Note: Descriptions are shown in the official language in which they were submitted.
CA 02240452 1998-06-19
95/F 313 K W0
Description
Transition metal compound
The pre8ent invention relates to a tra~ition metal
compound and a process for it~ preparation and also to
its u-~e as a catalyst component in the preparation of
polyolef in~ .
The literature di6clo~es the preparation of polyolefins
u8ing soluble metallocene compounds in combination with
aluminox~ne~ or other cocatalyst~ w~i~h, owing to their
Lewi~ acidity, can convert ~he neutral tran~ition metal
compound into a cation and stabilize it (EP-A-129 368,
EP-A-351 392).
Me~allocenes and semisand~ich compound~ are of great
interest not only for th~ polymerization or
oligomerization of olefins. They can also be used as
hydro~enation, epo~idation, i~omerization and C-C
coupling catalyst~ ~Chem. Rev. 1992, 92, 965-994).
WO 96/23004 di~clo6es certain boron-containing transition
metal compounds.
of great interest are tran~ition metal compounds which
have xufficient activity in re~pect of the abovedescribed
~ield~ of application.
It is an object of the present invention to provide a
transition metal compound and an economical and environ-
mentally friendly process for i~s preparation.
This object is achieved by a compound having the formulaI
LnAmMxk ( I )
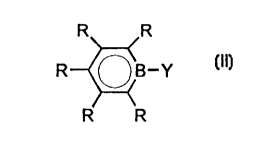
where ~ is a boratabenzen~ ligand of the formula II
- -
CA 02240452 1998-06-19
95/F ~13 K WO - 2 -
R R
R ~ ~_y
R R (Il)
whe~e ~he radic~l~ R are identical or dif$erent and are
each a hydrogen atom, a Cl-C20-group, preferably a C~-C10-
group ~uch as a Cl-C~0-alkyl group or a C6-Cl0-aryl group,
and two adja~ent radicals R together with the atoms
connecting them can form a ring ~y~tem, and Y is a
hydrogen atom, a Cl-C20-group, preferably a C~-C~0-group
~uch a~ a C~-C10-alkyl group or a C6-C10- aryl group, a
~alogen atom, an oR2, SR2, NR22 or PR22 radical, where R2 i~
a halogen atom, a C1-C1O-group such a~ a C~-C,~-alkyl
1~ group, preferably a C2-C~-alkyl grOup, or a C6-C1O-aryl
group and two radical-~ R2 can form a ring ~ystem,
A is a ~ ligand such a~ cyclopentadienyl which can be
either substituted or un~ub~tituted and adjacent sub~
tuent~ on the cyclopentadienyl ligand can form a ring,
M is a metal of group IVb of the Periodic Table of the
Elements and
X are identical or different and are eac~ a hydrogen
atom, a C1-C40-~roup ~uch as a cl-C20-alkyl group, a ~-C~0-
alkoxy group, a C~-C20-aryl group, a C2-Cl2-alkenyl group,
a C,-C~0-arylalkyl group, a C7-C40-alkylaryl group, an OH
group, a halogen atom or ~R22,
n is 1 or 2,
m is 0 or 1 and
k is an integer from 1 to ~,
2s where the sum of n+m~k is 3 ox 4.
L and A can be joined to one another by mean~ of a
bridge. When n is 2, ~ can be identical or dlfferent
The ~ridge is pre~erably
CA 02240452 1998-06-19
95/F313 K WO - 3 -
~ ~ RX ' , Rx RX
2 - ~2 ~2 --C C--
RY , F~l~ RY ' Rr RY
~ Rs
--O--I Z o-- C
I
RY R~ RY Rr R~
RX RX ~X f~ TX RX
O ~-- - C--~ C C--C--
RY ' RY RY ' RY R~ RY
~BRX, ~AIR~, -Ge-, -O-, -S-, ~SO, >SOz, ~NRX, ~CO, ~pRx or
~P(O)RX, where Rx and RY are identical or di~ferent and
are each a hydrogen atom, a halogen atom or a Cl-C,O-group
~uch as a C1-C20-alkyl group, a Cl-C1O-fluoroalkyl group, a
Cl-C1O-alkoxy group, a C~-Cl,-aryl group, a C6-C1O-fluoroaryl
group, a C6-C~O-aryloxy group, a C2-C1O-alkenyl group, a
C7-C~O-arylalkyl group, a C~-C40-alkylaryl group or a C~-C~O-
arylalkenyl group and M~ i~ silicon, germani~m or ~in.
Examples of bridges are groups (M2RXRY)y~ where Ma i~
carbon, silicon, germanium or tin and Rx and RY are
identical or different and are each a Cl-C20-hydrocarbon
group such as Cl-C~O-alkyl or c6-C~4-aryl and y is 1 Qr 2,
e.g. CH2, CH2CH2, CH ( CH3 ) CH2, C ( CH3 ) ( C6H5 ), C ( C6H5 )
CH(C~H~)C(CH3)z~ C(CH3)2, C(CH3) (C6H5), C(C6H5)2~ (CH3)2$i~
~CH3) 2Ge, tCH3) 2Sn, (C6H5) 2Si, (C6Hs) (CH3) Si, (C6H5) 2Ge,
~C6H5)2Sn, (CH2)~Si, CH2$i(CH3)z~ o-C6H4 or 2,2'-(C6H4)2
CA 02240452 1998-06-19
95/F 313 K WO - 4 ~
The pre8ent invention accordingly provides a tran~ition
metal compound which contains as a ligand at lea~t one
substituted or unQubxtituted boratabenzene group and is
described by the formula I.
A preferred ~mho~ nt of the invention is a compound in
whic~ M i~ an element of group IVb of the Periodic Table
of the Elements, in parti~ular zirconium.
