Note: Descriptions are shown in the official language in which they were submitted.
CA 02237~48 1998-0~-13
WO 97/18306 PCTi~ C/'05011
CRF Analo~s and their Use in Photoaffinit~ ~abelin~ of CRF
RecePtor~3
The present invention refers to CRF or analogs
thereof bearing a photoactivatable moiety and a label and
their use in detecting CRF receptors and binding proteins
and identifying and characterizing their binding site.
Corticotropin-releasing factor ~CRF) appears to
integrate the endocrine, autonomic, immunologic and
behavioral responses to stress in the CNS. The 41 residue
polypeptide (1) was originally characterized on the basis of
its hypophysiotropic activity stimulating the release of
adrenocorticotropic hormone (ACTH) (2), which is known to
stimulate the secretion of glucocorticoids from the adrenal
cortex. It is generally accepted that CRF is the main
regulator of the hypothalamus-pituitary-adrenal (HPA) axis
leading to the release of glucocorticoids after exposure to
stress.
The various functions of CRF in the endocrine,
autonomic and immunologic system are mediated by a variety
of receptor proteins. Many of these receptors have been
studied and investigations concerning the binding affinity
and biopotency of several homologues of the CRF family have
been performed.
CRF exhibits its activity through G protein-coupled
receptors. C~F receptor, type 1 (CRFR1), mainly found in
pituitary and brain was independently cloned from human,
mouse and rat brain, and a human Cushing's corticotropic
cell tumor (3-6). cDNAs coding for two splice variants of
CA 02237~48 Iss8-0~-l3
WO97/18306 PCT~P96/OSOlI
CRF receptor, type 2 (CRFR2a and CRFR2b), were cloned from
brain, heart, and skeletal muscle (7-lO). Recently, it has
been proposed that urocortin (Ucn), a naturally occurring
CRF analog, is the endogenous ligand to CRFR2 (11).
Besides the CRF receptor, a 37 kDa CRF binding
protein has been characterized. This protein which is not
homologous to any known splice variant of CRFRl or CRFR2 was
demonstrated to bind human/rat C~F (h/rCRF) but not ovine
CRF (oCRF) with high af~inity (12). The very potent CRF
antagonist astressin, cyclo(30-33)tD-Phel2, Nle21'38, Glu30,
Lys33]h/rCRF-(12-41), with its amino acid seguence based on
h~rCRF exhibited similar binding affinity to CRFRl as found
for h/rCRF but did not bind to the CRF binding protein (13).
The biopotency of astressin to inhibit CRF mediated ACTH
release in an in vitro pituitary cell culture assay was
attributed to the built-in lactam bridge at the end of the
presumable ~-helical part of the peptide stretching from
amino acid 5-36 in h/rCRF (13, 14). Surprisingly, the
potency of h/rCRF to stimulate ACTH secretion in pituitary
cells was not significantly increased when the same lactam
bridge motif was introduced into the peptide. It was
therefore assumed that the N-terminus of CRF is responsible
for CRF receptor activation and induction of ~-helicity
along the whole molecule (13). Recently, in binding studies
with COSM6 cells transiently expressing chimeric receptors
of rCRFRl and the rat growth hormone releasing factor
receptor (rGRFR), it was shown high affinity binding of
astressin to the N-terminus of rCRFRl (rCRFRN). The CRF
peptide agonists h/rCRF and urocortin still produced cAMP
production when bound to rCRFRN/rGRFR but to a lower extent
than CRF stimulation of rCRFRl (15). A study on the
characterization of another seven tra~hrane spanning G
protein-coupled receptor clearly indicated different binding
sites for agonist and antagonist binding of Gonadotropin-
releasing hormone by site-directed mutagenesis (16).
CA 02237~48 1998-0~-13
WO97/18306 PCT~P96/05011
Considering the numerous important ~unctions of CRF
and in order to further investigate agonist and antagonist
binding of CRF to its receptors, it would be helpful to
identify the amino acid sequence directly involved in CRF
binding and to investigate the cell biological fate of the
CRF receptor and the binding protein after ligand linkage by
means of a CRF analog serving as a label covalently linked
to proteins binding CRF with high affinity.
Chemical cross-linking with tl25I]TyrO oCRF has been
proved not to be suitable to characterize the actual binding
site since the cross-linking efficiency is very low and
subse~uent chemical and enzymatic cleavages result in the
removal of the label from the cross-linked CRF receptor.
Several CRF receptor cross-links with molecular
weights in the range of 58,000-75,000 have been
characterized applying bifunctional reagents to membranes of
bovine anterior pituitary membranes (17), AtT-20 mouse
pituitary tumor cells (18), rat brain, and anterior
pituitary (19,20). However, all CRF cross-links reported to
date were obtained with an extremely low yield (<1%).
Labeling through monofunctional photoaffinity probes
is expected to provide higher yields than labeling with
chemical cross-linking methods using bifunctional reagents.
Additionally, photoactivation is assumed to be superior over
thermal activation, because highly reactive species such as
carbenes and nitrenes can be selectively formed after
irradiation uder mild conditions. The carbenes or nitrenes
formed can insert into X-H bonds and thereby attack groups
that are normally inert to chemical affinity labeling (21).
A prerequisite for all experiments using a
photoaffinity labeling (PAL) technique is that the
photoactivatable ligand binds with high affinity to the
receptor and that the receptor is not destroyed or
CA 02237~48 l998-0~-l3
WO97/18306 PCT~P96/05011
deactivated by the light used to activate the label (21,22).
Recently, a new class of photoactivatable compounds, the
aryldiazirines, has been introduced, which allows
photochemical decomposition under mild conditions (23).
Thus, the t~chnical problem underlying the present
invention is to provide CRF or analogs thereof which bind
efficiently and with high affinity to the receptor resulting
in an irreversible labeling of the receptor.
The solution to said technical problem is provided
by the embodiments characterized in the claims.
Accordingly, the present invention provides CRF or
analogs thereof bearing a photoactivatable moiety and a
label.
