Note: Descriptions are shown in the official language in which they were submitted.
CA 02243277 1998-07-1~
W 097/26027 PCTrUS97/00843
I I IBRICOUS AND RF~DILY BONI:)AB! F CATHFTF~ SHAFT
- R''.CKGROUN~ C~F THF INVENTION
This invention relates to catheters for performing intrav~scul~r
5 procedures such as percutaneous transluminal coronary angioplasty (PTCA)
and more specificaliy to elongated sha~ts for such catheters.
PTCA is now one of the most widely used treatment modalities
for heart disease. The procedure basically comprises advancing a dilatation
catheter, having an inflatable balloon on its distal extremity, into the
10 patient's coronary anatomy over a guidewire until the balioon of the
dilatation catheter is properly positioned across the lesion to be dilated. Once
properly positioned, the dilatation balloon is infiated with liquid to a
predetermined size at relatively high pressures, e.g. up to 20 atmospheres
or more, to expand the arterial passageway. Generally, the inflated
SUE~;TITUTE SI~EET ~F~ULI~ 26)
CA 02243277 1998-07-1~
WO 97/26027 PCT/US97/00843
diameter of the balloon is approximately the same diameter as the native
diameter of the body lumen being dilated so as to complete the dilatation
but not overexpand the artery wall. After the balloon is finally deflated, blood
flow resumes through the dilated artery and the dilatation catheter
can be removed therefrom.
In most PTCA procedures, a guiding catheter having a
preshaped distal tip is first percutaneously introduced into the
cardiovascular system of a patient by means of a conventional Seldinger
technique and advanced therein until the preshaped distal tip of the guiding
10 catheter is disposed within the aorta adjacent to the ostium of the desired
coronary artery. The guiding catheter is twisted or torqued from its
proximal end, which extends out of the patient, to guide the distal tip of the
guiding catheter into the desired coronary ostium. Once the guiding
catheter is in proper position within the patient's vasculature, the dil~t~tion
1~ catheter with a guidewire slidably disposed within an inner lumen of the
dilatation catheter is positioned within the inner lumen of the guiding
catheter. The guidewire is first advanced out the distal tip of the guiding
catheter seated in the coronary ostium into the patienrs coronary artery
and directed to the region of the patient's coronary anatomy where the
20 procedure is to occur. A torque may be applied to the proximal end of the
SUBSTITUTE SHEET (F~ULE 26~
-
CA 02243277 1998-07-1~
W O 97/26027 PCT~US97100843
guidewire, which extends out of the proximal end of the guiding catheter,
to guide the curved or otherwise shaped distal end of the guidewire into a
desired branch of the coronary artery. The advancement of the guidewire
within the selected artery continues until it crosses the lesion to be dilated.
5 The dilatation catheter is then advanced over the previously advanced
guidewire, until the balloon on the distal extremity of the dilatation catheter
is properly positioned across the lesion which is to be dilated.
Current intravascular catheter designs are limited by the need to
incorporate conflicting characteristics. For example, most dilatation catheters
10 are designed to be introduced into a body lumen over an in-place guidewire
which is slidably received within an inner lumen within the
catheter. As such, it is desirable to ",i"i"li~e the friction between the
guidewire and the surface of the inner lumen of the catheter by
constructing the catheter from a lubricous material such as a high density
15 polyethylene. However, lubricous polymeric materials frequently lack other
desirable properties, including, for example, the ability to readily bond to
incompatible polymeric materials such as polyethylene terephthalate and
nylon. Due to the high inflation pressures (up to 300 psi or more)
associated with coronary balloon angioplasty, it is imperative to provide a
20 ~trorg bor;d be.~v~veer, or;e or more erd~ of the dilatatior. balloor, ar.d the
SUB5T~Tl)~E S~IEET ~P~IJLE 26~
CA 02243277 1998-07-1~
W 097/260Z7 PCTAJS97/00843
catheter shaft. Polyolefin balloons can be effectively fusion bonded to a
polyethylene shaft but ballo~5ns made of nylon and other polyamide
materials, and balloons made of polyesters such as polyethylene
terephthalate do not easily bond to polyolefinic materials. Nylon and
5 polyethylene terephthalate balloons usually require surface treatment and
the use of a suitable adhesive to bond to polyolefin materials such as
polyethylene. The additional manufacturing steps of surface treatments and
incorporating and curing an adhesives, greatly complicate the manufacturing
process and can introduce significant quality control problems. A catheter
10 shaft should also have adequate strength for pushability and resistance to
buckling or kinking. As another example, it may be desirable to provide a
catheter shaft with elastomeric properties to improve flexibility. However,
most lubricous materials are not elastomeric.
