Note: Descriptions are shown in the official language in which they were submitted.
CA 02244164 1998-07-23
W O 97/26831 PCTAUS97/01198
TISSUE SEPA ~ ATIn N CA N N ULA WITH
~ISSECTIO N PR OBE A N D M ET H ~ D
BACKGRO~nND OF THE INVENTION
1. Related ~pplication
This is a continuation-in-part application of pending application Serial No.
08/50~,494 entitled "TISSUE SEPARATION CANNIJLA AND METHOD" filed on July
13, 1995, by Albert K. Chin, and the subject matter hereof is related to the subject matter
of application Serial Number 08/421,481 entitled "CANNULA ASSEMBLY AND
METHOD FOR PROGRESSIVELY DISSECTING TISSUE" filed on April 12, 1995 by
l 0 Albert K. Chin, which prior applications are assigned to the same assignee as the
present application.
. Field of the ~nvention
The present invention relates generally to a tissue separation cannula used for
forming an elongated cavity in tissue planes particularly along the course of a small
blood vessel, and more specifically relates to a cannula having an endoscope forcontinuously visualizing the blunt dissection site through a tissue separating member
which is transparent and has a tapered shape and is selectively removable from the
carmula to facilitate dissection of tissue adjacent a blood vessel.
3. Descrtption of Backgrot~nd Art
Present rnethods for the formation of an elongated cavity involve the use of blunt
probes that are pushed through body tissue to accomplish the tissue dissection. The
force exerted b~; the passage of mechanical probes may lead to blood vessel avulsion
and trauma to tissue and internal organs.
The problem becomes acute when dissecting and harvesting blood vessels
having a small diameter of about 3 to 8 mm. The techniques which are used for
dissection of larger blood vessels such as the aorta are not applicable since the aorta is
located in the retroperitoneum, bounded by the peritoneum on one side and the psoas
muscle on the other side. An everting balloon placed in the infrarenal space located just
below the kidney will track easily down the length of the aorta along a natural cleavage
plane when inflated.
An everting type of balloon encounters difficulties when dissecting tissue
adjacent a smaller-diameter vessel. This is due to the presence of less distinct planes
that exist between small diameter blood vessels and the tissue that surrounds these
vessels, as compared with the aorta and the tissue that surrounds the aorta. Forexample, if an everting balloon is placed adjacent to the saphenous vein in the leg, it
usuallv skews dissection upon inflation rather than track along the vein. This is due to
CA 02244164 1998-07-23
W O 97/26831 PCTrUS97/01198
the amorphous nature of the fat and connective tissue that surrounds the saphenous
vein.
Everting balloon catheters are known which are used for arterial dilation. (See,for example, U.S. Patent No. 4,479,497 (Fogarty et al., 10/30/84) and U.S. Patent No.
S 4,863,440 (Chin, 9/5/89)).
Double lumen everting balloon catheters, such as those disclosed in the Fogarty
et al. '497 and the Chin '440 patents, have a through-lumen that slidably receives an
endoscope. However, an endoscope used in conjunction with those disclosed catheters
is unable to monitor the dissection process, since the endoscope lies within the central
10 lumen proximal to the everting balloon. As the balloon everts from the catheter, the
internal inflation pressure squee2:es the walls of the balloon and closes off the distal
viewing channel. Also, the area that requires monitoring during balloon dissection is
located at the advancing front of the everting balloon. This area colle~onds to the
balloon/tissue interface that is sub3ect to forces which cause tissue separation. Thus, an
15 endoscope in the central lumen of existing double-lumen, everting balloon catheters is
unable to view the area of tissue separation, since a double layer of balloon membrane
lies between the endoscope and the tissue and blocks the endoscopic line of sight. This
double layer obscures and distorts the viewing area of tissue separation.
~ndoscopes have been disclosed for use in optical trocars such as in U.S. Patent20 No. 5,385,572 ~Nobles et al., 1/31/95) and EP 0 642 764 A1 (Sauer et al., published
3/15/95) and in harvesting blood vessels such as in U.S. Patent No. 5,373,840
(Knighton, 12/20/94). The Nobles et al. '572 patent and the Sauer et al. '764
application disclose the use of sharp-tipped, metal cutting elements which extend
outwardly from an endoscope positioned in the trocar. Control of the dissection is
'75 difficult because visualization of the vessel is obscured by the collapse of the tissue
planes into the area between the cutting element and the endoscope. Furthermore, the
risk of side vessel avulsion or trauma to the vessel is greatly increased by theorientation of the outwardly extending cutting elements.
The endoscope disclosed in Knighton '840 has a lateral dimension of a size
30 sufficient to accommodate the blood vessel being harvested and at least one tool for use
in harvesting the blood harvested. However, the failure of the endoscope to enlarge a
cavity adjacent the blood vessel obscures viewing of the dissection area and
manipulation of the ~ressel therein. The position of the viewing image relative to the
tissue dissection area could obscure the identification of side vessels leading to an
35 increased risk of vessel avulsion. Since the vessel is retrieved through the center of the .
endoscope, all side vessels must be severed for the endoscope to advance and the length
of the vessel thus retrieved is limited substantially by the length of the body of the
endoscope.
,,
CA 02244164 1998-07-23
WO 97/26831 PCT~US97/01198
An instrument for penetrating body tissue, as disclosed in U.S. Patent
No. 5,271,380 (Riek, et al.), is equipped with a tapered tip of transparent material for
viewing tissue penetrated by the tip using an optical unit which is positioned behind
the tip. An instrument of this type may include a separate illumination channel that
5 ends at the tip for illuminating tissue being penetrated.
