Note: Descriptions are shown in the official language in which they were submitted.
CA 02238005 2006-06-28
1
SILICON-CONTAINING BIOCOMPATIBLE MEMBRANES
FIELD OF THE INVENTION
This invention lies in the field of polymer chemistry in which the polymers
produced can be formed into membranes suitable for in vivo use.
BACKGROUND OF THE IIWENTION
Biosensors are small devices that use biological recognition properties for
selective analysis of various analytes or biomolecules. Typically, the sensor
will produce a
signal that is quantitatively related to the concentration of the analyte. To
achieve a
quantitative signal, a recognition molecule or combination of molecules is
often
immobilized at a suitable transducer which converts the biological recognition
event into a
quantitative response.
A variety of biosensors have been developed for use with numerous
analytes. Electroenzymatic biosensors use enzymes to convert a concentration
of analyte
to an electrical signal. Immunological biosensors rely on molecular
recognition of an
analyte by, for example, antibodies. Chemoreceptor biosensors use
chemoreceptor arrays
such as those of the olfactory system or nerve fibers from the antennules of
the blue crab
Callinectes sapidus to detect the presence of amino acids in concentrations as
low as
10 M. For a review of some of the operating principles of biosensors, see
Bergveld, et
al., ADVANCES IN BIOSENSORS, Supplement 1, p. 31-91, Turner ed., and Collison,
et al.,
Anal. Chem. 62:425-437 (1990).
Regardless of the type of biosensor, each must possess certain properties to
function in vivo and provide an adequate signal. First, the elements of the
biosensor must
CA 02238005 1998-05-15
WO 98/13685 PCT/US96/15386
2
be compatible with the tissue to which it is attached and be adequately
shielded from
adjacent tissues such that allergic or toxic effects are not exerted. Further,
the sensor
should be shielded from the environment to control drift in the generated
signal. Finally,
the sensor should accurately measure the analyte in the presence of proteins,
electrolytes
and medications which may interfere.
The prototype biosensor is the amperometric glucose sensor. There are
several reasons for the wide ranging interest in glucose sensors. In the
healthcare arena,
glucose sensors are usefu.l for glucose monitoring of patients with diabetes
mellitus.
Additionally, a working glucose sensor is required for the development of a
closed loop
artificial pancreas with an implanted insulin pump. A commercial interest
focuses on
sensors that can be used to monitor fermentation reactions in the
biotechnology arena.
From a scientific standpoint, interest is driven by the availability of a very
robust enzyme,
glucose oxidase, which can be used to monitor glucose, as well as the desire
to develop
model sensors for a wide variety of analytes.
Any amperometric glucose sensor or any oxido-reductase enzyme that uses
02 as a co-substrate and is designed for subcutaneous or intravenous use
requires both an
outer membrane and an anti-interference membrane. The requirement of two
distinct
membranes is due to the fundamental nature of the sensor as well as the
environment in
which the measurement is made.
A glucose sensor works according to the following chemical reaction
(Equation 1) :
OH OH
HO 0 GOX HH O
+ 0 2 + H 202
OH OH OHO
In this reaction, glucose reacts with oxygen in the presence of glucose
oxidase (GOX) to form gluconolactone and hydrogen peroxide. The gluconolactone
25 further reacts with water to hydrolyze the lactone ring and produce
gluconic acid. The
H202 reacts electrochemically as shown below (Equation 2):
H202 ...........> 02 + 2e +2FI+ (II)
CA 02238005 2006-06-28
3
The current measured by the sensor/potentiostat (+0.5 to +0.7 v oxidation
at Pt black electrode) is due to the two electrons generated by the oxidation
of the H,OZ.
Alternatively, one can measure the decrease in the oxygen by amperometric
measurement
(-0.5 to -1 V reduction at a Pt black electrode).
The stoichiometry of Equation 1 clearly demonstrates some of the problems
with an implantable glucose sensor. If there is excess oxygen for Equation 1,
then the
H202 is stoichiometrically related to the amount of glucose that reacts at the
enzyme. In
this case, the ultimate current is also proportional to the amount of glucose
that reacts with
the enzyme. If there is insufficient oxygen for all of the glucose to react
with the enzyme,
then the current will be proportional to the oxygen concentration, not the
glucose
concentration. For the sensor to be a true glucose sensor, glucose must be the
limiting
reagent, i.e. the OZ concentration must be in excess for all potential glucose
concentrations. For a number of conditions, this requirement is not easily
achieved. For
example, the glucose concentration in the body of a diabetic patient can vary
from 2 to 30
mM (millimoles per liter or 36 to 540 mg/dl), whereas the typical oxygen
concentration in
the tissue is 0.02 to 0.2 mM (see, Fisher, et al., Biomed. Biochem. Acta.
48:965-971
(1989). This ratio in the body means that the sensor would be running in the
Michaelis
Menten limited regime and would be very insensitive to small changes in the
glucose
concentration. This problem has been called the "oxygen deficit problem".
Accordingly,
a method or system must be devised to either increase the O2 in the GOX
membrane,
decrease the glucose concentration, or devise a sensor that does not use O2.
