Note: Descriptions are shown in the official language in which they were submitted.
CA 02241004 1998-06-19
WO 97/22251 PCT/US96/20494
COMPOSITTONS FOR MODULATING INTRACELLULAR INOSITOL
TRISPHOSPHATE CONCENTRATION AND USES TH~REOF
Background of the Invention
This invention relates to modulation of inositol
trisphosphate (InsP3) concentration in neurons.
Within the nervous system, information is conveyed
from one neuron to another by electrical signals that are
generated by the flux of ions, including calcium ions,
10 across the neuronal cell membrane. When certain cell
surface receptors are bound, calcium enters the cell
through selective channels and may also be released from
intracellular stores. The cell surface receptors
involved include those that are bound by excitatory amino
15 acids such as glutamate. Glutamate, and other agonists
(discussed below), bind metabotropic receptors that are
coupled to G proteins, and thereby instigate the
biochemical cascade that leads to the release of calcium
from intracellular stores.
There are seven immunologically distinct subtypes
of metabotropic glutamate receptors (M1 - M7). When
bound, two of these receptor subtypes, Ml and M5, produce
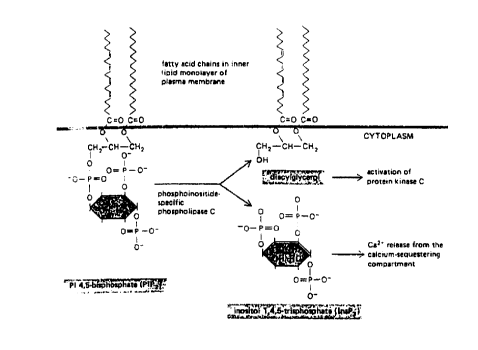
the second messenger InsP3 by stimulating
phosphoinositide-specific phospholipase C (hereinafter,
25 I'phospholipase C"), which converts phosphatidylinositol
bisphosphate, a lipid located in the plasma membrane, to
diacylglycerol and InsP3 (this reaction is illustrated in
Fig. 1).
In addition to L-glutamate, metabotropic receptors
30 are activated by L-aspartate and by the pharmacological
agonists quisqualate, ibotenate, and trans-ACP~
( trans- ( +-1 ) -1-amino-1,3-cyclopentanedicarboxylate;
Schoepp et al ., Trends Pharmacol. Sci., 11:508-515,
1990). L-aspartate and aspartate analogs also act as
35 agonists for metabotropic receptors expressed by neurons
in the ~rain (Porter et al., Neurosci. Lett., 144:87-89,
CA 02241004 1998-06-19
WO97/22251 PCT~S96~0494
1992). In addition to stimulating metabotropic
receptors, quisqualate and ibotenate stimulate ionotropic
receptors, which are coupled to ion channels (Watkins et
al., Trends Pharmacolo. Sci. 11:25-33, 1993). Thus, of
5 the excitatory amino acid receptor agonists, trans-ACPD
may be more selective for phosphoinositide-linked
metabotropic receptors (Desai and Conn, Neurosci. Lett.,
109:157-162, 1990). Pharmacological testing has also
shown that L-trans-pyrrolidine-2,4-dicarboxylate and
10 D,L-homocysteate stimulate receptor-coupled
phosphoinositide hydrolysis in rat brain tissue (Li and
Jope, Biochem. Pharmacol. 38:2781-2787, 1989).
Overstimulation of metabotropic receptors is
thought to occur in the course of several neurological
15 disorders. This overstimulation, and the resulting
increase in InsP3 production, increases intracellular
calcium to levels that produce severe hyper-functional
defects (see, for example, Thomsen et al., J. Neurochem.,
62:2492-2495, 1994) and eventual neurotoxicity and death
(Berridge, Nature, 361:315-325, 1993; Choi and Rothman,
Ann. Rev. Neurosci., 13:171-182, 1990). Specific
disorders associated with overstimulation of metabotropic
glutamate receptors in the brain include limbic seizures
(Tiziano et al ., Neurosci. Lett., 162:12-16, 1993) and
2S chronic neurodegenerative disorders such as Alzheimer's
disease, Huntington's disease, Parkinson's Disease, and
amyotrophic lateral sclerosis (ALS; more commonly known
as Lou Gehrig's Disease).
Another type of neuronal cell death, referred to
30 as programmed cell death or apoptosis, may be affected by
reduced activity of metabotropic glutamate receptors
(Copani et al., J. Neurochem. 64:101-108, 1995).
Similarly, inhibition of InsP3 is thought to mediate
neuronal apoptosis by reducing intracellular calcium.
CA 02241004 1998-06-19
WO 97/22251 PCT/US96/20494
Summary of the Invention
The work described herein is aimed at altering the
m~ch~n;sm(s) that regulate the concentration of InsP3 in
order to treat neurological disorders that are associated
5 with hyperactive function and neuronal cell death.
Accordingly, the present invention provides methods and
compositions for modulating the concentration of InsP3 in
neurons. Disorders associated with glutamate
excitotoxicity (either directly or indirectly) should be
10 particularly amenable to treatment with these
compositions and methods.
Brief Descri~tion of the Drawing
Fig. 1 is a diagram illustrating conversion of
phosphitidyl inositol 4,5,-bisphosphate (PIP2) into
15 diacylglycerol and inositol trisphosphate (InsP3).
Fig. 2 is a diagram of the structure of the (lyso)
sphingolipids sphingosine, lysosphingomyelin, and
lysocerebroside.
Fig. 3 is a bar graph illustrating the effects of
20 sphingosine and psychosine (lysocerebroside) on basal and
bradykinin-stimulated phosphoinositide signalling in PC-
12 cells. The bars marked A represent cells that were
untreated. The bars marked B represent cells that were
exposed to 10 ~M bradykinin for 30 minutes. The bars
25 marked C represent cells that were exposed to 100 ~g/ml
sphingosine for 30 minutes before treatment with an
excitotoxic agent.
Fig. 4 is a graphical representation of the
concentration effects of psychosine repression of
30 bradykinin-stimulated phosphoinositide signalling in PC12
cells.
Figs. 5A and 5B are scanned images at low (Fig.
5A) and high power magnification (Fig. 5B) of the CAl
CA 02241004 1998-06-19
WO 971222SI PCT/US96/20494
region of the hippocampus 7 days after infusion of 225 nm
quisqualate.
Figs. 6A and 6B are scanned images at low (Fig.
6A) and high power magnification (Fig. 6B) of the CA1
5 region of the hippocampus 7 days after infusion of
saline.
Figs 7A and 7B are scanned images at low (Fig. 7A)
and high power magnification (Fig. 7B) of the CA1 region
of the hippocampus seven days after treatment with 125 nm
10 psychosine and subsequent exposure to 225 quisqualate.
Fig. 8 is a bar graph illustrating the mean number
of convulsions or spasms (+SEM) across four groups of
treated animals. Q = quisqualate; P = psychosine;
SP = lysosphingomyelin.
Fig. 9 is a bar graph illustrating the mean
duration (in minutes) of three behaviors (+SEM): teeth
chatter, akinesia, and mobilizing. Q = quisqualate;
P = psychosine; SP = lysosphingomyelin.
CA 02241004 1998-06-19
WO 97/22251 PCT/US9612W94
Detailed Description
Sphingolipid is the name given to derivatives of
fatty acid-containing compounds of the long chain
amphiphilic amino alcohol sphingosine whose terminal
5 hydroxyl group is substituted by phosphoryl, glycosyl, or
other groups. Cationic sphingolipids that lack the fatty
acid component of the parent sphingolipid compound are
called lysosphingolipids. Free unsubstituted sphingosine
and the terminally substituted derivatives of
10 sphingosine: lysosphingomyelin (sphingosyl
phosphorylcholine), lysocerebroside (also called glycosyl
sphingosine or psychosine), lysosulphatides, and
lysogangliosides are all lysosphingolipids.