A preferred embo~;me~t of the invention i8 a compound in
which the radical~ R are identical and are ea~h a hydro-
gen atom, a Cl- C4 - alkyl group or ~ C6 - C10 - aryl group and Y
i~ a Cl-C~-alkyl group or NR2Z, where R2 is a C -C4-alkyl
group, preferably a Cz-C4-alkyl group.
A preferred embodiment of the in~ention is a compound in
which A is a substituted cyclopentadienyl ligand.
A preferred embodi~ent of the invention is a compound in
which X is Cl-C1O-alkyl or NRa2, where R2 i-~ a Cl-C4-alkyl
group, a C6-C~O-aryl group or a halogen acom, in
particular chlorine.
A preferred embodiment of the invention ie a compound in
which m - O or 1 when n = 1 and m - O when n ~ 2.
A i~ preferably a ~-ligand ~uch as cyclopentadienyl,
indenyl or fluore~yl, which can each be either
substituted or unsu~tituted.
~-~igands are preferably an u~ub~tituted cyclopenta-
dienyl group or sub~tituted cyclopentadienyl group whichpreferably bear one or more Cl-C~O-hydrocarbon radicals a3
sub~tituents, e.g. 2-methylcyclopentadienyl, methyl-tert-
butylcyclopentadienyl, tert-butylcyclopentadienyl,
i~opropylcyclopentadienyl, di~ethylcyclopentadie~yl,
trimethylethylcyclopent~;enyl, 5-phenylcyclopentadienyl,
diphenylcyclopentadienyl, ~n~Anyl, 2-me~hyli n~enyl, ~-
ethylin~nyl, 3-methylindenyl, 3-tert-butylin~yl, 2-
CA 02240452 1998-06-19
95/F 313 K WO - 5 -
methyl-4-phenylindenyl, 2-ethyl-4-phenylindenyl, 2-
methyl-4-naphthylinde~yl, 2-methyl-4-i~opropylindenyl,
benzlnfl~nyl, 2-methyl-4,5-benzindenyl, 2-methyl-a-ace-
naphthindenyl, ~-methyl-4,6-dii~opropylindenyl,
~luorenyl, 4-methylfluorenyl or 2,7-di-tert-butyl-
fluorenyl.
Particular preference i~ given to compound~ of the
formula I in which M i~ zirconium and X are identical and
are each a halogen atom, in particular chlorine.
Particular preference is given to compound~ of ~he
formula I i~ which L iQ a boratabenzene ligand of the
formula II and the radical~ R are preferably identical
and are each a hydrogen atom ~nd Y is preferably a C1-C~-
alkyl group ~uch aq methyl, ethyl, propyl, isopropyl or
butyl, or NRa2 in which R2 i~ a Cl-C,-alkyl group such a~
methyl, ethyl, propyl, isopropyl or butyl. When m i~ 1,
A is preferably a cyclopentadienyl ligand s~ch as cyclo-
pentadienyl, methylcyclopentadienyl, pentamethylcyclo-
pentadienyl or indenyl. Pre~erence i-~ al~o given to
compounds of the formula I in which X are identical and
are each a Cl-C4-alkyl group, in particular methyl, or a
C7-C~O-alkylaryl group, in particular benzyl, or a halogen
atom, in particular ch~orine, and n i~ 1 or 2 and m is O
or 1 when n i~ 1 and m is O when n is 2 and the ~um of
n+m+k can be 3 or 4.
Example~ of transition metal compound~ of the invention
are: -
(l-methylboratabenzene)~pentamethylcyclopentadienyl)-
zirconium dichloride
(1-butylboratabenzene)(pentamethylcyclopentadienyl)-
zirconium dichloride
(l-methylbora~benzene)(pentamethylcyclopentadien~l)-
dimethylzirconium
(l-butylboratabenzene)(pentamethylcyclopentadienyl)-
dibenzylzirconium
CA 02240452 1998-06-19
95/F 313 K WO - 6 -
[1-(dimethylamino)boratabenzene](pentamethylcyclo-
pentadienyl)-zirconium dichloride
[1-(diethylamino)boratabenzene](cyclopentadienyl)-
zirconium dichloride
[l-(dimethylamino)boratabenze~e](pentamethylcyclo-
pentadienyl)dimethylzirconium
~1-(diethylamino)boratabenzene](cyclopentadienyl)di-
benzylzirconium
(1-methylbora~abenzene)(cyclopentadienyl)zirconium
dichloride
(l-methylborat~h~n~ene)(methylcyclopentadienyl) 2 irconium
dichloride
(1-methylboratabenzene)(cyclopentadienyl)dimethylzir-
conium
(1-methylboratabenzene)(methylcyclopentadienyl)di-
benzylzirconium
bi~ methylboratabenzene)zirconium dichloride
bis(1-methylboratabenzene)dimethylzirconium
bis(l-methylborata~enzene)hafnium dichloride
bis(l-methylboratabenzene)dibenzylzirconium
(l-methylboratabenzene)zirconiumtrichloride
(1-ethylboratabenzene)zirconium trichloride
(l-methylboratabenzene)(indenyl)zirconium dichloride
(l-~ethylboratabenzene)(indenyl)dimethyl zirconium
(l-me~hylboratabenzene)(indenyl)dibenzyl zirconium
[1-(dimethylamino)boratabenzene~(indenyl)zirconium
dichloride
(1-methylboratabenzene)(pentamethylcyclopentadienyl)-
titanium dichloride
(1-methylbor~tabenzene)(cyclopentadienyl)titanium
dichloride
[1-(dimethylamino)boratabenzene](cyclopentadienyl)-
titanium chloride
bi~(l-methylboratabenzsne)titanium dichloride
bi~ methylboratabenzene)titanium chloride
(1-methylboratabenzene)titanium ~richloride
(1-methylboratabenzene)titanium dichloride
