Note: Descriptions are shown in the official language in which they were submitted.
WU 97/Z7483 CA 02241914 2004-10-15 p~/US97/01200
1
FI» of ~ nw»ITION
This invention relates to medical diagnostic test strips and, more
particularly, to a medical diagnostic test strip package having a desiccant
disposed in close
proximity to the reagents on the test strip such that single strip
manufacturing, packaging,
io shipping, and use is feasible.
BACKGROUND OF TI;iB IINVBNTION
Medical diagnostic test strips are used in a variety of applications. Such
strips are exposed to samples of blood, feces, or urine, for example, where
specific
reagents on the test strip are designed to detect various components in the
sample.
is Medical diagnostic test strips are specifically used to detect the level of
glucose in a
patient's blood. Such test strips must be exposed to an atmosphere that is
substantially
moisture-free until moments before use in testing a sample. The strips are
often highly
water absorbent, and absorption of moisture may alter test results.
Accordingly, the known industry practice for keeping the test strips dry is
2o to contain a plurality of~~such test strips in a container or vial. The cap
for the container
has a desiccant contained in it to absorb water from the air inside the
container. In this
manner, the strips within the container are kept dry. When ultimately used, a
docxor or
o#her testing personnel, . including a patient himself, removes the cap from
the container,
.ø ,extracts one of the test strips, and immediately places it in the sample
to be tested (or
as places a small quantity of the sample to be tested on the test strip).
Exposure of the strips
to the ambient atmosphere before use is thus miaimized to reduce water vapor
absorption
by the test strip.
CA 02241914 2004-10-15
w0 97/27483 PCT/US97lOiZ00
2
A disadvantage of this technique is that all of the strips within the vial
must
be used within a relatively short time after the vial is first opened or they
cannot be
reliably used. The time period is usually on the order of a few months.
Because of the
exposure to the ambient atmosphere upon opening, there is a risk that the test
strips will
s absorb enough moisture to render them ineffective in this amount of time
despite the
presence of the desiccant in the cap. In addition, there are manufacturing,
packaging, and
use complications resulting from the inclusion of multiple test strips in a
single vial
according to prior practice. It is therefore desirable to provide individual
test strips with
the ability to resist water absorption. This would provide efficiencies in
manufacturing,
to packaging, and using the strips.
SI1MMARY OF THB INVENTION
'The present invention involves packaging individual test strips such that the
test strips remain in a moisture-free environment. This is accomplished by
applying a
desiccant deposit directiy to the test strip and covering the test strip with
moisture barrier
is sheets, by using moisture barrier sheets having a desiccant deposit
attached to them, or by
forming a pouch with a desiccant deposit attached to an inner surface of the
pouch and
placing.the test strip within the pouch.
According to one aspect of the present invention, there
i~ provided a medical diagnostic test strip package
comprising: (a) a medical diagnostic test strip having a top
surfaces a reagent contained on a portion of said top surface,
and a bottom surface and (b) a first moisture barrier sheet
adhered to said top surface of said medical diagnostic test
strip, said first moisture barrier sheet having a desiccant
deposit adhered to a portion thereof such that said portion of
~ said first moisture barrier sheet with said desiccant deposit
thereon is integrally disposed on said portion of said medical
diagnostic test strip containing said reagent.
CA 02241914 2004-10-15
2a
According to another aspect of the present invention,
there is provided a medical diagnostic test strip package
comprising: (a) medical diagnostic test strip having (i) a top
surface, (ii) a reagent contained in a first portion of said
top surface, (iii) a desiccant deposit adhered to a second
portion of said top surface, said second portion adjacent to
said first portion, and (iv) a bottom surface; and (b) a first
moisture barrier sheet adhered to said top surface of said
medical diagnostic test strip.