Several approaches to solving the deficit problem have been attempted in the
past. The simplest approach is to make a membrane that is fully 02 permeable,
with no
glucose permeability and mechanically perforate it with a small hole that
allows glucose to
pass. Here the differential permeability is defined by the ratio of the small
hole area to the
total membrane area. Two significant problems with this method are first that
reproducibly making small holes is difficult and second and more serious, the
02
permeability is a strong function of the thickness of the membrane and
thickness is difficult
to control in mass production. Microporous membranes (U.S. Patent No.
4,759,828 to
Young et al. ) have also been tried with limited success.
Another problem with both the perforated membrane approach and the microporous
membrane approach is that the sensor electrodes and the enzyme layer are
exposed to body
CA 02238005 2006-06-28
4
fluids. Body fluids contain proteins that coat the electrodes leading to
decreased sensitivity
of'the sensor and enzymes (proteases) that can digest or degrade the sensor
active enzyme.
Another approach to the oxygen deficit problem is described by Gough
(U.S. Patent No. 4,484,987 ). The approach uses a
combination membrane with discrete domains of a hydrophilic material embedded
in a
hydrophobic membrane. In this case, the membrane is not homogenous and
manufacturing
reproducibility is difficult. Physical properties of the membrane are also
compromised. In
a similar manner, Gough (U.S. Patent No. 4,890,620 )
describes a "two dimensional" system where glucose diffusion is limited to one
dimension
while the oxygen diffusion is from both dimensions. This sensor is extremely
complicated
and manufacturing on a large scale is expected to be difficult.
Several other groups have used a homogenous membrane of a relatively
hydrophobic polyurethane and reported good results. See, for example, Shaw, et
al.,
Biosensors and Bioelectronics, 6:401-406 (1991); Bindra, et al., Anal. Chem.
63:1692
(1991); and Schichiri, et al., Horm. Metab. Resl. Suppl. Ser., 20:17 (1988).
In classical
diffusion experiments with these membranes, however, the glucose diffusion is
extremely
small. It is believed that the ability of these polyurethane layers to allow
glucose diffusion
is due to micro cracks or micro holes in these materials when applied as
membranes.
Still others have developed homogeneous membranes with both hydrophilic
and hydrophobic regions to circumvent the oxygen deficit problem. See, Allen
et al.,
U.S. Patent Nos. 5,284,140 and 5,322,063,
These patents describe acrylic and polyurethane systems,
respectively. Both of the membranes have hydrophilic and hydrophobic moieties
in the
molecule leading to limited control of oxygen and glucose permeabilities.
The key to stable, high sensitivity enzyme biosensors is that the sensor
output must be limited only by the analyte of interest, not by any co-
substrates or
kinetically controlled parameters such as diffusion. In order to maximize the
output
current (Equation 2) of the biosensor, oxygen diffusion should be as large as
possible
while maintaining oxygen excess at the reaction surface. Since the normal
concentration
of 02 in the subcutaneous tissue is quite low, maximization of the 02
diffusion coefficient
is desirable.
The membrane systems described in the literature as cited above attempt
only to circumvent the oxygen deficit problem by reducing the amount of
glucose diffusion
CA 02238005 1998-05-15
WO 98/13685 PCT/US96/15386
to the working electrode of the biosensor. There is a need for the membrane to
have
physical stability and strength, adhesion to the substrate, processibility
(ability to be
synthesized/manufactured in reasonable quantities and at reasonable prices),
biocompatibility, ability to be cut by laser ablation (or some other large
scale processing
5 method), and compatibility with the enzyme as deposited on the sensor. The
present
= invention fulfills these needs and provides other related advantages.
SUMMARY OF THE INVENTION
The present invention provides compositions which are biocompatible and
suitable for coating a biosensor. The compositions are polymers which are
formed into
membranes and can be prepared from:
(a) a diisocyanate,
(b) a hydrophilic polymer which is a hydrophilic diol, a hydrophilic diamine,
or a
combination thereof,
(c) a siloxane polymer having functional groups at the chain termini, and
optionally,
(d) a chain extender.
The membranes prepared from the above components will have a glucose diffusion
coefficient of from about 1 x 1079 cm2/sec to about 200 x 10-9 cm2/sec, a
water pickup of
at least about 25 % and a ratio of Doxygen/Dg,,,eose of about 5 to about 200.
In certain preferred embodiments, the functional groups present in the
siloxane polymer are amino, hydroxyl or carboxylic acid, more preferably amino
or
hydroxyl groups. In other preferred embodiments, the hydrophilic polymer is a
poly(ethylene)glycol which is PEG 200, PEG 400 or PEG 600. In still other
preferred
embodiments the diisocyanate is a isophorone diisocyanate, 1,6-hexamethylene
diisocyanate
or 4,4'-methylenebis(cyclohexyl isocyanate) and the chain extender is an
alkylene diol, an
alkylene diamine, an aminoalkanol or a combinations thereof.