(l-methylboratabenzene)(indenyl)titanium dichloride
~ dimethylamino)boratabenzene](indenyl)titanium
CA022404521998-06-19
95/F 313 K W0 - 7 -
chloride
[l-(diethylamino)boratabenzene](pentamethylcyclopenta-
dienyl~titanium dichloride
tl-(diethylamino)boratabenzene~lpentamethylcyclopenta-
dienyl)hafnium dichloride
[1-(diethylamino)boratabenzene]~pentamethylcyclopenta-
dienyl)zirconium dichloride
~1-(diethylamino)boratabenzene](pentamethylcyclopenta-
dienyl)dimethylzirconium
[1-(pyrrolidino)boratabenzene](pentamethylcyclopenta-
dienyl)zirconium dichloride
[l-(piperidino)borataben~ene](pentamethylcyclopenta-
dienyl)zirconium dichloride
tl-(pyrrolo)boratabenzene](pentamethylcyclopentadienyl)-
zirconium dichloride~1-(bi8trimethylsilylamino)boratabenzene~(pentamethyl-
cyclopentadienyl)zirconium dichloxide
tl-(diisopropylamino)boratabenzene](pentamethylcy
pe~tadienyl)zirconium dichloride
[1-(N-methylanilino)bora~Ahen7,~e](pentamethylcyclopenta-
dienyl)zirconium dichloride
[1-(diphenylamino)boratabenzene~(pentamethylcyclopenta-
dienyl)zirconium dichloride
tl-(pyrrolidino)borat~h-n7ene]~cyclopentadienyl)zirconium
dichloride
[l-(piperidino)boratabenzene](cyclopentadienyl)zirconium
dic~loride
[l-(pyrrolo)boratabenzene](cyclopentadienyl)zirconium
dichloride
[l-~bi~trimethyl~ilylamino)boratabenzene](cyclopenta-
dienyl)zirconium dichloride
[1-(diisopropylamino)boratabenzene](cyclopentadienyl)-
zirconium dichloride
[1-(N-methylanilino)boratabenzene~tcyclopen~adien~l)-
zirconium dichloridetl-(diphenylamino)~ora t aben z ene](cycl opent adienyl)-
zirconium dichloride
[l-(diphenylpho~phino) borat abenzene](pentamethylcyclo-
pentadienyl) titanium dichloride
CA 02240452 1998-06-19
95/F 313 K W0 - 8 -
[l-(~iphenylphosphino)boratabenzene~(pentamethylcyclo-
pentadienyl)zirconium dichloride
~l-(diphenylphosphino)boratabenzene3(pentamethylcyclo-
pentadienyl)dimethylzirconium
S [l-(di~ethylphosphino)boratabenzene](pen~amethylcyclo-
pentadienyl)zirconium dichloride
[1-(dibutylphosphino)boratabenzene](pen~amethylcyclo-
pentadienyl)zirconium dichloride
[1-(dii~opropylphosphino)bora~abenzene](pentamethylcyclo-
pentadienyl)zirconiu~ dichloride[1-(dicyclohexylphosphino)boratabenzene](pentamethyl-
cyclopentadienyl)zirconium dichloride
[1-(diphenylpho8phino)borat~benzene](cyclopentadienyl)-
zirconium dichloride
[1-(dimethylpho~phino)boratabenzene](cyclopentadienyl)-
zirconium dichloride
[1-(dibutylphosphino)boratabenzene](cyclopentadienyl)-
zirconium dichloride
E1-(diisopropylpho~phino)boratabenzene](cyclopenta-
dienyl)zirconium dichloride[1-(dicyclohexylphoQphino)boratabenzene]( cyc lopenta-
dienyl)zirconium dichlo~ide
bis[1-(diethylamino)boratabenzene]zirconium dichloride
bistl-(diethylamino)boratabenzene]dimethylzirconium
bis[1-(pyrrolidino)boratabenzene]zirconium dichloride
bis[1-(diphenylphosphino)boratabenzene~zirconium
dichloride
bi~[l-(dimethylphosphino)boratabenzene]zirconium
dichloride
[1-(diethylamino)boratabenzene](indenyl)zirconium
dichloride
~1-(diethylamino)boratabenzene](fluorenyl)zirconium
dichloride
~l-(~e~hylboratabenzene](fluorenyl)zirconium dichloride
[l-(diethylamino)boratabenzene]titanium trichloride
~1-(diethylamino)boratabenzene] titanium dichloride
bi~[l-(diethyla~ino)boratabenzene]ti~anium chloride
ethylenebis[4~ methylboratabenzene)~zirconium
dichloride
CA 02240452 1998-06-19
- 95/F 313 K W0 - ~ -
ethylenebi~[3~ methylboratabenzene)~zirconium
dichloride
ethylenebis[2-~1-methylboratabenzene)]zirconium
dichloride
ethylenebis(1-boratabenzene)]zirconium dichloride
ethylenebi~s-(1-dimethylaminoboratabenzene)~zirconium
dichloride
ethylenebi~3-(l-dimethylaminoboratabenzene)~zirconium
dichloride
ethylenebis~2-tl-dimethylaminoboratabenzene)]zirconium
dichloride
ethylenebis[4-~l-diethylami~oboratabenzene)]zirconium
dichloride
ethylenebi~[4-(l-diphenylphosphinoboratabenzene)]-
zirconium dichlorideethylenebis~4-(1-pyrrolidinoboratabenzene)]zirconium
dichloride
ethylenebi~[4~ methylboratabenzene)]titanium dichloride
ethylenebix[3~ methylboratabenzene)]titanium dichloride
ethylenebis~2-(1-methylbora~be~ene)]titanium dichloride
ethylenebis(1-boratabenzene)titanium dichloride
ethylene~(4-(1-methylboratabenzene))cyclopentadienyl~-
zirconium dich-loride
ethylene~(4-(1-~ethylboratabenzene))penta~ethylcyclo-
pentadienyl~zirconium dichlorideethylene[(4-(1-methylboratabenzene))indenyl]zirconium
dichloride
ethylene[(4-(1-~ethylboratabenzene))fluorenyl]zirconium
dichloride
3~ ethylene[(1-boratabenzene)cyclopentadienyl]zirconium
dichloride