It is disclosed herein that, at non-toxic
15 physiological levels, naturally occurring sphingosine
increases the production of InsP3 in brain neurons, while
naturally occurring lysosphingolipids, lysocerebroside,
and ~ysosphingomyelin (and no other naturally occurring
lysosphingolipids) potently repress InsP3. It is further
20 disclosed that lysosphingomyelin and lysocerebroside
potently and specifically repress the increase in InsP3
that is induced when neurons are exposed to excitatory
amino acids or their analogue agonists, such as ibotenate
and quisqualate. The repression of InsP3 by
25 lysosphingomyelin and lysocerebroside competes with the
stimulation of InsP3 by sphingosine.
The invention provides a method of modulating
InsP3 concentration in a neuronal cell of a mammal that
has, or is suspected of having, a disorder associated
30 with an abnormal concentration of InsP3. The method
involves administering to the mammal a composition
containing an isolated compound that modulates the
concentration of InsP3 and a pharmaceutically acceptable
carrier. The modulation produced by this method may
35 result in a decrease in InsP3 production (as can be
CA 02241004 1998-06-19
WOg7/22251 PCT~S96120494
caused by the compounds lysocerebroside or
lysosphingomyelin) or an increase in InsP3 production (as
can be caused by the compound sphingosine). The carrier
may consist of an excipient including buffers such as
5 citrate buffer, phosphate buffer, acetate buffer, and
bicarbonate buffer, amino acids, urea, alcohols, ascorbic
acid, phospholipids, proteins such as serum albumin,
gelatin, EDTA, sodium chloride, liposomes,
polyvinylpyrollidone, mannitol, sorbitol, glycerol,
10 propylene glycol, and polyethylene glycol (e.g., PEG-4000
or PEG-8000). The neuron that is contacted may reside
within the peripheral nervous system or the central
nervous system. Preferably, the neuron is within the
brain.
The composition described above may be
administered by any route known to skilled
pharmacologists. The route of administration may be, for
example, intra-arterial, intracerebral, intrapulmonary,
or transmucosal. Preferably, administration is by
20 subcutaneous, intramuscular, or intraperitoneal injection
and, most preferably, by intravenous injection.
If necessary, the compounds of the invention, or
compounds discovered by the method of the invention, can
be modified to increase the efficiency with which they
25 cross the blood brain barrier. In order to enable these
compounds to penetrate the blood brain barrier, they can
be delivered in encapsulated cell implants (e.g., those
produced by CytoTherapeutics, Inc., Providence RI; see
Bioworld Today 7: 6, December 2, 1996). Delivery of drugs
30 to the brain may also be accomplished using RMP-7~
technology (Alkermes, Inc., Cambridge, MA: see Business
Wire, "Third Major Agreement for Prolease Sustained
Release Drug Delivery System," (December 2, 1996) or
implantable wafers containing the drug (see PR Newswire,
35 "Implantable Wafer is First Treatment to Deliver
CA 02241004 1998-06-19
W097~2251 PCT~S96/20494
Chemotherapy Directly to Tumor Site, " September 24,
1996). The compositions may also be administered using
an implantable pump for direct administration into
intrathecal fluid (e.g., that made by Medtronic,
5 Minneapolis, MN; see ~enetic Engineering News,
"Neurobiotechnology Companies Focus Programs on Pain and
Neuroprotection," November 1, 1996).
The route of administration and the amount of
protein delivered will be determined by factors that are
10 well within the ability of skilled artisans to assess.
Furthermore, skilled artisans are aware that the route of
administration and dosage of a therapeutic substance may
be varied for a given patient until a therapeutic dosage
level is obtained. The dosage and length of any
15 treatment are known to depend on the nature and severity
of the disease and to vary from patient to patient as a
function of age, weight, sex, and general health, as well
as the particular compound to be administered, the time
and route of administration and other drugs being
20 administered concurrently. Skilled artisans will be
guided in their determination of the appropriate
therapeutic regime by, e.g., Gregoriadis (Drug Carriers
in Biology and Medicine, Academic Press) and Goodman and
Gilman (The Pharmacological Basis of Therapeutics, 6th
25 Edition). Skilled artisans can be guided further in
their determination of the correct therapeutic dosage by
assessing behavioral criteria, as disclosed in
Example VIII ! or by performing any standard test of a
patient's cognitive or motor skills. Typically, the
30 dosage of an InsP3 modulatory substance described herein
will range from 0.01 to loO mg/kg of body weight. More
preferably, sphingolipids such as sphingosine,
lysosphingomyelin, and lysocerebroside are administered
in the range from about 0.5 mg/kg body weight to 1.5
35 mg/kg body weight for each compound. It is expected that
CA 02241004 1998-06-19
WO 97/22251 PCT/US96/20494
-- 8
regularly repeated doses of the InsP3 modulatory compound
will be necessary over the life of the patient.
Alternatively, a neuron may be contacted in vitro.
A pharmaceutical composition containing a compound
5 that modulates InsP3 in mammalian neuronal tissue and a
pharmaceutically acceptable carrier is another embodiment
of the invention. This composition can modulate the
concentration of InsP3 by either increasing or decreasing
the concentration of InsP3 within a neuron, such as a
10 neuron within the brain, to a concentration that is
sufficient to treat a disorder that is associated with
abnormal InsP3 production.
When an increase in intracellular InsP3 is sought,
the isolated compound of the invention is a
15 lysosphingolipid which may be, but is not limited to,
sphingosine. When a decrease in intracellular InsP3 is
sought, the isolated compound of the invention is a
lysosphingolipid which may be, but is not limited to,
lysosphingomyelin or lysocerebroside. Preferably, the
20 mechanism by which the composition of the invention
modulates inositol trisphosphate concentration is by
modulating the activity of phosphoinositidase-specific
phospholipase C. The composition can be used to modulate
InsP3 in a neuron in vitro or in vivo.
When the invention provides a composition
containing a lysosphingolipid in a pharmaceutically
acceptable carrier, the lysosphingolipid is preferably
characterized as being the D-erythro isomer and having:
(1) a trans-4 double bond, (2) a net positive charge, (3)
30 an aliphatic chain of 8 or more carbon atoms linked in
series, and (4) a net neutrally charged substituent on
the oxygen atom of carbon atom 1. The substituent may
be, but is not limited to, any of the following:
hydrogen, monosaccharide, disaccharide, trisaccharide,
35 polysaccharide, phosphorylcholine, and phosphoryl
CA 02241004 1998-06-19
WO97~2251 PCT~S96/20494
ethanolamine. Preferably, agents fitting these
parameters and occurring at physiological, ng/mg protein
levels (Kolesnick, J. Biol. Chem., 264:7617, 1989), are
the naturally occurring lysosphingolipids sphingosine,
5 psychosine, and lysosphinqomyelin. Saturation of the
double bond of sphingosine by hydrogenation produces the
compound sphinganine which has less than one third the
membrane insertion potential of sphingosine. Compounds
such as N-acetyl sphingosine or ceramide, in which the
lO amino group is amidated and rendered non-ionic,
apparently have little or no physiological effect in this
system. A cationic free base, 4-trans-enic amphiphile
provides physiological activity. Ionically neutral l-0-
substitution provides inhibitory activity.
