Note: Descriptions are shown in the official language in which they were submitted.
CA 02239089 1998-05-28
WO 97/46268 PCT/US97/09468
-1-
IMPLANTABLE MEDICAL DEVICE
Technical Field
This invention relates generally to medical devices and, particularly, to
medical devices that are implantable either partly or completely into a human
or
veterinary patient.
Background of the Invention
It has become common to treat a variety of medical conditions by
introducing an implantable medical device partly or completely into the
esophagus,
trachea, colon, biliary tract, urinary tract, vascular system or other
location within a
human or veterinary patient. For example, many treatments of the vascular
system
entail the introduction of a device such as a stent, a catheter, a balloon, a
wire guide,
a cannula, or the like. However, when such a device is introduced into and
manipulated through the vascular system, the blood vessel walls can be
disturbed or
injured. Clot formation or thrombosis often results at the injured site,
causing
stenosis or occlusion of the blood vessel. Moreover, if the medical device is
left
within the patient for an extended period of time, a thrombus often forms on
the
device itself, again causing stenosis or occlusion. As a result, the patient
is placed
at risk of a variety of complications, including heart attack, pulmonary
embolism, and
stroke. Thus, the use of such a medical device can entail the risk of
precisely the
problems that its use was intended to ameiiorate.
Another problem associated with implantable medical devices and, more
particularly, to partly implanted medical devices such as catheters
percutaneously
introduced into the vascular system of a patient for long-term hemodialysis or
drug
infusion is the risk of infection. This risk is also present with
hyperalimentation
(intravenous feeding) catheters which are percutaneously introduced into the
patient.
K
The urinary tract is another system of the patient in which an urethral
catheter such
as a weli-known Foley catheter is introduced into the patient's bladder via
the urethra
for the drainage of urine.
A recent attempt to reduce the risk of infection has been to coat the outer
surface of the device with a bioactive material and/or pharmacologically
active
CA 02239089 2005-11-09
-2-
ingredient such as an antibiotic. Various coatings including antibiotics have
been
utilized, but have been found to disperse or dissipate from the coating in a
relatively
short period of time. Although effective in short-term implantation, such
coatings are
typically ineffective for extended duration placement such as with
hemodialysis, drug
infusion, or urinary tract catheters, which can be implanted in the patient
for two to
three years at a time.
The applicant has tested a partly implantable device containing an inner,
elongated tube with an elongated outer sheath coaxially positioned around the
inner
tube, and with an intermediate space established between them. A mixture of
drugs
having different diffusion rates through the sheath and tube were positioned
such as by
injection, into the intermediate space. It was found that the higher diffusion
rate drug
quickly diffused through the inner tube and outer sheath without the benefit
of the lower
diffusion rate drug therewith for concomitantly combating the risk of
infection.
Summary of the Invention
Certain exemplary embodiments can provide a medical device for implantation
or partial implantation within a patient, the device comprising two tubes or
layers
comprised of a base material, one tube or layer within the other, and a
plurality of
pharmacologically active ingredients contained within the device, at least
part of one of
the active ingredients being in a region between the two tubes wherein another
of the
active ingredients diffuses at a slower rate than that of said one of the
active
ingredients, and wherein the said one and said other active ingredients are
distributed
within the device in such a way, and at least one of the tubes are formed such
that the
active ingredients diffuse to or from an outer surface of the device
approximately
simultaneously.
The present invention is intended to provide a device in which at least two
treatment materials reach the external or outer surfaces simultaneously. The
implantable or partly implantable medical device includes a first elongated
member or
tube and a second elongated member or tube positioned adjacent to or within
the first
member or tube. A pharmacologically active ingredient is positioned between
and in
communication with the first and second elongated members. At least one of the
first
and second elongated members is permeable to the pharmacologically active
CA 02239089 2005-11-09
2a
ingredient for diffusing the pharmacologically active ingredient therethrough.
A
bioactive material preferably with a base material such as of at least one of
the first and
second elongated members is also provided, and the selected member(s) is
permeable
to the bioactive material for diffusing the bioactive material therefrom or
therethrough.
When the pharmacologically active ingredient material includes a mixture of
ingredients, the bioactive material can advantageously include one of the
slower
diffusion rate ingredients of the mixture, which is included in the base
material of the
CA 02239089 1998-05-28
WO 97/46268 PCT/US97/09468
-3-
selected member(s). This slower diffusion rate ingredient is then
advantageously
more readily accessible to the tissue surrounding the device for concomitant
treatment with the other higher diffusion rate ingredient(s) of the mixture.