According to still another aspect of the present invention,
there is provided a medical diagnostic test strip package
comprising (a) a medical diagnostic test strip having a top
surface and a reagent contained in a first portion of said top
surface; and (b) a pouch containing said medical diagnostic
test strip, said pouch having an inner surface and a desiccant
deposit adhered to a section of said inner surface, wherein
said inner surface of said pouch is disposed proximate said
top surface of said medical diagnostic test strip such that
said desiccant deposit is adjacent to said reagent of said
test strip.
According to yet another aspect of the present invention,
there is provided a method of preventing water absorption by a
medical diagnostic test strip having a top surface, a bottom
surface and a portion of said top surface containing a
reagent, said method comprising the steps of: (a) covering
said top surface with a first moisture barrier sheet; (b)
covering said bottom surface with a second moisture barrier
sheet; and (c) integrally disposing a desiccant deposit in
proximity to said portion of said top surface of said medical
diagnostic test strip containing the reagent.
CA 02241914 2004-10-15
WO 97/27483 PG"fIUS97101Z00
2b
BRI>~ DBSCRIPZZON OF THE FIG~JRES
Fig. 1 is a perspective view of a first exemplary embodiment of the present
2o invention;
Fig. 2 is a perspective view of a second exemplary embodiment of the
present invention;
Fig. 3 is a partially cut away perspective view of a third exemplary
embodiment of the present invention;
2s Figs. 4 and 5 are perspective views of moisture barrier sheets used in an
exemplary embodiment of the present invention;
Pig. 6 is a perspective view of a reagent pad used with a test strip in an
exemplary embodiment of the present invention; and
CA 02241914 2004-10-15 ra.,tW~'J7lUllW
3
Fig. 7 is a schematic view of a system for making a medical diagnostic test
strip package according to an exemplary embodiment of the present invention.
DBTAiI.ED DESCRIPTION OF THfi INVENTION
According to the present invention, individual medical diagnostic test strips
~ s are packaged in a moisture-free environment by disposing a desiccant in
close proximity
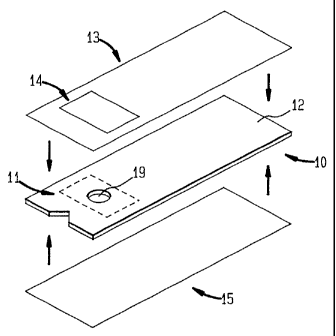
to the reagents used on the test strip. Fig. 1 shows a first exemplary
embodiment of the
present invention. - In Fig. 1, test strip 10 is shown having reagent pad 11
attached to it.
Reagent pad 11 contains the particular compounds necessary to perform the
desired
detection test for a sample in a particular application. In some cases,
depending on the
lo particular reagents used, reagent pad 11 changes color upon exposure to the
test sample.
This color change can be observed and possibly measured to yield information
regarding
the presence or amount of the component being measured. In other cases, other
measurable or observable characteristics of reagent pad 11, such as
reflectance, may
change upon exposure to the test sample and thereby yield the desired
information
is regarding the sample.
8xemplary test strips and reagent pads used to measure the amount of
glucose in the blood, and the manufacture and use of such test strips and
reagent pads, are
described in U.S. Patents No. 4,935,346; No. 5,049,487; and No. 5,304,468,
Alternatively, reagent pad 11 may have the structure shown in Fig. 6. In
Pig. 6, upper liner 60 is typically formed of polyethylene terephthalate (or
FE1~ and has
a conventional cold or hot melt adhesive on its bottom surface 64. A reagent
paper Iayer
61, having the roquired reagetris for a particular application on it, is
adhered to bottom
'j 2s surface 64 of upper liner 60. A double-sided tape layer 62 is used to
adhere reagent
paper layer 61 to lower liner 63, which is typically formed of PET. After
assembly of
the composite layers shown in Fig. 6, reagent pad 11 is adhered to test strip
10 by
conventional cold or hot melt adhesives placed around the edges of upper
surface 65 of
upper liner 60. Test strip 10 has a hole 19 formed in if to access reagent pad
11.
