Note: Descriptions are shown in the official language in which they were submitted.
CA 02240237 1998-06-10
Culture mediu~
~he present invention relate~ to a culture
medium for the detection o~ streptococcus
mutans in saliva or in plaque ~ith the
o charac~eristics of the generic part of patent
claim 1 a~ well a~ to an a~alysis ~et with the
characteri~ticR of the generic p~rt of patent
claim 18.
In the field of p~eventive denti~try it i~
known to ex~ine in the saliva or the plaque of
the teeth the pre~ence of streptococcu~ mutan~
and to determin~ eheir quantitati~e
concentration. In order to carry o~t ~uch a
quali~tive a~d quantitative detection a given
amount of ~aliva or an ~mount of plaque having
been ~cratched off the teeth iB applied onto a
culture medium, where~y in this case the known
mitis sali~ariu~ agar iB often used.
For the e~tabli~hment of a diagnosis,
particularly for estimating the risk of
cariogenicity, it is nece~ary to determine the
concentration of lactobacill~ in the ~aliva
and/or in the plaque, whereby in thi~ ca~e a
CA 02240237 1998-06-10
culture medium i~ al~o u~ed. A sample of the
~aliva, re~pectively of the plaque, i~ applied
onto thi~ culture medium based on a rogo~a agar
a~ thi~ i~ al~o de~cribed a~ove for the
detection o~ the ~treptoco~u~ mutan~, ~o that
the i~cuba~ed culture medium can be visually
evaluated on a quantitati~e and qualitative
basi~ after a corre~ponding ~ime of incubation.
The culture ~edium used herefore and ba~ed on a
o ueual ~ogOsa agar ha~ the following
componition:
Agar a~ar 19 g
Tryptone 10 g
Yea~t extract 5 g
Glu~o~e 20 g
Pota~ium hydrogenpho~p~ate6 g
Ammonium hydrogencitrat~ 2 g
Manganese~ ulphate l-hydr~te0.1 g
Iron-II-~ulph~te 7-hydr~te 0.03 g
Tween ~0 1 g
For the manufacture of the ready-to-apply
rogo~a culture medium the afore indicated
compo~ent~ are added to 1 l of water.
For the establi~hment of an impecca~le
CA 02240237 1998-06-10
dia~no~i~, par~ichl~ly for defining concrete
step~ to a~oid the risk of cariogenicity, it i~
moreover nece~ary to mea~ure the buffering
capacity o~ the ~aliva. In the analyei~ ~et
being available nowaday~ this buffering
capacity i~ mea~ured by ~prinkling a tect strip
which is ~oaked with an indicator ~ith ~aliva,
whe~eby the nece~a~y color change o~ the test
st~ip i~ ~hen vi~ally de~er~ined after an
exposure time of about two minutes. Hereby the
test strip used herefore, however, only allow~
a coar~e mea~uring of the buffering capacity
being defined a~ pH-value, so that the
measuring of the buffering capacity i6 not ~afe
and depend~ o~ the skill o~ the corre~ponding
u~er.
The pre~ent in~ention ha~ t~e obje~t of
di~posin~ a ~ulture medium of the indicated
sort which make~ po~ible a ~afer statement
about the concentration of ~treptococcus
mutan~.
Thi~ object i~ realized by mean~ of a culture
medium with the ~ignlficant characteristic~ of
patent claim 1.
CA 02240237 1998-06-10
The inventive culture medium of the detection
of streptococcus mutans in saliva and/or in
plaque provides t~at the inventive culture
medium contain-~ at least one ~ugar in addition
S to one agar, where~y ~he ~a~ ratio of the agar
to the at lea~t one sugar varies between l:o.
and 1:12.. In other ~ord~, the thu~ inventive
culture ~edium differs from the known culture
medium ~n ~hat way tha~ the inve~tive culture
lo m~dium compri~e-~ at least one sugar in the
afore indicated ma~ ~atio.
It was ~urprisingly notcd that the inventive
culture medium show~ a high ~electivity
con~erning ehe ~treptococcus mutanB, whereby
thie high ~electivity for ~treptococcus mutan~
i~ referred to the ~act that, in contra~t to
u~ual culture mediums being available in trade,
~he inventive culture medium additionally
comprises a sugar. Compared to u~ual culture
mediumfi thi~ impro~ed ~ele~ivity o~ the
inventive culture medium is expre~ed in that
the inventive culture medium ~hows a
~ignificantly higher germ yield of
~treptococcu~ mutan~, which was con~irmed by
135 Qaliva isolates o~ 55 children. This
supplemen~ary sugar additive doe~ not impede
CA 02240237 1998-06-10
the preparing of a ready-to-apply culture
medium from the inventive culture medium,
~hereby for the ~imple and rapid preparation
the inventive dry culture medium is only taken
up into the re~lred a~ount of water.
The inventive culture medium can also be
manuf~tured in a particularly ~i~ple ~anner
since here~ore it i~ only neces~ary to mix a
~uitable agar with the required ~ugar amount,
whereby the mixing of the agar with the ~ugar
can be carried out not only during the
manufacture o~ a ready-to-apply culture medium,
me~ning duri~g the taking up of the agar and of
~he ~u~ar into water, but al~o by mixing the
dry agar with the at lea~t one sugar.
A ~ir~t e~h~A~ent of the inventive culture
medium provide~ that hereby the ma~ ratio of
the agar to the at least one -~ugar lie~ between
1:3.8~ and 1:4 2~. ~ereb~ it wa~ noted that
thiY preferred embodiment of the inventive
culture ~edium wa~ taken up into water in a
particularly ~imple and rapid manner, ~o that a
ready-to-apply culture medium can be
manufactured corre~pondingly ~imple and rapid.
CA 02240237 1998-06-10
A ~urther embodiment of the inventive culture
medium ~howing an extremely high selecti~ity
for the detection o$ ~treptococcu~ mutans
provides that hereby the ma~s ratio of the agar
~o ~he at lea~t one ~ugar varie~ between 1:5.1
and 1:10. Althou~h t~ia embodiment of the
inventive aulture ~edium has an extremely hig~
Bugar concentrAtion it could ~e aurpriai~gly
noted that the ~treptococcu~ mutans extracted
o from hu~an ~ali~a or pla~ue grew perfectly and
~e~ectively on a culture medium being mixed
~ith su~ar in such a ~ay.