SUMMARY OF THE INVENTION
The present invention provides a carmula for bluntly dissecting an elongated
cavity in tissue particularly along the course of a vessel in a human or anintal body.
The cannula includes a tubular body having proximal closed end and distal blunt end
l O and at least one lumen extending the length of the body. The cannula also includes an
endoscope having a lighted, viewing end disposed in the lumen near the distal end of
the body, and includes a transparent, tissue separating member, or blunt tip,
substantially covering and selectively removable from the distal end of the body. The
tissue separating member or blunt tip disposed on the distal end of the body includes
15 internal walls that taper to a cusp to reduce visual distortion through the endoscope
that is optically aligned with the tip.
A rnethod is also disclosed for bluntly dissecting an elongated cavity particularly
along the course of a vessel using a cannula. The method includes the steps of: bluntly
dissecting an initial cavity; separating the tissue by advancing the cannula along the
20 cavity with continuous, visual observation; repeating the prior step of separating the
tissue at least until the cavity is sufficiently elongated to advance a balloon therein; and
successively inflating and deflating a balloon within the cavity to enlarge the cavity
along the course of the vessel. Following dissection of the cavity along the vessel, a
counterincision is made at the far end of the cavity, for example, to place a second blunt
25 tip balloon trocar and to allow introduction of dissection instruments. The tip of the
cannula is advanced out of the body through the counterincision, and the tapered tip is
detached leaving the cannula body in the dissected cavity. The endoscope residesinside the cannula body, and the endoscope and cannula body are selectively
positioned as a single unit inside the dissected cavity to facilitate isolating and
3() harvesting the vessel. The method further may include removing the cannula, then
maintaining the elongated cavity using insufflated gas through a balloon cannula that
seals the incisions against gas leakage, or using a structural balloon, or a mechanical
structural support within the dissected cavity.
In another embodiment of the present invention, the method includes the steps
3~ of bluntly dissecting an initial cavity; sealing and inflating the cavity; and separating
the tissue along the cavitv assisted by continuous, visual observation while under
inflation until the cavity is sufficientlv elongated.
CA 02244164 1998-07-23
W O 97/26831 PCTrUS97/01198
The isolated vessel, such as the saphenous vein, may be harvested and removed
for use as a coronary artery or peripheral vascular bypass graft, or may be left in place
as an in-situ femoropopliteal or femoral-distal graft. The side branches of the vein are
ligated, clipped, or occluded in both applications. In the case of an in-situ graft, the
S valves in the vein are disrupted by means of a valvulotome.
CA 02244164 1998-07-23
W O 97126831 PCTrUS97/01198
BRIEF DESCRIPTION OF TE~E DRAWINGS
FIGURE .l is a partial, longitudinal cross-sectional view of a cannula of the present
invention illustrating the profile of a tissue separating member affixed thereto;
FIGURE 7is an isolated, cross-sectional view of another embodiment of the tissue5 separating member having a blunt spherical tip with a straight tapered section suitable
for use with the cannula of the present invention;
FIGURE 3 is an isolated, cross-sectional view of another embodiment of the tissue
separating member having a blunt tip with a curved tapered section suitable for use
with the cannula of the present invention;
FIGURE~ is an isolated, cross-sectional view of another embodiment of the tissueseparating me].nber having a hemispherical shape suitable for use with the cannula of
the present invention;
FIGURE 5 is a flowchart of one embodiment of the method of separating tissue
using the cannula of the present invention;
FIGURE ~ is a flowchart of another embodiment of the method of the separating
tissue;
FIGURE 7 is a partial side view of a patient's leg with the advancement of a
flexible cannu~a of the present invention through an incision;
~ IGURES 8A AND 8B are partial side sectional views showing alternative
20 embodiments of detachable blunt tips positioned at the distal end of the cannula;
FIGUREC 9A AND 9B are pictorial side views showing, respectively, assembled and
dissembled configurations of another alternative embodiment of the present invention;
FIGURE 10 is a cross sectional view of cannula of FIGURES 9A and 9B;
FIGURES 11A~ 11B and 11c are, respectively, side and sectional views of an
25 alternate dissection probe that may be used with the cannula sh~:swn in FIGURES 9A and
9B;
FIGU~ES 11D and 11E are end views of the dissection probe in orbital positions
about the cannula body;
FIGURES 12A~ 12B and 12C are, respectively, simplified anatomical side sectional30 and front sectional views of the human body;
FIGURES 13A and 13B are side views illustrating, respectively, retracted and
extended configurations of another embodiment of the tissue separating cannula of the
present invention;
CA 02244164 1998-07-23
WO 97/26831 PCT~US97/01198
FIGURE14 is a partial pictorial view of a viewing, multiple-clip applier for use in
the method of the present invention;
FIGURE 15 is a flow chart of an artery isolating procedure according to the present
invention;
S FIGURE 16 is a flow chart illustrating the procedures involved with the cannula of
FIGURES 9~ and 9B; and
FIGURE 17 is a flow chart illustrating the procedures involved with the cannula of
FIGURES 13~ and 13B.
DESCRIPTION OF THE PREFERRED EMBODIMENT
In accordance with one embodiment of the present invention, a cannula includes
a tubular body having proximal closed end and distal blunt end, at least one lumen
extending the length of the body, an endoscope having a lighted, viewing end disposed
in the lumen near the distal end of the body, and a transparent, tissue separating
l ~ member substantially covering the distal end of the body and selectively removable
from the distal end. The present invention also includes methods for using such a
cannula for separating tissue to form an elongated cavity along the course of a small
blood vessel and subsequently harvesting the blood vessel, or using the blood vessel as
an in-situ graft.