CA 02241914 1998-06-30
WO 97/27483 PCTlUS97/01200
4
The top surface 12 of test strip 10 has a top sheet 13 adhered to it. Top
sheet I3 is adhered to test strip 10 by any conventional cold adhesive or hot
melt adhesive
known in the art. Fig. 4 shows an exempiary construction for top sheet 13. In
Fig. 4,
top sheet 13 is shown to have a two-part composite structure. Upper composite
Layer 40
s is formed of foil to provide a moisture barrier. Upper composite layer 40
has a
conventional cold or hot melt adhesive on its bottom surface 42. The adhesive
is used to
attach upper composite layer 40 to a Lower composite layer 41.
Lower composite layer 41 is a microperforated layer typically made of
parchment paper or MylarO film. Lower composite layer 41 also has a
conventional cold
to or hot melt adhesive on its bottom surface 43 to attach lower composite
iayer 41 (and
hence top sheet 13) to test strip 10. Lower composite layer 41 has a desiccant
deposit 14
adhered to it. Desiccant deposit 14 comprises a silica gel (or other particle
remover such
as a molecular seive or an oxygen absorber) dispersed in a medium suitable for
suspending the silica gel and allowing adhesion of desiccant deposit 14 to
lower composite
is layer 41. In the exemplary embodiment shown, the medium comprises vinyl
acetate and
a hot meat adhesive. As shown in Fig. 1, desiccant deposit 14 is located
within top sheet
13 such that, upon application of top sheet 13 to test strip 10, desiccant
deposit I4 is
adjacent to reagent pad l I.
Fig. 1 shows a bottom sheet I5 adhered to the side of test strip 10 opposite
2o upper surface 12. Bottom sheet 15 has an exempiary construction shown in
Fig. S. In
Fig. S, lower composite layer 50 is made of foil and forms a moisture barrier.
Upper
surface 52 of lower composite layer 50 has a conventional cold or hot melt
adhesive on it
to adhere lower composite layer 50 to upper composite layer 51. Upper
composite Layer _
51 is a microperforated layer typically formed of parchment paper or Mylar~
film, and
is has an upper surface 53 with a conventional cold or hot melt adhesive on it
to adhere
upper composite layer 51 (and hence bottom sheet 15) to test strip 10. '
Top sheet 13 and bottom sheet 15 are moisture barriers that prevent any ,
water from contacting, and being absorbed by, test strip 10. In the event that
test strip 10
is exposed to any moisture, desiccant deposit 14 absorbs the moisture rather
than reagent
CA 02241914 1998-06-30
WO 97127483 PCT/US97/01200
pad 11. Desiccant deposit 14 may also alternatively be applied to bottom sheet
15, or to
both top sheet 13 and bottom sheet 15.
Test strip 10, with top sheet 13 and bottom sheet 15 adhered to it and with
desiccant deposit 14 in top sheet 13, may be manufactured and shipped
individually,
s along with other test strips. An exemplary manufacturing process is
schematically
illustrated in Fig. 7. As shown in Fig. 7, press rolls may be used to produce
a test strip
package according to the present invention. Roll 70 is a rolled length of the
composite
reagent pad 11 described in connection with Fig. 6. Roll 71 is a rolled length
of
polyvinylchloride (or PVC) strip stock of which test strip 10 may be formed.
Roll 70 is
to unwound and cut into reagent pads 11 of appropriate size. Reagent pads 11
are then
pressed onto PVC test strip material unwound from roll 71. Simultaneously,
roll 73 of a
length of upper sheet 13, and roll 72 of a length of bottom sheet 15 are
unwound, and
sheets 13 and 15 are pressed onto the top surface 12 and the bottom surface
16,
respectively, of the length of test strip material with reagent pads 11.
To use test strip 10, the doctor or other testing personnel peels off top
sheet
13 and bottom sheet 15, much like with a Band-Aid~ bandage, and inserts test
strip 10
into a sample for testing (or places the sample on test strip 10}.