The term agar i~ ~ed above and ~ubsequently,
whereby thi~ term means particularly the
mucilage ext~acted and dried ~rom red seaweed
a~ well a~ highly molecular galac~o~ides,
pa~ticularly ~ B of galaktoae-~ulphate
e~ters, a~ far as ~pecia~ agar~ are
~ub~equently not mentioned.
In respec~ to the sugar contained in the
inventive culture medium it i~ to be noted that
thi~ sugar can be a monoaaccharide and/~r a
mono~accharide derivative. Ho~ever the
in~entive culture medium preferably compri~ea
a~ sugar a di~accharide, particularly aucroae
CA 02240237 1998-06-10
and/or ~ eucro~e dexivative.
Depending on the corre~ponding field of
application, the inventive culture ~ediu~
consists in the simplest cafie only of the u~ual
agar and the a~ore described sugar in the
mentioned mass ratios. If such an embodiment i,Q
then applied by skilled epecial staff a~, for
example, in correspondin~ ho~pitals, it i8 only
necessary to take up the inventive culture
~edium into a coxre~po~;n~ amoun~ of w~ter, so
that then corre~ponding dishes, for example
Petri dishes, can be filled with this ready~to-
apply, gel-like cu~ture ~edium. In order to
~a~ely and reproducibly distingui~h the
streptococc~ m~ea~s ~ulti~a~ed in the ~aliva
or in the plaque and the additionally and
eventually cultivated streptô~occu~ mutan~,
particularly the s~eptococcus para~ang~is and
~o the streptococcus sallvarius, aft¢r incubating
with the saliva, re~pe~tively with the plaque,
and after the ~ubsequen~ lncubation, it is
recl_. n~d to carry out a dilution ~erie~
belng u~ual for ~icrobiological e~Am~n~tions.
Hereby the saliva, respectively the plaq~e, is
dilu~ed by a phy~iological salt eolution to a
ten~h ~o thousandth part of its original
CA 02240237 1998-06-10
concentration, which cause~ an evident and
effective BUppres~ion of th¢ gro~th of the
a~ore mentioned strep~ococcus and of
lactobacillu~, actinomycete~, ~taphylococcus or
gram-negative bacterla. Thi~ again leads to a
particularly safe and reproduci~le detection
and vicual quantification of the ~treptococcus
mutans.
A particularly simple and safe detection of
strep~ococcus mutans which can renounce on the
afore mentioned dilution ~eries without showing
a fal~ification of the re~ult provide~ that
hereby an inventive culture ~edium iR used
w~ich co~pri~e~ ~t le~t o~e peptide antibiotic
co~plex in addition to t~e agar and to the at
least one sugar, wh~reby the concentration of
this peptide antibiotic complex varies between
0.001 ~ by weight and 0.0005 % by weight
relative to the total weight of the agar and
the added ~ugar.
The afore de~cribed e~bodiment of the inventive
culture medium particularly comprises as
peptide antibiotic complex the bacitracin being
ava~lable i~ t~de. In order to m~nufacture
from thl~ embodiment of the inventive culture
CA 02240237 1998-06-10
me~ium a ready-~o-apply culture medium it is
only nece~ary to ta~e up the agar containing
the at lea~t one sugar and the bacitracin into
the ~equired amo~n~ o~ water. It was
S particularly noted that such a rea~y-to-apply,
inventivç c~lt~re medi~ then effectively
suppre~e~ the growth of a mixed ~lora,
particula~ly the ~th of ~ep~ococcu~
salivariuR and/or streptococcu~ para~angui~,
lo without reducing the yield of strepeococcus
~utan~ in a ~tati~tically ~ignificant ~a~ner.
In re~pect to such ~aliva ~mp~e~ or plaque
samples ~ic~ ~ompri~e~ al~o other
~treptococcus type~ in addition to the
~treptoco~cu~ mutan~, thic e~h~;ment of the
inventive culture ~ediu~ makee it thu~ po~sible
to easily and effective~y distingui~h ~he
streptococcus mutans and the other
streptococcus or ~he afore mentioned other
microor~ani~m~.
A p~rticularly ~uitable agar being particularly
u~ed in the inventive culture medium is the
~l~eady initially mentioned mitiB ~alivariu9
agar.
Hereby a typical miti~ ~alivarius a~ar aj it iY
CA 02240237 1998-06-10
available in trade and cold by the firm DIFCO,
Augeburg, ha~ ehe following compo~ition
Bacto trypto~e 8 g to 12 g,
particularly 10 g
Proteose peptone no. 3 3 g to 7 g,
particularly 5 g
Proteose peptone 3 g to 7 g,
particularly 5 g
lo ~acto dextro~e 0.5 g to 1.5 g,
particularly 1 g
~acto ~ucrose 30 g to 60 g,
particularly 50 g
Pota~ium hydrogenpho~phate 3 g to S g,
particularly 4 g
~ye, particularly O.oS g to o.09 g,
trypan blue particularly 0.075 g
Dye, parti~ularly 0.0006 g to o.ooo1 g,
bacto cryetal violet particularly 0.008 g
~acto agar 13 g to 17 g,
particularly 15 g
For manufacturing a ready-to-apply culture
medium the afore mentioned components are
~eirred into 1 1 of water.
In order to facilitate particularly the
CA 02240237 1998-06-10
~pplication of the inventi~e culture medium a
further embodiment o~ the inventive culture
medium provides that the culture medium iB
applied onto one ~ide of an inert ~upport (dip-
~lide; solid c~rrie~). For ~he detection of
~treptococcu~ mu~an~ in ~aliva it i~ only
nece~ary to remove the s~erile packed culture
medium being appl ied onto an inert ~upport,
pareicularly onto a plastic ~upport, from the
lo s~erile packing and to add the saliva sample or
the plaque ~ample to be ex~ined to thi~
culture medium, ~ that the corre~ponding
st~eptococcu~ ~utan~ ~oncen~ration can be
detected after a corre~ponding ~terile
incubation.
