Note: Descriptions are shown in the official language in which they were submitted.
CA 02244076 1998-07-23
WO 98/23324 PCT/US97/21864
_ 1 _
RADIO FREQUENCY DILATOR SHEATH
Technical Field
This invention relates generally to medical devices and, in particular, to a
dilator sheath using electrical energy to separate encapsulating tissue from
an
implanted cardiac electrical lead.
background of the Invention
While cardiac electrical leads typicalEy have a useful fife of many years,
over time pacemaker and defibrillator leads fail. Unfortunately, by the time
they fail,
they have become encapsulated by fibrotic tissue against the heart itself or
the wall
of the vein. Encapsulation is especially encountered in areas where a device
has
caused tissue injury. Encapsulation is the body's healing response to protect
surrounding tissue from further injury. Scar tissue may also form due to
continual
device-related mechanical stresses (i.e., excessive pressure), infection, or
inadequate
blood supply to the site. The fibrotic tissue is tough and makes it difficult
to remove
the lead from the patient without causing trauma to the heart or great
vessels. For
example, when small diameter veins through which a pacemaker lead passes
become
occluded with fibrotic tissue, separating the lead from the vein can cause
severe
damage to the vein such as dissection or perforation.
To avoid this and other possible complications, some useless cardiac leads
are simply left in the patient when the pacemaker or defibritlator is removed
or
replaced. However, such a practice can incur the risk of an undetected lead
thrombosis or pulmonary embolism. Such a practice can also impair heart
function,
as multiple leads can restrict the heart valves through which they pass.
Furthermore,
such a lead can later become infected.
There are, of course, many other reasons why removal of a useless lead
is desirable. For example, if there are too many leads positioned in a vein,
the vein
can become totally occluded. Multiple leads can be incompatible with one
another,
interfering with the pacing or defibrillating function. An inoperative lead
can migrate
during introduction of another adjacent lead, and mechanically induce
ventricular
arrhythmia. Some recalled leads include J-shaped retention wires that have
been
CA 02244076 1998-07-23
WO 98/23324 PC~YUS97/21864
-2-
known to fracture and protrude through the insulation, causing several
reported
deaths. Other potentially life-threatening complications can require the
removal of
the lead as welt. For example, removal of an infected pacemaker lead is
considered
mandatory in the presence of septicemia and endocarditis. Other necessary ,
indications such as pocket infection, chronic draining sinus, and erosion can
lead to
significant morbidity if the lead is not removed.
Unfit recently, manual (or direct? traction, weighted (or sustained? traction,
and open-heart surgery/thoracotomy have been the most common methods of
removing useless or infected cardiac leads. Manual and weighted traction
involve the
risk of tearing the myocardium and are largely ineffective for leads
extensively
encased in fibrotic tissue. This procedure is atso ineffective in patients
with multiple
leads when these leads become scarred together at common fibrous binding
sites.
The risks and trauma associated with an open surgical approach are obvious.
Yet
another method of transvenously extracting a cardiac lead is by the use of a
grasping
device, such as a forceps or basket that is positionable around the outer
surface of
a lead or fragments of a lead. The use of forceps or a basket for tead
withdrawal is
complicated by the fact that the lead should first be freed from any
encapsulating
material surrounding it along its path. Furthermore, tearing of the myocardium
or
vessels can result during attempted extraction. Many of these problems were
overcome by the development of a system of tools and methods for transvenous
extraction of pacemaker leads and other elongated objects such as catheters.
Many
of these tools and procedures were developed with the assistance of Cook
Pacemaker Corp., Leechburg, PA, as evidenced by U.S. Patent Nos. 4,988,347;
5,013,310; 5,011,482; 4,943,289; 5,207,683; 5,507,751; 5,632,749; and
corresponding foreign patents. The preferred method involves positioning a
lead
removal tool or "locking stylet" inside the coiled wire of the lead to engage
the coil.
Once the locking stylet is positioned inside the coif, reinforcement is
provided and
extraction forces are concentrated at the lead tip. By using a sheath to apply
,
countertraction at the embedded tip as the lead is extracted, damage to the
myocardium can be largely avoided.
CA 02244076 1998-07-23
WO 98/23324 PCTfUS97121864
-3-
Typically, the locking stylet alone does not provide the tensional force
required to safely extract the lead due to excessive fibrotic or scar tissue
that has
encapsulated the lead against the vessel or myocardial wail. Dilator sheaths
formed
from plastic or metal tubes can be used to disrupt and separate the
encapsulating
tissue. Commonly, two coaxial dilator sheaths are positioned over the lead and
advanced therealong for loosening the lead from the fibrotic tissue on the
vein wall.
Plastic sheaths are flexible for bending around the natural anatomical
curvatures of
the vascular system. A problem with the plastic dilator sheaths is that the
leading
edge of the dilator sheath is weak and can lose its edge and buckle onto the
lead
during use. As a result, the plastic dilator sheath can become damaged and
unusable
before the lead is loosened from the fibrotic tissue. Furthermore, the tips of
the
flexible plastic sheaths can deform when subjected to tough fibrotic tissue.
This
problem is further heightened when the sheath is bent around a vessel curve:
Metal
dilator sheaths provide a sharp leading edge for encountering fibrotic tissue.
A
problem with some metallic dilator sheaths is that they are relatively
inflexible and
resist bending around natural anatomical curvatures. As a result, a metallic
dilator
sheath can be difficult or impossible to advance toward the distal end of the
pacemaker lead without injuring or obliterating the vein. Flexible metallic
dilator
-sheaths have been developed to address the problems associated with plastic
sheaths and rigid metal sheaths. While very effective for their intended use,
even
metal sheaths are inadequate for the toughest fibrotic tissue and
calcification in a
vessel. The tensile strength of the fibrous tissue increases with time.