In this context, the term "analog" encompasses any
variant or fragment of CRF which retains CRF ligand binding
activity.
In a specific embodiment the photoactivatable moiety
and the label are adjacent to each other.
The photoactivatable moiety should preferably be of
such quality that the photoaffinity labeling can be
performed under mild conditions at a suitable wavelength.
Examples of the photoactivatable moiety are the 4~ azi-
2,2,2-trifluoroethyl)-benzoyl residue or the phenylalanine
analog thereof.
The label can be a radioactive marker, e.g. 125I, or
a fluorescent marker, e.g. fluorescein, or via biotin which
interacts with avidin carrying a fluorescent group.
Preferred emboA; ents of the invention are a CRF
agonist, 4-(l-azi-2,2,2-trifluoroethyl)benzoyl-[125I]-
tyrosineOoCRF (compound 3), and CRF antagonists based on the
amino acid se~uence of astressin carrying the 4-(1-azi-
2,2,2-trifluoroethyl~-benzoyl (ATB) residue and a histidine
or tyrosine by choice for specific radiolabeling, e.g. ATB-
cyclo~30-33)[l25I-His13, Nle21,38 &lu30 Ala32
Lys33]h/rCRF-(13-41) (compound 6) and ATB-cyclo(30-
CA 02237~48 1998-0~-13
WO 97/18306 PcT/~;l r~
33)[Nle2l~38 Glu30 125I-Tyr32, Lys33]h/rCRF-(13-41)
(compound 7).
The synthesis of the compounds of the invention can
be performed by linking the photoactivatable moiety, e.g.
ATB, to the CRF or CRF analog and subsequent labeling, e.g.
iodination.
For example, the synthesis of compounds 4 and 5 is
performed by linking 4-(1-Azi-2,2,2-trifluoroethyl)benzoic
acid to cyclo(30-33)[Nle21~38, Glu30, Ala32, Lys333h/rCRF-
(13-41) and cyclo(30-33)[Nle21~38 Glu30 Tyr32
Lys33]h/rCRF-(13-41). Cyclization of the peptides on the
resin prior to coupling of the phenyldiazirine to the N-
terminus of the peptides is chosen because of the probable
sensitivity of the diazirine group towards
tetrakistriphenylphosphine palladium ~0) (23). Subsequent
iodination with 125I at histidinel3 or tyrosine32 furnishes
compounds 6 and 7 with a specific activity of 82 TBg/mmol,
respectively.
In a preferred embodiment of the invention the
[125I]TyrOoCRF analog bears the 4-(1-azi-2,2,2-
trifluoroethyl)benzoyl residue at its N-terminus, where the
disturbance of ligand binding is supposed to be ;ni~l (2,
24, 25). The i ~~;ate proximity of the photoactivatable
part to the radioactive tracer in the molecule facilitates
the identification and purification of peptide fragments
after photoaffinity labeling experiments. CRF-R1 with a
molecular weight of approximately 75kDa was detected with
the new CRF analog in HEK 293 cells, permanently transfected
with the CRFRl gene.
The compounds of the invention can be used for the
detection of CRF receptors and binding proteins and for the
identification of the binding site of these proteins. The
photoaffinity labeling technique of the present invention is
CA 02237~48 1998-0~-13
W097/18306 PCT~P96/05011
advantageous towards chemical cross-linking methods when
identifying the ligand binding site within a receptor
molecule as on irradiation of the photoactivatable ligand, a
highly reactive short living species is formed, which then
irreversibly binds with high yield to its receptor. The
affinity tagged receptor polypeptide identified by the label
is stable so that it can be further purified, e.g. by HPLC.
It can then be cleaved into fragments, and the binding site
can be identi~ied by amino acid sequence analysis.
Brief descriPtion of the fiqures
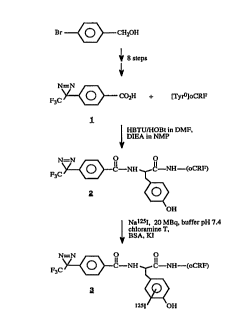
FIG. l. Synthetic route for the photoactivatable
diazirine l according to document (26) and its linkage to
TyrOoCRF 1-41 to generate 2 and its l25iodinated analog 3.
FIG. 2. (A.) Displacement of [l25I-TyrO]oCRF bound
to membranes ~rom transfected HEK 293 cells by oCRF (~) or
ovine photoCRF 2 (o). Data are the mean of triplicates of a
representative experiment. (Inset) Scatchard plots of the
binding of oCRF (~) and ovine photoCRF 2 (o).
(B). Stimulation of intracellular cAMP accumulation
in transfected HEK 293 cells by oCRF (~ ) and ovine photoC~F
2 (o). Data are the mean from duplicate of a representative
experiment. The error bars represent the SEM and are not
shown when smaller than the symbol size.
Fia. 3. Stimulation of intracellular cAMP accumu-
lation in Y79 cells by oCRF (~), ovine photoCRF 2 ( a), and
ovine photoCRF 2 (x) in the presence of lOO nM recombinant
human [D-Phel2, Nle2l~33]CRF-~l2-4l). Data is the mean ~ SEM
values (bars) of duplicates of a representative experiment.
FIG. 4. Photoaffinity cross-linking of ovine l25I-
photoCRF 3 to HEK 293 cell membrane homogenates. Lanes: 1-5,
extracts of cells stably transfected with cDNA coding for
rCRFRl; 6 and 7, extracts of nontransfected HEK 293 cells.
CA 02237~48 1998-0~-13
WO97/18306 PCT~P96105011
Radioactive ovine photoCRF was bound in the absence of oC~F
(lanes l, 5, and 6) or in the presence of lO0 nM (lane 2), l
~M (lane 3), lO ~M (lanes 4 and 7) oCRF or l ~M vasoactive
intestinal peptide (lane 5). Fifty micrograms of total
membrane protein was labeled with approximately lO0,000 cpm
of ovine l25I-photoCRF and incubated (37~C, 30 min.) in the
presence (lane 9) or absence (lane 8) of 2000 units of
PN&ase.