In particularly preferred embodiments, the diisocyanate is 1,6-hexamethylene
diisocyanate, the hydrophilic polymer is PEG 400 or PEG 600 and is present in
an amount
of about 17 to about 32 mol %(relative to all reactants), and the siloxane
polymer is
CA 02238005 2007-04-25
6
aminopropyl polysiloxane having a molecular weight of about 2000 to about 4000
and is
present in an amount of about 17 to about 32 mol% (relative to all reactants).
The present invention further provides an implantable biosensor for
measuring the reaction of an analyte, preferably glucose, and oxygen, the
biosensor having
a biocompatible membrane as described above.
In accordance with an illustrative embodiment of the present invention, there
is provided a biocompatible membrane formed from a reaction mixture of: (a) a
diisocyanate, said diisocyanate comprising about 50 mol % of the reactants in
said mixture;
(b) a hydrophilic polymer which is a member selected from the group consisting
of a
hydrophilic polymer diol, a hydrophilic polymer diamine and combinations
thereof; (c) a
siloxane polymer having functional groups at the chain termini; and (d) a
chain extender,
wherein said chain extender is selected to provide said biocompatible membrane
with
additional physical strength, but does not substantially increase the glucose
permeability of
the membrane, said membrane having a glucose diffusion coefficient of from
about 1 x 10-9
cm'/sec to about 200 x 10-9 cmz/sec, a water pickup of at least 25% and a
ratio of Do,y,en
/DgiL1CO5e of from about 5 to about 200.
In accordance with another illustrative embodiment of the present invention,
there is provided a biocompatible membrane described herein, wherein said
functional
groups are members selected from the group consisting of amino, hydroxyl and
carboxylic
acid.
In accordance with another illustrative embodiment of the present invention,
there is provided a biocompatible membrane described herein, wherein said
hydrophilic
polymer is a poly(ethylene)glycol selected from the group consisting of PEG
200, PEG 400
and PEG 600.
In accordance with another illustrative embodiment of the present invention,
there is provided a biocompatible membrane described herein, wherein said
diisocyanate is
a member selected from the group consisting of isophorone diisocyanate, 1,6-
hexamethylene diisocyanate and 4,4' -methylenebis(cyclohexyl isocyanate).
In accordance with another illustrative embodiment of the present invention,
there is provided a biocompatible membrane described herein, wherein said
chain extender
is selected from the group consisting of an alkylene diol, an alkylene
diamine, an
aminoalkanol and combinations thereof.
CA 02238005 2007-04-25
6a
In accordance with another illustrative embodiment of the present invention,
there is provided a biocompatible membrane described herein, wherein said
diisocyanate is
1,6-hexamethylene diisocyanate, said hydrophilic polymer is selected from the
group
consisting of PEG 400 and PEG 600 and is present in an amount of about 17 to
about 32
mol %, and said siloxane polymer is aminopropyl polysiloxane having a
molecular weight
of about 2000 to about 4000 and is present in an amount of about 17 to about
32 mol %.
In accordance with another illustrative embodiment of the present invention,
there is provided an implantable biosensor for measuring the reaction of an
analyte and
oxygen, said biosensor having a biocompatible membrane described herein.
In accordance with another illustrative embodiment of the present invention,
there is provided an implantable biosensor described herein, wherein the
analyte comprises
glucose.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 illustrates polymerization reactions of a diisocyanate with a
poly(alkylene) glycol or a diamino poly(alkylene oxide) which results in a
polyurethane or
polyurea, respectively.
Figures 2 and 3 provide the structures of certain aliphatic and aromatic
diisocyanates which are useful in forming the membranes described below.
Figure 4 provides the structures of a number of hydrophilic polymers
including poly(alkylene) glycols and diamino poly(alkylene oxides) which are
used in
polymers described below.
Figure 5 provides the structures of certain silicones which are useful in
forming the membranes described below.
Figures 6 and 7 provides synthetic procedures for the preparation of some
silicone polymers used in the present invention.
Figure 8 provides the structures of some chain extenders which are useful in
the present compositions. This include aliphatic diols, diamines and
alkanolamines and
further include some aromatic diols and diamines.
Figure 9 is an infrared spectrum of a polyurea composition prepared in
accordance with the present invention.
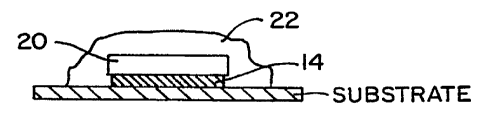
Figure 10 illustrates portions of a glucose sensor which can be coated with a
membrane of the present invention. Figure l0A is a schematic top view of a
glucose
sensor having electrodes covered with a polymer composition of the invention.
Figure lOB
is a sectional side view of a working electrode of the sensor which is covered
with layers
of an enzyme and a polymer composition of the invention.
Figure 11 is a graph showing sensor output in various glucose solutions as a
function of time.