Eventually the
tissue can even differentiate into cartilage or bone. Attempted separation of
difficult
fibrotic tissue can cause mechanical trauma to the vessel. Data show that 5.4%
of
all attempted lead extractions are not successful and 7.5% are only partially
successful, almost entirely due to the presence of excessive scar tissue. Lead
fragility is another problem and generally escalates over time when a lead has
a
design flaw or has been structurally compromised.
U.S. Patent No. 5,423,806 of Dale et al. discloses a laser catheter for
ablating encapsulating tissue during the extraction of pacemaker leads. Using
directed high energy to burn, desiccate, or melt the tissue encapsulating the
lead can
CA 02244076 1998-07-23
WO 98/23324 PC'1'/US97/21864
-4-
reduce the length of the procedure and increase the number of leads that can
be
extracted. In the practiced embodiment of 5,423,806, optical fibers are
arranged
circumferentially around an open lumen through which the lead passes. One
problem
with this embodiment is that tissue can be readily cored and plug the internal
lumen ,
of the device, thus making forward or reverse movement of the device extremely
difficult. The laser device is used in combination with a plastic outer sheath
and
tracks over the lead as the distal tip of the laser burns through any
obstructive tissue
surrounding the lead. Partly due to the difficulty in visualizing the
treatment site, a
significant disadvantage of this approach is the risk of burning though the
vessel wall
or myocardium. This is especially a problem if sufficient tension is not
constantly
maintained on the lead during the procedure, allowing the distal tip of the
laser to
angle toward the wall of the vessel or myocardium. This could pose an
unacceptable
risk for the large number of lead extractions that are elective procedures and
do not
involve Life-threatening indications.
Alternative embodiments of the_ laser catheter suggested by the Dale
reference include having the optical fibers grouped on one side of the
catheter or
utilizing a single fiber. Either would permit more precise ablation of scar
tissue
surrounding the lead if the point of ablation can be manipulated and
selectively
rotated away from the vessel wail. It is suggested that a stylet could be
inserted into
an additional lumen of the catheter to facilitate rotational control. While
providing
the physician with control over the point of ablation during the procedure
should
reduce the risk of accidentally penetrating the vessel wall, the effectiveness
of the
laser catheter is stilt limited by the fragility of the optical fibers. Given
the tendency
of optical fibers to break when subject to lateral bending or rotational
forces, current
laser catheter designs are not particularly torqueable. An annular arrangement
of
optical fibers, with its disadvantages, is used that does not require that the
catheter
be rotated. However, even when merely navigating a laser catheter through a
tortuous angle, breakage can occur that can result in the catheter burning
through .
itself or the cardiac lead insulation due to the large amount of heat
generated. These
disadvantages, along with the much higher cost, limit the laser catheter as an
alternative to manual sheaths.
CA 02244076 1998-07-23
WO 98!23324 PCT/LTS97/21864
_5_ _
Summary of~he Invention
The foregoing problems are solved and a technical advance is achieved by
a medical device for separating an elongated structure such as an electrical
cardiac
lead implanted in biological tissue. The medical device comprises an inner
elongated
a .J:1..,+...- 1-.g +Hl-..~..:r, c.+~i anrJ .~ nrI~ n~ a.~ o ewte~nrlinn Iran
itmrlin~iIv
J UIIaIVI jllGaLl1 IIQVIIIg a d~.7~Gi1 GiIV ~1W Ca -I,JGiS.7Gigir vnwlluuy
~vllgnw411fGa~~y
therethrough. The medical device further comprises an electrical conductor
positioned about the distal end and passage of the inner elongated sheath. The
passage of the sheath is sized and configured for placement of an elongated
structure, such as an electrical cardiac lead, implanted in biological tissue,
such as
a vessel leading to or from the heart. When energized, the electrical
conductor
electrically separates or ablates biological tissue from the elongated
structure
implanted therein and placed in the passage of the inner dilator sheath.
Advantageously, the distal end of the inner elongated dilator sheath is at
least
partially beveled for mechanically loosening and separating encapsulated
tissue from
the elongated electrical lead. As a result, the electrical conductor and the
mechanical
configuration of the inner dilator sheath work in concert with each other to
provide
separation of extremely tough encapsulating tissue and stubborn calcification
deposits from the elongated electrical structure. In addition, the sheath can
disrupt
the fibrous tissue bands which commonly bind multiple cardiac leads together.
The
beveled distal end also includes a transverse face that advantageously
positions the
electrodes of the electrical conductor (therein) so as to establish and
maintain an
electrical, tissue ablating arc therebetween. The tissue ablating arc also
advantageously maintains a necessary gap between the obstructive tissue and
the
end of electrodes as the dilator sheath is eased forward.
The radio frequency dilator sheath further includes an outer dilator sheath,
which also advantageously has a beveled distal end that is coaxially
positioned over
the inner dilator sheath for providing coordinated longitudinal and rotational
movement with the inner dilator sheath for separating encapsulating tissue
from an
implanted lead.
In the preferred embodiment, first and second electrical conductors are
advantageously positioned in the waH of the inner dilator sheath and about the
distal
CA 02244076 1998-07-23
WO 98/23324 PCT/US97/2I864
- 6 -
end thereof. When connected to a source of radio frequency energy, an
electrical arc
of radio frequency energy is selectively established between the conductors
for
heating, cutting, ablating, or wetting encapsulating tissue and calcification
deposits
away from the implanted lead. The electrical conductors preferably have a
tungsten
electrode tip so as to prevent deterioration of the conductor with an
electrical arc
emanating therefrom. The electrode tip is conveniently connected via a
connector
sleeve to a supply conductor which exits the inner dilator sheath about the
proximal
end thereof.