FIG 5. (A) Plot of radioactivity of membrane
components covalently labeled with ovine l25I-photo CRF 3
and purified with RPHPLC. (B) Pooled fractions were
subjected to SDS/PAGE in 7.5% gels.
Agonist bindinq studies using compounds 2 and 3
A. Preliminarv experiments
Preliminary experiments with the diazirine function
of l were performed in order to optimize the photo-affinity
labeling experiments with 3 on CRFRl transfected ~EK 293
cell membranes. The photolysis proceeded with a half-life of
lO0 s, and after 12 min all diazirine was converted to its
carbene or diazo valence isomer (80% carbene, 20% diazo
valence isomer ~26)). The photolysis was performed at a
wavelength of 360 nm using a W Stratalinker (Stratagene)
equipped with five 15 watts lamps and monitored with a W
spectrophotometer (Beckman DU650 spectrometer, Fullerton).
At a distance of 14 cm from the lamps, l was photolyzed (c =
1 mM in ethanol, V = 380 ~l, 1 ml quartz cuvette) with first
order kinetic and a half-life of lO0 s at 4 C. The same
results were obtained when photolyzing 3 after incubation
with membranes in different time intervals and analyzing the
photoproduct with SDS/PAGE.
CA 02237~48 l998-0~-l3
WO97/18306 PCT~P96/05011
B. Bindinq and cAMP assay
For the determination of the binding affinity and
the biological potency of ovine photoCRF 2, a permanent cell
line was established from HEK 293 cells stably transfected
with cDNA coding for rCRFRl. A pool of HEK cell clones was
employed in the following experiments. Binding results
obt~;n~ with individual ~EK cell clones did not differ
significantly from the results of binding experiments with
the cell clone pool. Scatchard analysis indicated that oCRF
was bound with a Kd value of 7.8 + 6.3 nM at a high-affinity
site and a Kd value of 137 + 90 nM at a low-affintiy site.
The BmaX values of 30 fmol/~g and 347 fmol/~g of protein,
respectively, indicated a high efficiency of expression. A
similar Kd value of 5.6 + 2.6 nM (BmaX = 12 fmol/~g of
protein) was found for ovine photoCRF 2 displacing tl25I-
TyrO]oCRF. Scatchard analysis indicated only binding of
ovine photoCRF to the high-affinity site (Fig. 2A).
Application of oCRF or ovine photoCRF to the transfected HEK
293 cells stimulated the accumulation of cAMP in a dose-
dependent manner. EC50 values of 0.5 + O.2 nM and O.4 i 0.l
nM were determined for oCRF and photoCRF, respectively (Fig.
2B). Non-transfected cells did not show significant binding
or cAMP accumulation. This observation was also confirmed by
photoaffinity-labeling experiments. Ovine l25I-photoCRF 3
did not bind to membranes of non-transfected HEK 293 cells
(see Fig. 4). In experiments with membrane preparations from
human Y79 retinoblastoma cells, known to carry an endogenous
functional CRF receptor (27), a Kd value of 2 nM (BmaX =
0.l9 fmol/~g of protein) was found for oCRF or ovine
photoCRF 2. In Y79 cells, only a high-affinity site was
detected for either CRF analog. Ovine photoCRF and oCRF
stimulated cAMP a~l lation in Y79 cells with EC50 values
of 2.3 ~ O.5 nM and l.3 + O.6 nM, respectively ~Fig. 3).
Statistical analysis of the described binding and cAMP data
with the program ANOVA revealed no significant differences
CA 02237~48 1998-0~-13
WO 97/18306 PCT/EPgG/OS011
between the Kd and EC50 values for oCRF and photoCRF. The
specificity of the stimulatory action of ovine photoCRF was
- further demonstrated by the observation that this peptide
exhibited lower stimulatory potencies in the presence of the
specific CRF antagonist recombinant human [D-Phel2,
Nle21~38]CRF-(12-41) For the antagonist an apparent
inhibitory constant (Ki) of 10.3 + 5.0 nM was found (Fig.
3).
C. PhotoaffinitY-Labelinq Experiments
Since it had been found (17,28) that ~SA interferes
with the labeling of the receptor, freshly prepared tracer 3
was stored free of any carrier protein, and photoaffinity-
labeling experiments were performed in buffer solutions in
the absence of BSA. A 75 kDa cross-link was identified with
SDS/PAGE after irradiation at 360 nm of a mixture of ovine
125I-photoCRF 3 and membranes of HEK 293 cells permanently
transfected with rCRFRl (Fig. 4). No cross-link could be
identified without light activation at 360 nm. Using
commercially available r I-Tyr }oCRF and
disuccinimidyltartrate, a 75 kDa protein was labeled in
chemical cross-linking experiments. Binding of ovine 125I-
photoCRF to the receptor could be efficiently inhibited by
addition of 1 ~M oCRF but not 1 ~M vasoactive intestinal
peptide, in agreement with the assumed specificity of this
photoprobe. As mentioned above, no photoaffinity cross-
linking of ovine 125I-photoCRF to nontransfected HEK 293
membranes was detected. Deglycosylation of the 75 kDa
protein cross-link with PNGase generated a 46 kDa protein
detected by SDS/PAGE (Fig. 4).
In a preparative photoaffinity-labeling experiment,
membrane proteins cross-linked to ovine 125I-photoCRF were
purified by RPHPLC. It was found by SDS/PAGE analysis that
the radioactive fractions that were eluted after the void
volume contained the 75 kDa CRFRl protein cross-link (Fig.
5). To calculate the yield of the cross-linking procedure,
CA 02237~48 1998-0~-13
WO97/18306 PCT~P96/05011
labeled receptor was divided by the radioactivity of ovine
l25I-photoCRF specifically bound to the H~K cell membranes
that served as starting material. On this basis, a yield of
at least 20-30% was estimated.