ethyleneL(l-boratabenzene)indenyl~zirconium dichloride
ethylene[(l-boratabenzene)fluorenyl]zirconium dichloride
ethylenebis(1-methylaminoboratabenzene)]zirconium
dichloride
ethylenebis~l-phenylphosphino~oratabenzene)]zirconium
dichloride
ethylenebis(1-oxyboratabenzene)]zirconium dichloride
ethylenebi~l-thioboratabenzene)]zir~onium dichloride
CA 02240452 1998-06-19
95/F 313 R WO - 10 -
dimethylsilanediylbi~4-(1-methylboratabenzene)]zirconium
dichloride
dimethyl~ilanediylbis~4-(l-dimethylaminoboratabenzene)]-
zirconium dichloride
dimethylsilanediylbiQ~4-(1-diphenylpho~phinoborata-
benzene)]zirconium dichloride
dimethylsilanediylbi~[(l methyleneboratabenzene)~-
zirconium dichloride
dimethyl~ilanediylbi~[(l-methylaminoboratabenzene)~-
zirconium dichlo~idedimethylsilanediylbis[(l-oxyboratabenzene)~zirconium
dichloride
dimethylsilanediyl[(4-(1-methylboratabenzene)) cyclopenta-
dienyl]zirconium dichloride
lS dimethylsilanediyl[(4-(1-methylboratabenzene))penta-
~ethylcyclopentadienyl]zirconium dichloride
dimethyl3ilanediyl[(4-(l-methylboratabenzene))indenyl]
zirconium dichloride
dimethylsilanediyl[(4-(l-methylborar~hen7.ene))fluorenyl]-
zirconium dichloridedimethyl~ilanediyl[(4-(l-dimethylAmjno~oratabenzene))
cyclopentadienyl]zirconium dichloride
dimethylsilanediyl[(4-(1-diphenylpho6phinolborata-
benzene))cyclopentadienyl]zirconium dichloride
dimethylsilanediyl[(3-(1-dime~hylaminoboratabenzene))-
cyclopentadienyl~zirconium dichloride
dimethylsilanediyl~(2-~l-dimethyl~minohoratabenzene))-
cyclopentadienyl]zirconium dichloride
dimethylsilanediyl[(l-methylaminoboratabenzene))cyclo-
pentadienyl]zirconium dichlorideisopropylidenyl[(4-(1-dimethylaminoboratabenzene))cyclo-
pentadienyl]zirconium dichloride
isopropylid~nylbi~[s-(l methylboratabenzene)]zirconium
dichloride ~
35 The invention provideQ a proce~ for preparing the navel
transition metal compound-~ h~ving the formula (I). The
process is illustrated by the synthesis schemes below ~or
co...~ounds of the formulae IV, v and VI. I~ these
CA 02240452 1998-06-19
- 95/F 313 K WO - 11 -
~ormulae, R, Y, M and X are defined as in formulae I and
II. A i~ a li~and ~uch as cyclopentadienyl, indenyl or
fluorenyl, each of which may be either ~ubstituted or
un~u~tituted. Ml i~ a metal of main group Ia of the
Periodic Table of the ~lement~.
For example, in a proces~ for preparing a compound havi~g
the formula (I), a compound having the formula (III)
reacts wi~h MXl where 1 i~ an integer from 3 to 5.
In an alternative proce~s for preparing a compound having
the formula (I), a compound having the formul~ ~V) reacts
with A-Ml'.
In a further alternative proces~ for preparing a compound
having the formula (I), a compou~d having the formula
~III) reacts with AMX~
R~ R
~Q ~ R
~ r
~' R~--r
R
In an alternative proce~R for preparing a compound having
CA 02240452 1998-06-19
- 95~F 313 K WO - 12 -
the formula ~I), a compound having the formula (VII)
reacts with MX, where 1 is an integer from 3 to 5 and Z
is an element of group IVa of the Periodic Table of the
Element~ and R3 are identical or different and are each a
hydrogen atom, a Cl-C2D-group,- for example a Cl-C20-
hydrocarbon radical such as a C1-C20-alkyl group or a
C6-C20-aryl group, or ~wo adjacent radical~ R3 together
with the atoms connecting them can form a ring y~tem.
Preferably Z i~ ~ilicon, germanium, tin or lead and R3
are identical and are particularly preferably each a Cl-
Clc-alkyl group 8uch a~ methyl, ethyl, i~opropyl or butyl
or a C~-C1c-aryl group such a~ phenyl.
In an alternative process for preparing a compound having
the formula (I), a compound having the formula
react~ with AMX1-l
R R
R~Y
R R ZR,~ R
R R~~
R~ y
t~l ~
R R
~Y
R 11
Y
q R
~he compound~ of the formulae III and VII can be prepared
CA 02240452 1998-06-19
~ 95/F 313 K WO - 13 -
by literat~re methods (Organometallics 1995, 14, 471).
The conver~ion of the compound~ of the formula III into
the de~ired tran~ition metal complexes i~ k~own in
principle. For this purpose, the monoanion of the formula
III i~ reacted in an inert solvent with the corresponding
metal halide ~uch as zirconium tetrachloride. A is a
li~and Yuch aQ cyclopentadienyl, indenyl or fluorenyl
which can eac~ be either ~ubstituted or un~ubstituted.
S~itable solvents for the reaction are aliphatic or
aromatic ~olvents such as h~nP or toluene, ether
solvent~ such as ~etrahydrofuran or diethyl ether or
halogenated hydrocarbons such as methylene chloride or
halogenated aromatic hydrocarbons ~uch as o-dichloro-
benzene.