In another illustrative embodiment, the electrical conductor or conductors
are positioned in longitudinal recesses formed in the outer surface of the
inner dilator
sheath about the distal end thereof. The electrode tip is positioned in the
recess and
fixedly positioned therein with a biocompatible material, such as a medical
grade
adhesive or epoxy. An outer wrap, such as a shrink-wrap tube, is positioned
around
the inner dilator sheath as well as the electrical conductors to fixedly
position and
mechanically support the remaining portion of the electrical conductors along
the
remaining length of the inner dilator sheath. As previously suggested, an
outer
coaxial dilator sheath is also used in combination with this alternative
embodiment
for separating encapsulating tissue from an implanted elongated structure.
In yet another embodiment of the radio frequency dilator sheath, a plurality
of electrical conductors, for example, three, are positioned in the wail of
the inner
dilator sheath and are selectively energized in pairs or simultaneously to
provide
further circumferential electrical separation of encapsulated tissue from the
implanted
lead positioned in the main passage of the dilator sheath.
The inner and outer coaxial dilator sheaths of the present invention each
preferably comprises an elongated tubular member of a biocompatible material,
the
inner sheath having a high temperature resistance or high continuous use
temperature
preferably over 500° F. In the preferred embodiment, the outer coaxial
dilator sheath
comprises a polypropylene material, whereas the inner dilator sheath comprises
a
radiopaque polytetrafluoroethylene material. By way of example, the radiopaque
material can include bismuth, barium, bismuth carbonate, platinum, tungsten,
or any
other commercially available radiopaque material. Other high-temperature
resistant
CA 02244076 1998-07-23 _
WO 98/23324 PCT/LTS97/21864
-7-
biocompatible materials having a heat deflection temperature of, for example,
500° F, include fluorinated ethylene propylene, polyetheretherketone,
2
polyetherimide, polyphenyisulfone, and polyimides.
Preferably, the electrical conductors of the radio frequency dilator sheath
r
include a high temperature electrode tip or a material such as tungsten so as
to
advantageously prevent deterioration of the conductor due to the electrical
arc
emanating therefrom during separation of tissue from the imptanted structure.
One or more conductors can extend over or in the distal end of the inner
sheath. Each conductor can be located on the outer surface, or in a recess on
the
outer surface, or in a passageway at the distal end region of the inner
sheath.
Brief Description of the Drawing
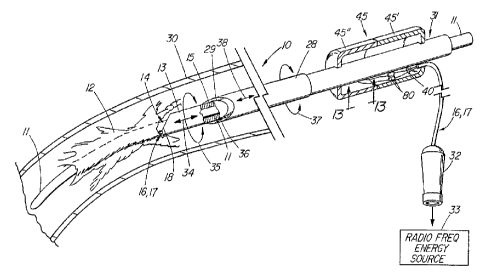
FIG. 1 depicts a pictorial view of a preferred embodiment of an illustrative
radio frequency dilator sheath of the present invention;
FIG. 2 depicts an enlarged pictorial view of the distal end of the dilator
sheath of FIG. 1;
FIG. 3 depicts an enlarged distal-end view of the dilator sheath of FIG. 1;
FIG. 4 depicts an enlarged and partially sectioned side view of the distal
end of the dilator sheath of FIG. 1;
FIG. 5 depicts an end view of an alternative embodiment of the dilator
sheath of FIG. 1;
FIG. 6 depicts an enlarged pictorial view of another illustrative embodiment
of the dilator sheath of FIG. 1;
FIG. 7 depicts an enlarged distal-end view of the dilator sheath of FIG. 6;
FIG 8 depicts an enlarged and partially sectioned side view of the distal
end of the dilator sheath of FIG. 6;
FIG. 9 depicts a block diagram of a radio-frequency generator system
connected to the medical device of FIG. 1;
FIG. 10 is a schematic diagram of the radio-frequency generator system
of FlG. 9;
FIG. 1 1 depicts a block diagram of still another embodiment of a radio-
frequency generator system connected to the medical device of FIG. 1;
CA 02244076 1998-07-23
WO 98/23324 PCT/US97/21864
_ g _
FIG. 12 depicts a block diagram of yet another embodiment of a radio-
frequency generator system-connected to the medical device of FIG. 1;
F(G. 13 depicts an enlarged and partially sectioned bottom view of the
dilator sheath of FIG. 1 taken along line 13-13;
FIG. 14 depicts an enlarged pictorial view of the distal end of an alternative
embodiment of the dilator sheath of FIG. 1;
FIG. 15 depicts an enlarged and partially sectioned side view of the distal
end of an alternate embodiment to the dilator sheath of FIG. 1; and
FIG. 16 depicts an enlarged and partially sectioned side view of the distal
end of an alternate embodiment to the dilator sheath of FIG. 6;
FIG. 1 depicts a pictorial view of a preferred embodiment of an illustrative
medical device such as a radio frequency dilator sheath 10 for separating an
encapsulated elongated structure such as a cardiac electrical lead 1 1 from
biological
tissue 12. Electrical cardiac lead 11 such as from a pacemaker or
defibrillator is
initially implanted in a blood vessel 30 extending to or from the heart. After
a period
of time, the elongated structure of the cardiac lead typically becomes
encapsulated
by fibrotic biological tissue 12 against the wall of the vessel or surrounding
tissue.
To remove the encapsutated cardiac lead from the vein of a patient, a dilator
sheath
1 O is used that includes inner and outer coaxial dilator sheaths 13 and 28
that are
coaxially positioned over the lead and advanced therealong for mechanically
separating the lead from the encapsulating fibrotic tissue 12 on the vessel
wall.
Inner elongated sheath 13 has a distal end 14 that includes an at least
partially
beveled distal end 18 for mechanically loosening and separating lead 11 from
the
encapsulating tissue as the inner sheath is advanced longitudinally back and
forth and
rotated about the lead as indicated by arrows 34 and 35. Inner elongated
dilator
sheath 13 also has a passage 15 extending longitudinally therethrough, which
is
sized and configured for placement therein of elongated structure 1 1
implanted in
vessel 30. Similarly, outer dilator sheath 28 includes a beveled distal end 36
for
loosening and separating the encapsulating tissue from the lead with the aid
of
circular and longitudinal movement as indicated by arrows 37 and 38. Outer
dilator
CA 02244076 1998-07-23
WO 98/23324 PCT/US97/21864
_g_
sheath 28 also has a passage 29 extending longitudinally therethrough, which
is
sized and configured for placement therein of inner dilator sheath 13 and
elongated
electrical structure 1 1.