Fig. 2 illustrates a second embodiment of the present invention. In Fig. 2,
test strip 10 has desiccant deposit 14 applied directly to it. Desiccant
deposit 14 has the
zo same composition as described in connection with the exemplary embodiment
of Fig. 1.
With the exemplary embodiment shown in Fig. 2, top, sheet 22 and bottom sheet
23 are
applied to the top and bottom surfaces 12 and 16, respectively, of test strip
10. Top sheet
22 and bottom sheet 23 are moisture barriers, and may have a composite
construction as
described in connection with bottom sheet 15 in the exemplary embodiment of
Fig. 5.
2s In use, top sheet 22 and bottom sheet 23 are peeled away from test strip 10
a
by the doctor or other testing personnel. Test strip 10, with desiccant
deposit 14 adhered
thereto, is then inserted into the sample for testing (or the sample is placed
on reagent pad
11 of test strip 10) .
CA 02241914 1998-06-30
WO 97/27483 PCT/CTS97/01200
6
Alternatively, desiccant deposit 14 may comprise a hot melt adhesive with
a desiccant dispersed in it. Any known hot melt adhesive and desiccant can be
used for
this purpose. The adhesive rnay serve an adhesive function, for example, to
help secure
tap sheet 22 or bottom sheet 23 to test strip 10. Alternatively, the adhesive
may be
1
s allowed to dry before application of top sheet 22 or bottom sheet 23 and
serve only as a
carrier for the desiccant and not as an adhesive.
Fig. 3 illustrates a third embodiment of the present invention. In Fig. 3,
test strip 10 with reagent pad 11 is shown with a pouch 32 ~ovhich is designed
to contain
test strip 10. Pouch 32 is formed of top sheet 33 and bottom sheet 34 which
are adhered
io together along three of their edges to form seals 35, 36, and 37. The
remaining edge of
top sheet 33 and bottom sheet 34 on one side of the pouch is closed using a
reclosable
ziplock opening 38. Reclosable ziplock opening 38 allows access to the
interior of pouch
32. Desiccant deposit 14 is formed on the inside of the pouch on top sheet 33,
bottom
sheet 34, or both. Desiccant deposit 14 may have the same composition as
described
is above in connection with Fig. 1. Top sheet 33 and bottom sheet 34 are
moisture barriers
that may have the composite structure described for bottom sheet 15 in
connection with
Fig. 5. In use, the doctor or other testing personnel opens reclosable ziplock
opening 38
and removes test strip 10. Test strip 10 is then immediately placed in a
sample for testing
(or the sample is placed on reagent pad 11 of test strip 10).
2o Alternatively, desiccant deposit 14 may comprise a hot melt adhesive with
a desiccant dispersed in it. Any known hot melt adhesive and desiccant can be
used for
this purpose. The adhesive may serve an adhesive function, for example, to
help secure
top sheet 33 or bottom sheet 34 of pouch 32 to the test strip. The adhesive
also may be
allowed to dry before insertion of test strip 10 into pouch 32 and serve only
as a carrier
2s for the desiccant and not as an adhesive.
In the embodiments of the present invention discussed above, only one
desiccant deposit is illustrated in each case. Additional desiccant deposits
may be
included in any of the embodiments as necessary for a particular application.
~ ..... ~..~.~.... CA 02241914 2004-10-15 aara,a,muva~w
7
BXAMPLB 1
A desiccant deposit was formed' by dispersing different amounts of silica
gels in vidyl acetate by mixing the components in a comrentional mixer until a
- homogeneous mixture was produced. The deposits were spread over a three
square inch
s area using a draw-down bar by applying several thin layers over one another,
allowing
each layer to dry before applying the next layer. The weight of the deposits
were
measured at various times over a seven day period. Over the first four days,
there was
40 % relative humidity. The results are tabulated below. The increase in
weight of the
deposits reflects the amount of moisture absorbed. The data show good moisture
io absorption by the deposits.