By way of example, the pharmacoiogically active ingredient
advantageously and preferably includes a mixture of minocycline and rifampin,
which
is positioned between and in communication with the first and second elongated
members of the implantable medical device. Minocycline has a lower diffusion
rate
than that of rifampin, and as a result, is included as the bioactive material
in the base
material in either one or both of the first and second elongated members. The
higher
diffusion rate rifampin permeates through the permeable base material of the
elongated members and is diffused with the lower diffusion rate minocycline
for
concomitant treatment of tissue surrounding the outer surface of the catheter.
The thickness of the base material is advantageously selected to, in effect,
slow down the diffusion of the higher diffusion rate ingredient so that the
higher and
lower diffusion rate ingredients are diffused from the medical device
concomitantly
for treatment of the tissues surrounding the device.
In one aspect of the invention, the implantable medical device includes a
catheter having an outer elongated member with a passage extending
longitudinally
therein and an inner elongated member whose outer surface forms a passage
extending longitudinally therein. The inner member is positioned in the
passage of
and surrounded by the outer elongated member. A pharmacologically active
ingredient such as minocycline, rifampin, or a mixture thereof is positioned
in the
passage of the outer elongated member. As a result, the pharmacologically
active
ingredient is positioned between and in communication with the outer and inner
elongated members. At least one of the outer and inner members, and preferably
the
outer member, is permeable to the pharmacologically active ingredient.
Applicant's improvement comprises including or mixing a bioactive material
in the base material in at least one of the outer and inner members, which is
permeable for diffusing therefrom or therethrough the bioactive material
and/or the
pharmacologically active ingredient from the intermediate space. The bioactive
CA 02239089 1998-05-28
WO 97/46268 PCT/US97/09468
-4-
material can advantageously include the slower diffusion rate ingredient such
as
minocycline of a pharmacologically active ingredient mixture.
In a preferred embodiment of the invention, the base material of the outer
and inner members is silicone having a durometer in a range of 30 to 90 on the
Shore
A Hardness Scale. Preferably, the base material silicone has a durometer of 65
and
is formed from a powder mixture with 7% minocycline by weight. A
pharmacologically active ingredient such as a 50:50 mixture by weight of
rifampin
and minocycline is positioned between the outer and inner coaxial members.
In another aspect of this invention, the 50:50 mixture by weight of
rifampin and minocycline is included or mixed with a base material of an
intermediately positioned member or layer to a concentration of 7% by weight.
The
base material of the intermediately positioned member or layer can also
include
silicone, which is readily positioned or formed with the outer and inner
coaxially
positioned members. Advantageously, the outer member is in contact with the
tissue
of the patient of which fluid from the patient permeates through the catheter
for
diffusing the pharmacologically active ingredient and/or the bioactive
material from
the catheter.
In the preferred embodiment of the invention, an inner elongated member
tube comprises a base material of silicone having 7% by weight minocycline
included
therein. An intermediate member or layer is positioned or formed over the
inner
member tube. The intermediate member or layer preferably includes a base
material
such as silicone and a 7% by weight mixture of 50:50 rifampin and minocycline.
The outer elongated member tube aiso preferably comprises a base material of,
for
example, silicone and 7% by weight minocycline, which is positioned over the
intermediate layer and inner member tubes. The overall wall thickness of the
catheter is approximately one-quarter of that of the outside diameter of the
catheter.
As a result, the passage or lumen of the catheter is approximately one-half of
its
outside diameter. The wall thickness of the inner and outer elongated member
tubes
or layers is approximately the same, whereas the thickness of the intermediate
layer
is approximately two and one-half times that of either the inner or outer
elongated
CA 02239089 1998-05-28
WO 97/46268 PCT/US97/09468
-5-
member tubes or layers. The outside diameter of the preferred catheter falls
within
a range of 3 French to 18 French (.039" to .236").
Brief Descriation of the Drawina
FIG. 1 depicts a cross-sectioned end view of a preferred illustrative
embodiment of the implantable medical device of the present invention;
FIG. 2 depicts a cross-sectioned end view of another preferred embodiment
of the implantable medical device of the present invention; and
FIG. 3 depicts a partial, sectioned side view of the implantable medical
device of FIG. 2.