Inner and outer coaxial dilator sheaths 13 and 28 are biocompatible
material tubes, which are laterally flexible for bending around the natural
anatomical
-- curvatures of the vascutar system. Although beveled distal ends 18 and 36
of the
dilator sheaths provide mechanical separation of the lead from most
encapsulating
fibrotic tissue, tough fibrotic tissue or calcification deposits present a
significant
problem for separation and often cause damage to the leading edge of these
beveled
distal ends. As a result, medical device 10 also includes at least one
electrical
conductor such as a bipolar pair of electrical conductors 16 and 17 that are
positioned about distal end 14 and passage 15 of inner elongated dilator
sheath 13.
This electrical conductor pair extends longitudinally along the inner
elongated sheath
and exits therefrom about proximal end 31 of the sheath. The exiting of
electrical
conductors 16 and 17 about proximal end 37 of the inner sheath is mechanically
supported by hollow plastic handle 45 that is provided to facilitate
manipulation of
the dilator sheath. The handle that is comprised of two cupped parts 45' and
45"
can be made from a wide variety of polymers, including commercially available
potyamides (nylon), acetal, or acrylonitrile butadiene styrene (ABS). The
handle also
provides physical protection for the connection of the supply conductor wires
40 to
the remaining proximal end wires of the electrical conductors 16 and 17. The
corresponding wires are each joined with solder and further secured with short
pieces
of heat shrink tubing 80. The electrical conductors 16 and 17 are secured to
the
handle with an adhesive such as silicon where they exit therefrom.
FIG. 13 depicts an enlarged and partially sectioned bottom view of the
dilator sheath of FIG. 1 along the line 13-13. Within the hollow plastic
handle 45,
the supply conductor wires 40 exit the sheath 13 through a pair of
longitudinally
. offset ports 81 and 82 that communicate with respective first and second
electrical
conductor passages 22 and 23 within the sheath wall 41.
Returning to FIG. 1, electricat conductors 16 and 17 proximally terminate
in an electrical connector 32, which connects to a commercially available
source 33
CA 02244076 1998-07-23
WO 98/23324 PCTIUS97/21864
- 10-
of radio frequency energy. Radio frequency energy is selectively applied to at
least
one electrical conductor, which can be either unipolar or bipolar, and
delivered to
distal end 14 of the inner dilator sheath. An arc of electrical energy is
established
at the distal end of the inner sheath between electrical conductors 16 and 17
and
separates, ablates, melts, or cuts encapsulating tissue 12 or calcification
deposits
from the cardiac electrical lead. As a result, the delivery of radio frequency
energy
to the distal end of the inner elongated dilator sheath is used singly or in
combination
with the mechanical configuration of the inner and outer coaxial sheaths to
separate
encapsulating biological tissue 12 from implanted electrical cardiac lead 1 1
.
FIG. 9 depicts a block diagram of radio-frequency generator system 46
connected to medical device 1 O of FIG. 1 via electrical conductors 16 and 17.
The
radio-frequency generator system includes a commercially available source 33
of
radio-frequency energy connected to dilator sheath 1 O via well-known
impedance
matching network 47. Electrical conductors 16 and 17 extend longitudinally
through
elongated inner sheath 13 and terminate at distal end 14 thereof for
establishing an
arc of electrical energy therebetween. Electrical conductors 16 and 17 have a
real
impedance of approximately 2000 Ohms. Typically, commercially available radio-
frequency energy sources have an output impedance of approximately 100 Ohms.
impedance matching network 47 matches the different impedances of the
electrical
dilator sheath conductors 16 and 17 to that of the radio-frequency energy
source
33. The impedance matching network minimizes power loss between the dilator
sheath and energy source and permits monitoring of the current and voltage
applied
to the dilator sheath when an arc of electrical energy has been established
between
the electrical conductors at the distal end of the dilator sheath. To monitor
the
various levels of current and voltage applied to dilator sheath 10, a well-
known
current-to-voltage converter 48 is positioned in the radio frequency generator
system
between impedance matching network 47 and energy source 33, as shown. The
radio frequency current flowing between the impedance matching network and the
energy source through current-to-voltage convertor 48 is transformed to
generate
a voltage signal representative of the current flowing to the dilator sheath.
This
voltage signal is applied to current monitor 49, which then applies a signal
to
CA 02244076 1998-07-23
WO 98/23324- 1'CTlUS97/21864
-11-
indicator lamp 50 via well-known LED driver circuit 51. Current monitor 49 is
set to
detect the amount of current flowing to dilator sheath 10. When an arc of
electrical
energy is established between electrical conductors 16 and 17 at the end of
dilator
sheath 10, a relatively Earge amount of radio frequency current flows in the
conductors. This large amount of current is indicative of when the dilator
sheath is
ablating or cutting encapsulating tissue. As a result, the level of this
current is
monitored and the current monitor 49 adjusted to light indicator lamp 50 when
an
arc of electrical energy is established between the electrical conductors.
This visual
indication signals the attending physician that ablation of encapsulating
tissue is
occurring. This signal is in addition to the tactile feel of the dilator
sheath as it is
advanced along the implanted cardiac lead.
When an arc is not established between the distal ends of electrical
conductors 16 and 17, the amount of current flowing in dilator sheath 10 as
well as
radio-frequency generator system 46 is much less. The current monitor detects
the
drop in current and extinguishes indicator lamp 50.