CA 02238005 1998-05-15
~
WO 98/13685 PCTIUS96/15386
7
DETAILED DESCRIPTION OF THE INVENTION
The following abbreviations are used herein: dl, deciliter; DEG, diethylene
glycol; DMF, dimethyl formamide; PBS, phosphate buffered saline; THF,
tetrahydrofuran;
DI, deionized; PEG, poly(ethylene)glycol; HDI, 1,6-hexane diisocyanate (1,6-
hexamethylene diisocyanate); TMDI, 2,2,4,4-tetramethyl-1,6-hexane diisocyanate
and
2,4,4-trimethyl-1,6-hexane diisocyanate; CHDI, 1,4-cyclohexane diisocyanate;
BDI, 1,4-
cyclohexane bis(methylene isocyanate); H6XDI, 1,3-cyclohexane bis(methylene
isocyanate)
or hexahydro metaxylene diisocyanate; IPDI, isophorone diisocyanate; and
Ht2MDI, 4,4'-
dicyclohexylmethane diisocyanate.
As used herein, the term "polyurethane/polyurea" refers to a polymer
containing urethane linkages, urea linkages or combinations thereof.
Typically, such
polymers are formed by combining diisocyanates with alcohols and/or amines.
For
example, combining isophorone diisocyanate with PEG 600 and aminopropyl
polysiloxane
under polymerizing conditions provides a polyurethane/polyurea composition
having both
urethane (carbamate) linkages and urea linkages.
Biocompatible Membranes
As noted above, requirements for a glucose sensor intended for in vivo use
is that the supply of oxygen in the vicinity of the sensing element not be
depleted.
Additionally, the glucose should diffuse to the sensor at a controlled rate.
This does not
mean that a glucose sensor membrane need have an extremely high permeability
to
oxygen. Instead, the membrane should control the relative rates of diffusion
of oxygen
and glucose to the sensor so that the local concentration of oxygen is not
depleted.
Additionally, the glucose sensors intended for in vivo use must also be
biocompatible with
the body, and they must be able to function in an environment in which acids
are present
as well as proteins which can interfere with a sensor. Thus, the enzyme(s)
used in such
sensors must be protected from degradation or denaturation, while the elements
of such
sensors must be protected from molecules which would foul the sensors or their
accuracy
will decrease over time.
In one aspect, the present invention provides a biocompatible membrane
formed from a reaction mixture of:
CA 02238005 1998-05-15
WO 98/13685 PCT/US96/15386
8
(a) a diisocyanate, said diisocyanate comprising about 50 mol % of the
reactants in
said mixture;
(b) a hydrophilic polymer which is a member selected from the group consisting
of
a hydrophilic diol, a hydrophilic diamine and combinations thereof; and
(c) a silicone polymer having functional groups at the chain termini.
Optionally, the reaction mixture will contain a chain extender. The membrane
formed
using the polymerized mixture of the above components will have a glucose
diffusion
coefficient of from about 1 to about 200 x 10-9 cmZ/sec, a water pickup of at
least 25 %
and a ratio of Doxygcn/Dg,,,.se of from about 5 to about 200.
Depending on the selection of components, the polymer used in forming the
biocompatible membranes will be a polyurea, a polyurethane or a
polyurethane/polyurea
combination. Figure 1 illustrates some of the polymerization reactions which
result in the
compositions of the present invention.
Membrane components
The homogeneous membranes of the invention are prepared from
biologically acceptable polymers whose hydrophobic/hydrophilic balance can be
varied
over a wide range to control the ratio of the diffusion coefficient of oxygen
to that
of glucose, and to match this ratio to the design requirements of
electrochemical glucose
sensors intended for in vivo use. Such membranes can be prepared by
conventional
methods by the polymerization of monomers and polymers noted above. The
resulting
polymers are soluble in solvents such as acetone or ethanol and may be formed
as a
membrane from solution by dip, spray or spin coating.
The diisocyanates which are useful in this aspect of the invention are those
which are typically those which are used in the preparation of biocompatible
polyurethanes. Such diisocyanates are described in detail in Szycher, SEMINAR
ON
ADVANCES IN MEDICAL GRADE POLYURETHANES, Technomic Publishing, (1995) and
include both aromatic and aliphatic diisocyanates (see Figures 2 and 3).
Examples of
suitable aromatic diisocyanates include toluene diisocyanate, 4,4'-
diphenylmethane
diisocyanate, 3,3'-dimethyl-4,4'-biphenyl diisocyanate, naphthalene
diisocyanate and
paraphenylene diisocyanate. Suitable aliphatic diisocyanates include, for
example, 1,6-
hexamethylene diisocyanate (HDI), trinnethylhexamethylene diisocyanate (TMDI),
trans-
1,4-cyclohexane diisocyanate (CHDI), 1,4-cyclohexane bis(methylene isocyanate)
(BDI),
CA 02238005 1998-05-15
WO 98/13685 PCT/US96/15386 ~
9
1,3-cyclohexane bis(methylene isocyanate) (H6XDI), isophorone diisocyanate
(IPDI) and
4,4'-methylenebis(cyclohexyl isocyanate) (H12MDI). In preferred embodiments,
the
diisocyanate is isophorone diisocyanate, 1,6-hexamethylene diisocyanate, or
4,4'-
methylenebis(cyclohexyl isocyanate). A number of these diisocyanates are
available from
commercial sources such as Aldrich Chemical Company (Milwaukee, Wisconsin,
USA) or
can be readily prepared by standard synthetic methods using literature
procedures.