Detailed Description
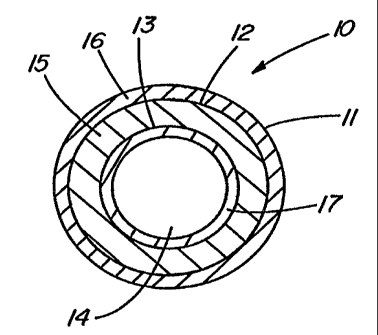
FIG. 1 depicts a cross-sectioned end view of a preferred illustrative
embodiment of implantable medical device 10 such as a catheter having an
outer,
elongated member tube 11 with passage 12 extending longitudinally therein.
Alternatively, outer elongated member tube can be simply a first layer 11 of
material.
Positioned preferably concentrically in passage 12 is inner elongated member
tube
13 with passage 14 extending longitudinally therein. Again, alternatively, the
inner
elongated member tube can be simply a second layer 13 of material adjacent
first
layer 11. A tube or layer 15 of a pharmacologically active ingredient is
positioned
between and in communication with the outer and inner elongated member tubes
or
layers 11 and 13. The pharmacologically active ingredient is any drug,
medicament,
or agent or mixtures thereof, for treating infections or other physiological
afflictions
encountered with or due to the placement of such implantable medical devices.
Preferabiy, this pharmacologically active ingredient includes one or more
drugs,
agents, or medicaments for concomitantly minimizing or treating the infection
or
affliction. Preferably, this pharmacologically active ingredient would include
a 50:50
mixture by weight of minocycline and rifampin. Minocycline has a lower
diffusion
rate than rifampin and, as a result, is also preferably mixed in the base
silicone
material 16 of outer member tube 11 as or part of the bioactive material. The
minocycline of the pharmacologically active ingredient is also preferably
included in
the base silicone material 17 of inner elongated member tube 13. Minocycline
7%
by weight in a powdered form is mixed with a powdered form of silicone and a
CA 02239089 1998-05-28
WO 97/46268 PCT/US97/09468
-6-
solvent to form a liquid that is extruded into outer and inner member tubes 11
and
13. Inner elongated member tube 13 is positioned in passage 12 of outer
elongated
member tube 11. A desired length of the member tubes is cut to form the
overall
length of the catheter. One end of the catheter tubes is bonded together with
a
medical grade silicone adhesive. By way of example, outer elongated member
tube
is approximately .125" in diameter with a wall thickness of approximately
.007".
Inner elongated member tube 13 has an inner diameter of approximately .062"
with
a wall thickness of .007". The pharmacologically active ingredient comprising
a
50:50 mixture by weight of rifampin and minocycline is positioned by being for
example, poured, or injected into the intermediate space between the inner and
outer
elongated member tubes 13 and 11. As a result, the wall or layer thickness of
the
pharmacologically active ingredient mixture is approxiniately .017". The
overall wall
thickness of the catheter is approximately .031 ".
Base silicone material 16 and 17 of the outer and inner members is a
silicone material having a durometer in a range of 30 to 90 on the Shore A
Hardness
Scale. Preferably, the minocycline and silicone mixture also has an overall
durometer
of 65 on the Shore A Hardness Scale. Base silicone material 16 and 17 is
commercially available from the NU-SIL Corporation of Carpinteria, CA.
FIG. 2 depicts a second preferred embodiment of implanted medical device
10 such as a catheter with outer elongated member tube 11 and inner elongated
member tube 13 positioned in passage 12 of outer elongated member tube 1 1. An
intermediate layer or tube 18 of a base material such as silicone is
positioned
between outer and inner elongated member tubes 11 and 13 and in the
intermediate
space therebetween. Base material 16, 17 and 19 of outer, inner and
intermediate
layer tubes 11, 13 and 18 is a medical grade silicone material from the NU-SIL
Corporation. The 7% minocycline is included in base material 16 and 17 of
outer
and inner member tubes 11 and 13. Base silicone material 19 includes a
bioactive
material such as a 50:50 mixture by weight of rifampin and minocycline. This
bioactive material mixture is 7% by weight of the base silicone material 19.
The wall
thickness of the inner, outer and intermediate layer tubes is as previously
described
CA 02239089 1998-05-28
WO 97/46268 PCT/US97/09468
-7-
to permit the higher diffusion rate of rifampin to mix and permeate through
the layers
and out the inner and inner surfaces of the catheter concomitantly.