FIG. 10 is a schematic diagram of impedance matching network 47,
current-to-voltage converter 48, current monitor 49, and LED indicator lamp 50
and
driver circuit 51 therefor. Impedance matching network 47 includes a well-
known
radio-frequency transformer 54 using ferrite toroid cores, such as F240-77
cores,
which are commercially available from Amidon Inc., Anaheim, California.
Primary
winding 55 of the transformer includes approximately 1 1 turns of wire
connected to
conductors 52 and 53 from energy source -33. Secondary winding 56 of the
transformer is approximately 50 turns of wire connected to electrical
conductors 16
and 17 of the dilator sheath. This provides a turns ratio of approximately
4.5:1,
which will match the 100 Ohm impedance of the energy source to the 2000 Ohm
impedance of the dilator sheath with minimal power loss. As is well known, the
voltage and current is transformed between the primary and secondary windings
of
the transformer in a 4.5:1 ratio. The impedance ratio is transformed per the
square
of the turns ratio, such as 20:1, which matches the impedance of the two
devices.
Current-to-voltage converter 48 is connected to primary winding 55 of
radio-frequency transformer 54 between radio-frequency energy source 33 and
CA 02244076 1998-07-23
WO 98/23324 PCTlLTS97/21864
- 12-
impedance matching network 47. Current-to-voltage converter 48 includes a
radio-
frequency transformer 57 having a ferrite toroid core commercially designated
F50-
61, which is also available from the Amidon Corporation. The primary winding
58
of this transformer is one turn, whereas the secondary winding 59 is a four-
turn
winding. This 4:1 turns ratio is to convert the large current flowing through
the
w- -primary winding to a voltage signal that is applied to current monitor 49.
Current monitor 49 is connected to the single, secondary winding turn of
transformer 57 and includes peak detector 60 connected in series to threshold
voltage detector 61. Both of these circuits are welt known electrical
circuits. Peak
detector 60 includes diode 62, such as commercially available diode IN914
connected in series to the input of threshold voltage detector 61. Connected
in
parallel to the output of diode 62 are capacitor 63 of, for example, .05 ,uf,
and load
resistor 64 of, for example, 6.8M Ohms.
Threshotd voltage detector 61 .includes a comparator 65, such as
commercially available operational amplifier CA3160E, connected in series
through
load resister 66 of, for example, 1 OOK Ohms, to the input of LED and driver
circuit
51. One input of the comparator is connected to peak detector 60, whereas the
other input of the cornparator is connected to a voltage divider potentiometer
67,
such as a 20K Ohm, ten-turn potentiometer connected between ground and a 9
volt
source. The dilator sheath is connected to the energy source and energized to
cause
an arc of electrical energy to be conducted between conductors 16 and 17 at
the
distal end of the dilator sheath. The potentiometer of the threshold voltage
detector
circuit is adjusted to cause indicator Tamp 50 to light, indicating that an
electrical arc
has been established. The dilator sheath is then put in contact with the
tissue to
cause the arc to extinguish. Should the indicator tamp still be lit, the
potentiometer
is again adjusted to extinguish the indicator lamp.
LED and driver circuit 51 includes well-known FET switch 68, such as
commercially available VN2222, which is connected in series between load
resistor .
69 of, for example, 1 K Ohms, and light emitting diode 70. The output of the
threshold voltage detector turns the switch on and off, permitting current to
flow
CA 02244076 1998-07-23
WO 98/23324 PCT/LTS97/21864
-13-
through the light emitting diode 70, causing indicator lamp 50 to light and
extinguish
as previously described.
FIG. 12 depicts another embodiment of radio-frequency generator system
46 connected to dilator sheath 10 of FIG. 1. In this embodiment, dilator
sheath 10
includes a temperature sensor 71, such as a commercially available thermistor,
positioned at distal end 14 of the dilator sheath adjacent the distal ends of
conductors 16 and 17. This thermistor and electrical conductors 72 and 73 are
longitudinally positioned through the dilator sheath in a manner similar to
those of
electrical conductors 16 and 17. When an arc of radio-frequency electrical
energy
is established between electrical conductors 16 and 17, thereby separating,
ablating,
melting, or cutting encapsulating tissue, heat is generated by the electrical
arc and .
the severed tissue. The heat generated by the electrical arc and the severed
tissue
is sensed by temperature sensor 71, which transmits an electrical signal
indicative
thereof to radio-frequency generator system 46. Alternatively, or in
combination
with thermal sensor 71, an optical fiber or other sensing device can be
positioned at
distal end 14 of dilator sheath 10. These additional or alternative sensors
are used
to monitor or indicate when an electrical arc has been established between
conductors 16 and 17 for dissecting or removing encapsulating tissue from the
encapsulated lead.
RF generator system 46 includes radio-frequency energy source 33 that
is connected to dilator sheath 10 through impedance matching network 47.
Impedance matching network 47 includes components as previously described.
Generator system 46 also includes a temperature monitor circuit 74 which is
connected to thermal sensor 71 via conductors 72 and 73. Temperature monitor
circuit 74 is a well-known circuit for lighting and extinguishing indicator
lamp 50, as
previously discussed, and for indicating the presence and absence of an
electrical arc
between dilator sheath conductors 16 and 17. Temperature monitor circuit 74
can
also be used to provide feedback to radio-frequency energy source 33 to
regulate the
amount of energy applied to dilator sheath 10.
F1G. 11 depicts still another embodiment of radio-frequency generator
system 46 connected to dilator sheath 1 O of FiG. 1. Electrical conductors 1 6
and
CA 02244076 1998-07-23 -.