The quantity of diisocyanate used in the reaction mixture for the present
compositions is typically about 50 mol % relative to the combination of the
remaining
reactants. More particularly, the quantity of diisocyanate employed in the
preparation of
the present compositions will be sufficient to provide at least about 100% of
the -NCO
groups necessary to react with the hydroxyl or amino groups of the remaining
reactants.
For example, a polymer which is prepared using x moles of diisocyanate, will
use a moles
of a hydrophilic polymer (diol, diamine or combination), b moles of a silicone
polymer
having functionalized termini, and c moles of a chain extender, such that x =
a+ b + c,
with the understanding that c can be zero.
A second reactant used in the preparation of the biocompatible membranes
described herein is a hydrophilic polymer. The hydrophilic polymer can be a
hydrophilic
diol, a hydrophilic diamine or a combination thereof. The hydrophilic diol can
be a
poly(alkylene)glycol, a polyester-based polyol, or a polycarbonate polyol (see
Figure 4).
As used herein, the term "poly(alkylene)glycol" refers to polymers of lower
alkylene
glycols such as poly(ethylene)glycol, poly(propylene)glycol and
polytetramethylene ether
glycol (PTMEG). The term "polyester-based polyol" refers to a polymer as
depicted in
Figure 4 in which the R group is a lower alkylene group such as ethylene, 1,3-
propylene,
1,2-propylene, 1,4-butylene, 2,2-dimethyl-1,3-propylene, and the like. One of
skill in the
art will also understand that the diester portion of the polymer can also vary
from the six-
carbon diacid shown. For example, while Figure 4 illustrates an adipic acid
component,
the present invention also contemplates the use of succinic acid esters,
glutaric acid esters
and the like. The term "polycarbonate polyol" refers those polymers having
hydroxyl
functionality at the chain termini and ether and carbonate functionality
within the polymer
chain (see Figure 4). The alkyl portion of the polymer will typically be
composed of C2
to C4 aliphatic radicals, or in some embodiments, longer chain aliphatic
radicals,
cycloaliphatic radicals or aromatic radicals. The term "hydrophilic diamines"
refers to any
of the above hydrophilic diols in which the terminal hydroxyl groups have been
replaced
CA 02238005 1998-05-15
WO 98/13685 PCT/US96115386 ~
by reactive amine groups or in which the terminal hydroxyl groups have been
derivatized
to produce an extended chain having terminal amine groups. For example, a
preferred
hydrophilic diamine is a "diamino poly(oxyalkylene)" which is
poly(alkylene)glycol in
which the terminal hydroxyl groups are replaced with amino groups. The term
"diamino
5 poly(oxyalkylene" also refers to poly(alkylene)glycols which have aminoalkyl
ether groups
at the chain termini. One example of a suitable diamino poly(oxyalkylene) is
poly(propylene glycol)bis(2-aminopropyl ether). A number of the above polymers
can be
obtained from Aldrich Chemical Company. Alternatively, literature methods can
be
employed for their synthesis.
10 The amount of hydrophilic polymer which is used in the present
compositions will typically be about 10% to about 80% by mole relative to the
diisocyanate which is used. Preferably, the amount is from about 20% to about
60% by
mole relative to the diisocyanate. When lower amounts of hydrophilic polymer
are used,
it is preferable to include a chain extender (see below).
Silicone polymers which are useful in the present invention are typically
linear, have excellent oxygen permeability and essentially no glucose
permeability.
Preferably, the silicone polymer is a polydimethylsiloxane having two reactive
functional
groups (i. e, a functionality of 2). The functional groups can be, for
example, hydroxyl
groups, amino groups or carboxylic acid groups, but are preferably hydroxyl or
amino
groups (see Figure 5). In some embodiments, combinations of silicone polymers
can be
used in which a first portion comprises hydroxyl groups and a second portion
comprises
amino groups. Preferably, the functional groups are positioned at the chain
termini of the
silicone polymer. A number of suitable silicone polymers are commercially
available from
such sources as Dow Chemical Company (Midland, Michigan, USA) and General
Electric
Company (Silicones Division, Schenectady, New York, USA). Still others can be
prepared by general synthetic methods as illustrated in Figures 6 and 7,
beginning with
commercially available siloxanes (United Chemical Technologies, Bristol,
Pennsylvania,
USA). For use in the present invention, the silicone polymers will preferably
be those
having a molecular weight of from about 400 to about 10,000, more preferably
those
having a molecular weight of from about 2000 to about 4000. The amount of
silicone
polymer which is incorporated into the reaction mixture will depend on the
desired
characteristics of the resulting polymer from which the biocompatible membrane
are
formed. For those compositions in which a lower glucose penetration is
desired, a larger
CA 02238005 1998-05-15
WO 98/13685 PCT/US96/15386
11
amount of silicone polymer can be employed. Alternatively, for compositions in
which a
higher glucose penetration is desired, smaller amounts of silicone polymer can
be
employed. Typically, for a glucose sensor, the amount of siloxane polymer will
be from
10% to 90% by mole relative to the diisocyanate. Preferably, the amount is
from about
20% to 60% by mole relative to the diisocyanate.