Antaqonist bindinq studies usinq com~ounds 4 to 7
A. ~inding and cAMP assay
For the determination of the binding affinity and
the biological potency of the photoactivatable CRF
antagonists 4 and 5, a HEK 293 cell line, stably transfected
with cDNA coding for rCRFRl, and the human Y79
retinoblastoma cell line, expressing an endogenous CRF
receptor (CRFRl), were used. The results are shown in Table
I. Scatchard analysis indicated high and low affinity
binding of oCRF (Kdl = l.l + 0.7 nM; Kd2 = l.l + l.3 ~M) and
astressin (Kdl = 0.9 + l.O nM; Kd2 = l.6 + l.6 ~M) to
membrane homogenates of Y79 cells. Compound 4 exhibited
similar binding characteristics as astressin (Kdl = 0.6 +
0.5 nM; Kd2 = 3.4 + 2.2 ~M). Compound 5 showed decreased
binding affinity to CRFRl in this cell line (Kdl = 26 + 23
nM). Similar results were obtained when oCRF, astressin and
compounds 4 and 5 were bound to membrane homogenates of
transfected HEK 293 cells with a Kd value of 3.3 + 0.5 nM,
7.7 + 2.6 nM, 3.2 + 2.7 nM and 12 + 3.6 nM, respectively.
Only oCRF showed binding to a low affinity site with a Kd
value of 147 + 78 nM in this cell line. Application of oCRF
to the Y79 cells and HEK 293 cells stimulated the
accumulation of cAMP in a dose dependent manner with EC50
values of 3.8 + 2.6 nM and 0.4 + O.l nM, respectively. Ovine
CRF stimulated cAMP production could be e~iciently
inhibited in the presence of 5 nM antagonist in Y79 cells.
An inhibitory constant (Ki) of 0.5 + 0.3 nM, l.0 + 0.3 nM
and 6.0 + 2.8 nM was determined for astressin and compound 4
and 5, respectively. Similar results were obtained when oCRF
stimulated cAMP accumulation in transfected HEK 293 cells
CA 02237~48 l998-0~-l3
WO97/183~6 PCT~P96/05011
11
was inhibited in the presence of 100 nM CRF antagonist. A Ki
value of lOl + 92 nM, 51 + 52 nM and 497 + 72 nM for
astressin and compounds 4 and 5 were obtained. Application
of a higher dosis of CRF antagonist to observe significant
reduction of oCRF stimulated cAMP production in HEK 293 was
necessary because of a fifty times higher expression of high
affinity receptors in transfected HEK 293 cells (oCRF: BmaXl
= 16 + 6 fmol/~g; Bmax2 = 197 + 15 fmol/~g) when compared
with the Y79 cells (oCRF: BmaXl = 0.3 + 0.3 fmol/~g; BmaX2
= 35 + 57 fmol/~g). Non-transfected cells did not show
significant binding or cAMP accumulation. This observation
was also confirmed by photoaffinity labeling experiments.
Compound 7 did not bind to mem~ranes of non-transfected HEK
293 cells. Statistical analysis of the described binding and
cAMP data with the program ANOVA revealed no significant
differences between the Kd and Ki values for astressin and
compound 4. Both peptides exhibited high potency to reduce
the stimulatory potency of oCRF to produce cAMP in
transfected HEK 293 cells and Y79 cells. Compound 5,
however, revealed 5-10 times lower potency to inhibit cAMP
production in both cell lines when compared to astressin or
compound 4 which was consistent with its decreased binding
affintiy to CRFR1.
B. PhotoaffinitY labelinq exPeriments
As described above, the freshly prepared tracer 7
was stored free of any carrier protein, and the
photoaffinity labeling experiments were performed in buffer
solutions in the absence of BSA. A 66 kDa cross-link was
identified with SDS PAGE after irradiation at 360 nm of a
mixture of compound 7 and membranes of HEK 293 cells
permanently transfected with rCRF~l. No cross-link could be
identified without light activation at 360 nm. Binding of
compound 7 to the receptor could be efficiently inhibited by
addition of 1 ~M ATB-cyclo(30-33)[Nle21~38, Glu30, Tyr32,
Lys33]h/rCRF-(3~-41) (compound 5) but not 1 ~M vasoactive
CA 02237~48 1998-0~-13
WO97/18306 PCT~P96/05011
12
intestinal peptide (V~P~ in agreement with the assumed
specificity of this photoprobe. As mentioned above, no
photoaffinity cross-lin~ing of compound 7 to non-transfected
HEK 293 membranes was detected. Deglycosylation of the 66
kDa protein cross-link with PNGase generated a 38 kDa
protein detected by SDS PAGE.
Thus, the compounds of the invention can be used for
the specific irreversible labeling and tracking of receptors
in various tissue membranes, of CRF binding proteins, as
well as in cytological investigations using a fluorescent
analog of 2, 4 or 5, e.g. on cell sorting, receptor
internalization, trafficking.
The invention is illustrated by the following
examples.
ExamPle 1
8ynthesi~ o~ 4-(l-azi-2,2,2-trifluoroethyl)benzoic aci~
In the dark, 420 mg of 4-(l-azi-2,2,2-
trifluoroethyl)benzyl alcohol (l.9 mmol; 44 ~ overall yield
starting with 4-bromobenzyl alcohol in a seven step
~ynthesis) (26) was dissolved in l.4 ml of dioxane and 12 ml
of 0.2 N aqueous KOH. Then, KMnO4 (462 mg; 2.9 mmol) was
added in portions and the mixture was stirred for 2 hr at
ambient temperature. The precipitated MnO2 was removed by
filtration, washed several times with methanol and the
combined filtrates were concentrated under reduced pressure.