WO 98/23324 PC~'l(TS97/21864
- 14-
17 extend to distal end 14 of inner elongated dilator sheath 13. In this
particular
embodiment, generator system 46 includes radio-frequency energy source 33
connected to dilator sheath 10 via impedance matching network 47 as previously
described. Impedance monitor circuit 75 is connected across the output of .
impedance matching network 47 to detect changes in impedance of the dilator
sheath due to the presence and absence of an electrical arc between electrical
conductors 16 and 17 at distal end 14 of the sheath. As previously suggested,
the
differences in impedance detected by impedance monitor 75 are used to energize
and
extinguish indicator lamp 50 during the presence and absence of the electrical
arc at
1 O the distal end 14 of the dilator sheath. In addition, the impedance
monitor circuit can
be connected to radio-frequency energy source 33 to provide a feedback signal
thereto for regulating the amount of energy applied to the dilator sheath in a
well-
known manner.
In addition, it is contemplated that radio-frequency energy source 33 can
be controlled with impedance monitor 75, temperature monitor 74, or current
monitor 49 to provide a targe pulse of electrical energy to the distal end of
the dilator
sheath. This large pulse of electrical energy is applied via electrical
conductors 16
and 17 so as to cause the tissue fluids about the encapsulated lead to enter a
gaseous state, thereby essentially exploding the tissue away from the
encapsulated
lead.
FIG. 2 depicts an enlarged pictorial view of distal end 14 of inner dilator
sheath 13 of medical device 10 of FIG. 1. FIG. 3 depicts an enlarged distal
end view
of inner dilator sheath 13 of medical device 1 O of FIG. 1. The inner
elongated dilator
sheath 13 is configured in the form of an elongated tubular member 26 with
main
passage 15 extending longitudinally therethrough. However, the inner dilator
sheath
and its main passage can take on any cross-sectional shape such as square,
rectangular, elliptical, triangular, etc., or any combination thereof. As
previously
indicated, main passage 15 is sized and configured for placement therein of
the
elongated structure of the electrical cardiac lead. Also inctuded in tubular
member
26 is first and second electrical conductor passages 22 and 23 also extending
longitudinally in wall 41 of the tubular member. Electrical conductor passages
22
CA 02244076 1998-07-23
WO 98/23324 PCTlLTS97/21864
-15-
and 23 have respective electrical conductors 16 and 17 fixedly positioned
therein,
with the aid of biocompatibte material 24 such as a commercially available
medical
grade adhesive or epoxy. One example of medical grade epoxy is Hysol~ epoxy,
which is available from the Dexter Corp., Olean, NY. Tubular member 26 of
inner
dilator sheath 13 is formed from a high temperature biocompatible polymer
material
which is capable of withstanding the temperatures resulting from the
generation of
an electrical arc between electrical conductors 16 and 17. Preferably, tubular
member 26 of inner dilator sheath 13 comprises polytetrafluoroethylene (PTFE),
which is radtopaque due to the addition of a radiopaque material 27 such as,
for
example, bismuth, barium, bismuth carbonate, platinum, tungsten, or any other
commercially available radtopaque material. As is well known, PTFE is also a
lubricious material. Other high temperature resistant biocompatible materials
having
a heat deflection temperature in excess of, for example, 500° F and
suitable for the
tubular member of dilator sheath 13 include fluorinated ethylene propylene
(FEP),
polyetheretherketone (PEEK), potyetherimide (PE1), potyphenytsulfone (PPS),
and
polyimides. The use of other biocompatible material having lower heat
deflection
temperatures is also contemplated depending on the particular application and
the
heat generated by the electrical conductors. Should tubricity become a concern
with
any of the biocompatible materials, a hydrophillic coating can be applied to
the
surface thereof.
By way of exampte, tubular member 26 of inner dilator sheath 13 is
approximately 19 inches long and has an outer diameter of approximately .155
inches with a main passage inner diameter of approximately .1 13 inches. Wall
41
of tubular member 26 has a minimum outer wall thickness of approximately .021
inches. Electric cardiac leads typically have an outer diameter of
approximately .100
inches, which is readily accommodated by the diameter of main passage 15 of
the
sheath. The diameter of main passage 15 can be readily adapted to facilitate
the
placement of electric cardiac leads as small as .060 inches and as large as
.125
inches. The diameter of the main passage is selected so to provide a clearance
of
no more than .020 inches. This tolerance minimizes, if not eliminates, the
collection
of tissue inside the dilator sheath, which can block up the main passage
thereof.
CA 02244076 1998-07-23
WO 98/23324 PCT/US97/21864
-16-
Electrical conductor passages 22 and 23 are formed in the thicker portion of
wall 41
with a diameter of approximately .022 inches. Outer dilator sheath preferably
comprises a polypropylene material and is approximately 13 inches long with an
outside diameter of .233 inches ana an inside diameter of .208 inches. Beveled
distal ends 18 and 36 form a 45 ° angle with respect to the
longitudinal axis of
sheaths 13 and 28. Beveled distal edge 18 of the inner dilator sheath 13 is
truncated to form a transverse face 89 whereby the electrical conductors 16
and 17
are flush with the distal edge of the sheath. The transverse face is formed
with the
electrical conductors extending out from beyond the distal end of the sheath
and
then cutting the distal portion of the distal beveled edge with a diamond saw
such
that the transverse face or surface 89 is perpendicular to the longitudinal
axis of the
sheath.
FIG. 4 depicts an enlarged and partially sectioned side view of distal end
14 of inner dilator sheath 13 of medical device 1 O of FIG.1 . As depicted,
the distal
end of electrical conductor passages 22 and 23 are counterbored to a diameter
of
approximately .030 inches and to a depth of approximately .3 inches.
Electrical
conductors 16 and 17 each include an electrode tip 44 of a high temperature
electrical conductor material such as, for example, tungsten for generating a
radio
frequency electrical arc therefrom. Electrical conductor 16 also includes
connector
sleeve 39 of, for example, stainless steel, which is connected to a low
resistance
electrical conductor 40 of, for example, No. 2840/7 stranded and twisted
copper
wire available from the Alpha Wire Company, Elizabeth, NJ. Electrical
connector
sleeve 39 is approximately .2 inches long with an inner diameter of
approximately
.021 inches and an outer diameter of .028 inches. Tungsten electrode tip 44 is
approximately .2 inches long with a diameter of approximately .020 inches.
Half of
the electrode tip is positioned in connector sleeve 39. The tungsten electrode
tip is
formed from a pure tungsten wire such as used with T1G welding electrodes,
which
are commercially available from any welding supply shop. The connector sleeve
is ,
mechanically crimped to the tungsten tip and electrical supply conductor 40.
Electrical conductor 16 is first positioned through conductor passage 22 and
exits
at or in the vicinity of the proximal end of the tubular member. The connector
sleeve
CA 02244076 1998-07-23
WO 98/23324 PCT/ETS97/21864
-17-
and tungsten electrode tip are then pulled into the counterbored portion of
the
conductor passage and fixedly positioned therein with biocompatible material
24
such as a medical grade adhesive or epoxy as previously indicated.
As depicted in FIGs. 2 and 3, electrical conductors 16 and 17 are
positioned in conductor passages 22 and 23, respectively, in the thicker
portion of
wall 41 of tubular member 26. Center-to-center spacing of the electrical
conductors
and conductor passages is preferably in the range of .090 to .100 inches with
a
maximum spacing of .150 inches and a minimum spacing of .010 inches.
FIG. 5 depicts an end view of an alternative embodiment of medical device
10 of FIG. 1 in which inner dilator sheath 13 includes at least three
electrical
conductor passages extending through wall 41 of tubular member 26. These three
conductor passages include previously described conductor passages 22 and 23
along with conductor passage 42 for positioning electrical conductors 16, 17
and 43
therein, respectively. As previously described, inner sheath passage 15 is
utilized for
positioning the electrical cardiac lead therein. In operation, an electrical
arc is
established between central conductor 17 and outer conductor 16 or,
alteratively,
between central conductor 17 and outer conductor 43, either simultaneously or
alternatively with the other conductor pair. The electrical arcs established
therebetween are used to establish a broader base of radio frequency energy
for the
separation of tissue from the electrical_cardiac lead. It is also contemplated
that
electrical conductors be positioned entirely around the circumference of the
inner
dilator sheath.
FIG. 6 depicts an enlarged pictorial view of another illustrative embodiment
of inner dilator sheath 13 of medical device 10 of FIG. 1. In particular, this
embodiment of inner dilator sheath 13 positions electrical conductors 16 and
17 in
outer surface 19 thereof. FIG. 7 depicts an enlarged distal end view of inner
dilator
sheath 13 of medical device 10 of FIG. 6. To fix the relative position of
electrical
conductors 16 and 17 in the outer surface of the dilator sheath, recesses 20
and 21
are formed in outer surface 19. Electrical conductors 16 and 17 are positioned
in
respective outer surface recesses 20 and 21 and fixedly positioned therein
with
biocompatible material 24 such as a medical grade adhesive or epoxy, as
previously
CA 02244076 1998-07-23
WO 98/23324 PCT!l1JS97/2I864
- as -
described. The biocompatible adhesive or epoxy is applied over the electrical
conductors and around the circumference of the dilator sheath just about the
distal
end thereof. To fixedly position the remaining portion of electrical
conductors 16 and
17 with respect to dilator sheath 13, outer wrap 25 is positioned around the
electrical conductors and elongated tubular member 26.
As previously indicated, inner elongated dilator sheath a 3 is configured in
the form of an elongated tubular member 26 with main passage a 5 extending
longitudinally therethrough. Furthermore, electrical conductors 16 and 17 each
include a tungsten electrode tip 44, a connector sleeve 39 and a supply
conductor
40. In this particular embodiment, the length of the tungsten tip is increased
to
approximately .75 inches with the diameter of recesses 20 and 21 being
maintained
at approximately .022 inches. The proximal end of the recesses are enlarged to
accommodate connector sleeve 39. Outer wrap 25 is preferably a high
temperature
shrink-wrap tube of a KYNAR° material available from Pennwalt Corp.,
Philadelphia,
PA. This shrink-wrap tube preferably has an inner diameter of . a 87 inches
with a
wall thickness of .005 inches and is heated to shrink around the conductors
and
inner dilator sheath a 3. This shrink-wrap material shrinks about the inner
dilator
sheath with temperatures in the range of 450° F to 500° F. The
outer wrap tube 25
provides mechanical strength to electrical conductors 16 and 17 so as to
minimize
breakage or movement during separation of tissue from the electrical cardiac
lead.
The center-to-center spacing of electrical conductor 16 and a 7 is maintained
as
previously described with respect to the first embodiment.
FIG. 8 depicts an enlarged and partially sectioned side view of distal end
14 of inner dilator sheath 13 of medical device 1 O of FIG. 6. As depicted,
electrical
conductor a 6 includes electrode tip 44 and supply conductor 40 interconnected
by
connector sleeve 39. Electrode tip 44 is positioned in recess 20 and fixedly
positioned therein with biocompatible material 24 and outer wrap tube 25.
Distal
end a 4 includes beveled distal end 18 which makes an angle of approximately
45 _
degrees with respect to the longitudinal axis of the dilator sheath with a
transverse
face 89 at the distal tip perpendicular to the longitudinal axis of the
dilator sheath.
This beveled distal end is formed after the electrical conductors,
biocompatibfe
CA 02244076 1998-07-23
WO 98/23324 PCT/LTS97/21864
-19-
material, and outer wrap tube are positioned around tubular member 26. The
transverse face is formed using a diamond saw to remove the most distal
portion of
the beveled distal end such that electrical conductors 16 and 17 are flush
with the
distal end of the sheath.
FIG. 14 depicts an enlarged pictorial view of the distal end of an alternative
embodiment of the dilator sheath of F1G. 1. In this embodiment_ a thir~ ImmAh
uz
formed in wail 41 of sheath 13 through which dye or other fluid or material
can be
injected or aspirated. Also depicted is an alternative embodiment in which
electrical
conductors 16 and 17 are of different diameters. Still further depicted, are
alternative embodiments of electrode tip 44 such as conductor tips 84-88 which
provide selected examples of alternative tip shapes that can be used including
concave 84, chisel 85, rounded 86, truncated 87 and conical 88. These tips can
either be slightly protruding, flush, or slightly recessed with respect to
distal
transverse face 89 of the distal end 14 of the dilator sheath. if the
electrical
conductor tips are protruding or recessed too much, there can be difficulty in
establishing or maintaining an electrical arc for cutting. The method of
placing the
specially shaped conductor tips 84-88 is the same as in the preferred
embodiment
except that the transverse face 89 is created prior to threading the supply
conductor
wires 40 into the first and second electrical conductor passages 22,23.
FIGs. 15 and 16 depict enlarged and partially sectioned side views of the
distal end of an alternate embodiment of the dilator sheath of FIGs. 1 and 6,
respectively. In each of these embodiments, the beveled distal end 18 is not
truncated distally as in the preferred embodiment, forming a transverse face
or
surface such that the electrical conductors are flush with the distal end. In
these
alternate embodiments, each electrode tip 44 is slightly recessed from the
face of the
beveled distal end 18 which terminates at a sharp edge extending distal to the
electrode tips. The sharp beveled edge can be used advantageously to
mechanically
disrupt scar tissue along the path of the lead, thus complimenting the action
of the
radio frequency energy.
In operation, electrical conductors 16 and 17 are connected to a
commercially available source of radio-frequency energy commonly found in
CA 02244076 1998-07-23
WO 98/23324 ~'CT/iFS97/2I864
-20-
hospitals, such as the Model Force FX .RF Generator available from Valleyiab,
Boulder,
CO. This eiectrosurgicai unit (ESU) is capable of delivering approximately 50
to 100
Watts of power with a radio-frequency fundamental of approximately 500 KHz
(500
KHz ~ 10 KHz) modulated by 120 Hz (65%120 Hz modulation) to the tungsten
electrode tips. The radio-frequency dilator sheath 1 O of FlG. 15 was tested
with a
similar Valieylab generator (Model SSE2L) on an anesthetized sheep with the
heart
exposed through the right side of the ribs. With a setting of 2 on the
electrosurgical
generator, connective tissue was cut with 100 mA. Also at a setting of 2,
muscle
outside the rib cage was cut with 80-100 mA. The electrosurgicat generator was
then set to 2.5 to cut a fat pad on the outside of the pericardium without any
stimulation of the heart. The heart could be stimulated through the
pericardium at
this setting. The next set of cuts were performed by placing the dilator
sheath on
the exterior surface of the right ventricle. The data obtained is shown in
Table A,
below.
TABLE A
Electrical
Characteristics
of Bipolar
Sheath Cutting
the Ventricular
Surface
ESU Knob Current Voltage Power impedance
Setting (mA) (Vrms) (Watts) fL2)
2. 5 180 360 65 2000
3 160 520 83 3250
4.5 190 580 1 1 O 3050
5 205 540 111 2630
All of these cuts caused stimulation of runs of ventricularly-based ectopic
beats;
however, no fibrillation occurred.
In another sheep, the sheath was used intravascularly to remove an atrial
lead implanted 6 months; scar tissue was freed by electrosurgical dissection
in the
superior vena cave and atrium. fn a third sheep, scar tissue along a coronary
sinus
lead implanted 12 months was freed by electrosurgical dissection in
combination
with sheath rotation within the coronary sinus. Injection of contrast media
confirmed
CA 02244076 1998-07-23
WO 98/23324 PCTtUS97/21864
-21 -
integrity of the vessel after extraction. No complications arose from the use
of the
electrosurgical dissection sheath.
As a result of these animal experiments, the dilator sheath of this invention
demonstrated its separation of encapsulating tissue from an electrical cardiac
lead
positioned in the vessel leading to the heart. The impedance of the radio
frequency
dilator sheath needs to be approximately 100 ohms so as to match the impedance
of most commercially available electrosurgical units. A well-known impedance
matching transformer or circuit as previously described can be used to match
the
impedance of the electrical conductors to that of the electrosurgical unit.
It is to be understood that the above-described radio frequency dilator
sheath is merely an illustrative embodiment of the principles of this
invention and that
other dilator sheaths may be devised by those skilled in the art without
departing
from the spirit and scope of this invention. In particular, the dilator
sheaths have
been described as being comprised of a high temperature resistant polymer or
copolymer plastic material. However, any temperature resistant material is
contemplated and can include combinations of dilator sheaths made of a metal
or a
combination of metal and plastic material. The shape or configuration of the
dilator
sheaths can be accommodated for any elongated structure shape and/or the
vessel
or tissue in which the elongated structure is implanted. Furthermore, the
outer
dilator sheath can be withdrawn from the proximal end of the inner dilator
sheath as
long as the electrical conductors are positioned so as not to interfere with
its
removal. In particular, the conductor passages can extend throughout the
entire
Length of the dilator sheath, thus allowing the electrical conductors to exit
through
the wall of the dilator sheath at its proximal end. The electrical conductor
connector
would also have to be removable or small enough so as to facilitate placement
through the passage of the outer sheath. Otherwise, it is contemplated that
the
outer coaxial dilator sheath is positioned over the inner sheath from the
distal end
thereof. As previously suggested, the radio frequency dilator sheath of this
invention
is preferably of a bipolar configuration; however, a unipolar construction is
fully
contemplated with an electrical return path established through the patient
and
exterior to the surface of the patient. It is also contemplated that an
additional lumen
CA 02244076 1998-07-23
WO 98/23324 PCT/CTS97/21864
-22-
can be included in the inner dilator sheath to inject contrast media or
medicaments
into the vessel or tissue.