Na]+
1 H-NMR : (270MHz;DMSO-d6) : S -0.18 (3H, s), -0.16 (3H, s), -0.14 (3H,s), -
0.12
(3H,s), 0.00 (9H,s), 0.64 (9H, s), 0.65 (3H, s), 0.67 (9H, s), 1.01(4H, m),
1.14 (3H,
CA 02237368 2007-02-14
-33-
d, J = 6.6), 1.40 (2H, m), 3.58 (1 H, t, J = 4.9), 3.79 (1 H, m), 3.87 (2H,
m), 4.20
(1 H, m), 5.43 (1 H, d, J = 3.6), 7.88 (1 H, br.s)
Example 45:
5'-deoxy-2',3'-bis-O-(tert-butyldimethylsilyll-5-cyanocytidine
To a stirred solution of 5'- deoxy-2', 3'-bis-(O-tert-butyidimethylsilyloxy)-5-
iodocytidine (153 mg, 0.263 mmol) in DMF (5 ml) NaCN (34.3 mg, 0.70 mmol)
was added at room temperature. After stirring for 1 day, the reaction mixture
was concentrated under reduced pressure. The crude product was dissolved in
EtOAc, and then washed with water and brine. The extract was dried over
Na2SO4 and filtered. The filtrate was concentrated under reduced pressure. The
crude product was purified by flash chromatography on Si02 (eluent : EtOAc) to
give 5'-deoxy-2',3'-bis-O-Oert-butyldimethylsilyl)-5-cyanocytidine as a pale
yellow
solid. (71.1 mg, 56 % yield)
FAB-MS : (m/z) 481 [M+H]+
1 H-NMR :(270MHz; DMSO-d6) : 8-0.04 (3H, s), 0.00 (3H, s), 0.02 (3H,s), 0.76
(9H, s), 0.82 (9H, s), 1.21 (3H, d, J = 6.3), 3.81 (1 H, m), 4.05 (1 H, t, J =
5.0), 4.71
(1 H, t , J = 5.0), 5.65 (1 H, d, J = 5.3), 6.41 (1 H, s), 7.69 (1 H, br.s),
7.85 (1 H, br.s)
Example 46: Preparation of 2'.3'-di-O-acetyl-5'-deoxy-5-vinylcytidine
To a solution of 2',3'-di-O-acetyl-5'-deoxy-5-iodocytidine, (1.6 g, 3.66
mmol) in 10 ml DMF were added Pd2(dba)3 (67 mg, 0.073 mmol) and tri-2-
furylphosphine (85mg, 0.366mmol) and tri-n-butyl(vinyl)stannane ( 2.1 ml,
7.318mmol ) under Ar atmosphere at room temperature. After stirring for 19h,
tri-
n-butyl(vinyl)stannane ( 2.1 ml, 7.318mmol ) was added to the reaction
mixture,
and then the reaction mixture was warmed up to 40_C with stirring for 24hr.
The
solvent was removed in vacuo, and the residue was purified on a column of
silica
gel ( eluent : ethyl acetate - CH2CI2 : MeOH = 95 : 5 ) to give 2',3'-di-O-
acetyl-5'-
deoxy-5-vinylcytidine (1.13g, 92 %) as colorless solid:
MS:FABMS : (m/z) 338 [M+H]+
'H-NMR :( 270MHz; DMSO-d6 ): d 1.33 (3H, d, J= 6.3 ), 2.05 ( 3H, s), 2.06 (
3H,s),4.05(1H,quin.,J=6.3),5.14(1H,d,J=10.8),5.16(1H,t,J=6.6),
5.54 ( 1 H, d, J = 17.2 ), 5.53 ( 1 H, dd, J = 6.9, 5.9 ), 5.73 ( 1 H, d,
J=4.3),6.55
( 1 H, dd, J = 17.2, 10.8 ), 7.20 ( 1 H, br. s ), 7.57 ( 1 H, br. s ), 7.88 (
1 H, s )
CA 02237368 1998-05-12
-34-
Example 47: Preparation of 5'-deoxy-5-vinylcytidine
To a solution of 2',3'-di-O-acetyl-5'-deoxy-5-vinylcytidine (111 mg, 3.29
mmol) in 5
ml of methanol was added 1 N NaOH (0.32 ml, 0.32 mmol) at room temperature.
After stirring for 1 h, 1 N HCI (caØ3 ml) was added to the reaction mixture,
and
then the reaction mixture was concentrated under reduced pressure. The residue
was purified by solid phase extraction ( MEGA Bond Elute LRC, eluent : H20 -
H20: MeOH = 1: 1, step gradient) to give 5'-deoxy-5-vinylcytidine (82 mg, 98
%)
as colorless solid:
MS:LC-MS: (m/z) 253.9 [M+H]+
1 H-NMR:(270MHz;DMSO-d6):d1.29(3H,d,J=6.3),3.68(1H,m),3.86(
1H,m),4.08(1H,m),4.97(1H,d,J=5.9),5.12(1H,d,J=11.1 ),5.28(
1H,d,J=5.3),5.50(1H,d,J=17.2),5.70(1H,d,J=3.6),6.58(1H, dd,J=
11.1, 17.2 ), 7.10 ( 1 H, br.s ), 7.42 ( 1 H, br.s),7.64(1H,s)
The following examples illustrate pharmaceutical preparations containing a
compound provided by the present invention.
Example A:
Interlocking gelatin capsules each containing the following ingredients were
manufactured in a manner known perse:
5'-Deoxy-5-ethynyl-N4-(n-pentyloxycarbonyl)-
cytidine 40 mg
Lactose 70 mg
Corn starch 25 mg
Magnesium stearate 1 mg
Crospovidone 4 mg
140 mg
CA 02237368 1998-05-12
-35-
Example B:
Interlocking gelatin capsules each containing the following ingredients were
manufactured in a manner known per se:
5'-Deoxy-5-fluoro-N4-(n-pentyloxycarbonyl)-
cytidine 100 mg
5'-Deoxy-5-ethynyl-N4-(n-pentyloxycarbonyl)-
cytidine 10 mg
Lactose 70 mg
Com starch 25 mg
Magnesium stearate 1 mg
Crospovidone 4 mg
210 mg
Example C:
Tablets each containing the following ingredients were manufactured in a
manner known per se:
5'-Deoxy-5-ethynyl-N4-(n-pentyloxycarbonyl)-
cytidine 40 mg
Lactose 70 mg
Magnesium stearate 3 mg
Crospovidone 7 mg
Povidone 10 mg
130 mg
If necessary, the tablet is film-coated with hydroxypropylmethyl cellulose,
talc
and colorant.
Example D:
Tablets each containing the following ingredients were manufactured in a
manner known per se:
CA 02237368 1998-05-12
-36-
5'-Deoxy-5-fluoro-N4-(n-pentyloxycarbonyl)
-cytidine 300 mg
5'-Deoxy-5-ethynyl-N4-(n-pentyloxycarbonyl)-
cytidine 20 mg
Lactose 70 mg
Magnesium stearate 3 mg
Crospovidone 7 mg
Povidone 10 mg
186 mg
If necessary, the tablet is film-coated with hydroxypropylmethyl cellulose,
talc
and colorant.