A~ already repeatedly mentioned above, a ready-
to-apply cult~re medium can be eaoily and
rapidly manufactured from the inventive culture
medium, ~he~eby ~uch a ready-to-apply culture
medium preferably ha~ gel-like con~i~tency. ,
whereby water can be added to the culture
medium in order to produce the gel-like
con~i~tency. For generating the gel-like
consi~tency t~e dry inventive culture ~edium
comprising ~he agar, preferably the miti~
salivariu~ agar, ~hich contain~ the at lea,~t
CA 02240237 1998-06-10
12
on~ ~ugar and, if necee~ry, the peptide
anti~iotio ~o~plex, pre~erably the bacitracin,
ie only taken up into water, whereby ~he
de~ired gel-like con~i~tency can then be
ad~usted ~y varying ehe amount of the added
water.
Shch a gel-like development of the ready-to-
apply culture ~ediu~ preferably co~pri~es
between 70 g to 150 g of the agar, peptide
antibiotic the miti~ ~alivariuB agar, between
42 g and 1.8000 g of the at lea~t one BUgar and
a~ much water as i~ required, prefe~ably
between 888 g and 100 g, for ~an~acturiny the
gel-like con~i~tency. If, in addition to the
gel-like and ready-to-apply culture medium, the
afore ~e~cribed peptide antibiotic complex,
preferably the bacitra~in, i~ to be added, the
amount of this peptide antibio~ic complex,
preferably the amount of the bacitracin, then
variee ~etween ~.~ mg and 12 mg, e~ch xelati~e
to the total amount of the at lea~t one ~u~ar
and of the agar, paxticularly the mitis
~alivarius agar.
In order to fa~ ate the determination
preci~ne~ of the cultivated ~treptococcu~
CA 02240237 1998-06-10
mutan~ in re~pect to the afore de~cri~ed and
~ub~equently described e~odiments of ~he
inve~tive culture medium in which the culture
medium iB applied on~o an iner~ ~upport, a
further development o~ the inventive culture
~edium provides that hereby the inert ~upport
is dy~d with dark color, preferably dyed black,
or that it con~ist~ o~ a corresponding dark
plastic ~terial.
Another ~m~o~iment of the inventive cultu~e
me~iu~ being at the ~ame time B~ table for the
de~ection of streptococ~us mueans and of
lac~obacillu~ or yeast fungus provides that the
in~enti~e ~ulture ~edium whi~h comprises the at
lea~t one ~4gar ~nd, if ~eceu~ary, the peptide
antibiotic co~plex in addition ~o the agar,
particularly the mitis ~alivarius agar, i~
applied onto one side of the i~ert support,
preferably a plastic ~upport, wherea~ on the
other side of the inert support a further
culture ~ediu~ for the detection of
laceobacillus in the saliva and/or in the
plaque or a further culture medium for the
deteation of yea~t ~ungus in the ~aliva and/or
~he plaque i~ depo~ited. Hereby such an
embodiment of the in~entive ~ulture medium
CA 02240237 1998-06-10
allows that the concentration ~f ~treptococ~us
mutan~ and la~tobacillus or of str~ptococcus
mutan~ and ye~et fungus ~n the ~aliva and/or in
the plaque bei~g required for the Carie~
prophylaxi~ can ~e concurrently determined with
one sing~e te~t, 50 that ~uch a development of
the inventive cultuze ~edium can ~e applied
al~o particularly for the rapid and safe
diagno~i~ in the denti~t'~ practice.
In the afore de~cribed development of the
in~entive cult~re medium by which lactobacillu.
can be determined conc~rrent~y with
~treptococcus mutan~ a culture medium ~a~ed on
a rogo~a agar i~ 6elected a~ further culture
medium, whereby ~uch a culture mediu~ then ha~
a chemical conetruction a~ initially described.
In a particularly advantageou~ development o~
the ~fore de~ribed embodi~ent of the
inventive culture mediu~ the further culture
medium comprises at least one sugar in addition
to the u~ual rogoca agar or to ~he cul~ure
medium for the detection of yea~t fung~,
whereby ~hi~ a~ leaet one ~ugar preferably i8
gluco~e, ~ucro~e and/or a gluco~e derivative,
re~pectively a ~ucrose derivative. Particularly
CA 02240237 1998-06-10
if the further culture medium contalns ~he
rogo~a agar or the culture medium for ~he
detection of yea~t fungu~ and the at lea~t one
~ugar in a ma~s ratio of between l:0.~ and 1:6,
S then su~h modi~ied further culture medium ~how~
a h~gh selecti~ity and yield of lactobacillus,
recpectively of yea~t fung~, ~o that ~hi~
e~bodiment allow~ a preci~e and reproducible,
qualitative ~nd qu~ntitA~ive de~ermination o~
lactobacillu~, re~pectively yea~t fung~, in
the ~al iva a~d/or i~ the plaq~e.
If in the a~ore de~cribed embodimen~ one ~ide
of the inert ~pport i~ coated with the culture
medium for the de~ection of the streptococcuq
mutans and the other ~ide of the inert ~upport
i~ coated with the culture mediu~ for the
detection of the yea~t funguB in the ~aliva
and/or in the plaque, thi~ e~bodiment of the
inventive culture medium then allow~
particularly for children b~ing under ~our
~ears old, preferably howev~r for the a~e of
le~ ~han three year~, a particularly easy and
concurrent deter~ination of an eventual
pre~ence of yeajt fungu~ in the ~aliva or in
the pla~ue of the~e children in addition to
~treptococcu~ mutans. Especially in the ca~e of
CA 02240237 l998-06-lO
16
children infected ma~ively with carie~,
partic~larly with the nur~ing bo~tle ~yndrome,
it wa~ noted that yea~t fungu~ are important
indlaator germ~. Hereby a culture medium for
the detec~ion of yea~e fungu~ with the
following compo~ition is preferably u~ed:
Chromopeptone 8 g to 12 g
Glucose 15 g to 25 g
lo Chloramphenicol 0.3 g to 0.7 g
Dye mixture (chro~ogen mix) 1.5 g to 2.S g
Agar 10 g to 20 g
The agar 601d under the trade name "BL
CHROMagar Candida~ ~Manufacturer: ~ecton
~ickin~on 6~b~, Heidel~erg~ i~ particularly
~uitable for the detection of yea~t ~ungu~.