The invention provide~ for the use of the compound ha~ing
the formula I as a catalyst component in ~he
polymerization of olefins. The present invention
accordin~ly provides a process for preparing a polyolefin
by polymerization of one or more olefin~ in the pre~ence
2 0 of a transition metal compound of the ~ormula I . For the
purposes of the invention, the term polymeriza~ion refer~
to both homopolymerization and copolymerization.
In the process of the in~ention, preference is given to
polymerizing one or more olefin~ of the formula R'-CH=CH-
R~ where R~ and Rb are iden~ical o~ different and are eacha hydrogen atom or a ~ydrocarbon radical having from 1 to
2~ carbon atom~, in particular from 1 to 10 carbon atoms,
or Ra and Rb together with the atoms connecting them form
one or more rings. Examples of such olefi~ are l-olefins
ha~ing 1-20 carbon atom~, for example ethylene, propene,
1-butene, l-pentene, l-hexene, 4-methyl-1-pentene or l-
octene, styrene, cyclic or acyclic diene~ ~uch as 1,3-
butadiene, isoprene, 1,4-hexadiene, norbornadiene,
vinylnorbornene, s-ethylidenenorbornene or cyclic mono-
olefins such a~ nor~ornene or tetracyclododecene. In theproceyy o~ the in~ention, pre~erence is given to homopo-
CA 02240452 1998-06-19
- 95/F 313 K WO - 14 -
lymerizing ethylene or propylene or copolymerizing
ethylene with one or more acyclic 1-olefins having ~rom
3 to 20 carbon atoms, for example propylenç, and/or one
or more diene~ havi~g from 4 to 20 carbon atoms, for
5 examlple 1, 3-butadiene .
The polymerization i-~ preferably carried out at a
temperature of from -60 ~o 250~C, more preferably 20 to
70~C, particularly preferably from 50 to 20~C. The
pressure is preferably from 0.5 to 2000 bar, particularly
preferably ~rom 4 to 64 bar, very particularly prefera~ly
from 5 to 64 bar.
The polymerization can ~e carried out in solution, in
bulk, in su~pen~ion or in the gas pha-~e, continuously or
batchwise, in one or more ~tage~.
The catalyst used in the proces~ of the invention
prefera~ly comprise~ a ~ran~ition metal compound. It i~
also po~sible ~o use mix~ure~ of two or more transition
metal compound~ ~r mixture~ with metallocenes, for
example for preparing polyolefins having a broad or
multimodal molecular weight distribution.
In principle, a suitable cocatalyst in the proce~s of the
invention is any compound which, owing to i~ Lewis
acidity, can eonvert the neutral transition metal
compound in~o a cation and Qtabilize the latter ("labile
coordination"). Furthermore, the cocatalyst or the anion
formed therefrom 6hould undergo no further reactions with
the cation formed (EP-A-427 6g7). A6 cocatalyst,
pre~erence i~ given to using an aluminum compound and/or
a boron compound.
The boron compound preferably has the formula R5XNH4.xBR~,
RsxpH~-xBR6~ R53CBR6~ or BR63, where x i~ from l ~o, 4,
preferably 3, and the radical~ R5 are identical or
different, preferably identical, and are C~-Cl0-alkyl or
C6-ClR-aryl or two radicals R5 together with the atoms
CA 02240452 1998-06-19
95/F 313 R WO - 15 -
connecting them ~orm a ring, and the radicals R6 are
identical or different, preferably identical, and are
C6-Cl~-aryl which may be sub~tituted by alkyl, haloalkyl
or fluorine. In particular R5is ethyl, propyl, butyl or
phenyl and R6 is phenyl, pentafluorophènyl, 3,5-bi~-
(trifluoromethyl)phenyl, mesityl, xylyl or tolyl (EP-A-
2~ 003, EP-A-2~7 004 and EP-A-426 63~).
The cocatalyst used is preferably an all~m~ m compound
~uch as aluminoxane and/or an aluminum alkyl.
The cocataly~t used i~ particularly preferably an
aluminoxane, in particular one of the formula VIIa for
the linear type and/or the formula VIIb for ~he cyclic
type,
~ R~ -
¦ ~ R~
~ O- Al O Al ~ ~n~J)
R~ _ _ D R4
Al O ~llO)
_ _ p~2
where, in the ~ormulae VIIa and VIIb, the radicals R~ are
identical or different and are each hydr~gen or a Cl-C20-
hydrocarbon group such as a C1-Cle-alkyl group, a C6-C1,-
aryl gr~up or benzyl and p is an integer from 2 to 50,
preferably from 10 to 35.
The radical~ R4 are p~eferably identical and are hydro-
gen, methyl, isobutyl, phenyl or benzyl, particularly
preferably methyl
I~ the radical~ R4 are different, then they are
pre~erably me~hyl and hydro~en or alternati~ely methyl
CA 02240452 1998-06-19
~ 95/F 313 K W0 - 16 -
and isobutyl, with hydrogen or i~obutyl preferably ~eing
present in a proportion of from 0.01 to 40~ by number (of
the radicals R4).
The methods of preparing the al~mi~o~nes are known. The
exact -~patial structure of the al~m~nox~ne~ not known
(J. Am. Chem. Soc. (1993) 115, 4971). For example, it i~
concei~able that chains and rings can joi~ to ~or~ larger
two-dimen~ional or three-dimen~ional ~tructure~.
Regardless of the method of pr~paration, all al~;nox~e
~olution~ ha~e in common a varying content of unreacted
alu~nl~ starting compound which i9 pre-~ent in free form
or a~ adduct.
It is pos~ible to preacti~ate the tran~ition metal
compound u~ing a cocatalyst, in particular an alumin-
oxane, prior to use in the polymerization reaction. Thissignificantly increa~e~ the polymerization acti~ity. The
preactivation of the transition metal compound i~
preferably carried out in ~olution. The transition metal
compound i~ here preferably di~olved in a solution of
the aluminoxane in an inert hydrocarbon. Suitable inert
hydrocarbo~ are aliphatic or aromatic hydrocarbons.