A method of identifying a compound that modulates
the production of InsP3, preferably in a neuron, is also
provided by the invention. In this method, a neuron is
contacted, either in vitro or in vivo with a compound and
inositol, such as tritium-labeled inositol, under
20 conditions sufficient to modulate InsP3 production. The
amount of inositol trisphosphate produced is then
measured. Any change in the concentration of InsP3 can
be measured by standard techniques known to one of
ordinary skill in the art. For example, one can monitor
25 the amount of radiolabelled InsP3 produced from
prelabelled inositol starting material. Alternatively,
symptoms of a mammal having a neural disorder can be
monitored. The compound identified by this method of the
invention may modulate an increase or a decrease in InsP3
30 production. The method of identifying a compound that
modulates neuronal inositol trisphosphate concentration
may also include contacting the neuron with a second
compound that modulates InsP3.
CA 02241004 1998-06-lg
WO97/22251 PCT~S96120494
-- 10 --
The invention also provides for a first and a
second compound contacting a neuron under conditions
sufficient to modulate InsP3 production.
In addition to neurons, the nervous system
5 contains neuroglia, such as astrocytes, Schwann cells,
and microglia, which contribute to repair processes in
the nervous system and lend support to the neurons.
Neuroglia and neurons are phenotypically distinct cell
types. For example, astrocytes have major adrenergic
(rather than excitatory amino acid) metabotropic
receptors and, in these cells, InsP3 production is
enhanced by sphingosine and inhibited by psychosine
(Ritchie et al., Biochem. Biophys. Res. Commun.,
186:790-795, 1992). In addition, the way in which InsP3
15 controls calcium ion concentration and signalling in
astrocytes is distinctly different from that in brain
neurons: in astrocytes, sphingolipid modulation of InsP3
production occurs by ~-adrenergic stimulation of InsP3
production via a G protein intermediate that is inhibited
20 by treatment with pertussis toxin (Ritchie et al.,
supra ) . Furthermore, InsP3 produced in astrocytes is
communicated through gap junctions to other cells,
whereas in neurons, InsP3 remains within the cell where
it modulates the concentration of Ca2+.
Applicants have shown that sphingosine increases
the basal level of InsP3 in brain neurons independently
of stimulation of excitatory amino acid receptors by any
agonists (the effect of sphingosine ~eing downstream from
the receptors). Because the excitatory amino acid
30 analogues are structurally and functionally unrelated to
sphingolipids (as reflected ~y their action at a distinct
point in the signalling pathway), these analogues do not
predict the present invention.
The drug chlorpromazine (lO-(3-
35 dimethylaminopropyl)-2-chlorphenothiazine) raises the
CA 02241004 1998-06-19
WO97~2251 PCT~S96/20494
-- 11 --
level of InsP3 in rat C6 glioma cells (Leli and Hauser,
Biochem. Biophys. Res. Commun., 135:465-472, 1986).
However, chlorpromazine is unrelated structurally and
functionally to the sphingolipid compositions of the
5 present invention, and therefore does not predict the
present invention.
Sphingosine and several sphingosine derivatives
inhibit a protein kinase C isoform that is activated by
diacylglycerol and phorbol esters in non-neuronal cell
10 types such as lymphocytes, neutrophils, granulocytes,
Chinese hamster ovary cells, and platelets. (Hannum and
Bell, Trends Biochem. Sci., 20:73-77, 1987; and Grove and
Maestro, Biochem. Biophys. Res. Commun., 151:94-99,
1988). However, it is disclosed herein that the effects
15 of sphingosine on brain neurons are independent of
protein kinase C inhibition: the protein kinase C
inhibitor staurosporine does not influence the effects of
sphingosine, lysocerebroside, or lysosphingomyelin on
InsP3 production in brain neurons that are responsive to
20 excitatory amino acids. As a result, the protein kinase
C-mediated pathway and the phospholipase C-mediated
pathway for controlling intraneuronal calcium ion
concentration are independent. Consequently, compounds
that affect the protein kinase C pathway are not
25 predictive of the effects of compounds, such as the
sphingolipids of the present invention, on phospholipase
C pathway regulation.
A synthetic analog of the amino acid glycine ((S)-
4-carboxy-3-hydroxyphenyl glycine) blocks a metabotropic
(but not ionotropic) amino acid receptor and is thereby
thought to render protection against seizures (Thomsen et
al ., supra ) . However, blockage of glutamate-responsive
metabotropic receptors by therapeutic glutamate
antagonists is highly impractical because these
35 antagonists are generally toxic (Michel and Agid, J.
CA 02241004 1998-06-19
WO 97n2251 PCT/US96/20494
Neurosci. Res., 40:764-775, 1995). In contrast, the
compositions and methods of this invention contain
naturally occurring lysosphingolipids that act downstream
from the cell surface receptor. These substances are
5 associated with a reduced risk of toxicity.
It is a significant advantage of the invention
that naturally occurring compounds within a non-toxic
physiologic range are used, limiting the possible dangers
to the patient and undesirable side effects that
10 accompany treatment with non-natural, synthetic
pharmaceutical compounds. The lysophingolipids are
metabolized and cannot accumulate to dangerous levels in
patients, as is the case for the synthetic receptor
blockers. Also, the lysosphingolipids used herein do not
15 rely upon liver detoxification and kidney excretion so
their use will have minimal risk of liver or kidney
damage. In addition, receptor blockage disturbs normal
brain function, which may be avoided by targeting
phospholipase C and allowing receptor function to
20 regulate neuronal ion balance. A further advantage of
the invention is that the naturally occurring compounds
of the invention are readily available, making use of the
present invention potentially much less expensive to the
patient than the use of synthetic pharmaceutical
25 preparations.
An object of the invention is to provide methods
and compositions that can be used to treat mammalian
conditions associated with glutamate excitotoxity. These
conditions include, ~ut are not limited to, acute
30 conditions stemming from brain anoxia or ischemia, brain
seizure activity, and chronic conditions such as
Alzheimer's disease, Parkinson's Disease, Huntington's
disease, and amyotrophic lateral sclerosis.
Other features and advantages of the invention
35 will be apparent from the following detailed description,
CA 02241004 1998-06-19
W097n22~1 PCT~S96/20494
- 13 -
and from the claims. Although methods and materials
similar or equivalent to those described herein can be
used in the practice or testing of the invention, the
preferred methods and materials are described below.
The finding that lysosphingolipids influence InsP3
production which, in turn, controls the concentration of
calcium ions in neurons, led to the compositions and
methods described herein for controlling aberrant InsP3
production in various neuronal disorders. Exemplary,
10 non-limiting compositions and methods that can be used to
carry out the invention are described below.
ExamPle 1: Preparation of Neuronal Cell Cultures
Primary cultures of neurons were prepared from the
telencephalon of white Leghorn chick embryos on the
15 eighth day of their development (Rosenberg et al.,
.Biol. Chem. 267:10601-10612, 1992). The neurons were
seeded in 24-well plastic tissue culture plates that had
been coated with L-polylysine. The density of the
neurons was 105 cells per well, and they were cultured in
20 Dulbecco's modified Eagle's medium/Ham's high glucose F-
12 medium (1:1, vol:vol) containing 500 ng/L codium
selenite, 500 ~g/L transferrin, and 165 pmole/L EGF. The
cultures were maintained at 37~C under 5% CO2 in air for
4 days, by which time the cells had differentiated into
25 neurite-bearing cortical granulocytic neurons. These
cultures of mature embryonic neurons were used for
phosphoinositide hydrolysis assays (as described in
Example 2).
PC12 cells, from a rat adrenal pheochromocytoma
30 cell line, were used as a model of neurons from the
peripheral nervous system. These cells were obtained
from a common laboratory cell culture stock and grown in
plastic culture flasks in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 5~ heat-inactivated horse
CA 02241004 1998-06-l9
WO 97/22251 PCT/USg6/20494
-- 14 --
serum and 10% heat-inactivated fetal calf serum (PC12
cells are also available from the American Type Culture
Collection under the Accession number CRL 1721). The
cells were grown at 37~C in 5% C02 in air until a
5 confluent monolayer formed. The cells were dispersed
into 24-well plastic tissue culture plates at a density
of 105 cells per well and used for the phosphoinositide
hydrolysis assays (as described in Example 2).