In ord~r to manufactu~e ~ re~dy-to-apply
culture medium for the detection of yeast
fungu~ the afore indi~ated component,s are taken
up into one liter of deionized water, so that
gel-like culture medium i~ produced to which,
if de~ired, further amounts of ~ugar of the
described concentration range~ can be added.
In order to facilitate the application of the
CA 02240237 1998-06-10
inert ~uppor~ ~oated on one side with the
inventi~e ~ulture medium and coated on the
other ~ide with the further c~lture medium, a
further develop~ent of the inve~tivo cult~re
medium provides that the fuxther cult~re medium
ha~ a ge~-like con~i~ten~y, whereby the gel
coneain~ between 70 g and 250 g of the further
c~lture medium snd between 930 g and 750 g of
water.
pa~ticularl~ ~uitable and advantageous
development of the afore de6crib~d embodime~t
of ~he inven~ive culkure medium ~hich can be
applied in each denti~t'~ practice provide~
that the inert eupport which i~ co~ted on one
~ide with the gel-l~ke culture mediu~ on the
baei~ of the agar, preferably the miti~
~all~riu~ ag~r, containing at lea~t one ~ugar
and the peptide antibiotic complex,
particularly bacit~acin, and which is ~oated on
the other ~ide with the gel-like further
culture medium on the basis of the afore
described rogosa agar or the culture medi~m for
the detection of yea~t fungu~ i~ to be arranged
~ithln a ga~tight lockable ~torage ~e~el.
Hereby the ga~tight lockable storage ve~el
~erve~ for ~he ~terile ~torage and for the
CA 02240237 lgss-06-lo
18
transport of thi~ e~bodiment of the ~nventive
culture medium and guaranties at the same time
that, after the adding of the corre~ponding
~aliva ~ample~, respecti~ely plaque ~a~ples to
~oth culture mediums, the thus treated culture
~ediume can be incubated in the stora~e vessel,
where~y a C02 relea~ing ~able~, preferably an
alkali carbonate tablet, re~pectively an alkali
hydrogencarbonate tablet, can be arranged
~ithin ~he etor~e ve~el particularly during
the incubation. Hereby thi~ C02 reloa~ing
tablet cau~e~ the fact that a corre~ponding C02
atmo~phere i~ adju~ted ~ithin the gastight
lockable ~torage veesel during the incubation
time, whereby, on one hand, the selectivity of
the inventive culture medium for the
streptoco~cus mutan~ and the lactobacillus,
respectively the yeast ~ungu~ increa~ed
and, on the other hand, unde~ired side
reaction~ are excluded. Such ah em~odi~ent is
perfe~tly ~uitable for the concurrent detection
of ~treptococcus mutanB and lactobacillu~,
re~pectively yea~t ~ungus, in the dentist ' B
practices, whereby this development of the
Z5 inventive culture ~edium then allow~ a
reproducible and ~uantified determination
r~garding these ~icroorgani~m~.
CA 02240237 1998-06-10
lg
The present invention rela~e~ further~ore to a
analy~i~ ee~ for the ~imple e~tablishment of a
diagnosis, particularly for evaluating the ri~k
S of cariogenicity.
Thi~ object i~ reali~ed according to the
inven~ion by an analysis set havin~ the
significant ~aracte~ietic~ of patent claim 18.
The inventive analysis set comprises a culture
medium being arranged o~ one side of ~n inert
support and being of the afore de~çribed ~ort
for the det~ction of streptococcus mutans,
whereas the other side of the inert support is
co~ted ~ith the fu~ther culture mediu~ baeed on
the afore de~cribed rogosa agar for the
detec~ion of lactobacillus or with a culture
- mediu~ of the detection of yea~t fungus.
ln a development of the in~entive analysis set
the analysis #et can prefera~ly comprise ~t
leaet one carpule filled with an acid,
pref~ral~ly with ~ O . 005 n hydrochloric aoid, a8
~ell as at least one cannula.
The inventive analy~is 8et ~hows a range of
CA 02240237 1998-06-10
ad~antage~. Fir~t of all it i~ to be noted that
the afore deecribed in~en~ive analy~i~ set
allow~ a preci~e qualit~ti~e and quantitative
a~ well as concurrent determination of
~treptococcu~ mutan~ and lactoba~lllus,
respectively yeast fungue, in ~aliva an~/or in
pla~u~. In addition to that the afore mentioned
development of the in~entl~e analyRi~ Bet can
make possible a determination o~ the buffering
lo c~p~city of the ~ali~a. Thi~ is achieved by
taking up a defined amount of saliva into the
carpule bein~ filled with an acid, prefe~ably
with a O.OOS n hydrochloric acid, ~o that after
mlxing the ~aliva with the acid the pH-value of
thi~ liquid mixture can be easily determined,
whereby the pH-v~lue i~ a quantitative
indication o~ the bu~fering capacity o~ the
ple.
In order ~o ~ke ~p ~he s~liva into the carpule
filled with ~he a~id the carpule of the
inventive analy~i~ aet i8 ~it into a usual
~edi~al ~yring~. The cannul~ contai~ed i~ the
analysi6 ~et is mounted onto the ~yringe, 50
that then the carpule i~ filled wlth a given
amount of the ~aliva sample, xe~pectively the
pl~que ~uapen~ion, ~y mean~ of the ~yringe by
CA 02240237 1998-06-10
pulling back the ~yri~ge pi~ton to a given mark
or to a gi~en stopper. After ~ixin~ the ~ample
with the acid the evaluation can be realized by
interpreting the color change. After ~he
determination o~ the concentration of
streptococcus mutan~, lactobacillu~ or yeast
fungus and even~ally o~ ~he buffe~ing capacity
the inventi~e analy~ln set can be easily
~asted.
A ~urther development of the a~ore de~cribed
inventive analy~i~ set p~ovides that the
analynis set co~pri~eC at lea~t one c02
~eleasing tablet. Hereby the C02 relea~ing
tablet e~enti~lly c~use~ that, after
incubating the cultu~e ~edium ~o~ the detection
of streptococcus mutans and the furthe~ c~lture
mediu~ ~or the detection of lactob~eillus,
respec~ively for the detection o~ yea~t fungu~,
the i~ert ~upport coated with those two cult~re
medium iB ~tored in a ga~tight, lockable
~torage vessel, whereby the C02 relea~ing
tab~et i~ a~anged within this gastight ~torage
ve~el during ~he incubation. Thi~ C02
relea~ing tab~et cau~es the ga~tight storage
ve~el i~ ~et under a corresponding C02
atmosphere durin~ the incu~atlon, ~o that, on
CA 02240237 1998-06-10
one hand, the selectivity for the detection of
the ~treptococcu~ mu~an~ and lactob~cillu~ i~
~o~re~pondingly increased and, on the oeher
hand, unde~ired ~ide reactions are e~cluded
becau~e of the CO2 atmo~phere.
In a further e~odi~ent of the inventive
a~ly~i~ set ehe analysi~ ~et comprise~ ~t
least one paraffin piece and~or a pH-value
i~dicator. Hereby thix paraffin piece ~erves
for the ~illing of ~he corre~po~ding patient
whose saliva, respectively plaque i~ to be
ex~m;n~d in res~ect to the presence of
~treptococcus mu~an~ and lacto~cillus,
re~pectively yea~t fungu~, ¢hew~ thi~ paraffin
piece for a given time before the execution of
the co~resp~d~ng ~ ion, in order to thus
animate the salivation, whereas the pH-value
indicator i~ employed to determine the pH-valu~
of the saliva, a~ thi~ iB de~ribed later on.
In order to furtherly ~implify the
determination of the buffering capacity of the
saliva in the afore described embodimen~ of the
~5 inventive analy~ e~, a further development
of the inventive analysis set provides that
hereby the carp~le ¢ompri~es also an indicator,
CA 02240237 1998-06-10
particularly a liquid indicator, in addition to
the acid being pareicularly contained in ~he
carpule in a given amount and concentration.
Hereby it is rendered po~ible to directly
def ine the buf f ering capacity of the saliva
sample by interpreting the color change after
the filling o~ a 8ali~a ~ample, respectively a
pla~ue ~uBpen~ion, intb the carpule, whereby
~he safety and reproducibility of the
determination of the bu~fering capa~i~y iB
fu~therly i~proved.
It is ~l~o pos~i~le to provide the analysis set
with a pH-value indicator a~, for example, a
liquid i~dicator, with a ~olid indicator and~or
with a indicator strip, 80 that hereby t~e
buffered ~aliva can be removed from the carpule
in order to then deter~ine its pH-value.
The afore described indicator preferably i~ a
liquid ~niver~al indic~tor, whereby thiP liquid
universal indicator has a pH-value indicating
range of between pH 4 and pH 10.
In order to ~acilitate the vi-~ual defini~ion of
the buffering capacity of the ealiva,
re~pectively of the plaque suspeneion, , it is
CA 02240237 1998-06-10
24
particularly advantageou~ that the analy~is ~et
containe color ~cale, eo that the bu~ering
capacity of the corre~pon~in~ ~ample can be
de~ermined in a particularly ea~y and rapid
s manner by vi~ually compaxing the color ~c~le
with the obta$ned color yield of the çarpule
which i~ filled with the ~aliva and which
~ontain~ the univer~al indicator a~ ~ell as the
afore described acid.
Another advantageou~ development of the
inventive analy~i~ set provide~ ~hat the
analy~is ~et comprises at lea~t on~ ma~k which
can be arranged on the inert support, so that
by thi~ ~a~k the cul~ure mediu~ which is
arranged on on~ side of ~he inert ~uppor~ and
which i~ based on the modified agar,
pa~ticulaxly the mitiu ~alivarius ~gar, and/or
the ~urther culture ~edium which i~ arranged on
the other ~ide of ~he inert ~upport and which
is ~a~ed on the rogo~a agar, re~pectively for
~he detec~ion of ye~t fu~gu~, can be
~ubdivided into single ~ection~. This
~mbodiment of the inventive analy~i~ see makes
it thus poosibl~ to optionally subdivide one
c~lture ~edium or both culture medium~ in
single 8ections, 80 that single mouth areas or
CA 02240237 l998-06-lO
particularly dental area~ can be specifically
examined fo~ streptococcuR mutane and
l~ctobacillu~ or yea~ fungu~ with one single
te~t. Herefore it is only ~ecessary to apply
S thi~ 6ample removed from the mo~h area,
re~pectively from the ~pecific tooth, onto the
section of the co~re~rond; ngly ~ubdivided
c~lture ~edlum, ~o that after the inc~bation
the microbiological ~ondition6 can easily be
defined in re~pect to their concentration of
streptococcu~ mutan~ and/or lac~oba~illus or of
~t~e~ococcu~ mutan~ and~or yeast fungus. T~i~
maQk i~ preferably provided wit~ an in~c~iption
part, ~o that an a~ign~en~ of the obtained
valuee to ~pe~ial mo~th ~reaB, re~pe~tively
dental area~, can ~afely be carried o~t.
In another em~odiment of the inventive anal~sis
set this analy~is eet compri~e~ moreover a
calibrated ve~el for taking up the saliva
Here~y it i~ ~ade po~sible to measure a further
parameter during the ~aliva e~-nAtion,
whereby thic para~eter ig the ealiva volume
generated by the patient by chewing the afore
de~cribed paraf~in piece for a ~iven time.
A further advantageoue develop~ent of the
CA 02240237 1998-06-10
inventive analy~ et provide~ that the
a~aly~i~ qet compri~e~ ~oreover a di~po~able
~yringe. Hereby thi~ ~ispoeable syring~ ~erve~
to inject saliva or a plaque ~u~pen~ion into
~he carpule filled ~ith the acid, preferably
~lth the 0.005 n hydrochloric acid, and wi~h
the univercal indlcator, whereby the amount o~
the injected ~aliva can be determined
optionally in the disposable ~yringe or in a
particularly advantageou~ manner by a mark
located on the carpule up to which mark the
gliding plug i~ di~placed in the axially
oppo~ite direction o~ the piercable membrane.
Thi8 embodiment o~ the analy8i~ -~et guaranties
that each u~er of the inventive analy~ et
find~ ~ll in~tru~en~ required for the
dete~mina~ion of the buf~e~in~ capacity of
saliva in thi~ analysi~ set, ~o that ~he
corre~ponding analyei~ can be carried out in a
p~r~iclllarl~ ~imple ~nd ~apid and thul3 ~ery
economical manner.
The afore described embodiments o~ the
inventive cult~re medium in which the culture
~edium, re~pectively the afore de~cribed
fur~her culture medium, i~ applied each onto
one ~ide of an inert ~upport can be ufied in a
CA 02240237 1998-06-10
particularly ~imple, rapid and reproducible
manner for the dete~tion of ~treptococcus
mutan~ on one hand and for the detection of
lactobacillu~ or yea~t fungu~ on the other
hand. In thi~ detection method firat of all a
saliva ~a~ple or a pla~ue ~ample i~ taken from
th~ patient and applied onto the culture medium
for the detection of otreptococcue ~utans a~
well as onto the further culture medium for the
detection of lactobacillu~, respectively ye~t
fungus. The culture medium~ thus loaded with
the ~ali~a sample are then transmiteed
p~eferably i~ a ~a~tight, lockable ve~sel
having been event~ally ~llled before with a CO2
atmo~phere into a suitable incubator, so that
the corre~po~ding ~torage ve88el i8 then
inçuba~ed for a given incubation time,
preferably between 24 and ~8 hours at a
temperature o~ 37 nc + 4 ~C.
After e~pi~ation of the incubation time a
vi~ual evaluation and ~uantification of the
streptococcu~ mutan~ cultivated on ehe first
culture m~dium arranged on one side of the
inert ~upport as well as of the lactobacillus,
re~pectively the yeast fungus, cultivated on
the further culture medium arranged on the
CA 02240237 1998-06-10
other ~ide of the inert support. By a vi~u~l
comparison with the corresponding ~tandard~ the
con~ent~ation o~ strepto~oc~u~ mu~ans and
lactobacillu~, re~pectively of ~treptococcu~
mutans and yeast fungu~ ~an then be quantified,
as thi~ i~ u~ual in microbiology.
Advantageou~ development~ of the inventive
culture medium a~ well as of the analysis set
lo are indi~ated in the ~ubclai~s.
The inventive culture medium and the inventive
analy~i,q ~et are .qubsequently explained by an
example in connection with the drawings.
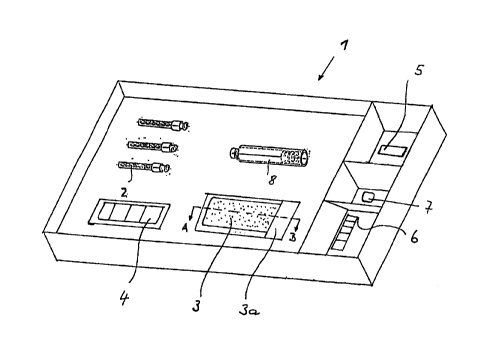
Fi~ure 1 show~ a ~chematical, partly
per~pective embodiment of the
ana~yui~ ~et;
Figure 2 Bhow~ the cul~ure medium in a
se~tional view along the line
A-B in figure 1, whereby the
culture medium is contained in
the analy~i~ Ret according to
figure 1 and arranged on an
inert ~upport;
Fi~ure 3 show~ an enlarged vie~ of the
ma~k ~hown in ~igure 1 in a top
CA 02240237 1998-06-10
view;
~ig~re 4 ~how~ a view of the culture
medium b~ing arranged on a
~upport, whereby this view can
be compared with figure 2. The
culture medium ha~ the m~k
~ho~n in figure 3; and
Figure 5 shows a sectional view of a
carpule .
In the figures 1 ~o 5 the same part~ are
xeferred to the corre~ponding number~.
A~ ~hown in fi~ure 1 ~he analy~ et 1 fir~t
of all compri~es a corre~ponding amount of
cannula~ 2, ~hereby the embodiment ~hown in
~igure 1 ex~mpl~rily indi¢~te~ three ca~ula~
The analy~ et 1 moreover compxi~e~ an amount
of carpules 8 which corresponds to the amount
of the cannula~ z, ~he~e~y in ~ igure 2 one
single ca~pule 8 i~ only examplarily ~hown. Th~
- more detailed con~txuction of thi~ carpule 8 i~
subse~ùently explained by figure 5.
In addition to the carpule B and the cannulas 2
the analy~is set co~prise~ furthermore a
corre~ponding amount of inert plastic supports
CA 02240237 1998-06-10
3 a coated wi~h culture mediums, whereby these
plactic Bupport~ 3 a co~ted with culture
mediums 3 are explained more detailed in the
figuree 2 and 4. The analysis ~et al~o
compri~e~ a mask 4 being schematically ~hown in
figu~e 1, where~y the ~a~k 4 i6 de~cribed more
detailed by the figure 3. The analysis set 1
furthermore co~pri~ number of paraffin
blocks 5 which corre~pond~ to the number of
cannula~ 2 and a number of C02 releasing tablet
7 which corre~pond~ to the number of pl~etic
support~ 3 a, whereby in ~igure 1 on~y one
paraf~in block 5 and one C02 relea~ing tablet 7
i~ examplarily ~hown. Moreover the analy~i~ set
1 compri~e'~ a ~lor ~cale 6.
As shown be~t in ~he sectional view~ of the
figures 2 and ~, each inert pla~tic 6upport 3 a
compri~e~ a grip ~ection 13, whereby a dieh-
~h~ped area 14 iB directly adjoined to both
sides of the grip section 13. Hereby the di~h-
shaped area 1~ co~p~i~e~ a ridge-like ~ide wall
sec~ion 15, where~y only ~he front-~ide ridge
sections 15 are shown in the figure~ 2 and 4,
The di~h- ~haped ~rea 14 ~erve to take ~p the
culture medium 3 which i~ nu~bered ~ith 16,
CA 02240237 1998-06-10
re~pectively with 17, in the ~igure~ 2 and 4,
~hereby one of the cultu~e mediums 16,
re~pectively 17, i8 the afore described miti~
sallvarius agar and the other culture medium
17, re~pectively 16, i~ th~ al~o a~ore
de~c~ibed rogo~a a~ar or i~ a culture medium
for the detection of the yeast ~ungu~. The
inert plastic suppor~ 3 a is arranged within a
gastight lockable storage ve~xel 18, whereby
the ~torage ven~el 18 i~ clo~ed up with the
grip ~ec~ion 13.
It surely i~ po~ible to form the inert pla~tic
~upport 3 a in such a way that an in~erior ~rip
section 13 is ~onnected with the di~h-shaped
area 14, eo th~ ~he ~hole pla~tic ~upport 3 a
i~ arranged within the ~torage ve~çl 18 and
corre~pondingly ~lo~ed up ~ith a ~crew cap.
However thi~ embodiment i~ not 6hown in the
figure~ 2 and 4.
The culture medium being arranged on the di~h-
shaped area 14 and bein~ ~ased on a ~odi~ied
miti~ salivariu6 agar i~ s~ch a oulture medi~m
16 a8 it i~ manu~actured as ~ollows.
~ir~t of all a purcha~able mitis salivarius
CA 02240237 1998-06-10
agar a~, for example, from DI~C0 i~ ~hosen,
whereby the pu~ha~able mitis salivariu~ agar
contains the following compo~ents:
Bacto tryptose 20 g
Proteose peptone no. 310 g
Proteo~e peptone 10 g
Bacto dextro~e 2 g
Bacto ~ucro~e 100 g
Potas~ium hy~ e~pho~phate 8 g
Trypan blue 0.15 g
Bacto crystal violet0.0016 g
~acto agar 30 g
360 g sucrose (Merck 1.07651) and 3 mg
bacitracin (Sigmar ~-0125) are added to the
fir~t half o~ this dry culture medium bein~
sub8equently named culture medium I, and 600 g
sucro~e (Merck 1.07651) and 5 mg bacitracin
(Sigmar b-0125) are added to the eecond half of
the dry culture medium being ~ub~equently named
culeure medium II, whereafter ~he culture
medium~ I a~d II are mixed. The culture mediums
I and II are ~hen ~olved in deionized water
under the ~ormation of one liter of ready-to-
apply culture ~ediums I and II ~ereafter the
water i~ boiled. Then the culture mediums are
CA 02240237 1998-06-10
~terilized at lZl ~~ for lS ~inute,~. After
having aooled down, the gel-like culture
medium~ are applied onto the inert plastic
support 3 a whi~h i~ a black p~a~ic aupport.
Culture medium I then ha~ a raeio of miti~
~alivariu~ agar to ~he added ~ucroee of
1:~.996, wherea~ cultur~ medium II has a ratio
of mitis salivarius agar to the a~ded sucrose
lo of 1:6.661.
For the manufacture of the culture medium 17
the u~ual rogosa agar i~ used as starting
~ateri~l, whereby the corre~ponding ro~o~a agar
lS ha~ the already abovc indicated fo~lowi~g
compo~ition:
Agar agar 19 g
Tryptone lO g
Yeast eXtract S g
Gluco~e 20 g
Pota6sium hydrogenphosphate6 g
Ammonium hydrogencitrate 2 g
Mangane~e~ sulphatc l-hydrate0.1 g
Iron-II-~ulpha~e 7-hydrate0 03 g
Tween 80 l g
CA 02240237 1998-06-10
This rogo~a agar i~ ~olved in 1 1 of water
after h~vlng added 1.32 ml of glacial acetic
acid. After ~ol~ing the rogo~a agar one liter
of a gel-like to pa~ty composition is produced
which is applied onto the dish-~haped area 1
of the inert plastic support 3 a whereon it
adhere fi .
The figure 3 shows the enlarged and schematical
lo view of a mask 4, whereby the ~a~k 4 is formed
in ~uch a way that rect~ngular free spaces 25
between the ridges are formed, whereby four of
such free spaces 25 are examplarily -~hown in
figure 3.
on their bottom side the ridge~ ~hich extend
~ro~s~i~e to the longitudinal ridge~ 2~a have
wedge-e~aped c~te~ 28 ~figure 4), ~hereby
the~e wedge-~haped cutters 28 cause the
division of the culture medium into fields 26,
a~ this i~ s~own in figure 4 for the culture
medium 16. In orde~ to fix ~he ~k ~ on the
ine~t pla~tic support the mask 4 ha s
corre~ponding ~la~p section~ 29 which can ~ear
in with the ridge-like side wall sections 15.
In figure 5 a carpule 8 is ~chematically ~hown,
CA 02240237 1998-06-10
whereby the carpule 8 ha~ a piercable mem~ane
31 at it~ top Ride and a piston 3~ at itB
boteom side, whereby the piston 32 can be
~xially ~hifted i~ ~he ~i~e~tion of the arrow
33 and vice-ver~ igure 5). A 0.005 n
hydrochloric acid and a ~olution of the
univer~al indicator are located in the inner
space 30 of the carpule 8, whereby the volume
of ~oth BolutionB ha~ the t~tal value of 1.27
ml. A limiting element 34 iB arranged at the
bottom ~ide of the carpule 8 in ~uch a way that
the a~ial ~hif~in~ of the pi~ton 32 in the
direation of the arrow 33 is limited by mean~
of thi~ limiting ele~ent 34. Hereby it i~
gu~ra~teed that a maximum total v~l~me 4~ 1, 7
ml of liquid i~ taken up into the inner space
~0 of the carpule 8 during the shifting of the
pi~ton 32 in the direction o~ the arrow 33.
If the u~er want~ to employ the afore described
analyei~ ~et ~hown in figu~es 1 to 5 the
corre8~n~; ng patien~ has to chew firstly the
paraffin block 5 taken from the analy~ib ~et 1
for a gi~en time pe~iod (generally for five
minutes). Hereby the ~ali~ation of the pa~ient
i9 animated, ~o that a corre~ponding measuring
cylinder can detect and then measure the spit
CA 02240237 1998-06-10
Oue ~aliva amount.
For ~he ~etermination of the buffering capacity
of the ~ali~a, the u~er of the analysis ~et l
take~ a carpule 8 from the analy~ et 1,
whereby the carpule 8 is filled with the
mixture of hydrochloric acid and univer~al
indica~or (measuring range pH 4 to pH lo). This
c~rpule B i~ then flt into a usual ~yringe,
whereby a ca~ula 2 taken from ~he analysi~ set
1 i~ mounted onto the syrlnge. The carpule ~it
inco the syrlnge then takes up a given amount
of ~liva by a correeponding movement of the
~yrin~e piston which gear8 in with the piston
32 ~figure 5) arranged within the carpule B,
whereby the volume of the 6aliva e~ual,~ ~
quarter of the volume of the carpule content.
The axial ~hifting of the ~hiftabl~ pi~ton 32
i~ then limited by the limiting element 34, so
that it is guaranteed that always the same
amount of ~aliva i~ taken up into the carpule
8.
The carpule 8 being addltionally filled with
~aliva ie then removed from the syringe and ~he
dispo~able cann~la belng mounted onto the
~yringe and having a length o~ 3 2 mm and
CA 02240237 1998-06-10
diameter of o.9 mm is removed and wasted.
The carpule 8 removed from the syringe, the
carpule 8 being a glacs carpule, then
S immediate~y allows a vi~ual evaluation of the
colo~ change of ~he carpule content, whereby
thi~ color change i9 quantified by the vi~ual
comparlson with the color scale 6 of the
analysi~ set 1. Herewith the corresponding u.Rer
lo then define~ the bu~ering capacity of the
saliva.
A furth~r sali~a sample is then applied onto
c~lture medium 16 and another saliva sample
onto cul~ure ~ediu~ 17 (figure 2), where~y the
pla~tic ~upport 3 a coated with the culture
mediums 16 and 17 is introduced into the
storage ve~el 18 after applying the saliva
~amplç~. Befor~ that a C02 releaRing tablet 7
~0 was removed from the analy~is eet 1 and
introduced into the ~torage ve~el 18.
Afte~ ga~tightly clo~ing the storage vessel 18,
ehe storage ve~eel 18 is then transmitted into
2S an incubato~ together with ~he culture medium~
16 and 17 being incubated wi~h ~aliva and being
arranged within the ~torage ve~el 18, whereby
CA 02240237 1998-06-10
3~ .
the corre~ponding storage ve~el i~ then
incu~ated, for example, for 24 or ~8 hour~ a~ a
temperature of 37 ~C.
After expiration of ~he inaubation time the
vi~ual e~aluation and quantification of the
cultur~ mediums l~, re~pectively 17, whereby
the cul~i~ated streptocoacus mutan~ ~e well a~
the lacto~a~ill~ cultiv~ted on the further
lo culture medium are clearly vi~ible Bince they
~tand out again~t the ground in re~pect to the
color. The concent~ation of ~trep~ococcu~
m~tan~ and lactobacillu~ can then be detected
by ~ vi~ual compari~on with the ~t~n~ard, as
lS thic i~ u~ually done in microbiolo~y.
If, in contra~t to that, the ~er wants to
examine ~pecial mouth areaR or ~ingle teeth in
re~pect to the pre~ent microorgani~m~, he
2~ arrangc~ two or three mask~3 4 of the analy~3i8
Be~ 1 on the culture medium 16 and/or on the
culture medium 17, a~ thi~ is shown in f igure 4
~or the culture medium 16. Hereby aingle fielda
26 are generated on ~he corre~pondi~g c~lture
medium a~, for example, o~ the culture medium
16 in figure 4, ~here~y the~e fields 26 are
~traightway incubated with the locally removed
CA 02240237 1998-06-10
:~9
~aliva qample~, respectively ~he plaque
su~pensions. Hereafter the further proce~sing
i~ carried out a~ it i~ described above.
~he e~ential advantag~ of this way of
proce~ing 1~ the fact that all locally removed
~ample~ are examined at the ~ame time in the
~ame analysi~, BO that corre~ponding to that a
~e~hodical fault can be excluded. ~his
lo e~eentially contribut~ to increa~ing the
preci~enes~
In order to proo~ that the afore described
miti5 salivarius agar (culture mediums I and
II) which additionally contains the sugar in
dif~erent concentrations i~ clearly superior to
the u~ual miti8 ~alivariu~ agar, a large,
clinical ~tudy with ~5 children ~nd 13~ ~aliva
isolate~ was carried out. Hereby the usual
miti8 Balivarius agar wa~ compared to the
culture ~edium~ I and II containing the ~ugar,
whereby the ~iti~ salivariu~ agar containing
the sugar ha~ the compo~ition as it is
de~cribed abo~e for th~ culture mediums I and
Z5 II.
In the ~cope of ehi~ ~tudy it wa~ noted that
CA 02240237 1998-06-10
the germ yield of ~treptococcus mutans wa~
clearly and ~ignificantly higher for ~he
culture m~dium I and II containing ~ugar than
the ge~m yield of ~ereptococcu~ mutans for ~he
ueual mitis sallvarius agar. In comparieon with
the cult~re medi~m I the culture medium I I had
a higher germ yield of ~trep~ococcu~ mutan~.