It is intended that the term bioactive material includes any material that is
molecularly interactive with the fluids, cells, proteins or tissues of an
animal including
humans to augment the diagnosis, treatment or prevention of any physiologic or
pathologic condition. It is further intended that this term includes
therapeutic and
diagnostic agents such as, for example, drugs, vaccines, hormones, steroids,
proteins, previously described agents, complexing agents, salts, chemical
compounds, polymers, and the like.
The base silicone material is a powdered material that is mixed with the
bioactive material and/or the pharmacologically active ingredient in a well-
known
solvent. The mixture is then extruded at low temperatures with the solvent
evaporating therefrom as the silicone material cures. This low temperature
silicone
is utilized so as not to evaporate the pharmacologically active ingredient
and/or the
bioactive material.
FIG. 3 depicts a partial, sectioned side view of medical device 10 of
FIG. 2. Outer and inner elongated member tubes are likewise shown with
intermediate tube or layer 18 positioned therebetween and in communication
therewith. Passage 14 of the catheter is approximately one-half the outside
diameter
of catheter 10. As previously discussed, the overall wall thickness of
catheter 10
is approximately .062". The outside diameter of the catheter is again .025".
Intermediate tube or layer 18 is approximately two and one-half times the wall
thickness of inner and outer elongated member tubes 13 and 11. Inner elongated
member tube 13 is first extruded, with intermediate tube or layer 18 extruded,
namely positioned, thereover. Outer elongated member tube 11 is then extruded
over the intermediate and inner elongated member tubes. The intermediate layer
is
thus positioned between the inner and outer tubes.
With continued reference to FIGs. 1-3, implantable medical device 10 of
the present invention comprises at least one bioactive material mixed with
base
material 16, 17 and/or 19 of outer, inner and/or intermediate layers 13, 11
and 18.
In an alternative embodiment, at least one bioactive material can also be
posited on
CA 02239089 1998-05-28
WO 97/46268 PCT/US97/09468
-8-
outer surface 20 of intermediate layer 18. The other surfaces of the outer,
inner, and
intermediate layers or the layers themselves can either contain no bioactive
material
or one or more different bioactive materials. In this manner, one or more
bioactive
materials or drugs may be delivered, for example, with a vascular stent or
catheter,
to the blood stream from the lumen surface of the stent, and a different
treatment
can be delivered on the vessel surface of the stent. A vast range of drugs,
medicaments and materials can be employed as the bioactive material in one or
more
base material layers 16, 17 and 19, so long as the selected material can
survive
exposure to the placement or extrusion process or to a vacuum drawn during
vapor
deposition or plasma deposition. Particularly useful in the practice of the
present
invention are materials which prevent or ameliorate abrupt closure and
restenosis of
blood vessels previously opened by stenting surgery or other procedures.
Thrombolytics (which dissolve, break up or disperse thrombi) and
antithrombogenics
(which interfere with or prevent the formation of thrombi) are especially
useful
bioactive materials when the implantable medical device 10 is a vascular
stent.
Particularly preferred thrombolytics are urokinase, streptokinase, and the
tissue
plasminogen activators. Particularly preferred antithrombogenics are heparin,
hirudin,
and the antiplatelets.
Urokinase is a plasminogen activating enzyme typically obtained from
human kidney cell cultures. Urokinase catalyzes the conversion of plasminogen
into
the fibrinolytic plasmin, which breaks down fibrin thrombi.
Heparin is a mucopolysaccharide anticoagulant typically obtained from
porcine intestinal mucosa or bovine lung. Heparin acts as a thrombin inhibitor
by
greatiy enhancing the effects of the blood's endogenous antithrombin lll.
Thrombin,
a potent enzyme in the coagulation cascade, is key in catalyzing the formation
of
fibrin. Therefore, by inhibiting thrombin, heparin inhibits the formation of
fibrin
thrombi. Alternatively, heparin can be covalently bound to the outer layer of
implantable medical device 10. Thus, heparin would form the outermost layer of
implantable medical device 10 and would not be readily degraded enzymatically,
and
would remain active as a thrombin inhibitor.
CA 02239089 1998-05-28
WO 97/46268 PCT/iJS97/09468
-9-
Of course, bioactive materials having other functions can also be
successfully delivered by the device 10 of the present invention. For example,
an
antiproliferative agent such as methotrexate will inhibit over-proliferation
of smooth
muscle cells and thus inhibit restenosis of the dilated segment of the blood
vessel.