Example 2: Measurement of Inositol TrisphosPhate
10 Production bY a Phosphoinositide Hydrolysis Assay
Receptor-stimulated and basal unstimulated
hydrolysis of phosphoinositides was measured by the
following procedure. In order to label the cellular
phosphoinositides metabolically, the primary cultures of
15 chick cortical neurons (described in Example 1) were
incubated overnight with 0.5 ml Dulbecco's modified
Eagle's medium containing 1 ~Ci [3H]myo-inositol per
well. The cells were then washed twice with 1 ml
Dulbecco's phosphate-buffered saline (PBS) and 0.5 ml of
20 PBS containing 4.5 g/L glucose was added. Sphingosine,
psychosine, lysosulphatide, or lysosphingomyelin,
prepared as the hydrochloride salt dissolved in water,
were then added to produce the desired extracellular
concentration in specific wells, and the 24-well plates
25 were swirled gently on a rotary shaker for 1 hour at
23~C. The cells were then washed twice with 1 ml PBS,
and pre-incubated for 15 minutes at 37~C with 0.5 ml PBS
containing 10 mM LiCl and the compound to be tested was
added. After 30 minutes, the experiment was terminated
30 by adding 1 ml of ice-cold methanol to each well and
transferring the material in each well to polypropylene
tubes that contained 0.4 ml water and 1 ml chloroform.
The tubes were vortexed thoroughly, then centrifuged at
500 x g for 5 minutes to separate the aqueous and
35 chloroform phases. For each sample, a 1.5-ml aliquot of
CA 02241004 1998-06-19
WO 97/222sl PCT/US96/20494
the upper (aqueous) phase was applied to a small column
containing BioRad AGlX8 resin (formate form). Free
[3H]inositol and [3H]glycerophosphoinositol were washed
through the column with 5 ml of a solution containing
5 5 mM sodium borate and 60 mM sodium formate. The total
phosphorylated [3H] inositol fraction was eluted from the
column for radiometric scintillation spectrometry
analysis with 3 ml 1.0 M ammonium formate/0.1 M formic
acid.
In addition, a 0.5 ml aliquot of the lower
(chloroform) phase of each sample was analyzed
radiometrically by scintillation spectrometry in order to
determine the ~uantity of lipid-bound [3H]m~o-inositol.
Estimation of phosphoinositide hydrolysis by
15 phospholipase C is based upon the quantity of
[3H]inositol phosphates produced, and is expressed as the
percentage of the latter relative to total free and
componential [3H~inositol (dpm ammonium formate + dpm
chloroform fractions).
ExamPle 3: InsP3 Production in Neurons
A. Inositol tris~hosphate p~oduction in PCl2 Cells
PC12 cells display a strong phospholipase C-linked
metabotropic receptor response to bradykinin, an
effective pain producing agonist. As with the other
25 neuronal cells, sphingosine tonically upregulated the
basal, unstimulated level of InsP3 production in PC12
cells. Psychosine and lysosphingomyelin strongly inhibit
InsP3 production in PC12 cells and, even at relatively
moderate levels, can entirely block the sensitivity of
30 PC12 cells to bradykinin. These findings are shown in
Fig 3.
The degree of InsP3 inhibition is a function of
the log of the psychosine concentration for a fixed time
of exposure (Fig. 4). Exposing PC12 cells to 50 ~M
CA 02241004 1998-06-19
WO97/22251 PCT~S96/20494
- 16 -
exogenous psychosine for 30 minutes was sufficient to
reduce a metabotropic response to lO ~M bradykinin to
half maximum.
B. InsP3 Production in Cultured Neurons
Cultured primary chick cortical neurons that were
prelabeled with t3H]myo-inositol were examined for
enhanced InsP3 signalling in response to various agonists
including ~lutamate, aspartate, and the metabotropic
Ml/M5 receptor agonists ibotenate and quisqualate. As
lO shown in Table l, sphingosine, lysocerebroside, and
lysosphingomyelin potently modulated InsP3 signalling.
Lysosulphatide was without inhibitory effect in this
system. Thus, sphingosine tonically enhanced the basal,
unstimulated InsP3 level in cortical neurons, while
15 psychosine and lysosphingomyelin blocked this enhancement
whether it was caused by sphingosine or by metabotropic
glutamate receptor agonists.
CA 02241004 1998-06-19
WO 97/22251 PCT/US96/20494
_ 17 --
~o 3
U~ ~ C,
0 o
,:~ . - C --I ~ + -
~ ~ V o
+~ I C. Il
o ~ .~1 ~v ~ Q
0_ ~ ~ 0
o ~ ~;,, a~ ~ ~
~, o+1 0 ~ 0
~1~ o o +1 1~ J
~ ~ o ~r 0~
o ~ o ~ ~: 3 ~
+l O ~ dP
~V _
~o ~ ~ '
a5 U~ O N +l O ~1 ~ ~' N ,t ~
O ~ +~ +1 ,~, ~ O
,~ O N O _~ O ~ o
~~ 0 11 5~ r
~ ~ O L, ~t
o\~ ~ U~
rf N C
O ~ ~ O
~~rl ~ t)
- _ O ~
~ +l ~V O I
J ~ ~ O ~N O
U, o U~ ~ + C'
o O --L IV
p~ O ~ I O ~
V
O ~ ~
J ~ ~ V
o
N O
u~ C N
~ o ~ ~ r~ o ~ ll
o _I ~v u~ In ~ ~ O ~ ~ C
~ .~o o
~V o ~ o ~ V ~ ~: ~ lV
o ~ o
+l ~ +l ~ l ~ U I
U l.q ~ r~ ~ +l ~ +1 O -- ;n ~ O o
-,: o a~ o c~ ~ o _ o ~
S:' H r ~ ~ o u. o ~ o ,~ V
C, ~ . O .-, o ~
t~ 1. N R ~ ~ c r
H c)t ~ ~ ~ ~ O n ~ c~ -
d Cl P~ 11 ~n
o ~
~1 ~1
CA 0224l004 l998-06-l9
WO 97/22251 PCT/US96/20494
-- 18 --
ExamPle 4: Monitorinq Lysosphingo~ipid Content of
Neurons
Agonist-binding to metabotropic receptors
influences the levels of lysosphingolipid in neuronal
5 membranes. However, there is no convenient methodology
available for the measurement of lysosphingolipid content
in cultured neurons. As a result, a sensitive method of
fluorometric tracing has been devised and is described
herein. The measurement of lysosphingolipid content in
10 cultured neurons is based on the stable fluorescence-
tagging procedure using 4-fluoro, 7-nitrobenzofuran
(NBDZF) described by Nozowa et al J. Neurochem. 59:607-
609, 1992). The procedure was applied to: resting
neurons; neurons exposed to 100 ~M metabotropic receptor
15 agonist for 1 hour; resting neurons exogenously enriched
in lysosphingolipids; and neurons exogenously enriched in
lysosphingolipids and exposed to metabotropic receptor
agonist for 1 hour. The neurons were scraped in batches
of 107 (1 Petri dish, approximately 8 cm in diameter, is
20 equivalent to 2 mg membrane protein) and subjected to
anaylsis.