United States Patent No. 5,304,134 to Kraus et al., which is
15 hereby incorporated in its entirety by reference, attempts to provide a
solution to the poor bonding of lubricous by providin~ the catheter shaft
with an inner tubular member havlng a lubricous proximat portion and a
non-lubricous, bondable distal portion. However, this approach does not
represent a complete solution, because the lubricous proximal portion must
20 still be bonded to the non-lubricous distal portion. The Kraus et al. system
SUB~;TITI~TE SHEET ~RULE 26~
CA 02243277 1998-07-1~
W O 97/26~27 PCTrUS97/00843
also requires that some portion of the guidewire lumen be formed from a
non- lubricous material which restricts guidewire movement within the
lumen.
A different approach involves forming the dilatation balloon as
5 an integral portion of the catheter shaft itself, but this requires the balloon and
the shaft to be formed from the same material, which is not always desirable
because the property requirements for the balloon and the shaft can be quite
different, particularly for dilatation catheters for PTCA .
Accordingly, there remains a need to provide a catheter shaft
10 having a lubricous inner surface defining a guidewire lumen while allowing
an easy, secure bond with a dilatation balloon or other catheter components
formed of non-lubricous polymeric materials. The present invention
s-dli~r,es these and other needs.
SUMMARY C)F THF INVFlaTION
The present invention is directed to an intravascular catheter,
such as a balloon dilatation catheter for performing angioplasty procedures,
which has a shaft or shaft segment which is both lubricous and is capable
of readily bonding to other catheter components such as a balloon forrned
20 of essentially non-lubricous polymers.
~UBSTITUT~ S~EET (RULE 26)
CA 02243277 1998-07-1~
W 097/26027 PCTrJS97/00843
In accordance with the present invention, the catheter shaft or
catheter shaft segment is formed of a polymeric blend comprising at least
about 30% by weight, preferably at least about 50% by weight of a
lubricous polymeric component, not more than about 60%, preferably not
5 more than about 40% of a bonding polymeric component and up to about
30%, preferably not more than about 10% of a polymeric component for
compatiblizing the lubricous and bonding components. Optionally, up to 25%
by weight, usually not more than about 10% by weight of the blend should be
a catalytic material to facilitate cross linking the shaft material
10 after forming the product. The lubricous component and the bonding
component must be compatible or capable of bein~ made compatible. As
used herein the term "compatible" and words of similar import mean that
two polymer materials readily form an inli,-,ate mixture when they are melt
processed together. Usually, they are miscible when both are in a molten
1 5 condition.
In one presently preferred embodiment, the catheter or
catheter segment is formed of a blend of about 50% to about 80%
polyethylene (a lubricous component), about up to about 50% of a
copolyester such as Hytrel~) (the bonding component) and up to about 50%
20 of a compatiblizing agent such as an acrylate. The polymer components are
SlJBSTITUTE SHFET ~P~ULI~ 26~
- CA 02243277 1998-07-1~
W O 97/26027 PCT~U~,97/00843
i"li",~lely-mixed and extruded into a tubu,ar product which is utilized as the
inner tubular member of an intravascular catheter. The surface defining an
inner lumen of the tubular member has a kinematic frictional coefficient of
about 0.08 to about 0.3 on a smooth glass. A balloon formed of PET
5 readily fusion bonds to the outer surFace of the tubular member.
These and other advantages of the invention will become more
apparent from the following detailed description of the invention when
taken in conjunction with the accompanying exemplary drawings.
IFF DF-~CRIPTION OF THF DRAWINGS
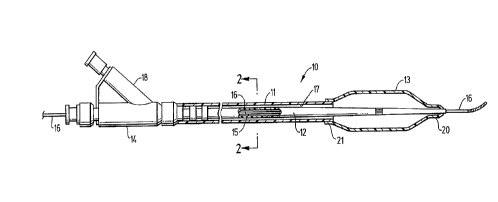
Fig. 1 is an elevational view, partially in section, of an over-the-
wire dilatation catheter having an inner tubular member embodying features
of the invention.
Fig. 2 is a transverse cross section of the embodiment shown
in Fig. 1 taken along the lines 2-2.
Fig. 3 is an elevational view, partially in section, of the distal
section of a rapid exchange type dilatation catheter having an inner tubular
member embodying features of the invention.
SUBST~TlJTE SHEET (R.,ILE ~6
CA 02243277 1998-07-1~
W 097/26027 PCT~US97/00843
- Fig. 4 is a transverse cross section of the embodiment shown
in Fig. 4 taken along the line~ 5-5.
Fig. 5 is an elevational view, partially in section, of an alternative
embodiment wherein the distai section of the catheter shaft is formed of an
5 extrusion of a polymerblend.