The antiproliferative is desirably supplied for this purpose over a period of
about four
to six months. Additionally, localized delivery of an antiproliferative agent
is also
useful for the treatment of a variety of malignant conditions characterized by
highly
vascular growth. In such cases, the device 10 of the present invention could
be
placed in the arterial supply of the tumor to provide a means of delivering a
relatively
high dose of the antiproliferative agent directly to the tumor.
A vasodilator such as a calcium channel blocker or a nitrate will suppress
vasospasm, which is common following angioplasty procedures. Vasospasm occurs
as a response to injury of a blood vessel, and the tendency toward vasospasm
decreases as the vessel heals. Accordingly, the vasodilator is desirably
supplied over
a period of about two to three weeks. Of course, trauma from angioplasty is
not the
only vessel injury which can cause vasospasm, and the device 10 can be
introduced
into vessels other than the coronary arteries, such as the aorta, carotid
arteries, renal
arteries, iliac arteries or peripheral arteries for the prevention of
vasospasm in them.
A variety of other bioactive materials are particularly suitable for use when
the structure 12 is configured as something other than a coronary stent. For
example, an anti-cancer chemotherapeutic agent can be delivered by the device
10
to a localized tumor. More particularly, the device 10 can be placed in an
artery
supplying blood to the tumor or elsewhere to deliver a relatively high and
prolonged
dose of the agent directly to the tumor, while limiting systemic exposure and
toxicity.
The agent may be a curative, a pre-operative debulker reducing the size of the
tumor,
or a palliative which eases the symptoms of the disease. It should be noted
that the
bioactive material in the present invention is delivered across the device 10,
and not
by passage from an outside source through any lumen defined in the device 10,
such
as through a catheter employed for conventional chemotherapy. The bioactive
material of the present invention can of course, be released from the device
10 into
any lumen defined in the device, or to tissue in contact with the device and
that the
CA 02239089 1998-05-28
WO 97/46268 PCT/US97/09468
-10-
lumen may carry some other agent to be delivered through it. For example,
tamoxifen citrate, Taxol or derivatives thereof, Proscar , Hytrin , or
Eulexin can
-
be applied to the tissue-exposed surface of the device for delivery to a tumor
located,
for example, in breast tissue or the prostate.
Dopamine or a dopamine agonist such as bromocriptine mesylate or
pergolide mesylate is useful for the treatment of neurological disorders such
as
Parkinson's disease. The device 10 could be placed in the vascular supply of
the
thalamic substantia nigra for this purpose, or elsewhere, localizing treatment
in the
thalamus.
A wide range of other bioactive materials can be delivered by the device
10. Accordingly, it is preferred that the bioactive material contained in or
posited on
the layer 18 includes at least one of heparin, covalent heparin, or another
thrombin
inhibitor, hirudin, hirulog, argatroban, D-phenylalanyl-L-poly-L-arginyl
chloromethyl
ketone, or another antithrombogenic agent, or mixtures thereof; urokinase,
streptokinase, a tissue plasminogen activator, or another thrombolytic agent,
or
mixtures thereof; a fibrinolytic agent; a vasospasm inhibitor; a calcium
channel
blocker, a nitrate, nitric oxide, a nitric oxide promoter or another
vasodilator; Hytrin
or other antihypertensive agents; an antimicrobial agent or antibiotic;
aspirin,
ticlopidine, a glycoprotein llb/Ilia inhibitor or another inhibitor of surface
glycoprotein
receptors, or another antiplatelet agent; colchicine or another antimitotic,
or another
microtubule inhibitor, dimethyl sulfoxide (DMSO), a retinoid or another
antisecretory
agent; cytochalasin or another actin inhibitor; or a remodeling inhibitor;
deoxyribonucleic acid, an antisense nucleotide or another agent for molecular
genetic
intervention; methotrexate or another antimetabolite or antiproliferative
agent;
tamoxifen citrate, Taxol or the derivatives thereof, or other anti-cancer
chemotherapeutic agents; dexamethasone, dexamethasone sodium phosphate,
dexamethasone acetate or another dexamethasone derivative, or another anti-
inflammatory steroid or non-steroidal antiinflammatory agent; cyclosporin or
another
immunosuppressive agent; trapidal (a PDGF antagonist), angiopeptin (a growth
hormone antagonist), angiogenin, a growth factor or an anti-growth factor
antibody,
or another growth factor antagonist; dopamine, bromocriptine mesylate,