The residual alkaline solution was extracted with ether,
acidified to pH 2-3 with lN aqueous H2SO4 and extracted
again with ether. The organic layer was washed neutral with
water, dried with anhydrous Na2SO4 and the solvent was
evaporated in vacuo. The product was crystallized from
hexane and yielded 230 mg o~ l (l.0 mmol; 53%) :m.p. 123-
125 C, decomp. with foam (N2); lH-NMR (CDCl3, TMS) 7.72
CA 02237~48 1998-0~-13
W097/18306 13 PCT~P96/05011
(AABB, 4H, Ar-H); 13C-NMR (CDC13, TMS) 28.46 (m, J = 41 Hz),
121.85 (m, J = 274 Hz), 126.49 (m, J = 1.3 Hz), 130.32 (m, J
- 2.9 Hz), 130.54 (s), 134.78 (s), 170.81 (s); 19F-NMR
(DMSO-d6, CFC13)-64.00; W (ethanol) ~ (~ )348 nm (248); MS
t m/z (rel. intensity) 229 (100, [M-H]~), 201 (21, [M-N2]+),
157 (51), 137 (8); HRMS calcd. for CgH5N2F3O2 229.0249,
found 229.0228.
~xample 2
~ynthesis of 4~ Azi-2,2,2-trifluoroethyl)benzoyl-tyro-
sine0oCRF 1-41 (2).
In the dark, 26 mg of 1 (0.11 mmol) in 0.2 ml of NMP
were activated by 0.2 ml of 0.45 M HBTU/HOBt in DMF (6 min.)
and 0.1 ml of 2 M of DIEA in NMP (2 min.). 83 mg of peptide
resin (7.00 ~mol side chain protected [TyrO]oCRF 1-41 on
TentaGel S RAM resin; capacity 0.22 mmol/g) were ~dded and
the mixture was reacted for 15 min. The resin was filtered
off, washed three times with 0.5 ml of NMP, added to 750 ~1
o~ cleavage mixture (75 ~g of crystalline phenol, 25 ~1 of
EDT, 50 ~1 of thioanisole and 50 ~1 of dH2O, 1 ml of TFA)
and stirred for 1.4 hr. The resin was filtered off and the
peptide precipitated in 20 ml of ice cold ether. After
filtration, the crude peptide was dissolved in 2 ml of TFA
and 50 ml of 20% MeCN in 0.1 % TFA/water and lyophilized. 21
mg of 38 mg crude product was purified by preparative
reversed-phase ~PLC~and yielded 2.7 mg of 2 (0.54 ~mol,
14~ SI MS calcd. 5045.7; found 5045.1. Analytical RP-HPLC
was performed on a Vydac C18 silica gel column (0.46 x 25
cm, 5 ~m particle size, 30 nm pore size) with solvents
~ A:0.1% TFA/water and B: 80% MeCN in 0.1~ TFA/water, flow
rate: 1 ml/min, 40% ~ for 5 min, then 40-90% B for 25 min.Rt
= 19.62 min).
CA 02237~48 1998-0~-13
WO 97/11~306 PCT/EP96/O5011
14
F~ample 3
8ynthesis of ~ -Azi-2,2,2-trifluoroethyl)benzoyl-Cl25I]-
tyrosineOoCRF 1-41 ~3).
2 was iodinated with slight modifications according
to literature (29). To a tube containing 4 ~l of a 100 ~M
solution of 2 in 0.01N ~OAc in dH2O, the following reagents
were added in a certain order: 10 ~1 of 0.5 M phosphate
buffer, pH 7.4, approximately 20 MBq of 125I (IMS 30,
Amersham, UK), 12.5 ~g of chloramine T in 5 ~1 of 0.05 M
phosphate buffer, 15 s later the reaction was stopped by
adding 10 mg of BSA in 100 ~1 of 0.5 M phosphate buffer and
1 mg of KI in 100 ~1 of 0.05 M phosphate buffer. The mixture
was pipetted onto a ~ond Elut C18 cartridge (Varian
Associates), prewetted with 5 ml of MeOH, then 5 ml of 0.1 %
TFA/water. Five milliliters of dH2O followed by 5 ml of 0.1
~ TFA/water were passed through the column in order to
separate the iodinated peptide from free iodine and BSA. The
iodinated peptide was then eluted from the column by the
addition of 5 ml of 80% MeCN in 0.1 % of TFA/H2O. The volume
of the peptide fraction was reduced to approximately 200 ~1
with a Speed Vac (Christ) and loaded onto a Vydac C18 silica
gel column (0.46 x 25 cm, 5 ~m particle size, 30 nm pore
size) and eluted with solvents A (0.1 % TFA/water) and B
(80% MeCN in 0.1 % TFA/water) and a flow rate of 1 ml/min.
Elution was performed with 45% B for 5 min, then 45-95% B
for 25 min. The retention time for 3 was Rt = 17.36 min. A
Beckman 171 Radioisotope Detector equipped with a liquid
scintillator flow cell was used. The specific activity of
the peptide: 82 TBq/mmol. The peak tubes of radioactivity
were pooled and ~-mercaptoethanol was added to a final
concentration of 0.5 M. The iodinated tracer 3 (Fig. 1) was
stored in aliquots at -20~C and typically used for binding
assays and photoaffinity labeling experiments for 2 months.
CA 02237~48 1998-0~-13
WO97/1~306 P~T~P96/05011
~rle 4
8ynthesis of ovine CRF, cyclo(30-33)[D-Phel2, Nle21'38,
Glu30, Ly 33]h/rCRF-(12-41) (Astre~in), ATB-cyclot30-
33)[Nle2l~38 GlU30, Ala32, Lys33]h/rCRF-~13-41) (compound
_cyclO(3o-33)tNle2l~3g~ GlU30, Tyr32, Ly~33]h/rCRF-
(13-41) (~ ~d 5)
The CRF peptides were synthesized with Fmoc
chemistry on TentaGel S RAM resin (0.1 mmole scale, Rapp,
Tubingen, F.R.G.) with a model ABI 433A peptide synthesizer
(Applied Biosystems). After cleavage of the peptides from
the resin, the crude peptides were purified by preparative
reverse-phase HPLC (RPHPLC) performed on a Waters Prep Nova-
Pak H~ C18 silica gel column (~ x 30 cm, 6-~m particle size,
6-nm pore size) with a mixture of aqueous 0.1%
trifluoroacetic acid (TFA) and MeCN. The mass spectra of the
purified peptides were measured with ESI (electrospray ion)
MS on a Micromass AutoSpec-T tandem mass spectrometer.