Fig. 6 is a transverse cross section of the embodiment shown
in Fig. 6 taken along the lines 7-7.
nFTAI5 Fn DESCRIPTION OF THF INVFI~ITION
Reference is made to Figs. 1 and 2 which illustrate a balloon
dilatation catheter 10 embodying features of the invention. Generally, the
call ,eler 10 comprises an outer tubular member 1 1, an inner tubular
member 12, a dilatation balloon 13 on a distal portion of the caLl,eter and
,an adapter 14 on the proximal end of the catheter. The inner tubular
15 member 12 has a guidewire receiving inner lumen 15 which slidably
receives guidewire 16. The outer surface of the inner tubular member 12
and the inner surface of the outer tubular member 11 define an annular
inflation lumen 17 which is in fluid communication with the interior of
balloon 13 and side arm 18 of adapter 14.
SUBSTITUT~ SHEE~ ~IILE 26)
CA 02243277 1998-07-1~
W O 97/26027 PCTrUS97/00843
The distal skirt 20 of balloon 13 is bonded, preferably fusion
bonded, to the exterior of th~ inner tubular member 12 and the proximal
skirt 21 is fusion bonded to the exterior of the outer tubular member 11.
The fusion bonds are preferably formed by applying laser energy to the
5 exterior of the skirts 20 and 21 which ca-lses the interface between the
skirts and the exterior of the outer and inner tubular members 12 and 13.
In one presently preferred embodiment, both the outer and inner tubular
members 11 and 12 are formed of a polymer blend in accordance with the
invention.
Figs. 34 depict another embodiment of the invention directed
to a rapid exchange type dilatation catheter 30. The catheter 30 includes a
relatively stiff proximal shaft 31 formed of hypotubing and a relatively
flexible distal shaft section 32. The distal shaft section 32 includes an inner
tubular member 33, an outer tubular member 34 and a dilation balloon 35.
15 The inner tubular member 33 has a guidewire receiving inner lumen 36
which is in fluid communication with a distal guidewire port 37 in the distal end
of the catheter 10 and a proximal guidewire port 38 disposed a short distance,
e.g. about 10 to about 45 cm from the proximal end of the
balloon 35. The proximal shaft 31 comprises a metallic hypotube 40 (e.g.
20 stainless steel or NiTi alloys) and an outer polymer jacket 41 formed of
SUB~ITUTE SHEET ~ E ~6~
CA 02243277 1998-07-1~
W 097/26027 PCTAUS97/00843
suitable poiymer material such as high density polyethylene. The distal end
32 of the hypotube 40 is truncated and fits into the interior of the outer
- tubular member 34 and bonded thereto by suitable adhesive 42. Support
tube 43, preferably formed of polyimide, is disposed between the inner and
outer tubular members 33 and 34 and defines inflation lumen 44. As
shown in more detail in Figs. 4 and 5, the outer tubular member is partially
bonded to the inner tubular member 33 and partially to the support tube 43.
A filler material 46, such as 75/25 high density/low density polyethylene, is
disposed between the outer tubular member 34 and the support tube 43.
In the embodiment of Figs. 3-4 the inner tubular member 33 is
rc ~" ,ed of a polymer blend in accordance with the present invention. The
distal skirt 47 of balloon 35 is fusion bonded to the exterior of the inner tubular
member 33 as in the previously discussed embodiment shown in
Figs.1 and 2. The proximal skirt 48 of the balloon 35 forms the outer
tubular member 34 and is formed of essentially the same material as the
balloon. In an alternative embodiment not shown the outer tubular member
34 may be a member separate and distinct from the balloon and formed of
a polymer blend in accordance with the present invention. In this latter
case the proximal skirt of the balloon 35 is fusion bonded to the exterior of the
outer tubular member.
SUB~TITl iTE SH~ R~JLE ~t3)
CA 02243277 1998-07-1~
WO 97/26027 PCTrUS97/00843
- Figs. 5 and 6 illustrate yet another embodiment of the
invention wherein the catheter 50 has a distal shaft 51 which is of a dual
lumen construction and is formed by extruding a poiymer blend in
accordance with the present invention. A tubuiar extension 52 extends
5 through the interior of the dilatation balloon 53 and has a distal guldewire
port 54 in its distal end. The balloon 53 has a distal skirt 55 fusion bonded
to the distal end of the tubular extension 52 and a proximal skirt 56 fusion
bonded to the distal shaft 51 as shown in the drawings.
A presently preferred polymer blend includes about 65% high
10 density polyethylene, about 30% Hytrel(~) (available from Dupont) and about
5% ethylene methyl acrylate such as Lotryl 24MA005 (available from Elf
ATOCHEM). This blend readily fusion bonds to polyethylene terephthalate
and has a coefficient of friction of about 0.1-0.2.
Although individual features of embodiments of the invention
15 may be shown in some of the drawings and not in others, those skilled in
the art will recognize that individual features of one embodiment of the
invention can be combined with any or all the features of another
embodiment. A variety of modifications can be made to the present
invention without departing from the scope thereof.
1 1
SUBSTITUTE SHEET ~RU~E 2~3
. ' i~'t~.A; ,~-t=! ! - ~ ~yt~