ABSORPTION OF MOISTURE
BY A COATING CONTAINING SILICA GEL
% of Original Weight2 Days 4 Days 7 Days
Sample Silica (grams) (grams] (grams) (grams)
Gel
#1 50 % .303 .303 .304 .306
#2 50 % 1 .303 .305 .306 .307
#3 50 % . .318 .318 . 320 .349
#4 35 % .313 .313 .314 .314
#5 35 % ' .317 .317 .318 .319
#6 35 % .319 .320 .321 . 323
i~I,2,3: 50% 7-10 micron Grace Syloid*AI, 1 silica gel in B--15 vinyl acetate
is homopolymer (available from Air Products), ethanol used as the solvent.
#4,5,6: 3596 7-10 micron Grace 5~oi~'~~:-1 silica gel in B-IS vinyl acetate
homopolymer (available fire Air Products), 'ethanol used as the solvent.
* Trade-mark
CA 02241914 1998-06-30
WO 97/27483. PCT/US97l01200
EXAMPLE 2
A desiccant deposit was made by dispersing 41 % by weight 50 mesh silica
gel (non-indicating) in a conventional hot melt adhesive. Three samples were
produced
and deposited as drops on foil for moisture absorption testing. The results
are tabulated
s below.
Sample number: 1 2 3
Resin weight (g): 4.45 4.14 4.I7
Total sample weight (g): 5.01 4.78 4.71
Moisture absorbed after 5 0.02 0.03 0.03
days (g):
Moisture absorbed after 10 0.05 0.05 0.04
days (g):
Moisture absorbed after 17 0.08 0.08 0.08
days (g):
Moisture absorbed after 21 0.09 0.09 0.09
days (g):
EXAMPLE 3
A desiccant deposit was made by dispersing 40 % by weight 50 mesh silica
to gel (non-indicating) in H.B. Fuller HM 1072 hot melt adhesive. Three
samples were
produced and deposited as drops on foil for moisture absorption testing. The
results are
tabulated below.
CA 02241914 1998-06-30
WO 97/27483 PCTlUS97/01200
9
Sample number: [ ~ ~ 2 ~ 3
Resin weight (g): 5.39 4.05 4.14
Total sample weight (g): 6.09 4.74 4.73
Moisture absorbed after 5 days (g): 0.08 0.05 0.04
Moisture absorbed after 0.12 0.09 0.08
days (g):
Moisture absorbed after 0.16 0.11 0.09
i7 days (g):
Moisture absorbed after 0.19 0.14 0.10
21 days (g):
EXAMPLE 4
Another desiccant deposit was made by dispersing by weight
40 % 50 mesh
s silica gel {non-indicating) in H.B. Fuller HM Three samples
1072 hot melt adhesive.
were produced and deposited as drops on foil for
moisture absorption testing. The results
are tabulated below.
Sample number: 1 2 3
Resin weight (g): 3.90 4.46 3.45
Total sample weight (g): 4.58 5.04 4.05
Moisture absorbed after 5 days (g): 0.08 0.06 0.05
Moisture absorbed after 10 days (g): 0.13 0.12 0.09
Moisture absorbed after 17 days (g): 0.16 0.14 0.12
Moisture absorbed after 21 days {g): 0.17 0.17 O.I2
CA 02241914 1998-06-30
WO 97/27483 PCTlLTS97/01200
EXAMPLE 5
A desiccant deposit was made by dispersing
40 ~ by weight 50 mesh silica
gel in Eco hot melt adhesive. Three sited as drops on
samples were produced and depo
foil for moisture absorption testing. p
The results are tabulated below.
Sample number: 1 2 3
Resin weight (g): 4.15 3.47 5.06
Total sample weight (g): 4.70 4.06 5.62
Moisture absorbed after 5 days (g): 0.00 0.05 0.07
Moisture absorbed after 10 days (g): 0.05 0.11 O.I1
Moisture absorbed after 17 days (g): 0.13 0.13 0.16
Moisture absorbed after 21 days {g): 0.15 0.13 0.18
5
E~~AMPLE 6
A desiccant deposit was made by dispersing 40 °b by weight
indicating
silica gel in store-bought hot melt adhesive. Three samples were produced and
deposited
as drops on foil for moisture absorption testing. The results are tabulated
below.