In one group of embodiments, the reaction mixture for the preparation of
biocompatible membranes will also contain a chain extender which is an
aliphatic or
aromatic diol, an aliphatic or aromatic diamine, alkanolamine, or combinations
thereof (see
Figure 8). Examples of suitable aliphatic chain extenders include ethylene
glycol,
propylene glycol, 1,4-butanediol, 1,6-hexanediol, ethanolamine, ethylene
diamine, butane
diamine, 1,4-cyclohexanedimethanol. Aromatic chain extenders include, for
example,
para-di(2-hydroxyethoxy)benzene, meta-di(2-hydroxyethoxy)benzene, Ethacure 100
(a
mixture of two isomers of 2,4-diamino-3,5-diethyltoluene), Ethacure 300 (2,4-
diamino-
3,5-di(methylthio)toluene), 3,3'-dichloro-4,4'diaminodiphenylmethane, Polacure
740 M
(trimethylene glycol bis(para-aminobenzoate)ester), and methylenedianiline.
Incorporation
of one or more of the above chain extenders typically provides the resulting
biocompatible
membrane with additional physical strength, but does not substantially
increase the glucose
permeability of the polymer. Preferably, a chain extender is used when lower
(i. e. , 10-40
mol %) amounts of hydrophilic polymers are used. In particularly preferred
compositions,
the chain extender is diethylene glycol which is present in from about 40% to
60% by
mole relative to the diisocyanate.
= Membrane preparation
Polymerization of the above reactants can be carried out in bulk or in a
solvent system. Use of a catalyst is preferred, though not required. Suitable
catalysts
include dibutyltin bis(2-ethylhexanoate), dibutyltin diacetate, triethylamine
and
combinations thereof. Preferably dibutyltin bis(2-ethylhexanoate is used as
the catalyst.
Bulk polymerization is typically carried out at an initial temperature of
about 25 C
(ambient temperature) to about 50 C, in order to insure adequate mixing of the
reactants.
Upon mixing of the reactants, an exotherm is typically observed, with the
temperature
rising to about 90-120 C. After the initial exotherm, the reaction flask can
be heated at
from 75 C to 125 C, with 90 C to 100 C being a preferred temperature range.
Heating
is usually carried out for one to two hours. Solution polymerization can be
carried out in
CA 02238005 1998-05-15
WO 98/13685 PCT/US96/15386
12
a similar manner. Solvents which are suitable for solution polymerization
include
dimethylformamide, dimethyl sulfoxide, dimethylacetamide, halogenated solvents
such as
1,2,3-trichloropropane, and ketones such as 4-methyl-2-pentanone. Preferably,
THF is
used as the solvent. When polymerization is carried out in a solvent, heating
of the
reaction mixture is typically carried out for three to four hours.
Polymers prepared by bulk polymerization are typically dissolved in
dimethylformamide and precipitated from water. Polymers prepared in solvents
that are
not miscible with water can be isolated by vacuum stripping of the solvent.
These
polymers are then dissolved in dimethylformamide and precipitated from water.
After
thoroughly washing with water, the polymers can be dried in vacuo at about 50
C to
constant weight.
Preparation of the membranes can be completed by dissolving the dried
polymer in a suitable solvent and cast a film onto a glass plate. The
selection of a suitable
solvent for casting will typically depend on the particular polymer as well as
the volatility
of the solvent. Preferably, the solvent is THF, CHC13, CH2C1z, DMF or
combinations
thereof. More preferably, the solvent is THF or DMF/CHZC12 (2/98 volume %).
After
the solvent is removed from the films, the resulting membranes are hydrated
fully, their
thicknesses measured and water pickup is determined. Membranes which are
useful in the
present invention will typically have a water pickup of about 20 to about
100%, preferably
30 to about 90%, and more preferably 40 to about 80%, by weight.
Oxygen and glucose diffusion coefficients can also be determined for the
membranes of the present invention. Methods for determining diffusion
coefficients are
known to those of skill in the art, and examples are provided below. The
biocompatible
membranes described herein will preferably have a oxygen diffusion coefficient
(Doxygen) of
about 0.1 X 10-6 cm2/sec to about 2.0 x 10-6 em2/sec and a glucose diffusion
coefficient
(Dalõ~5) of about i x 10 9 cm2/sec to about 500 X 10-9 cm2/sec. More
preferably, the
glucose diffusion coefficient is about 10 x 10-9 cm2/sec to about 200 X 10-9
cm2/sec.
From the above description, it will be apparent to one of skill in the art
that
the discovery underlying the present invention is the use of silicon-
containing polymers,
such as siloxanes, in the formation of biocompatible membranes. The silicon-
containing
polymers are used in conjunction with (covalently attached to) hydrophilic
polymers for the
preparation of membranes in which the movement of analytes and reactive
species (e.g.,
oxygen and glucose) can be controlled by varying the amounts of each
component. The
CA 02238005 2006-06-28
13
membranes produced from these components are homogeneous and are useful for
coating a
number of biosensors and devices designed for subcutaneous implantation.