Preference i~ given to u~ing toluene.
The concentration of the al~ oxane in the solution is
in the range from about 1~ by weight to the saturation
limit, preferably from 5 to 30~ by weight, in each case
ba~ed on the total amount of solution. The transition
metal compound can be u~ed in the same concentration, ~ut
it is preferably u~ed in an amount of from 10-~ to 1 mol
per mol of aluminoxane. The preactivation time i~ from
5 minutes to 60 hours, preferably from 5 to 60 min~te~.
The preactivation is carried out at a temperature of from
-78 to 100~C, preferably from o to 70~C.
The tran~ition metal compound i8 preferably u~ed in a
concentration, ba-~ed on the ~ran~ition ~etal, of from 10~1
CA 02240452 1998-06-19
95/F 313 K W0 - 17 -
to lO-a mol, pre~erably from lO-' to 10-7 mol, of
tran~ition metal per d~ of sol~ent or per dm3 of reactor
volume. The aluminoxane is preferably used in a
concentration of from 1o-6 to 1o-l mol, preferably fro~ 10-
5 to 10-2, mol per dml of solvent or per dm~ of rea~tor
volume. The other cocatalysts mentioned are used in
approximately equimolar amount~ to the transition metal
compound. However, higher concentrations are alRo
posYible in principle.
To re~ove catalyst poisons pre~e~t in the ole~in,
purification using an aluminum compound, preferably an
al~m; ~l~m alkyl such ag trimethyla~lm;nl~m or trie~hyl-
al~minum, is advantageous. Thi~ purification can be
carried out either in the polymerization system itself or
the ole~in i~ brought into contac~ with the aluminum com-
pound and sub~equently separated off again before
addition to the polymerization system.
In the proceYs of the invention, hydrogen can be added as
a molecular weight regulator and/or to increa~e ~he
catalyst activity. This enables low molecular weight
polyolefin~ ~uch a~ waxe~ to be obtained.
In the process of the pre~ent inve~tion, the tran~ition
metal catalyst is preferably reacted with the cocataly~t
outside the polymerization reac~or in a separate step
using a suitable golvent. Application to a support can
also be carried out during thi~ procedure.
In the process of the invention, a prepolymerlzation can
be carried out with the aid o~ the transition metal
compound. For the prepolymerization, pre~erence is given
to using the (or one of the) olefin(s) used in the
polymerization.
The catalyst u~ed in the proce~s of the invention can be
~upported. Application to a support enables, for example,
the particle morphology of the polyolefin prepared to be
CA 02240452 1998-06-19
95/F 313 K W0
controlled. Here, the transition metal compound can be
f irst reacted with the ~upport and ~ub~equently wich the
cocatalys~. The cocataly~t can al~o be supported ~irst
and subsequently reacted with the tran~ition metal
co~pound. It is also possible to support t~e reaction
product of tran~ition metal compound and cocatalyst.
Suitable ~upport materials are, for example, ~ilica gel~,
aluminum oxide~, ~olid aluminoxane or other inorganic
~upport ma~erial~ such as magnesium chloride. Ano~her
lo ~uitable Qupport material i~ a polyolefin powder i~
finely di~ided form. The preparation of the ~upported
cocatalyst can be carried out, for example, as de~cribed
in EP-A-567 952.
If the polymerization is carried out as a ~uspen~ion or
solution polymeri~ation, an inert 601vent cu~tomary for
the Ziegler low-pres~ure process is used. For example,
the polymerization is carried out in an aliphatic or
cycloaliphatic hydrocarbon, for example propane, butane,
h~Y~n~, heptane, isooctane, cyclohexane, methylcyclo-
hexane. A petroleum or hydrogenated diesel oil fxactioncan al~o be u~ed. It i9 also po~sible to use toluene.
Preference i~ given to carrying out t~e polymerization in
the liquid monomer.
Before addition of the cataly~t, in particular the
-~upported catalyst system (compri~ing the tran~ition
metal compound of the invention and a ~upported
cocatalyst), another aluminum alkyl compound such as
trimethylaluminum~trie~hylaluminum~triisobutylalllm;nllm~
trioctylaluminum or i~oprenrlaluminum can be introduced
into the reactor to make the polymerization sy~te~ inert
(for example to remove catalyst poi~ons pre~ent in t~
olefin). ~iQ compound is added to the polymer~zatlo~
sy~tem in a concentration of from 100 to 0.01 mmol o~ Al
per kg of reactor conten~. Pre~erence is given, to
trii~obutylaluminum and triethylaluminum in a
concentration o~ from 10 to o.1 mmol of Al per kg of
reactor content~, This enableQ the molar Al/ml ratio to
CA 02240452 1998-06-19
95/F 313 K W0 - 19 -
be made -~mall in the synthesi~ of the ~upported catalyst
YyStem ~
If inert qolventR are used, the monomers are preferably
metered in in ga~eou~ or liquid form.
The specific tran~ition metal compound~ described in the
pre~ent invention are suita~le for the preparation of
polyolefins. The latter are suitable, in particular, for
producing shaped bodies such as filmR, plates or large
hollow bodieg (e.g. pipe~) and can al~o be used as
plasticizer and lubricant formulations, for melt adhesive
applications, coatings, seals, insulatio~, filler
compo~itions or ~ound insulation material~.
U6e of hydro~en or increasing ~he polymerization
tempera~ure make it p~ssible to obtain polyolefin~ having
15 a low molar ma6~ ~ e . g . waxe~, who~e hardnes~ or melting
point can be varied by means of the comonomer content.
Selection of the polymerization proce~ and the type(s)
of comonomer(s), ~nd also amount(s) of comonomer(~),
enable olefin copolymers having ela~tomeric properties to
be prepared, for example ethylene-propylene-1,4-hex~;ene
terpolymer~.
The following example~ illus~rate the invention.
Preparation and handling of organometallic compounds were
carried out with exclu~io~ of air and moi~ture under
protect~ve argon ga~ (Schlenk technique). All solvents
r~quired were freed of air and moi~ture ~efore u~e by
boiling for a number of hour~ over a suitable desiccant
and ~ub~equent di-~tillation under argon.
The Al/CH3 ratio in the al~m;nn~ane was de~ermined by
decomposing the sample with HzS04 and determining .the
volume of the hydrolysis gase~ formed under standard
conditions and by complexometric titration of the
aluminum in the then completely dis~olved ~ample by the
CA 02240452 1998-06-19
95/F 313 K W0 20 -
Schwarzenbach method. The compounds were characterized
using ~H-NMR, ~3C-NMR and IR spectroscopy.
Examples
Example 1
bis(l-Methylboratabenzene)zirconium dichloride
1 g of zirconium tetrachloride and 0.84 g of (1-methyl-
boratabenzene)lithium were ~u~pended in 20 ml of ~oluene
and ~tirred for three days at 100~C. The yellow
suspension obtained was filtered and the ~olvent wa~
removed under reduced pre~sure. The yellow solid o~tained
was washed with 5 ml of hexane. The yield of transition
metal compound wa~ O.B9 g (60~ of theory).
'~-NMR (CD2Cl2): 7.6, 6.6, 6.3 (each m, 5H, arom. H), 0.9
(s, 3H, CH3). Mas~ spectrum: 344 Mt~ correct
disintegration pattern.
Example 2
di(~-Chloro)tetra[h6-(1-methylboratabenzene)]dititanium
0.48 g of titanium trichloride and 0.~1 g of (1-methyl-
boratabenzene)lithium are su~pended in 10 ml of toluene
and ~tirred ~or ~hree day-~ at llO~C. The brown suspension
obtained is filtered and the filtrate is cooled to -30~C.
The bro~n solid obtained i~ wa~hed with 5 ml of h~x~n~,
giving the txansition metal compound in a yield of 60
(0.49 g).
Ma~ ~pectrum: 265 M+, correct disintegration pattern.
Example 3
~1-Methylboratabenzene)titanium trichloride
1.36 g of 1-~ethyl-6-(trimethylstannyl)-2,4-boracyclo-
h~y~nediene are dissol~ed in 10 ml of hexane and 1.1 g of
CA 02240452 1998-06-19
~ 9S/F 313 K W0 - 21 -
titanium tetrachloride are added at -10~C. After ~tirring
for 3 hour~, the red-violet ~olution i~ evaporated until
crystallization commence~ and stored for 12 hour~ at
-300C. The product is obtained in the form of deep violet
S cry~tals in a yield of 93~ (1.22 g).
H-NMR (CD2Cl2): 8.1, 7.0, 7.3 (each m, 5H, arom. H), 1.1
(8 , 3H, CH3). Ma~ spectrum: 244 Ml, correct disin-
tegration pattern.
Example 4
(1-Methylborataben 2 ene ) c yC lopentadienyltitanium
dichloride
0.57 g of cyclopentadienyltitanium trichloride are
dissolved in S ml of tetrahydrofuran and admixed at - ~ 0 ~ C
with a solution of 0.65 ~ of 1-methyl-6-(trimethyl-
stannyl)-2,4-boracyclohexanediene in 2 ml of tetrahydro-
~uran. After ~he Rolu~ion has been warmed to room
temperature, the precipitated dark green ery~tal~ are
filtered off; yield: 60~ (0.4 g).
lH-NMR (CD2Cl2): 7.8, 6.9, 5.9 ~each m, 5H, arom. H), 6.7
(m, 5H, Cp-H), 1.1 (s, 3H, CH~). Ma~s spe~trum: 274 M+,
correct diQintegration pattern.
Example 5
~ ethylboratabenzene)cyclopentadienyl~iraonium
dichloride ~
0.3 g of cyclopentadienylzirconium trichlorida is
~uQpended in 5 ml of toluene and ad~ixed at room
temperature with a solution of 0.3~ ~ of 1-methyl-6-
(trimethylsilyl)-2,4-boracyclohexanediene in 2 ~ml of
toluene. Af~er the solution has been heated for 3 hours
at ~OoC, it i~ cooled to -30~C and ~tored for 12 hour~at
thi~ temperature. Thi3 gives 0.26 g of the yellow
compound (70~).
lH-NMR (~D2C12): 7.7, 6.3, 6.~ (each m, SH, arom. H), 6.
CA 022404~2 l998-06-l9
9~/F 313 K wo - Z2 -
(m, 5H, Cp-H), 1.0 ~s, ~H, CH3). ~a~ ~pe~trum: 316 Ml,
correct disintegration pattern.
Example 6
bis(l-Methylboratabenzene)hafnium dichloride
0.32 g of hafnium tetrachloride i~ ~uspended in ~ ml of
toluene and admixed at room temperature with a solution
of 0.33 g of 1-methyl-6-(trimethylsilyl)-2,4-boracyclo-
hexanediene in 2 ml of toluene. After the ~olution ha~
been ~eated for 2 hours at 70~C, it is cooled to -30~C
and ~tored for 12 hours at thi~ temperature. ThiQ givee
0.37 g of the yellow compound (88~).
H-NMR (CD2C12): 7.6, 6.3, 6.6 (each m, 5H, arom. H), 5.8
(m, 5H, Cp-H), 1.0 (~, 3H, CH3) . Mas~ ~pectrum: 132 M+,
correct di~integration pattern.
Example 7
tl-Methylboratabenzene)(pentamethylcyclopentadienyl)-
hafnium dichloride
1.1 g of peneame~hylcyclope~tadienylhafnium trichloride
are ~uspended in 10 ml of toluene and admixed at room
temperat~re with a ~olution of 0.27 g o$ l-methylborata-
benzenelithium in 10 ml of toluene and 2 ml of dimethoxy-
ethane. After the ~olution has been heated for 3 hours at
110~C, the suspension is filtered and the filtrate is
cooled to -30~C and stored for 12 hours at this
temperature. Thi~ give~ 1. 0~ g of the pale yellow
compound (85~).