Total lipids were removed from the neuron samples
by repeated extraction with chloroform:methanol (2:1,
vol:vol). The lysosphingolipids were separated from
25 total neuronal lipid in 90 + 5 % recovery yield ~n = 10)
by the stepped chromatographic procedure of Van Veldhoven
et al. (Anal. Biochem. 183:177-189, 1989). The recovered
lysosphingolipids were stably tagged with NBDZF by the
rapid procedure of Nozowa et al. (supra). The
30 fluorescence tagged neuronal lysosphingolipids were
separated in parallel with reference standards NBDZF-Sph,
NBDZF-Psy, and NBDZF-Lsm on high performance silica gel
thin layer chromatography plates with acetonitrile:H20
(10:0.5, vol:vol). The highly fluorescent, well
35 separated bands were scraped from the plate. The
CA 02241004 1998-06-19
WO 97/22251 PCT/US96/20494
-- 19 --
fluorescence tagged lysosphingolipids are eluted from the
scraped silica gel with methanol:l N HC1 (1:0.02,
vol:vol). The fluorescence tagged lysosphingolipid
eluates were quantitated against reference standards by
5 fluorescence measurement in a Perkin-Elmer LS-5
Fluorescence Spectrometer.
Resting, unstimulated neurons contained 113 + 13
pmoles sphingosine, 50 + 10 pmoles lysocerebroside, and
s + 0.2 pmoles lysosphingomyelin per mg protein (n = 9).
10 Neurons stimulated with L-glutamate agonist, e.g. 100 ~M
ibotenate for 1 hour contained 85 + 3 pmoles sphingosine,
80 + 5 pmoles lysocerebroside and 52 + 2 pmoles
lysosphingomyelin per mg protein (n = 6). It appears
that a compensatory increase in the inhibitory
15 lysosphingolipids may occur during extended exposure to
agonist. Sphingosine is convertible in the cytoplasmic
face of cis-Golgi (Burger and DeMeer, Trends Cell Biol.,
2:332-337, l9g2) to lysocerebroside by transfer of a
glucosyl moiety from UDP-glucose to the l-0-position of
20 sphingosine by the action of
glucosyltransferase:lysocerebroside (psychosine) synthase
(Schwarzmann and Sandhoff, Methods in Enzymology,
38:319_341, 1987). An analogous system exists for
lysosphingomyelin synthesis by transfer of a
25 phosphorylcholine moiety from CDP-choline.
Neurons synthesize prodigious amounts of
sphingolipids with 92 mass % of the total cellular
sphingolipid located in the outer lipid bilayer of the
plasma membrane. Sialoglyco-sphingolipids are present at
30 the level of 40.8 + 1.9 ~g/mg cell protein, and
sphingomyelin is present at 12.1 + 3.0 ~g per mg cell
protein.
Sphingolipid content of neurons exposed to
sphingosine, lysocerebroside, and lysosphingomyelin was
35 examined as follows. A Petri dish containing
CA 02241004 1998-06-19
wo 97n2251 PCT/US96/20494
-- 20 --
approximately 107 neurons was incubated with 5 ~Ci
[3H]mYo-Ins in 3 ml DMEM overnight to prelabel the
phosphoinositide InsP3 may contain. The neurons were
washed with PBS and 3 ml of PBS containing 4.5 g/l
5 glucose was added. Preparations of sphingosine,
lysocerebroside, lysosphingomyelin (Sigma Chemical Co.,
St. Louis, MO) and combinations of each were prepared as
the hydrochloride salt dissolved in PBS. The
lysosphingolipids were added to the Petri dishes to
10 produce a concentration ranging from 0 - 150 ~M. The
dishes were held at room temperature for 0.5, 1, or 2
hours, then washed with PBS and incubated with 5 Units of
trypsin in glucose/PBS for 4 hours at 37~C.
Trypsinization removes non-specifically bound
15 lysosphingolipids that may adhere to extracellular plasma
membrane protein domains. Non-specific adherence between
cationic micelles of lysosphingolipids and the neuronal
glycocalyx is avoided by performing the experiments with
lysosphingolipid concentrations below the critical
20 micelle concentration. Neurons were analyzed for
lysosphingolipid content as described above.
Following exposure to 50 ~M sphingosine for 1
hour, the content of sphingosine in the neurons was
430 + 70 pmolestmg protein (n = 9). Following exposure
25 to 50 ~M lysocerebroside, the content of sphingosine was
220 + 45 pmoles/mg protein (n = 20), and exposure to 50
~M lysosphingomyelin resulted in 105 + 0.15 pmoles
sphingosine/mg protein (n = 9).
Example 5: Monitoring Metabotro~ic Rece~tor
Function
Metabotropic receptor function was examined in
intact neurons by assaying lysosphingolipid modulation of
calcium ion signalling. Approximately 1 X 106 cortical
neurons were cultured on rectangular L-polylysine-coated
35 coverslips and loaded with lysocerebroside or
CA 02241004 1998-06-19
WO 97/22251 PCT/US96/20494
-- 21 --
lysosphingomyelin to a level of 150 + 20 pmoles per mg
protein. The neurons were returned to complete Ham's
high glucose medium/DMEM (l:l, vol:vol) and infiltrated
with 5 ~M fura 2/AM (Molecular Probes, Eugene, OR). Fura
5 2 is a calcium chelator whose fluorescence absorbance
shifts to shorter wavelength upon calcium ion binding is
measurable in intact cells. A change in intraneuronal
calcium ion concentration was monitored as a change in
the fluorescence spectrum.
The neurons were exposed to 10 ~M glutamate
agonist (for example, ibotenate) for 30 minutes at 37~C.
The coverslips were then transferred to quartz cuvettes
in medium containing 250 ~M sulfinpyrazone to prevent
fura 2/AM diffusion. Fluorescence ratios were recorded
15 in a Perkin-Elmer LS-5 ~luorescence Spectrometer
thermostatted at 37~C at excitation wavelengths of 340
and 380 nm, and emission measurement at 500 nm. Calcium
ion signalling in agonist-stimulated neurons was
3.0 + 0.2 arbitrary units for ibotenate-exposed neurons.
20 In lysosphingomyelin and lysocerebroside-loaded neurons,
agonists elicited no calcium signalling measurably over
controls, indicating that agonist activity can be
effectively blocked by inhibitory lysosphingolipids.
Exam~le 6: Monitoring PhospholiPase C Activity in
the Presence of LYsos~hinqolipids
A. Nonitoring free PhosPholipase C
ActivitY in Solution
The effects of sphingosine, lysocerebroside, and
lysosphingomyelin on the kinetics of pure,
30 immunologically isolated phospholipase C isoforms beta,
delta, and gamma (which are commercially available) was
examined. For each isoform, 10 ~M sphingosine was added
to a reaction medium consisting of 1 ~g phospholipase C
isoform/ml, Tris-malate buffer (50 mM, pH 7.0) containing
35 100 ~M phosphatidyl inositol bis-phosphate tri-ammonium
CA 02241004 1998-06-19
wo97n225l PCT~S96/20494
- 22 -
salt, 0.01 ~Ci tritium labeled phosphatidyl inositol bis-
phosphate, 100 mM NaCl, 10 mM CaC12, and 5 mM 2-
merceptoethanol. The sphingosine induced an
approximately 2-fold increase in Vmax, a decrease in
5 calcium ion concentration required for optimal activity,
and no effect on KM for substrate phosphatidylinositol
bis-phosphate. Conversely, 10 ~M lysocerebroside or 5 ~M
lysosphingomyelin induced a 60% + 10% diminution in Vmax,
a 5-fold increase in calcium ion concentration required
10 for optimal activity, and no change in KM. These
observations indicate that the lysosphingolipids affect
the ability of calcium to activate phospholipase C and
operate on a regulatory domain of the enzyme.