Membrane-Coated Biosensors
Glucose sensors which utilize, for example, glucose oxidase to effect a
reaction of glucose and oxygen are known in the art, and are within the skill
in the art to
fabricate. See, for example, U.S. Patent Nos. 5,165,407, 4,890,620, 5,390,671
and
5,391,250. The present
invention depends not on the configuration of the biosensor, but rather on the
use of the
inventive membranes to cover or encapsulate the sensor elements.
In particular, the biocompatible membranes of the present invention are
useful with a variety of biosensors for which it is advantageous to control
diffusion of the
analytes/reactants to the sensing elements. Various such biosensors are well
known in the art. For example, other sensors for monitoring glucose
concentration of
diabetics are described in Shichiri, et al., :"In Vivo Characteristics of
Needle-Type
Glucose Sensor-Measurements of Subcutaneous Glucose Concentrations in Human
Volunteers," Horm. Metab. Res., Suppl. Ser. 20:17-20 (1988); Bruckel, et al.,:
"In Vivo
Measurement of Subcutaneous Glucose Concentrations with an Enzymatic Glucose
Sensor
and a Wick Method," Klin. Wochenschr. 67:491-495 (1989); and Pickup, et al.,:
"In Vivo
Molecular Sensing in Diabetes Mellitus: An Implantable Glucose Sensor with
Direct
Electron Transfer," Diabetologia 32:213-217 (1989).
The following examples are offered by way of illustration and are not meant to
limit the scope of the invention.
EXAMPLES
The materials used in the examples were obtained from the following
sources: isophorone diisocyanate
General Methods
(a) Membrane Preparation
CA 02238005 1998-05-15
WO 98/13685 PCT/US96/15386 ~
14
Membranes were prepared by casting films from a suitable solvent onto
glass plates using a parallel arm Gardner knife (Gardner Labs). The solvent
chosen will
depend on the particular chemical structure of the polymer. Typically, THF or
DMF/CHZCl2 (2/98 vol %) are used although chloroform is also useful as it is
readily
volatile. After removal of the solvent, the dried membranes were hydrated with
deionized
water for 30-60 minutes. The membranes were then removed and transferred to a
Mylar
support sheet. Wet film thicknesses were measured with a micrometer before
removal
from the support. Films were also cast from solution onto filtration membranes
of known
thickness. For the measurements provided below, it was assumed that the
membrane
material completely filled the pores of the filtration membranes and that the
thickness of
the filtration media is the thickness of the membrane.
(b) Diffusion constants
Diffusion constants were measured in a standard permeability cell (Crown
Glass Co., Inc.) maintained at 37 C, using Fick's relationship:
J = - D dC/dx
where J is total flux, D is the diffusion constant of the analyte of interest,
and dC/dx is the
concentration gradient across the membrane. The diffusion coefficient is a
physical
property of both the analyte of interest and the material in which it is
diffusing. Thus, D
is a property of the system under evaluation.
Oxygen diffusion constants (Do) were determined by securing the membrane
with two rubber gaskets between the two halves of a diffusion cell maintained
at 37 C,
and clamping the two halves together. Each side of the cell was filled with
phosphate
buffered saline (PBS, 0.15 M NaCl, 0.05 M phosphate, pH 7.4). One side was
saturated
with HPLC grade helium while the other side was saturated with room air
(assumed 20%
02). A calibrated oxygen electrode (Microelectrodes, Inc.) was placed in each
cell. The
oxygen electrode outputs were connected to a microcomputer-controlled data
acquisition
system and the oxygen concentration from both cells was recorded as a function
of time.
The curves of concentration vs. time were plotted and the diffusion
coefficients were calculated using the entire curve. Curve fits generally had
correlation coefficients (RZ) of
greater than 0.95.
Glucose diffusion constants (D,,) were determined as above except that one
half of the cell was filled with phosphate buffered saline containing 400
mg/dl of glucose.
The concentration of glucose in each half of the cell was measured at 5 minute
intervals
CA 02238005 1998-05-15
WO 98/13685 PCT/US96/15386
until equilibrium was achieved using a YSI glucose analyzer. As above, the
curves of
concentration vs. time were plotted and the diffusion coefficient was
calculated.
(c) Water pickup
Water pickup was determined gravimetrically at room temperature on films
5 which were less than 0.5 mm thick. After evaporation of the casting solvent,
films were
dried to constant weight at 50 C in vacuo, weighed, immersed in deionized
water for 24
hours, removed and blotted with filter paper, and weighed. Percent water
pickup was
determined from the formula:
% Pickup = (W,y - Wd)/Wd x 100
10 where W , is the weight of the swollen film and Wd is the weight of the dry
film.
EXAMPLE 1
This example illustrates a bulk polymerization method of polymer formation
carried out with isophorone diisocyanate, PEG 600, diethylene glycol and
aminopropyl
terminated polydimethyl siloxane.
15 Isophorone diisocyanate (4.44 g, 20 mmol, 100 mol%) was dried over
molecular sieves and transferred to a 100 mL round bottom flask fitted with a
nitrogen
purge line and a reflux condenser. PEG 600 (2.40 g, 4.0 mmol, 20 mol%),
diethylene
glycol (1.06 g, 10 mmol, 50 mol%) and aminopropyl terminated
polydimethylsiloxane
(15 g, 6.0 mmol, 30 mol%, based on a 2500 average molecular weight) were added
to the
flask. Heating was initiated using a heating mantle until a temperature of 50
C was
obtained. Dibutyltin bis(2-ethylhexanoate) (15 mg) was added and the
temperature
increased to about 95 C. The solution was continuously stirred at a
temperature of 65 C
for a period of 4 hr during which time the mixture became increasingly
viscous. The
resulting polymer was dissolved in 50 mL of hot THF and cooled. After cooling,
the
solution was poured into 5 L of stirring DI water. The precipitated polymer
was torn into
small pieces and dried at 50 C until a constant weight was achieved.
CA 02238005 1998-05-15
WO 98/13685 PCT/US96/15386
16
EXAMPLE 2
This example illustrates a solution polymerization method using 1,6-
hexamethylene diisocyanate, PEG 200 and aminopropyl terminated
polydimethylsiloxane.
Dried 1,6-hexamethylene diisocyanate (1.34 g, 8 mmol, 100 mol%) was 5 added to
a 100 mL 3-neck flask containing 20 mL of dry THF. PEG 200 (0.8 g, 4.0
mrnol, 50 mol %) was added with stirring followed by addition of aminopropyl
terminated
polydimethylsiloxane (10 g, 4.0 mmol, 50 mol%). The resulting solution was
warmed to
50 C and dibutyltin bis(2-ethylhexanoate) (about 15 mg) was added. After an
initial
temperature rise to 83 C, the mixture was warmed and held at 70 C for 12 hr,
during
which time the mixture had become very viscous. After cooling, the mixture was
poured
into 3 L of rapidly stirring DI water. The precipitated polymer was collected,
washed
with DI water (3X), torn into small pieces and dried at 50 C until a constant
weight was
obtained.
A membrane was prepared as described above. An infrared spectrum of the
product was obtained and is reproduced in Figure 9, exhibiting the expected
absorbance
bands (cm i).
EX-A,MPLE 3
This example provides the formulations and properties of representative
membranes.
Table 1 provides the five formulations for representative polymers which
were then formed into membranes. The polymers were prepared by solution
polymerization.
CA 02238005 1998-05-15
WO 98/13685 PCT/US96/15386
17
TABLE 1
Representative Polymer Formulations
Polymer Diisocyanate Poly(alkylene Aliphatic diol Siloxane
glycol)
1 1,6-Hexamethylene PEG 600 (20%) DEG (60%) Aminopropyl
(20%)
2 Isophorone PEG 600 (20%) DEG (50%) Aminopropyl
(30%)
3 1,6-Hexamethylene PEG 600 (50%) None Aminopropyl
(50%)
4 1,6-Hexamethylene PEG 400 (40%) None Aminopropyl
(60 %)
5 1,6-Hexamethylene PEG 600 (60%) None Aminopropyl
(40%)
Table 2 provides certain physical and chemical properties of the polymers
provided above.
TABLE 2
Physical Properties of Representative Polymers
Polymer Water Pickup (%) Doy- ~enm2 D lueose
( x 10-6 c/sec) ( x 10- cm2/sec)
1 28.5 1.21 18.5
2 31.3 0.57 55.7
3 44 1.50 105
4 57 1.22 13.5
5 71 1.45 155
CA 02238005 2006-06-28
18
EXAMPLE 4
This example illustrates the evaluation of a membrane-coated biosensor
constructed according to the present invention.
A membrane prepared from the polymer identified as 3 above was found to
have excellent mechanical properties as well as appropriate oxygen and glucose
diffusivities. The membrane was evaluated using a prototype glucose sensor
illustrated in
Figure 10A. According to Figure 10A, a sensor 10 was constructed having a
reference
electrode 12, a working electrode 14, and a counter electrode 16 deposited on
a polymeric
sheet 19. A series of bonding pads 18 complete the sensor 10. As shown in
Figure IOB,
the working electrode 14 was covered with a layer 20 of the enzyme glucose
oxidase and
the entire electrode array was coated with a layer 22 of the polymer 3 by dip
coating two
times from a 5 wt% solution of the polymer in THF. The sensor was connected to
a
commercial potentiostat (BAS Instruments, not shown) and operated with a
potential of
+0.6 volts between the working electrode and the reference electrode.
Glucose response is shown in Figure 11. As seen in Figure 11, the
response of the electrode system is linear over the physiological glucose
range, suggesting
relative independence of local 02 concentration. All of the other polymers
tested showed
similar behavior to the polymer identified as 3 and are acceptable as
membranes for
biosensor applications.
The above description is illustrative and not restrictive. Many variations of
the invention will become apparent to those of skill in the art upon review of
this
disclosure. Merely by way of example a variety of solvents, membrane formation
methods, and other materials may be used without departing from the scope of
the
invention. The scope of the invention should, therefore, be determined not
with reference
to the above description, but instead should be determined with reference to
the appended
claims along with their full scope of equivalents.