Sample number: 1 2 3
Resin weight (g): 4.31 3.91 3.99
Total sample weight (g): 4.82 4.48 4.57
Moisture absorbed after 5 days (g): 0.16 0.15 0.17
Moisture absorbed after 10 days (g): 0.22 0.86 0.24
Moisture absorbed after 17 days (g): 0.28 0.90 0.28
Moisture absorbed after 21 days (g): 0.31 0.93 0.30
CA 02241914 1998-06-30
WO 97127483 PCT/US97/0~200
I1
EXAMPLE 7
A desiccant deposit was made by dispersing 40
% by weight 50 mesh non-
indicating silica gel in store-bought hot melt Three
adhesive. samples
were
produced
and
deposited as drops on foil for moisture absorptiong.
testin The
results
are
tabulated
s below.
Sample number: 1 2 3
Resin weight (g): 3.793.91 4.45
Total sample weight (g): 4.344.48 5.I2
Moisture absorbed after 5 days (g): O. 0. 0. I3
I2 I5
Moisture absorbed after 10 days (g): 0.160.20 O. I9
Moisture absorbed after 17 days (g): 0.230.25 0.24
Moisture absorbed after 21 days (g): 0.240.27 0.25
EXAMPLE 8
A desiccant deposit was made by dispersi ng
40
%
by
weight
indicating
silica gel in a conventional hot melt adhesive.
Three samples were produced and
deposited as drops on foil for moisture absorptiong.
testin The
results
are
tabulated
to below.
Sample number: 1 2 3
Resin weight {g): 5.094.49 4.93
Total sample weight (g): 5.705.16 5.50
Moisture absorbed after 5 days (g): 0.040.05 0.04
Moisture absorbed after 10 days (g): 0.070.22 0.07
J
Moisture absorbed after 17 days (g): 0.100.23 0.10
Moisture absorbed after 21 days (g): 0.12O.I2 0.1I
CA 02241914 1998-06-30
WO 97/27483 PCT/US97/01200
I2
EXAMPLE 9
A desiccant deposit was made by dispersing 40 % by weight indicating ,
silica gel in FPC 725 hot melt adhesive. Three samples were produced and
deposited as
drops on foil. Additional silica gel was sprinkled on top of the drops.
Moisture
s absorption test results are tabulated below.
Sample number: ~ 1 ~ 2 ( 3
Weight of foil (g): 0.62 0.56 0.62
Weight of foil, resin, and geI (g): 5.75 6.85 7.20
Weight with sprinkled gel (g): 6.41 7.50 7.66
Moisture absorbed after 3 days (g): 0.35 0.38 0.10
Moisture absorbed after 7 days (g): 0.36 0.43 0.10
EXAMPLE 10
A desiccant deposit was made of FPC elt
725 hot m adhesive.
Three
samples were produced and deposited Silica sprinkled on top
as drops on foil. gel of
was
the drops. Moisture absorption test
results are tabulated below.
Sample number: 1 2 3
Weight of foil (g) : 0.54 0. 0.45
62
Weight of foil, resin, and gel (g): 4.23 6.91 3.50
Weight with sprinkled geI (g): 6.41 7.50 7.66
Moisture absorbed after 3 days (g): 0.13 0.26 0.07
r
Moisture absorbed after 7 days (g): O.I3 0.26 0.07
io
CA 02241914 1998-06-30
WO 97127483 . PCT/US97101200
z3
Although illustrated and described herein with reference to certain specific
embodiments, the present invention is nevertheless not intended to be limited
to the details
shown. Rather, various modifications may be made in the details within the
scope and
. range of equivalents of the claims and without departing from the spirit of
the invention.
s In particular, the invention is intended to incorporate moisture and
particle absorption
from any flat medical diagnostic product.