B. Monitorinq Phos~holipase C
ActivitY in Intact Membranes
Physiologically active phospholipase C is present
on the endofacial portion of the plasma membrane. A
procedure for examining its activity in an intact
membrane is provided. The neuronal membrane preparation
20 described below is useful for determining
lysosphingolipid effects on the phosphatidylinositol
kinase and phospholipase C activities in intact
membranes. One hundred Petri dishes of cultured cortical
neurons were incubated overnight with 50 ~Ci [3H]myo-
25 inositol per mL of culture medium. Ten Petri dish-
samples of cultured neurons (1 X 108 neurons; 23 + 2 mg
protein, n = 15) were pooled to produce 2.4 + 0.2 mg
plasma membrane by the following procedure. The
collected neurons were homogenized in 5 mL 0.32 M sucrose
30 and centrifuged at 1000 X g for 15 minutes. The
supernatant was layered on top of 2 ml 1.2 M sucrose and
centrifuged at 300,000 X g for 20 minutes. The
sedimented pellet was resuspended by sonication for
10 seconds in 50 mM Tris buffer (pH 7.4), containing
35 0.3 ~M CaCl2, 1 mM MgCl2, and 0.01% ascorbic acid.
CA 0224l004 l998-06-l9
WO 97/22251 PCT/US96/20494
-- 23 --
The suspended plasma membrane preparation was
apportioned into 250 ~1 samples, each providing
approximately 100 ~g membrane protein. Sphingosine,
psychosine, and lysosphingomyelin were added to each
5 plasma membrane sample. The samples were held at room
temperature for 3 hours, centrifuged at 300, OOO X g for
30 minutes, and the supernatant discarded. Samples were
analyzed for lysosphingolipid loading (i.e., membrane
lysosphingolipid content following exposure to exogenous
10 sphingolipid) by fluorescence tagging. Table 2 provides
the results of exposure of plasma membrane samples to 10
~M lysosphingolipids. Values are reported in pmoles
lysosphingolipid/mg plasma membrane protein. Values
obtained for isolated plasma membrane and intact neurons
15 are compared.
TABLE 2
HOURS OF INCUBATIO
Membrane Lysosphingolipid N
Source 10 ~molar 0 0.5 1.0 3.0
exposure 2.0
Plasma Sphingosine 105+5 113+15 140+15 165+20 175+25 D
5 Membrane O
Suspension Lysocerebroside 45+5 75+20 120+15 180+15 175+20 r
(Psy~ o
5+1 65+15 110+15 120+10 145+25 r~
Lysosphingomyelin
Intact Sphingosine 85+15 85+20 110+15 105+5 115+15 ~
neurons ~ O
Lysocerebroside 25+5 35+15 45+10 80+5 80+5
(Psy)
5+1 8+2 20+5 45+5 55+15
lysosphingomyelin
CA 02241004 1998-06-19
W097n2251 PCT~S96/20494
- 25 -
Phospholipase C activity in plasma membrane
samples in the presence of neuronal agonists is
determined as follows. Replicate series of control and
lysophingolipid-loaded neuronal plasma membrane
5 preparations pre-labelled with [3H]myo-inositol are
analyzed for phosphatidylinositol phosphate hydrolysis
and inositol phosphate release. Membrane samples
equivalent to 100 ~g membrane protein in 250 ~l buffer
(50 mM Tris buffer (pH 7.4), containing 0.3 ~M CaC12, 1
10 mM MgC12, 0.01~ L-ascorbate, and 1 ~M ATP) are incubated
for 30 minutes at 37~C in various concentrations of the
neuronal agonists glutamate (0-100 ~M), ibotenate, or
quisqualate. Reactions are terminated by the addition of
1 ml ice-cold methanol to each sample followed by
15 addition of 2 ml ice-cold chloroform. Diminution in
phosphatidylinositol phosphates and release of inositol
phosphates is estimated by thin layer chromatographic
analysis, as described for phosphoinositidyl kinase
activity analysis. Inositol phosphate release is plotted
20 against ~g lysosphingolipid per mg protein in the plasma
membrane preparations and analyzed by Fisher's Least
Squares Difference test. These data estimate
lysosphingolipid modulation of phospholipase C activity
in neuronal plasma membrane.
C. Monitorinq PhosPhatidYlinositol Kinase
Activity in Neuronal Plasma Membrane
Candidate compounds for use in modulating calcium
ion levels in cells via InsP3 production are evaluated in
terms of the enzymatic process they affect. The enzymes
30 phosphatidylinositol kinase and phospholipase C both
transfer from the cytoplasm to the endofacial lipid
bilayer of the plasma membrane for physiological
activity. To control for any affects of
phosphatidylinositol kinase activity in the plasma
35 membrane preparations, a procedure for monitoring
CA 02241004 1998-06-19
WO 97122251 PCT/US96/20494
-- 26 --
activity of this enzyme is provided herein along with a
procedure for monitoring phospholipase C activity. Also,
procedures for monitoring the activities of these enzymes
in the presence of candidate compounds is provided below.
5 In the following examples, naturally occurring
lysosphingolipids are tested.
Phosphatidylinositol kinase (control) and
lysosphingolipid-loaded neuronal plasma membrane samples
are used to test for phosphatidylinositol kinase
10 activity. The content of lysosphingolipids was increased
(in the lysosphingolipid-loaded membrane) by exposure to
exogenous lysosphingolipids and each sample contained the
equivalent to 100 ~g membrane protein. The membrane
samples are pelleted and resuspended in 250 ~l of buffer
(50 mM Tris buffer (pH 7.4) containing 0.3 ~M CaCl2, 1 mM
MgCl2, and 0.01% ascorbic acid) by sonication for 5
seconds. They are then cooled to 0~C and [32P]-ATP
(DuPont NEN, 1 TBeq/mmole) is added to 1 ~M. The samples
are then incubated at 20~C for 1 minute and placed in an
20 ice bath to halt phosphorylation. The samples are
vortexed with 1 ml ice-cold methanol:concentrated HCl
(20:1, vol:vol). Two ml of ice-cold chloroform and 1 ml
H2O are added with continuous vortexing. The samples are
centrifuged at 2000 X g for 10 minutes and the lower
25 phase is drawn off.
Samples are analyzed for phosphatidylinositol and
its mono- and bis-phosphate derivatives as a measure of
phosphatidyl inositol kinase activity. Aliquots of each
test sample, as well as reference standards of
30 phosphatidylinositol and the mono- and bis-phosphate
derivatives (Sigma Chemical Co., St. Louis, MO), are
assayed, for example, by thin layer chromatography.
Aliquots are chromatographed on silica gel G thin layer
plates (Merck) that have been pre-soaked in 1% potassium
35 oxalate dissolved in methanol:water (1:1, vol:vol), and
CA 02241004 1998-06-19
WO 97122251 PCT/US96/20494
-- 27 --
dried at 120~C for 1 hour. The plates are developed with
the following mixture of solvents:
chloroform:methanol:acetic acid:water at 40:15:12:13:8
(vol:vol). The developed plates are sprayed with
5 Molybdenum Blue reagent (Sigma) which visualizes lipid-
bound phosphoryl groups. Phosphatidylinositol,
phosphatidylinositol mono-phosphate, and
phosphatidylinositol bis-phosphate contents are analyzed
relative to membrane protein by scanning in a BioRad
10 Videodensitometer Model 620 coupled with the BioRad l-D
analyst program. Bands are then scraped and analyzed for
radioactivity in a Beckman LS 5800 Scintillation
Spectrometer.
Control and lysosphingolipid-loaded plasma
15 membrane activities are compared by plotting
phosphorylation labelling at tl/2 (1 minute) against pg
lysosphingolipid per mg membrane protein. Data are
analyzed by Fisher's protected least significant
difference test.