pergolide
CA 02239089 2005-11-09
-11-
mesylate or another dopamine agonist; 60Co (5.3 year half life), 192lr (73.8
days), 32P
(14.3 days), "'In (68 hours), 90Y (64 hours), ss'"Tc (6 hours) or another
radiotherapeutic agent; iodine-containing compounds, barium-containing
compounds,
gold, tantalum, platinum, tungsten or another heavy metal functioning as a
radiopaque agent; a peptide, a protein, an enzyme, an extracellular matrix
component, a cellular component or another biologic agent; captopril,
enalapril or
another angiotensin converting enzyme (ACE) inhibitor; ascorbic acid, alpha
tocopherol, superoxide dismutase, deferoxamine, a 21-aminosteroid (lasaroid)
or
another free radical scavenger, iron chelator or antioxidant; a 14C-, 3H-,
1311-, 32P- or
36S-radiolabelled form or other radiolabelled form of any of the foregoing;
estrogen
or another sex hormone; AZT or other antipolymerases; acyclovir, famciclovir,
rimantadine hydrochloride, ganciclovir sodium, Norvi,MCrixivan,Mor other
antiviral
agents; 5-aminolevulinic acid, meta-tetrahydroxyphenylchlorin, hexadecafluoro
zinc
phthalocyanine, tetramethyl hematoporphyrin, rhodamine 123 or other
photodynamic
therapy agents; an IgG2 Kappa antibody against Pseudomonas aeruginosa exotoxin
A and reactive with A431 epidermoid carcinoma cells, monoclonal antibody
against
the noradrenergic enzyme dopamine beta-hydroxylase conjugated to saporin or
other
antibody targeted therapy agents; gene therapy agents; and enalapril and other
prodrugs; Proscar , HytrinO or other agents for treating benign prostatic
hyperplasia
(BHP) or a mixture of any of these; and various forms of small intestine
submucosa
(SIS).
It is to be understood, however, that the above-described implantable
medical device is merely an illustrative embodiment of the principles of this
invention,
and that other devices and methods for using them may be devised by those
skilled
in the art, without departing from the spirit and scope of the invention. It
is to be
understood that the invention is directed to embodiments both comprising and
consisting of the disclosed parts. It is contemplated that only parts of the
device can
include the bioactive material and/or the pharmacologically active ingredient.
Furthermore, different parts of the device can include different bioactive
materials.
It is also contemplated that different sides or regions of the same part of
the device
can include different bioactive materials or layers.
CA 02239089 1998-05-28
WO 97/46268 PCT/US97/09468
-12-
The intermediate layers 15 and 18 can comprise a single or one treatment
material with a higher diffusion rate, with or without a base added thereto,
whilst
another treatment material with a slower diffusion rate is nearer to the outer
surface
of the device, to enable the treatment materials to achieve the objective of
reaching
the "outer surfaces" substantially simultaneously. The other treatment
material can
be located on the tubes or in the tubes or form parts of the tubes, and
because of
their particular positioning, they will reach the outer surfaces substantially
simultaneously with the said one treatment material.
Alternatively, the intermediate layers can each comprise a plurality of the
treatment materials. The latter can be mixed together preferably with a base
material, but the treatment materials can also be formed in layers with the
higher
diffusion rate treatment material being more concentrated at the center of the
intermediate layer.
This concentration effect can be achieved in a variety of ways, but it is
preferred that the inner-most layer be of the one material and the innermost
layer be
of the other material, and the layers in between be of appropriate mixed
percentages
with the percentages of the other material increasing in proportion as the
outermost
layer is approached.
These layers may or may not contain base material.
Any of the above devices may include outer tubes containing base material
and only siower diffusion rate material(s), but it is to be understood that
the tubes
can contain higher and slower diffusion rate materials with the slower rate
materials
being more in evidence nearer the said outer surfaces.
Channels can be formed in the device through either the intermediate layer
or through the inner and outer tubes, or both.
The channels can be permanently filled with treatment material or they can
be used to supply a further treatment material each as liquid to a particular
part of
a patient when the device is positioned within the patient.
Alternatively, a balloon can be attached to the outer surface of the outer
tube and inflated by further treatment material.