For the synthesis of the cyclized CRF analogs, amino
acid derivatives Fmoc-Glu(OAl~-OH and Fmoc-Lys(Aloc)-OH
(PerSeptive Biosystems GmbH, Hamburg, F.R.G.) were used. The
~ide-chain protected peptides were reacted with PdO[PPh3]4
in ~OAc/N-methylaniline/dichloromethane (v/v; Z:1:40) for
three hours and then cyclized with HOBt/HBTU in DMF and ~IEA
in NMP for eight hours. After removal of the N-terminal Fmoc
group with piperidine in NMP, 4-(1-azi-2,2,2-
trifluoroethyl)benzoic acid was linked to the N-terminus of
the peptide resin with HOBt/~BTU in DMF and DIEA in NMP in
the dark. The peptides were then cleaved from the resin and
purified by preparative RPHPLC. The purified CRF peptides
were subjected to analytical RPHPLC on a Vydac C18 silica
gel column (0.46 x 25 cm, 5-~m particle size, 30-nm pore
~ size) with solvents A (0.1% TFA in water) and B (80% MeCN
in 0.1% TFA in water) at a flow rate of 1 ml/min. The
samples were eluted with 5% B for 5 min. and then with a
linear gradient of 5-95% B in 30 min. (oCRF: ESI MS calcd
CA 02237548 1998-05-13
W097118306 PCT~P96/05011
16
4670.4, found 4669.2, Rt = 25.9 min; astressin: ESI MS calcd
3565.1, found 3563.1, Rt = 24.8 min; 4: ESI MS calcd 3562.1,
found 3561.1, Rt = 30.2 min; 5: ESI MS calcd 3654.2, found
3653.7, Rt = 2g.6 min).
ATB--oyclo~30--33) [125T--Hi813, ~Nle21,38 G11130 Ala32
Lys33]h/rCRF-S13-41) (r~~ _-u~d 6) and ATB-cyclo(30-
33)~N1o2l~38 Glu30 125I-Tyr32, Ly~33]h/rC~F-(13-41)
(Compound 7)
Compounds 6 and 7 were iodinated as described
(29,30). The peptides were partially purified with a Bond
Elut C18 cartridge (Analytichem, Harbor City, CA, USA) and
subsequently with RPHPLC performed on a Vydac C18 silica
gel column (0.46 x 25 cm, 5 ~m particle size, 30 nm pore
size) with solvents A ~0.1% TFA in water) and B (80% MeCN
in 0.1~ TFA in water) at a flow rate of 1 ml/min. The
samples were eluted with 45% B for 5 min. and then with a
linear gradient of 45-95% B in 25 min (6: Rt = 21.9 min;
7:Rt = 20.4 min). A Beckman 171 Radioisotope Detector
equipped with a liquid scintillation flow cell (Beckman,
Fullerton, CA, USA) was used to monitor radioactivity. The
specific activity of the peptides was 82 TBq/mmol.
E~ample 5
Transfection of HER 293 cells
Human embryonic kidney cells 293 (Graham, Smiley,
Russell & Naim, 1977) (supplied by Dr. C. Stevens and G.
Sharma, The Salk Institute, La Jolla) were grown in
Dulbecco's modified eagle medium (GIBC0 BRL, Gaitherburg,
MD, USA, cat. no.: 041-01885M) supplemented with 10% fetal
calf serum (Sigma, St. Louise, Mo, USA, cat. no.: F-7524)
and brought to a final concentration of 4 mM L-glutamine
(GIBC0 BRL, cat. no.: 043-05030), 0.45% glucose. They were
maintained as described (31). The rat CRFR1 gene fragment
CA 02237~48 1998-0~-13
WO97/18306 17 PCT~P96/05011
(1284 bp, BamHI, EcIT26II fragment) was subcloned into the
vector pcDNA3 (Invitrogen, San Diego, Ca, USA). The
recombinant plasmid (pCDNA3-rCRFl) was isolated, and
purified with the Qiagen plasmid preparation system ~Qiagen,
Hilden, Germany). The ligation sites were verified by DNA
sequence analysis.
HEK 293 cells were transfected with pCDNA3-rCRF-Rl
utilizing the calcium/BBS transfection method (32). Sixteen
hours after ~ransfection, the medium was removed and
replaced by selection medium (600 ~g/ml Geneticin in
medium). Cells were grown until confluent and split l:2 with
further selection. Following one to two weeks of growing
under selection conditions, all cells were geneticin-
resistant and grew normally.
~nle 6
Prep~ration of Cruae Membranes
The cells obtained according to Example 5 were
dislodged from the cell culture flasks with a cell scraper
into ice cold PBS buffer. The cells were precipitated at 150
g for lO min. at 4-C, resuspended in l x PBS buffer and
recentrifuged. The supernatant was entirely removed and the
wet weight of the cell pellet was determined. The cells were
suspended in 3 ml/g cells of C~F membrane buffer (50 mM
Tris/Cl, 5 mM MgC12, 2 mM EGTA, 500 ~l Trasylol (FBA, New
York, USA), l mM DTT, pH 7.4~ and treated for lO strokes
(each 2 s) with the medium sized polytron tool at power
level 5. The nuclei were precipitated for 5 min at 600 g in
the cold. The supernatant was carefully removed with a
~ Pasteur pipette and collected on ice. The pellet was
reextracted with the same amount of membrane buffer using
some strokes of the polytron. The nuclei were again
precipitated from this suspension as described. The combined
supernatants were centrifuged at lO,000 g for 15 min to
precipitate the membranes. The pellet was resuspended with 3
CA 02237~48 1998-0~-13
WO97/18306 PCT~P96/05~11
18
ml/g of cells in storage buffer (membrane buffer containing
20% glycerol) with lO strokes of a glass Teflon homogenizer.