Example 7: Methods of Moculatinq Inositol
Trisphosphate Production _n a Neuron i~ vivo f or
Treatment of a Neuronal D_sorder
Inositol trisphosphate production is modulated
in vivo by administering a compound, such as a naturally
25 occurring lysosphingolipid, to a mammal exhibiting
symptoms of a neuronal disorder associated with aberrant
InsP3 production. Disorders associated with undesirable
increases in InsP3 production have been reviewed above
and include acute brain hypoxia/ischemia and brain
30 seizure, while chronic disorders include Alzheimer's
disease, Huntington's disease, and amyotrophic lateral
sclerosis. A disorder associated with an undesirable
decrease in InsP3 production is neuronal apoptosis or
programmed cell death. Administration of the compound is
35 performed under conditions such that symptoms of the
CA 02241004 1998-06-19
W097~2251 PCT~S96120494
- 28 -
disorder are controlled to a desirable level or
alleviated.
A candidate compound for use in modulating InsP3
production in vivo is screened by administering the
5 candidate compound to an animal exhibiting a neuronal
disorder associated with aberrant InsP3 production. An
example of such a test animal includes, but is not
limited to, a DBA/2 mouse having audiogenic seizures
(Thomsen et a7., J. Neurochem. 6~:2492-2495, 1994;
10 incorporated by reference specifically to include a
method of administering a neuroactive compound to an
animal). Another in vivo screening model uses a gerbil
which has surgically occluded common carotid arteries,
which produces brain ischemia/anoxia (Matesik et al.,
15 J. Neurochem., 63:1012, 1994). Also incorporated by
reference as in vivo screening models are the focally
ischemic rat model (Zobrist et al., Stroke, 24:2002,
19~4) and the Alzheimer's disease model adult mouse
treated with beta-amyloid peptides (Hartmann et al.,
20 Biochem. Biophys. Res. Commmun., 194:1216, 1993).
Example VIII. In vivo Studies
Application of the specific metabotropic glutamate
receptor agonist, quisqualate, to the lateral ventricle
of the brain produces a dose-dependent behavioral
25 response and loss of hippocampal neurons in rats. At a
high dose (250 nmoles per animal), quisqualate produced
severe convulsions and death. At a moderate dose
(225 nmoles per animal), quisqualate produces moderate
convulsions and death in 60% of animals tested. At a low
30 dose (150 nmoles per animal), animals exhibit an increase
in activity and teeth chattering. Seven days after
application, both the moderate and high doses of
quisqualate produce severe loss of neurons in the
hippocampus. When these animals were pretreated with
CA 02241004 1998-06-19
WO 97/22251 PCT/US96/20494
125 nmoles of lysocerebroside (psychosine) or
lysosphingomyelin, the behavioral and histological
changes caused by quisqualate were absent. From this it
was concluded that lysocerebroside or lysosphingomyelin
5 are capable of prevent metabotropic glutamate
excitotoxicity in vivo. In addition, no behavioral or
neurotoxic effects were observed by doses of these
compounds as high as 125 nmoles per animal.
The animals used in this experiment were male F344
10 rats (Charles River Breeding Laboratories). The animals
were housed individually, exposed to a 12-hour light-dar~
cycle, and allowed free access to food and water. The
animals weighed approximately 250 g at the beginning of
each experiment and were weighed daily. In addition, the
15 animals were habituated to handling before each
experiment.
Before administering various compounds, the
animals were anesthetized with sodium pentobarbital
(50 mg/kg, i.p.) and positioned in a sterotaxic
20 instrument. A 26 gauge cannula was inserted into the
cerebral ventricle at the following co-ordinates: 1.0 mm
posterior to bregma, 1.5 mm lateral to the midline, and
4.0 mm below the dorsal surface of the neocortex.
Cann~las were capped and fixed in place with dental
25 cement. One week after cannulation, 10 ~1 of each of the
treatment drugs was infused, ICV (i.e. into the
ventricals of the brain), via a micropump at a rate of
10 ~l/min. For this procedure, the animals were lightly
restrained and unanesthetized. Following administration
30 of the compound, the infusion cannula remained in
position for one minute, after which the permanent in
situ cannula was capped.
The compounds administered were obtained from a
commercial supplier (Sigma Chemical Co., St. Louis, MO)
35 and dissolved in saline prior to injection. These
CA 02241004 1998-06-19
WO97/22251 PCT~S96/20494
- 30 -
compounds included quisqualate (injected at doses ranging
from lOO nmole/lO ~l to l ~mole/lO ~l), lysocerebroside,
and lysophingomyelin (which were injected at doses of
25 nmoles/lO ~l to 150 nmoles/lO ~l).
A. Histolo~ical AnalYsis
Seven days after the compounds were administered,
animals were sacrificed by transcardial perfusion with
neutralized, buffered 10% formalin-saline. Their brains
were removed, post-fixed overnight in 10% formalin-
lO saline, and sectioned along the coronal plane (into 50 ~m
thick sections) on a freezing microtome. The sections
were stained with cresyl violet to determine the extent
of cellular destruction.
A subset of the animals in each group was
15 sacrificed by transcardial perfusion with
4% paraformldehyde. The brains of these animals were
removed, post-fixed for 48 hours in 4~ paraformaldehyde,
placed in PBS, embedded in paraffin, and sectioned at
room temperature into 6 ~m thick setions along the
20 coronal plane.
Given that high concentrations of metabotropic
receptors are located in the hippocampus, and that the
hippocampus is particularly vulnerable to the effects of
a variety of insults includinq ischemia and hypoxia, this
25 region was examined for evidence of neuronal degeneration
due to glutamate excitoxicity. Histological analysis of
brain slices using cresyl violet staining confirm that
quisqualate (225 nmoles) injected ICV produces a
considerable loss of CAl pyramidal neurons in the
30 hippocampus (Figs. 5A and SB) compared to saline-injected
controls (Figs. 6A and 6B) and that pretreatment with
psychosine (125 nmoles) prevents the neuronal loss caused
by quisqualate (Figs. 7A and 7B~.
CA 02241004 1998-06-lg
WO97/22251 PCT~S96120494
- 31 -
B. Behavioral AnalYsis
Pilot studies identified several specific
behavioral measures that could be used to indicate
excessive activation of the metabotropic receptor (see
5 Table 3). These behaviors include convulsions, spasms,
teeth chattering, akinesia, and motor activity. The
experiments that follow were based on an analysis of
these behaviors over a two hour period after
administration of the compounds of the invention.
10 Behavioral observation was carried out by two trained
observers, who worked independently of one another and
who were blind to the experimental groups. The frequency
of convulsions and spasmsj and the duration of teeth
chattering, akinesia and mobilizing was recorded
15 continuously for 20 minutes prior to administration of
the compound of the invention and for 2 hours after the
treatment. Frequency and duration means were computed by
subtracting pre-infusion means from post-infusion means.
Some of the experiments that were performed first
20 were carried out to determine the amount of quisqualate
required to produce behavioral and histological changes
and the amount of lysosphingolipids that could
effectively prevent or reverse these effects. Table 3
outlines the behavioral responses of rats to various
25 doses of quisqualate (in~ected as described above) and
the associated histological changes. The behaviors are
listed as they occur chronologically. The limb clasp and
reflex tests indicate the degree of decortication. A
limb clasp or placement score of zero is normal, and a
30 score of 3 is severely abnormal.