H-NMR (CD2C12): 7.5, 6.2, 6.0 (each m, 5H, arom. H), 1.8
(s, lSH, Cp-CH3), 0.8 (x, 3H, CH3) . Mas~ spectrum 476 M+,
correcC di~integration pattern.
Polymerization
The catalysts of the formula (I) were used for PE
' CA 02240452 1998-06-19
- ss/F 313 K wo - 23 -
polymerization in a su~pen~ion polymerization in an
autoclave. Cataly#t I is bisll-methylboratabenzene)-
zirconium dichloride. Cataly-qt II i-~ methylborata-
benze~e~(pentamethyl~yclopentadienyl)zirconium
dichloride. Catalyqt II~ iq ~l-dimethylaminoborata-
~enze~e)(pentamethylcyclopen~adienyl)zirconium
dichloride.
Example ~
2.0 mg of cataly~t II (0.0052 mmol of Zr) were dissol~ed
under argon ~hile ~tirring in 1.5 ml o~ toluene in a
Schlenk tube. The ~olution was then activated by ~;n~
3.7 ml of 30~ ~trength MAO (17.69 mmol of Al; 2r:Al =
1:3402) from Witco and thi~ ~olution wa~ added to 750 ml
of ~xxsol loo/120. The catalyst solution wa~ tran6ferred
to an autoclave. In the autoclave, polymerization wa~
carried out for one hour at a tempera~ure of 70OC and a
stirrer ~peed of 750 rpm under an ethylene partial
pressure o~ 4 bar. From the yield of 145.72 g of PE,
which is formed as a fine p~wder, the catalyst activity
is calculated a~ 7006 g PE/mmol Zr/bar C2H~/h.
~mrles 9 to 13 were carried out similarly to Example a.
The changes from Example Y are ~hown in Table 1 below.
Table 1: Polymerization Examples 9 to 13
Example Cat. ~at. Cat. Al Zr:Al Pre~sure rield Cataly~t
[mg] ~mmol][mmol~ of C,H, tg] acti~rity~
[bar]
259I 1.8 0.0052 15.77 1:3033 4 12.26 586
10I 2.q 0.007023.89 1:3413 4 17.86 641~
11II 1.7 0.0044 16.72 1:3800 4 69.76 3982 "
12III 2.4 0.005821 99 1:3791 4 41.50 17B9
13III 2.8 0.006723.~9 1:3566 ~ 42.90 1~01
~Catalyst acti~ity: [g PE/mmol Zr/bar C2H4/h]
CA 02240452 1998-06-19
~ 95/F 313 K W0 - 24 -
Preparation of organoboron compound~
Example 14
1-Dimethylamino-6-(trimethylsilyl)boracyclohexa-2,4-diene
1.5 g of l-dimethylA~;nohoratabenzenelithium ~TMEDA) were
suspended in 50 ml o~ pentane and cooled to -75~C. 1.0 ml
of chlorotrimethyl~ilane wa~ added to the ~uspension, the
~ixture wa~ allowed to warm up to room temperature and
filtered, the pentane was removed under reduced pres~ure
and the product wa~ distilled at 103~C/12 mbar. The yield
of the organoboron compound wa~ 1.0 g (84~ of theory).
l~_NMR ~CDC13); 6.80, 6.~3, 6.09, 5.97 (each m, 4H,
olefin. H), 2.76, 2.64 (each ~, 6H, ~ (CH3) Z), 2.42 (d,
6.1 Hz, lH, CH), 0.1 (s, 9H, Si(CH3)3).
Mass spectrum~ 193 M', correct disintegration pattern.
Example 15
1-Chloro-6-(trimethylsilyl)boracyclohexa-2,4-diene
4.8 gofl-dimethylamino-5-(trimethyl6ilyl)boracyclohexa-
2,4-diene were dissol~ed in 10 ml of ~ethylene chloride
and cooled to -75~C. 29 ml of a 0.89 molar solution of
BCl3 in methylene chloride were added to the a~ove
sol~tion, the mixture wa~ allowed to warm up to room
temperature, the solvent wa~ removed undèr reduced
pressure and the product was distilled at 64-65~C. The
yield of the organoboron compound was 3.36 g (-73~ of
theory).
~H-NMR (CDCl3, 250 MHz): 7.5, 6.8, 6.45 (each br., 3H,
olefin. H), 6.47 (dd, ~.4 Hz, lH, olefin. H), 3.6 ~br.,
lH, CH), 0.12 (~, ~H, Si (~H3) 3) .
Mass spectrum: 1~4 M , correct disintegration pattern.
CA 02240452 1998-06-19
~ 95/F 313 K WO - 25 -
Example 16
Bis[(6-trimethylsilyl)~oracyclohexa-2,4-dien-1-yl] oxide
(ra~ and me~o ~orm)
100 ~l of diethyl ether were cooled to -75~C a~d 0.021 g
of water and 0.473 g of 1-chloro-6-(trimethylsilyl)bora-
cyclohexa-2,4-diene were added in ~ucce~io~. The mixture
waa allowed to warm up to room temperature, the ether was
~ lOved under reduced pressure and the crude product up
to 180~C/10-3 mbar wa~ condensed in a cooled receiver. The
yield of-the organo~oron compound was 0.32 g (8~ of
theory).
lH-NMR (CDCl3~: 7.35, 6.6, 6.3, 6.1 (each m, 8H, olefin.
H), 2.84, 2.78, 2.5~ (each d, 5.2 Hz, 2H, CH), 0.1, 0.9,
O.7 (each a, 18H, Si (CH3) 3) .
Mas~ ~pectrum: 314 M , correct di~integration pattern.