A micro BCA assay (Pierce, Rockford, USA) was performed with
2 ~l and 4 ~l of the suspension to estimate the total
protein concentration (about 2.5 ~g/~l). The membranes were
frozen in li~uid nitrogen and stored at -70 C until use.
~xam~le 7
Binding assays with oCRF, astressin ~ compounds 2, 4 and 5
To a tube containing the peptides (c = 0-l ~M) and
lO0,000 or 200,000 cpm, respectively, of [l25I-TyrO]oCRF in
200 ~l incubation buffer (membrane buffer supplemented with
BSA to l mg/ml), lO0 ~l of membrane suspension containing 25
~g of protein (HEK 293 cells) or lO0 ~g of protein (Y79
cells) was added. After incubation (l hr, 23-C), membrane
buffer (l ml) was added. After centrifugation at 14,000 x g
(4~C, 5 min), the pellet was washed twice with l ml of
membrane buffer. Radioactivity was measured with a 1470
WIZARD automatic gamma counter (Berthold, Hannover). Data
analysis was achieved with the non-linear curve fitting
program LIGAND.
Exam~le 8
a) Photoaffinity labeling experiments with 3
Photoaffinity labeling experiments were in principle
performed in the same manner as mentioned above except that
the incubation buffer used was without BSA. To a
concentration series of either oCRF (0, lO0 nM, l ~M, lO
~M) or VIP (l ~M) and 180,000 cpm of 3 per tube, HEK 293
membrane homogenates of either transfected or non-
transfected cells t75 ~g of protein/tube~ were added and
incubated for the indicated time. Before photolysis, the
pellets were washed three times, resuspended in 300 ~l of
CA 02237~48 1998-0~-13
WO 97/18306 PcT/~ G,'~l!;~ll
19
buffer and irradiated at 360 nm for 30 min (4~C, 8 cm
distance from the lamps). After photolysis, 1 ml of buffer
was added and the pellets were spinned out at 15,000 rpm for
5 min. The pellet was resuspended in 15 ~l of dH20 and 15 ~1
- of 2xSDS sample buffer and heated at 100~C for 5 min. The
samples were subjected to electrophoresis in a 7.5% SDS gel
and autoradiography developed on a BAS-IP NP 2040P imaging
plate with a Fujix BAS 2000 scanner (Raytest). Apparent
molecular masses were estimated from gel mobilities relative
to those of commercial markers (SDS-PAGE high range markers,
BioRad). Gel documentation was performed with the programs
TINA (Straubenhardt) and WINCAM (Cybertech).
b) Photoaffinity labeling experiments with compound 7
The photoaffintiy labeling experiments were carried
out like the binding assay except that no BSA was use.
Samples (25 ~g of protein/tube) were irradiated at 360 nm
for 30 min (4-C, 8 cm distance from the lamps) after
incubation with ligand (1 hr, 23-C). In some experiments the
photolabeled receptor was deglycosylated with PNGase (New
England Biolabs, Schwalbach). Samples were then heated
(lOO-C, 5 min) and subjected to SDS PAGE. Autoradiography
was carried out on a BAS-IP NP 2040P imaging plate.
Radioactivity was monitored with a Fujix BAS 2000 scanner
(Raytest, Straubenhardt). Gel documentation was accomplished
with the program TINA (Raytest).
Example 9
cAMP ~ti~ulation
HEK 293 and human Y79 retinoblastoma cells ~American
Type Cell Culture, Rockville) were incubated with different
CRF analogs in the presence of 1 or 5 mM 3-isobutyl-1-
methylxanthine (37-C, 30 min), respectively. The incubation
medium of the Y79 cells contained additionally 1 mg/m- BSA
CA 02237~48 l998-0~-l3
WO97/1~06 PCT~P96/05011
and 0.05 mg/ml ascorbic acid. When compound 2 or the
photoactivatable astressin analogs were used, all
experiments were performed in the dark. After removal of the
medium, cells were lyzed with aqueous 6% trichloroacetic
acid tl00-C, 5 min). The cell lysates were stored at -70-C
until assayed with a RIA ~it (Amersham, Little Chalfont).
Data analysis was achieved with the sigmoidal dose-response
curve fitting programs ALLFIT. Statistical significance was
determined across groups by one-way ANOVA.
Exam~le lO
Purification and characteriz~tion of the 75 kDa Protei~
Cros~-Link
Membrane protein (250 ~g) was labeled with l.l x 107
cpm of 3 (2.82 pmol). One-tenth of the sample was dissolved
in 50% ethanolic formic acid (l00 ~l) and subjected to
RPHPLC using a Vydac C4 silica gel column (0.46 x 25 cm,
5 ~m particle size, 30 nm pore size.) Elution was
accomplished with a mixture of aqueous 0.5% trifluoroacetic
acid and EtOH.
CA 02237548 l998-05-l3
W O 97/}8306 PCT~EP96/O5011
21
t
o a~ tn t--
~ _ ff ~ ~ ~H ~
X 3 o ~ -~ ~ t
o X
n
~D ~ ~ ~ O
c~ o O ~t r~
ff -H ~ ~ '¢
o o ~
o ~ ~
1~tn ~D r~
u o ~ ~ ~ o ~ ~
_~ t ~a
~ o tn
o ~ o t~
-H ~1 ~H ~H ~J
h
~t ~1 o o ~ O .C
el' ~
t _~ _t
._ ~ U
' ~ " o~t~
~ tn
o ~ h
o ~
~ ' C ~ .-t
.'t ~ ~ ._
~ Z Z ,~ 1
a I I _ tr~
O , ~ ~~ --t
~ , O
,~ ~ ~' e ~ d.
o !~
E~ Z ~ t~ ~
_
CA 02237~48 1998-0~-13
WO 97/18306 PCTlEP
22
Re~erences
1) Spiess, J., Rivier, J., Rivier, C. & Vale, W. (1981)
Proc. Natl. Acad. Sci. USA 78, 6517-6521
2) Vale, W., Spiess, J., Rivier, C. & Rivier, J. (1981)
Science 213, 1394-1397.
3) Vita, N., Laurent, P., Lefort, S., Chalon, P., Lelias,
J.-M., Xaghad, M., Le Fur, G., Caput, D. & Ferrara, P.
(1993), FEBS Lett. 335, 1-5.
4) Chen, R., Lewis, K.A., Perrin, M. H. & Vale, W. (1993)
Proc. Natl. Acad. Sci. USA 90, 8967-8971.
5) Perrin, M. H., Donaldson, C. J., Chen, R., Lewis, K. A.
& Vale, W. (1993) Endocrinology 133, 3058-3061.
6) Chang, C.P., Pearse II, R.V., O'Connell, S. & Rosen~eld,
M.G. (1993) Neuron 11, 1187-1195.
7) Lovenberg, T. W., Liaw, C. W., Grigoriadis, D. E.,
Clevenger W., ~h~ l ~rs , D. T., De Souza, E. B. &
Oltersdorf, T. ~1995) Proc. Natl. Acad. Sci. USA 92,
836-840.
8) Perrin, M., Donaldson, C., Chen, R., Blount, A.,
Berggren, T., Bilezikjian, L., Sawchenko, P. & Vale ~.
(1995) Proc. Natl. Acad. Sci. USA 92, 2969-2973.
g) ~;~h; -~to, T., Pearse II, R.V., Lin, C. R. & Rosenfeld
M. G. (1995) Proc. Natl. Acad. Sci. USA 92, 1108-1112.
10) Stenzel, P., Kesterson, R., Yeung, W., Cone, R. D.,
Rittenberg, M. B. & Stenzel-Poore, M. P. (1995)
Molecular Endocrinology 9, 637-645.
CA 02237~48 1998-0~-13
WO97/18306 PCT~P96/050l1
23
11) Vaughan, J., Donaldson, C., Bittencourt, ~., Perrin, M.
~ H., Lewis, K., Sutton, S., Chan, R., Turnbull, A. V.,
Lovejoy, D., Rivier, C., Rivier, J., Sawchenko, P. E. &
Vale, W. (1995) Nature 378, 287-292.
12) Sutton, S. W., Behan, D. P., Lahrichi, S. L., Kaiser,
R., Corrigna, A., Lowry, P., Potter, E., Perrin, M. H.,
Rivier, J. & Vale, W. W. (1995) Endocrinology 136, 1097-
1102.
13) Gulyas, J., Rivier, C., Perrin, M., Koerber, S. C.,
Sutton, S., Corrigan, A., Lahrichi, S. L., Craig, A. G.,
Vale W. & Rivier, J. (1995) Proc. Natl. Acad. Sci. USA
92, 10575-10579.
14) Lovejoy, D.A. (1996) Biochem. Cell. Biol. 74, 1-7.
15) Perrin, M. ~., Sutton, S. W., Berggren, W. T. & Vale, W.
W. (1996) Society for Neuroscience 22, poster 609.9.
16) Zhou, Wei, Rodic, V., Kitanovic, S., Flanagan, C. A.,
Chi, L., Weinstein, H., Maayani, S., Millar, R. P. &
Seal~on S. C. (1995) J. Biol. Chem. 270, 18853-18857.
17) Nishi~l~a~ E., Billestrup, N., Perrin, M., & Vale, W.
(1987) J. Biol. Chem. 2C2, 12893-12896.
18) Rosendale, B. E., Jarrett, D. B. & Robinson, A. G.
(1987) Endocrinology 120, 2357-2366.
lg) Grigoriadis, D. E. & DDe Souza E. B. (1988) J. Biol.
Chem. 263, 10927-10931.
20) Grigoriadis, D. E. & De Souza E. B. ~1989) Endocrinology
125, 1877-1888.
CA 02237~48 1998-0~-13
W097/18306 PCT~P96/OS011
24
21) Schuster, D. I., Probst, W. C., Ehrlich, G. K. & Singh,
G. ~1989) Photochem. Photobiol. ~9, 785-804.
22) Guillory, R. J. (1989) Pharmac. Ther. 41, 1-25.
23) Bayley, H. (1987) in Chemistry of Diazirines, ed. Liu,
M. T. H. (CRC Press, Boca Raton, FL~, Vol. 2, pp. 75-99.
24) Rivier, J., Spiess, J. & Vale W. (1983) Proc. Natl.
Acad. Sci. USA 80, 4851-4855.
25) Rivier, J., Rivier, C. & Vale, W. (1984) Science 224,
889-891.
26) Nassal, M. (1983) Liebigs Ann. Chem. 1510-1523.
27) Olianas, M. C., Lampis, G. & Onali, P. (1995) J.
Neurochem. 64, 402-407
28) ~iihm~nn, A., Kopke, A. K. E., Dautzenberg, F. M. &
Spiess J. (1996) Proc. Natl. Acad. Sci. USA 93, 10609-
10613.
29) Ruckert, Y., Rhode, W. & Fur~ert, J. ~1990) Exp. and
Clin. Endocrinology 96, 129-137.
30) Vale, W., Vaughan, J., Yamamoto, G., Bruhn, T.,
Dgouglas, C., DAlton, D., Rivier, C. & Rivier, J. (1983)
Meth. in Enzymol. 103, 565-577.
31) Graham, F.L., Smiley, J., Russell, W.C. & Naim, R.
(1977) Journal o~ gen. Virology, 36, 59-72.
32) Sambrook, J., Fritsch, E.F. & Maniatis, T. (1989)
Molecular Cloning, (Cold Spring Harbor Laboratory Press:
Cold Spring Harbor) 2nd Ed., chapter 16.33.