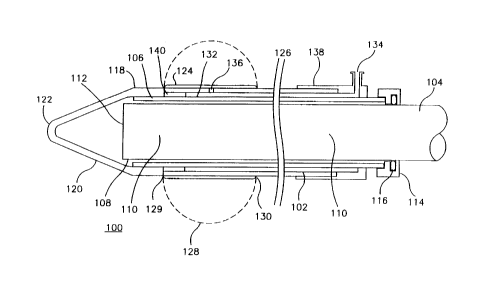
FIGURE 1 shows an embodiment of the cannula 100 of the present invention. The
cannula 100 includes a tubular body 102 having a proximal end 104 and a distal end
106. At least one lumen 108 extends the length of the body lQ2. Disposed in the lumen
108 is an endoscope 110 having a lighted, viewing end 112 near the distal end 106 of the
body. The other end of the cannula 100 has a proximal end cap 114 and an elastomeric
washer 116 that provides a pressure-sealed, sliding fit with the endoscope 110.
The carmula 100 also includes a transparent, tissue separating member or blunt
tip ~18 substantially covering the distal end 106 of the body. The tissue separating
member 118 has a tapered section 120 which angles toward a blunt, tissue-separating
tip 1''2 distal to the distal end 106 of the tubular body. The shape of the tissue
separating member 118 allows atraumatic dissection of a cavitv with sufficient control
and maneuverability to prevent tearing or puncturing of the nearby vessel. Typically,
the tip 122 has an outer radius of curvature of about .030" to about .10Q", and preferablv
of about .045". The length of the tapered section 120 of the tip is approximately .500" in
length. The tapered shape and blunt tip of the tissue separating member 118 thusallows deflection of branch vessels to the side of the cannula 100 without their avulsion,
CA 02244164 1998-07-23
W O 97/26831 PCT~US97/01198
upon forward advancement of the cannula 100 with reduced requirement of applied
axial force to advance the cannula and tip through tissue being dissected.
In tapers of uniform wall thickness with a rounded inner surface near the apex, it
has been found that a small circular spot of distortion exists in the center of the visual
S field of the endoscope, equivalent to the diameter of the rounded taper tip. This
distortion may be substantially eliminated by forming ~he transparent taper with an
inner profile that ends in a sharp point, or apex, while maintaining the outer profile as a
rounded tip with approximately a .045" radius. Undistorted visual imaging through
such tip thus allows the surgeon to track down the vessel, identify side branches, and
lO guide the device past the side branches. An optimal taper length of approximately 0.5"
facilitates cannula manipulation around side branches, and the ~re~ll~d configuration
of the tapered tip is illustrated in FIGURES8A and 8B.
Alternative embodiments of the present invention include other shapes for the
tissue separating member 118 which provide the necessary control and atraumatic
15 dissection. FIGURE 2 illustrates another embodiment of a tissue separating member 218
which substantially covers the distal end 106 of the cannula and provides a transparent
shield for the el~doscope 110. The tissue separating member 218 includes a tapered
section 220 integrally formed with a more blunt, spherical section 22~ at the distal tip
222 of the tissue separating member.
FIGURE 3 illustrates another embodiment of a tissue separating member 318
which substantially covers the distal end 106 of the cannula and provides a transparent
shield for the endoscope 110. The tissue separating member 318 includes a curvedtapered section 320 integrally formed with a blunt section 324 at the distal tip 322 to
form a duck-bi]l shape. The curved tapered section 320 can have convex or concave
shape.
FIGURE 4 illustrates another embodiment of a tissue separating member 418
which substantially covers the distal end 106 of the cannula and provides a transparent
shield for the endoscope 110. The tissue separating member 418 has a hemispherical
shape 424 coveIing the distal end 106.
Preferably, the tissue separating members 118, 218 have an overall length of
about 0.5 inches and a uniform wall thickness of about 0.06 inches along the entire
surface to allow visualization bv the endoscope without distortion of the image that
would result if a section of the wall is thickened or otherwise forms a lens. The wall
thickness of the tissue separating member may be contoured to form a lens for special
applications that require a magnified or otherwise distorted image, e.g. asymmetric,
fish-eyed image, or the like, transmitted by the endoscope. Suitable materials for
making the tissue separating member or blunt tip include polvcarbonate and anv
CA 02244164 1998-07-23
W O 97/26831 PCT~US97/01198
material which is sufficiently strong to separate tissue and sufficiently transparent to
allow visualization by the endoscope. As illustrated in FIGURES8A,8B,9A, and gs, the
tissue separating member or blunt tip may be attached by threads or ~ayonet-type twist
lock to the cannula for selective removal during procedures later descri~ed herein.
Referring again to FIGURE1~ the cannula 100 preferably includes a balloon 124
located at the distal end 106 on the exterior wall 126 of the cannula. The balloon 124
may be elastic or inelastic, although an elastomeric balloon is ~re~-led because it
achieves a smaller, smoother outer profile. Fully inflated (as shown in phantom 1Z8 in
FIGURES1~8A~ and 8B), the diameter of the balloon 124 is about 3 cm. Preferabl~, a
sleeve type of balloon 124 has both the distal end 129 and proximal end 130 of the
balloon secured to the exterior wall 126 of the cannula.
The balloon 124 is selectively inflated by supplying thereto via another lumen
132 a pressurized fluid, such as a gas or liquid, from an inflation port 134 to a hole 136
in the exterior wall 126 of the cannula between the proximal and distal ends 129, 130 of
lS the balloon to communicate with the interior thereof. A plunger device, such as a
manually-operated syringe, is suitable for connecting at the inflation port 134 to control
the inflation of the balloon 124. The lumen 132 is formed as another tubular body 138 in
a concentric arrangement with the body 102 to form a space 140 between the two
bodies. Another embodiment suitable of the present invention may include two lumens
108, 132 in a side-by-side arrangement. A~ itional lumens can be added in similar
manner to provide other functions such as irrigation and aspiration in known manner.
The present invention is illustrated using a sleeve type of balloon with the
cannula 100. Other balloon types are suitable for use with the present invention such
as, and not limited to, using an invertable balloon positioned in a separate lumen in the
cannula to assist in separating the tissue when inflated.
The cannula 100 may be manufactured from a variety of bioinert, substantially
inelastic materials, such as stainless steel, polyethylene, polyurethane, polyvinyl
chloride, polyimide plastic, and the like that preferably have a tensile strength of at
least 10,000 psi. Preferably, each lumen of the cannula 100 has a wall thickness of
between about 0.005 inch and 0.010 inch.
The endoscope 110 has an outer diameter of approximately 5.0 mm and an
endoscope may be permanently built into the cannula 100, or may be a separate device
that is advanced through the endoscope lumen 108, for example, through a sliding gas-
tight seal 805 configured as shown in FIGURE8A in conventional manne~. The
endoscope 110 is positioned within the lumen 108 with the tip in correct position to
allow unimpeded visualization through the transparent blunt tip of the surrounding
tissue and vessel outside of the cannula 100. A preferred endoscope 110 having a
CA 02244164 1998-07-23
W O 97/2683I PCTAUS97/01198
tubular diameter of about 5.0 mm is cornmercially available from Solos Endoscopy, Inc.,
at Norcross, Georgia, although other commercially-available endoscopes 110 as small as
1.00 to 1.75 mm in diameter may also be used.
Methods for bluntly dissecting an elongated cavity using the cannula of the
5 present invention are shown in the flow diagrams of FIGURES 5 and 6. Although the
blunt dissection of an elongated cavit~r along the course of a vessel is specifically
described, the present invention is ~enerally suitable for separating any tissue. For
example, the cannula may be used to track along the median nerve from an incision at
the patient's wrist, forming a cavity for surgical treatment of carpal tunnel syndrome.
l O The cannula allows visualization and tracking of the median nerve, preventing the
injury to the nerve which may occur if blind advancement of a balloon cannula were
used. Alternatively, the cannula of the present invention may also be used to dissect a
cavit~r ad~acent the mammary artery in the manner as later described herein.
The method illustrated in the flow diagram of FIGURE 5 includes the steps of
15 incising the skin and bluntly dissecting 501 through the subcutaneous tissue to the level
of the selected vessel or nerve. Blunt dissection is performed to separate the vessel from
adjacent tissue i-or a length of approximately 1 to 2 cm. The blunt dissection may be
perforrned with a pair of curved Metzenbaum scissors, using the tips of the scissc~rs to
cut and bluntly spread tissue in a plane ~etween the vessel and the adjacent tissue.
Preferably, a blunt tip balloon cannula is introduced into the space between thevessel and the overlying tissue. The ~alloon is then inflated to form a gastight seal
which seals 502 the dissection. A gas such as carbon dioxide is infused under pressure
via another lumen in the cannula having an external opening positioned distal to the
balloon. The natural perivascular plane around the vessel is expanded by the injected
gas, ~orming a tract along the course of the vessel. For a superficial vessel such as the
saphenous vein, the expanded tract is visible on the surface of the skin. The interior of
the expanded tract is not cleanly open but rather, includes gossamer-like strands of
connective tissue and fat, preventing unobstructed visualization and m~king hazardous
the passage of an endoscope along the tract adJacent to the vessel. If a conventional
endoscope is pushed into this connective tissue in an attempt to form a cavitv adjacent
to the vessel, the view through the con~rentional endoscope is blurred by the tissue that
contacts the viewing end of the conventional endoscope. A blurred view through the
~ conventional endoscope increases the potential for side branch avulsion during blunt
dissection of the perivascular tunnel.
The cannula 100 is inserted 503 into this dissected space. With the fiberoptic
endoscope 110 ~ontinuously visualizing down the course of the vessel, the cannula 100
separates the tissue by advancing forward 50~, probing between the vessel and the
adJacent gossamer perivascular tissue in the plane initiated b~r blunt dissection. The
CA 02244164 1998-07-23
W O 97/26831 PCTrUS97/01198
transparent, tissue se~arating member 118 allows the endoscope 110 to clearly visualize
a segment of the vessel at least equivalent to the length of the tapered section ~ 20.
If the blunt dissection along the course of the vessel is not sufficient to advance
the balloon 124 therein, the method returns 5~5 to the step of advancing the cannula 504
5 forward to continue the separation of tissue along the course of the vessel. When a
cavity of sufficient length has been formed by the cannula 100, the balloon lZ4iS
successively inflated and deflated 506 to enlarge the cavity to about 3 cm in diameter.
The method returns 507 to the step of separating the tissue ~y advancing the
cannula 504 and the step of selectively inflating and deflating 506 the balloon 124, as
l0 described above. Successive application of these steps forms a cavity along the entire
length of the vessel. Once the elongated cavity is complete, the carmula 100 is
completely retracted 508 from the elongated cavity.
The elongated cavity site is then maintained 509 in expanded form in accordance
with the method of the present invention. Following use of the cannula 100 to form an
l 5 elongated cavity along the course of a vessel, the cavity must be supported to allow
procedures to be performed on the vessel, such as vessel ~ ec~ion~ grafting of the
vessel, or vessel harvesting. A blunt tip trocar may be used to seal the entrance incision
and allow gas insuff}ation to maintain the cavity in expanded form. One blunt-tip
balloon trocar suitable for use herein is presently marketed by Origin Medsystems, Inc.
20 of Menlo Park, CA.
Another method of maintaining the cavity in expanded form includes making an
incision at the distal extent of the dissected cavity, and inserting a double rod system
through the cavity. The double rods are suspended via a laparoscopic mechanical
lifting device to maintain the cavity. This system allows instruments to be advanced
25 into the cavity via simple incisions, without the requirement for trocars with gas sealing
valves, as is the case with gas insufflation.
Alternatively, an inflatable structural balloon or mechanical structure may be
used to support the dissected cavity. For example, the cavity may be maintained by
mechanical retraction or by a mechanical finger-like retractor attached to a powered
30 lifting arm plus a separate flat balloon retractor used to displace the side wall of the
cavity. The endoscope 11û may be introduced behind the legs of the finger-~ike
retractor that connect to the mechanical lifting arm.
The vessel is completely dissected within the formed cavity, using laparoscopic
instruments such as graspers, scissors, hooks, and blunt probes. Side branches to the
35 vessel may be ligated using suture ties, clipped using titanium vessel clips, cauterized
using electrocautery, or a combination of these procedures. The dissected vessel is
CA 02244164 1998-07-23
W 097/26831 PCT~US97/01198
removed from the cavity for possible use as a conduit for an arterial bypass procedure,
or the vessel may be left in place to be used as an in-situ bypass graft.
In an al~ernate embodiment, the method of the present invention forms a small
diameter cavity, about 7 mm, along the entire length of the vessel before the cavity is
5 then enlarged. As illustrated in FIGURE5, the steps of making a blunt dissection 501,
sealing and inflating the dissection ~02, inserting 503 the dissection cannula 100 and
successively separating the tissue by advancing 504 the cannula 100 are perforrned as
described above. The alternate method, however, continues advancing 504 the
dissection cam~ula until the entire length of the elongated cavity is bluntly dissected to
l0 the small diam.eter of about 7 mm.
Only after the entire length of the elongated cavity has been bluntly dissected
does the alternate method include the step of inflating and deflating 506 the balloon 124
of the cannula to increase the diameter of the distal end of the elongated cavity to about
3 cm. The dissection cannula 100 is then retracted 508 partially by about the length of
15 the balloon 124. The alternate method then returns 510 to the steps of inflating and
deflating 506 tl~e balloon 124. The cannula 100 is again retracted 508 partially and the
method returns 510 to repeating the above steps until the entire length of the elongated
cavity has been en~arged to the r~i~rnet~r of the inflated balloon 124 which is typically
about 3 cm.
An altemate method involves making an incision down to the vessel. Blunt
dissection of the vessel from the adjacent tissue is performed for a 3-4 cm length. A
blunt tip trocar is placed in the incision, and gas insufflation is initiated.
The cannula 100 is inserted through the blunt tip trocar, and blunt dissection
using the balloon 124 is performed under the presence of gas insufflation in the cavity.
This technique provides a larger cavity for visl-~li7~tion during separation of the tissue,
since gas ins~lffl~tic)n is used from the onset of blunt dissection. However, a gas sealing
blunt tip trocar is required. If vessel dissection without gas insufflation is conducted,
and a double rod system is used to maintain the cavity, then the use of a blunt tip trocar
may be avoided.
Another method of the present invention forms a small diameter cavity along the
entire length of the vessel. The cavity is enlarged only by the initial inflation at the
blunt dissection site. As illustrated in FIGURE 6, the steps of making a blunt dissection
601, sealing and inflating the dissection 602, inserting 603 the cannula 100, and
successively separating the tissue by advancing 604 the cannula 100 are performed as
described abo-,-e. The cannula advances 604 until the entire length of the elongated
cavity is bluntlv dissected and expanded only by the inflating gas of the prior step 602.
CA 02244164 1998-07-23
W O 97/26831 PCT~US97/01198
The carmula 100 is then retracted 605 entirely from the elongated cavity. The dissection
site is maintained 606 in expanded form as described above.
The present invention includes methods particularly useful for harvesting vein.
In one method, an incision is made near the ankle, and the cannula is passed along the
sophenous vein up to the knee, or near the knee. Following balloon inflation to enlarge
this segment, an incision is made into the dilated cavity near its endpoint at the knee.
The incision at the knee is the approximate mid-point of the saphenous vein between
the ankle and the groin. The vein is isolated and the side branches ligated in this
segment between the ankle and the knee to harvest and remove this segment.
10The segment from the lcnee to the groin is then harvested. The cannula may be
passed from the same knee incision used for harvesting the vein from the lower leg, or a
separate incision down to the vein may be made slightly above the knee. Use of aseparate incision may be useful if the vein is overly curved or tortuous as it passes
around the knee. The cannula is advanced toward the groin, the balloon is inflated to
l 5 dilate the cavity, an incision is made into the dilated cavity at its groin end, and the
portion of the saphenous vein residing in the thigh is harvested.
~ s an alternate method for saphenous vein harvesting, the initial incision may be
made at the knee. The cannula is passed successively in both directions, toward the
ankle and toward the groin, from the same incision. Then additional incisions are made
20 at the ankle and at the groin to allow harvesting of the entire length of the saphenous
vein. The vein may be removed as a single strand, or it may be cut at the knee and
removed as two strands.
In some anatomic regions, it may be difficult to advance a rigid, straight tissue
separation device along the course of a vessel. Por example and referring to FIGU~E 7, if
25 the saphenous vein 700 is harvested by passing the cannula 702 from an incision 712 3ust
above the ankle, the presence of the medial malleolus 704 and the foot 706 may prevent
an otherwise rigid cannula from being angled upwards or sideways to follow the vein
700.
For such obstructed situations, the cannula 702 may be formed with a body
30 which is flexible or otherwise malleable, or is rigid with a pre-determined gradual arc,
as shown in FIGURE 7. The endoscope 708 that is inserted into the cannula 702 UP to the
tissue separating member 710 must also be flexible to facilitate shaping the flexible body ~.
within the curved cannula. Such conventional flexible fiberoptic endoscopes are
commercially available for use in gastrointestinal endoscopy.
3~In other embodiments of the method, and of the apparatus illustrated in FIGURES
8A and 8B, the tapered and transparent tip 8~3 of the cannula 800 may be removably
attached to the body of the cannula. At the cannula insertion site, a blunt tip balloon
12
-
CA 02244164 1998-07-23
W O 97/26831 PCTAJS97/01198
trocar (for exan:lple, as commercially available from Origin Medsystems, Inc.) is placed
to seal an incision and allow insufflation into the space to be dissected by the cannula
800 which is advanced through the blunt tip trocar along the course of the vessel. The
balloon on the cannula is selectively inflated to dissect a perivascular cavity. Following
5 dissection of the cavity along the vessel, a counterincision is made at the far end of the
cavity to place a second blunt tip balloon trocar and allow introduction of dissection
- instrurnents. The tip of the cannula is advanced out of the body through the
counterincision and the tapered tip 803 is detached, leaving the cannula body in the
dissected cavity. The endoscope resides inside the cannula body, and the
10 endoscope/cannula body is advanced as a single unit inside the working cavity to
isolate and harvest the vessel.
The detachable tip 803 may be attached to the cannula body 800 using a threaded
connection bet~reen the tip and the distal end of the cannula body, or a bayonet-type of
fitting my be used to lock the tip onto the cannula, with a slot in the tip engaging a pin
15 on the end of the cannula body.
The cannula of this embodiment with a detachable tip 803 has the advantages
compared with the embodiment of the cannula described with reference to FIGURE1
that the 5mm diameter endoscope used with the cannula often does not have sufficient
rigidity to allow it to be directed along the dissected working cavity for unobstructed
20 visualization. For example, in the lower leg, the curvature of the calf muscle impedes
visualization along the surgical cavity, and the endoscope must deflect muscle tissue to
allow it to view down the bore of the cavity. Flexion of the endoscope which is about
4~cm long and about 5mm diameter may prevent successful visualization. The cannula
which surrounds the endoscope according to the present invention has an 8mm outer
~5 diameter, and this larger diameter imparts rigidity to the endoscope/cannula system.
The cannula body may be constructed of stainless steel for a~ ihonAl rigidity.
Also, the ability to remove the tapered tip 803 via an incision at the opposite end
of the cavity results in a decreased number of passes of the cannula and endoscope up
and down the length of the cavity. This adds to the convenience of the procedure, and
30 decreases the potential for vessel injury by decreasing the number of full length passes
required through the dissected cavity.
Referrin~ now to FIGURES9A and 9B, retracted and dissembled configurations of
another embodiment of the cannula 900 of the present invention are illustrated.
Specifically, the outer body of the cannula 900 includes a sleeve-type balloon 901
- 35 secured to the body at proximal and distal ends thereof, as illustrated in FIGURES8A and
8B, for selective inflation via a lumen 1010 within the body that communicates
therewith, as illustrated in the sectional view of FIGU~E 10.
CA 02244164 1998-07-23
W O 97/26831 PCT~US97/01198
~ t the distal end of the body of the cannula gO0, a detachable, transparent blunt
tip 903 is shown in FIGURE9A retracted onto the distal end of the cannula body, and is
shown in FIGURE 9B mounted on push rod 906 and extended beyond the distal end ofthe cannula body 900 to expose the viewing end 905 of an endoscope, and a crescent-
shaped dissection probe 907. The probe 907 is mounted on shaft 909 to facilitateselective manipulation of the dissection probe 907 within the field of view of the
endoscope 905. As shown in FIGURE1O~ the push rod 906 may be non-circular within a
mating non-circular lurnen to retain the blunt-tip 83 in axial aligmnent as it is selectively
extended and retracted relative to the distal end of the cannula body 900. Also as
10 shown in FIGURE 10, the shaft 909 for supporting the dissection probe 907 may be
circular or cylindrical to facilitate both longitudinal and rotational positioning of the
dissection probe 9~7 via corresponding manipulations of the shaft 909 at the proximal
end of the cannula body 900. Alternatively, the shafts 906 and 909 may reside in slots of
suitable sectional shapes along the outer surface of the cannula body 900, with an
l S encircling sheath of heat-shrinkable PET plastic, or other bioinert plastic, to retain the
shafts 906,909 in captivated orientation along the cannula body 900.
The dissection probe 907 has leading and trailing edges thereof to facilitate
selective dissection of strands of cor nective tissue and lateral branch vessels along the
saphenous vein, or other vessel, to be harvested. The blunt tip 903 may thus be
20 selectively extended beyond the cannula body 903 (or the cannula body 900 may be
retracted relative to the tip 903) to expose the dissection probe 907 at a selected location
along a dissected cavity adjacent a vessel being harvested. Selective translational and
rotational manipulations may be achieved via similar manipulations of the shaft 909 at
the proximal end thereof to dissect connective tissues and lateral branch vessels along
~5 the course of the vessel being harvested. The dissection probe 907 and the blunt tip 903
may then be retracted into axial alignment with the cannula body 900, as shown in the
retracted configuration of FIGUR~ 9A. Surgical procedures involving the cannula 900 of
FIGURE9A are described later herein with reference to the flow chart of FIGURE16
Referring to the side and sectional views of FIGURES11A~11B, and 11c, the
30 dissection probe may be formed in separate, spiral-like segments 1101,1103 that are
axially spaced along the supporting shaft 909, for example, in the illustrated
configuration, to provide greater convenience in selectively by-passing or dissecting
connective tissue and lateral branch vessels along the course of a vessel being
harvested. Thus, as shown in FIGURES11D and 11E, the dissection probe 907 (or 1101
3~ and 1103) may be orbited about the axis of shaft 9~9, and the cannula body 900 mav be
rotated on its longitudinal axis to facilitate the dissection of the vessel away from
connecting tissue, and the traversal of side-branch vessels.
1~
CA 02244164 1998-07-23
W O 97/26831 PCT~US97/01198
Referring now to FIGURES12A and 12B, the simplified side and frontal
illustrations of the human anatomy disclose another operating envi,o~lment for the
apparatus and method of the present invention, for example, in preparing the internal
mammary artery for coronary artery bypass. Spe~ifi~lly, the combined blunt tip
5 cannu}a and dissection probe of the present invention permits a working cavity to be
formed along a vessel, and the vessel to be dissected and isolated, or otherwisemanipulated as later described herein, via a single incision. This decreases the number
of incisions required to harvest a vessel. The cannula and dissection probe of the
present invention facilitate harvesting the intemal mammary artery in the chest wall to
10 enable its use as a coronary artery bypass graft. The internal n~mmAry artery may be
harvested via a single subxiphoid incision, with the rectus muscle bluntly dissected to
expose the superior epigastric artery. The cannula tracks along the superior epigastric
artery which leads directly to the internal mammary artery that lies behind the ribs
lateral to the sternum. The internal mammary artery is dissected substantially in the
15 manner as prev-iously described up to its origin at the subclavian artery. Its side
branches are clipped and transected, and distally, it is tTansected to yield a free end
which is anastomosed to the coronary artery to complete the bypass.
As illustrated in FIGURES12A~12B and 12C, the internal m~mrn~ry artery (also
known as the internal thoracic artery) runs internal to the costal cartilages, lateral to the
20 sternum, descending to the interval between the sixth and seventh cartilages where it
bifurcates into the superior epigastTic artery and the musculophrenic artery. The
superior epigastTic artery lies within the rectus sheath. In its superior portion, it lies
behind the rectus abdominis muscle. The superior epigastric artery eventually
anastomoses with the inferior epigastric artery.
Since the superior epigastric artery lies in the abdominal wall within the rectus
sheath, it may be easily found, for example, using Doppler ultrasound, and isolated via
incision of the s~cin and blunt spreading of the rectus abdominis muscle overlying the
artery. The blunt tip, visual balloon dissection cannula previously described herein
may be placed next to the isolated section of superior epigastric artery, and passed
3û superiorly, following the course of the superior epigastric artery to its junction with the
internal mammary artery. An endoscopic working cavity may be formed along the
length of the internal mammary artery in the manner previously described herein,allowing side branch identification and interruption, and using vessel clips or bipolar
electrocautery closure followed by scissor transection. The dissected portion of the
internal mammary artery may then be used to revascularize diseased coronary arteries,
facilitated by the availability of the endoscopic working cavity thus formed along the
internal mammary artery.
1~
CA 02244164 1998-07-23
W O 97/26831 PCTrUS97/01198
This abdominal approach to internal mammary artery dissection is preferable to
a supraclavicular approach or an intercostal approach since the supraclavicular
approach is impeded by the presence of the subclavian artery and the aortic arch, and
dissection risks trauma to these vessels. In contrast, the intercostal approach gives
5 limited exposure, unless rib spreaders are used, and only a small portion of the internal
mammary artery is accessible from the side. The abdominal approach described herein
thus allows the entire length of the internal mammary artery to be exposed. By
initiating the dissection at the level of the superior epigastric artery, no vascular or bony
structures are present to impede the passage of the dissection cannula of the present
l O invention, thereby resulting in a safer approach to the internal mammary artery.
Specifically, with reference to the flow chart of FIGURE 15, the method of creating
a working space along the superior epigastric artery according to the present invention
includes forming an incision 1501 of the skin and blunt ~ ssection 1503 and spreading of
the rectus abdominus muscle overlying the artery. The balloon cannula as illustrated in
15 figures 1, or 8A,8B, or 9A, 9B, 13A~ 13B is inserted in the bluntly dissected cavity next to
the isolated section of the superior epigastric artery. The cannula is advanced 1505
along the course of the superior epigastric artery and the internal mammary artery by
the iterative sequence of advancing the cannula, visualizing dissection of tissue through
the transparent tip until resistance to tissue penetration is felt. The balloon is inflated to
2~ expand the cavity around the cannula adjacent the artery, and then deflated, and the
cannula is again advanced, and retracted and diverted and advanced as required to
properly track the course of the vessel substantially to its junction with the subclavian
artery. The tunnel or cavity thus formed along the artery facilitates side branch
identifications for subse~uent operative procedures, and in one embodirnent of the
5 process invention the cannula may be completely removed 1507 from the working
cavity thus formed by successively inflating and deflating the balloon to establish an
adequate working cavity as the carmula is completely withdrawn therefrom.
Next, the artery may be dissected from the cavity wall by placing 1509 a blunt tip
trocar in the working cavity at the abdominal incision, and the associated balloon is
30 then inflated. The cannula of the present invention including the dissection probe and
an endoscope positioned 1511 within a lumen of the cannula is positioned in the
working cavity through a gas tight port of the trocar, with the dissection probepositioned about the artery. The dissection probe is now translated and rotated 1513, as
illustrated in Figures 11D and 11E to free the artery from connective tissue. The axial
35 opening 908 in the perimeter of the dissection probe facilitates passing over lateral
branch vessels encountered along the course of the artery being isolated. When the
artery and side branches are completely free of connecting tissue, the cannula with
endoscope and dissection probe is removed 1515 from the working cavitv.
16
CA 02244164 1998-07-23
W O 97/26831 PCTAUS97/~1198
Thereafter, a viewing, multip~e-clip applier, as illustrated in FIGURE 14, including
a clip applier 1401 and an endoscope 1403, and having a circular cross section may be
inserted 1517 through the trocar port for placing two surgical clips on each side branch
of the isolated artery 1519, spaced sufficiently to divide the branch vessel therebetween.
5 After all side-branch vessels are clipped in this manner, the viewing, multiple-clip
applier is completely removed 1521. Alternatively, a two-port, trocar gas seal may be
attached to the blunt tip trocar and an endoscope may be inserted through one port
with a clip applier or electrocauterizer inserted through the other port for clipping or
otherwise occluding the side-branch vessels. The two-port seal for the blunt tip trocar
l O facilitates removal of only the clip applier and replacement thereof by scissor blades
that can be manipulated proximally to cut and divide 1523 each of the side branch
vessels between the clips that were previously placed or through electrocauterized
segment that WclS prepared while viewing through the endoscope 1403. Thereafter, the
scissor blades may be removed, and the internal mammary artery thus isolated may15 then be used to revascularize diseased coronary arteries 1525, for example, by grafting a
transsected free end of the isolated internal mAmm~ry artery to the left anterior
descending coronary artery downstream of a significant stenotic occlusion.
Referring now to FIGURES13A and 13~, there are shown side views of retracted
and extended configurations of another embodiment of the dissection cannula
20 according to the present invention. This embodiment includes a blunt, tapered tip 1301
which deflects to one side of the endoscope 1303 to allow visualization outside of the
cannula 1300 during vessel dissection and isolation. The dissection probe 1305 may
reside adjacent to the cannula body 1300, proximal to the tapered tip 1301, and extend
forward to dissect around the vessel being harvested and its side branches. The
25 dissection probe shaft 1307 may run through a separate lumen in the cannula body
1300. The canmlla body 1300 may contain an intrinsic curvature, and contain a port
1309 on the side of the cannula body near the tip, for exit of the endoscope 1303. The
cannula body which has a normally curved configuration, straightens out upon
introduction of a rigid, straight endoscope 13û3. Partial retraction of the endoscope
1303 allows the cannula to curve, and the endoscope is advanced through the side port
1309 in the cannula body 1300 to view outside of the deflected cannula tip 1301.
A surgical procedure involving the cannula 1300 shown in Figure 13 is illustrated
in the flow chart of FIGURE17~ Specifically, an initial incision and blunt dissection is
performed 1701 to prepare an initial dissected cavit~T. The cavity may then be sealed
3~ 1703 in conventional manner and inflated 1705 to facilitate insertion of the cannula 1300
that is inserted into the cavity 1707 through a conventional gas-tight seal. The cannula
is advanced 1709 and a perimeter balloon 128 on the cannula is inflated 1711 to expand
the dissected cavitv, and is then deflated to facilitate further advancement of the
17
CA 02244164 1998-07-23
W O 97/26831 PCTrUS97/01198
cannula and reinflation of the balloon. This sequence is repeated 1712, 1713 until the
dissected cavity of sufficient size or length is formed along the vessel of interest. The
dissected cavity is maintained 1715 by insufflation or mechanical traction or otherwise,
as previously described, and the cannula may be retracted 1717 to a selected location in
5 the cavity at which the endoscope 1303 may be retracted 1719 relative to the body of the
cannula 1300 in order to permit deflection of the blunt tip 13~1 away from the tip of the
endoscope, as shown in FIGURE 13B. Thereafter, the endoscope 1303 may be extended
1721 to view past the blunt tip 1301, and the dissection probe 1305 may also be
extended 1723 and manipulated within the dissected cavity to dissect the vessel of
10 interest 1725 from remaining connective tissue.
Referring now to the flow chart of FIGURE 16, a surgical procedure invoiving thecannula 900 shown in FIGURE9A includes making an initial incision and blunt dissection
1601 to form an initial dissected cavity. The cavity may then be sealed 1603 in
conventional manner and inflated 1605 to facilitate insertion 16û7 of the cannula 900
15 into the cavity through a conventional gas-tight seal. The cannula 900 is advanced 1609
and a perimeter balloon 901 on the cannula is inflated 1611 to expand the dissected
cavity, and is deflated to facilitate further advancement of the cannula, and reinflation
of the balloon. This sequence is repeated 1612, 1613 until the dissected cavity of
sufficient size or length is formed along the vessel of interest. The dissected cavity is
20 maintained 1615 in a manner as previously described, and the cannula 900 may be
retracted 1617 sufficiently within the dissected cavity to facilitate detaching and/or
extending 1619 the blunt tip 903 and facilitating extension 1621 of the dissection probe
907. One or more of the steps 1617, 1619, and 1621 may be repeated while dissecting
connecting tissue 1623 to harvest the vessel of interest.
2~ Therefore, the cannulas and dissection probes and associated surgical procedures
facilitate blunt dissection of a working cavity along a vessel of interest, withvisualization of the tissue being dissected through a blunt tip of transparent material
and selected optical configuration positioned on the forward end of the cannula.Selective remote deployment and remote manipulation of a dissection probe carried on
30 the cannula facilitates dissection of tissue around the vessel of interest and around side
branch vessels along the vessel of interest being harvested from within and along the
working cavity of dissected tissue.