CA 02241004 1998-06-19
W O 97~2251 PCTAUS96/20494
- 32 -
T~ble 3
DO5E BE~AVIOR ~T~Tn~cIcAL
Qui~qualate C~ANa~S
500 ~olo~ vocalization 10~B of neuron~
ln the piriform
and entorhinal
cort-x CA1
and dentate
gyrus reg$on~
of the
hirpoc-,
5 1 ~ol-~ ~s~a~e repeated full body convulsionQ ~B above
-~-vere ~cratching
-severe teeth chattering
-foaming at mouth
-d-ad 5-15 mins in lOO~ cases
250 nmoles -vocalization as above
-teeth chattering
-scratching
-localized ~pasms jerking/twitching
-full body convul~ions ~ev_~eJrepeated
-foaming at mouth
-r-spiratory di~tr~s
-abnG -1 pl~r~ ~-t and clasp reflex (3)
-d-ath in 75% of cases
225 ~ oles -t-eth chatter lo~s of Q 1
-hind leg paraly-i~ ~yL
-hyperactivity/walk in circles neuron~ in the
-full body convul~ions - moderate hippoC ,~s
-localized spa-m~ twitching/jerking
-episodic akine~ia/tonic immobility
-respiratory distre~s
-foaming at mouth
-abn~- -l pl~c ~ and clasp reflex (1-2)
-d~ath in 60~ of cases
200 nooles -t-eth chatter as a~ove
-occasional mild convulsions
-hyperactivity - circle walking
-epieo~ic akin-~ia/tonic immobility
-hind leg w~
n~ -1 limb cla~p and pl~ L reflex ~O)
150 nmoles -teeth chatter none O~C~L~- d
-localized twitches
-mild hyperactivity walking in circles
-hind leg weakne~ for 60 minutes
-nonmal pl~c --L and c14~p reflex (O)
10 100 nmoles -teeth chatter none observed
-mild scratching
-hind leg wE-kne~s 60 minute~
-normal placement and cla~p reflex ~01
The effective dose of quisqualate was determined
to be 225 nmoles, that of lysocerebroside was determined
to be 125 nmoles, and that of lysosphingomyelin was
CA 02241004 1998-06-19
PCTlUS96nO494
WO97/22~51
determined to be either 125 nmoles or 150 nmoles (the
higher doses appeared to be more reliable in pilot
testing).
The data shown in Table 4 represents the
5 observatiOnS of pilot studies aimed at determining the
effect (on behavior) of lysosphingolipids infused
directly into the lateral ventricles of the brain 30
minutes prior to infusion of quisqualate.
TABLE 4
10 DOSE ~E~AVIORAL aESPON~E
Psycho~ine 25 nmoles + Moderate convulsions, teeth chattering,
Quisqualate 200 n~oles akins~La and hyperactivity
Peychosine 50 nmole~ + Moderate convulsions, t-eth chatterin~,
Quisqualate 200 nmol~s akinesia and hyperactivity
15 P~ycl~osine 100 nmoles + Almost complete ~tt~nu~tion of Qui-qualate
Quisqualate 200 nmoles reBpon~e, ~ome t-eth chattering po~t-injection
Psychosine 125 nmoles + Complcte attenuation of Quisqualate ~s~unE~
Quisqualate 200 nmol~fi
P~ychoeine 125 nmol~s + ~ te attenuation of Quisqualate L~ -e
20 Qui~qual~te 225 nmole~
Psychosine 100 nmoles + Moderate convulsions, teeth chattering,
Quisqualate 250 nmole6 akin-sia Quisqualate markedly reduced
compared to typical Quisqualate re~ponse at
this dose
Psychooine 150 nmoles + Almo-t complete attenuation of Quisqualate
Quisqualate 250 nmo~e~ respon-e Psycho~ine produced ~hort term
~eda~ion at this dose
25 Ly-osphi-,_ y~in A~mo-t comp~ete attenuation of Qui-qualate
100 nmoles + re8pon8e, ~ome t~eth chattering post-injection
Quiequalate 225 nmol-~ and hyperactivlty
Ly~osp~ lin Complete attenuation of qui~gu~te Lc8~
1~0 nmole~ ~ LysoJphi-a~ ~lin produced mild ~e'-t jon at
30 Qui~qualate 225 nmol~s this do-e
The experiment began one week after ICV
cannulation. Animals (n = 6 per group) were randomly
allocated to one of 8 treatment y~OU~: (1) saline,
(2) quisqualate at 225 nmoles, (3) psychosine at
125 nmoles, (4) lysosphingomyelin at 125 nmoles, t5)
lysosphingomyelin at 150 nmoles, (6) quisqualate at
CA 02241004 1998-06-19
WO 97/222~1 PCT/US96/20494
-- 34 --
225 nmo}es and psychosine at 125 nmoles, (7) quisqualate
at 225 nmoles and lysosphingomyelin at 125 nmoles, and
(8) quisqualate at 225 nmoles and lysosphingomyelin at
150 nmoles. Following the baseline observation period,
5 animals received the first of two ICV infusions (all
infusions were of a 10 ~l volume that was administered
over a 1 minute period). For the first five groups
listed above, this consisted of saline (10 ~l/minute),
for the following 3 groups the infusions were psychosine
10 at 125 nmoles, lysosphingomyelin at 125 nmoles, or
lysosphingomyelin at 150 nmoles, respectively. Thirty
minutes later, all animals received the second infusion
which, for the first five groups listed above, consisted
of saline, quisqualate at 225 nmoles, psychosine at
15 125 nmoles, lysosphingomyelin at 125 nmoles, and
lysosphingomyelin at 150 nmoles respectively. The
remaining three groups received infusions of quisqualate
at 225 nmoles. Foll~wing the second infusion animals
were returned to their cages, and observed continuously
20 over the subsequent 2 hours.
During the observation period, two behavioral
analyses were carried out. The first on the frequency of
spasms and convulsions, the second on the duration of
teeth chattering, akinesia and motor activity. Figure 8
25 illustrates the mean number of convulsions or spasms
across 4 of the main treatment groups (quisqualate at 225
nmoles + saline; quisqualate at 225 nmoles + psychosine
at 125 nmoles; quisqualate at 225 nmoles +
lysosphingomyelin at 125 nmoles; and quisqualate at 225
30 nmoles + lysosphingomyelin at 150 nmoles). The remaining
4 control groups (saline; psychosine at 125 nmoles; and
lysosphingomyelin at 125 nmoles and 150 nmoles) have not
been illustrated, since animals in these treatment groups
displayed no convulsant behavior. A two way ANOVA, group
35 X response on the mean frequency of convulsions or spasms
CA 02241004 1998-06-19
WO 97/22251 PCT/US96/20494
revealed a significant group effect (F(7.24-9.75, p~.o5).
Multiple comparisons, using the Bonferroni adjustment,
revealed that there were significantly (p.'01) more
convulsions/spasms in the group injected with quisqualate
5 alone at 225 nmoles compared to all other groups.
Pretreatment with Psychosine (125 nmoles) or
Lysosphingomyelin (125 nmoles and 150 nmoles)
significantly (p-01) attenuated the number of convulsions
or spasms, although there was no significant difference
10 between the three treatment groups.
The second analysis was carried out on the
duration of teeth chattering, akinesia and mobilization.
Fig. 9 illustrates the mean duration of each of these
behaviors. A two way ANOVA, group X behavior, revealed a
15 significant group effect (F(7.34)=4.66, p=0.01) and a
significant behavior effect (F(2.68)=3.98, p=0.023).
Multiple comparisons, using the Bonferroni adjustment, on
the group effect revealed that animals injected with
quisqualate alone spent significantly (p'.01) more time
20 engaged in these behaviors than did animals injected with
the lysophingolids alone (psychosine at 125 nmoles and
lysophingomyelin at 125 nmoles and 150 nmoles) and
animals injected with quis~ualate 225 nmoles and
lysophingomyelin 150 nmoles. Multiple comparisons on the
25 behavior effect revealed that animals spent significantly
(p~.05) more time mobilizing compared to engaging in
teeth chatter.
Although the invention has been described with
reference to the presently preferred embodiments, it
30 should be understood that various modifications can be
made without departing from the spirit of the invention.
What is claimed is: