Note: Descriptions are shown in the official language in which they were submitted.
CA 02244576 2006-10-04
1
INHALATION DEVICE
Field of the Invention
This invention relates to devices for the administration of a powdered
substance by inhalation, and in particular, to a device for administering
powdered medicaments to the lungs of a user.
Background of the Invention
Various types of inhalers for delivering a medicament are known. For
example, United States Patent No. 3,938,516 (Mathes No. 1) discloses an
inhaler for delivering a powdered medicament. The device includes a mouth
piece which has provided therein an emptying chamber. A longitudinally
extending passageway for introducing air into the inhaler is connected to the
passageway. The inhaler also includes a hollow air stream tube which
extends preferably into an opened capsule containing a medicament. Upon
inhalation, air drawn through the air stream tube into the capsule assists in
causing the medicament to be expelled therefrom.
Mathes No. 1 states at column 4, lines 32-45, that "Quite obviously, no
single device will be suitable for all persons requiring administration of
powdered medicaments since, for example, people with differing lung
capacities are known to generate flow rates from about 301iters/minute or so
to about 120 liters/minute or so through inhalation devices of this and known
types. Nonetheless, the device of [Mathes No. 1] affords such variability,
through proper selection of the various design parameters, that a device,
embraced with the scope of [Mathes No. 1], can be designed for a particular
patient-generated flow rate to deliver the medicament according to a certain
CA 02244576 1998-08-04
2
set of pre-determined objectives (e.g., slow or fast administration, one or
more inhalations etc.)."
Accordingly, one of the disadvantages of Mathes No. 1 is that a single
device is not capable of being used with a variety of patients. In some cases,
the inhaler may be required for treating an individual who has a diminished
lung capacity. For example, an individual who may need to use the device
may suffer from, for example, emphysema or asthma, and may not be able to
generate a high flow rate of air. Therefore, the device of Mathes No. 1 would
have to be designed for someone who could only administer a dose slowly
due to their diminished lung capacity. Alternately, the device may be used by
someone who does not have a diminished lung capacity. Unless the device
is properly designed, the medicament will exit the inhaler at a rate such that
a
portion, if not substantially all of the medicament, will impact upon the
throat
and airways of the user and therefore not be drawn into the lungs for
absorption.
A further disadvantage of Mathes No. 1 is that, over the course of a
single inhalation, the concentration of the medicament in the air inhaled by a
user is uneven. This arises for two reasons. First, once the medicament is
withdrawn from the container, it is immediately transported through the
inhaler into the mouth or nose of the user. Therefore, little mixing of the
medicament in the air inhaled by the user occurs. This results in uneven
distribution of the powder in the air inhaled by the user and, to the extent
that
the medicament is drawn into the lungs of the user, the medicament will not
be distributed evenly throughout the lungs. Secondly, a substantial portion of
the medicament may be withdrawn from the medicament container and
entrained in the air upon initial inhalation. Accordingly, the medicament will
not be distributed throughout the entire lung of the user but will be
concentrated in that portion of the lungs of the user to which the first
portion
of the air inhaled on inhalation travels. (See also United States Patent Nos.
4,014,336 (Mathes No. 2); 4,005,711 (Glen No. 1); and 4,098,273 (Glen
No. 2).) In Glen Nos. 1 and 2, a deflector surface is used to direct a portion
CA 02244576 1998-08-04
3
of the incoming air into the medicament container so as to entrain the
medicament in the air which is inhaled by a user.
United States Patent Nos. 3,964,483 (Mathes No. 3) and 3,973,566
(Mathes No. 4) each disclose a device wherein the air entering the inhaler is
not aimed directly at the medicament in a medicament container. Instead,
turbulent air flow is created so as to draw the medicament out from the
container. These devices have the same disadvantages as Mathes No. 1.
Summary of the Invention
In accordance with the instant invention, there is provided an
inhalation device for use in delivering a powdered substance to a user. The
inhalation device comprises a holding portion for holding the substance, an
air entry passageway, a hold-up chamber and an air exit passageway. The
ai't entry passageway is sized and configured to direct air entering the
inhalation device at the holding portion and to fluidize the substance upon
inhalation by the user.
In one embodiment, the air entry passageway includes an inlet port, an
exit port and a cross-sectional flow area. The exit port is proximate to and
directed at the holding portion. The hold-up chamber is in flow
communication with the holding portion. The air exit passageway preferably
includes an inlet port in flow communication with the hold-up chamber, an exit
port and a cross-sectional flow area. The cross-sectional flow area of the
exit
passageway is preferably greater than the cross-sectional flow area of the air
entry passageway.
In a preferred embodiment of the invention, the inhalation device
includes a plurality of air entry passageways, with at least one of the air
entry
passageways directed at the holding portion. The number and cross
sectional flow area of the air entry passageways may be selected to cause
the air entering the inhalation device through the air entry passageways to
travel at a velocity to fluidize a major portion of the powdered substance,
preferably a medicament, upon the commencement of inhalation by the user.
5
CA 02244576 1998-08-04
4
In a more preferred embodiment, substantially all of the powdered substance
is fluidized upon commencement of the inhalation by the user. The combined
cross-sectional flow area of the plurality of air entry passageways is
preferably less than the cross-sectional flow area of the air exit passageway.
Accordingly, one advantage of the instant invention is that the air
entering the inhaler effectively forms a jet directed to impinge upon the
powdered substance in the inhaler so as to cause the substance to effectively
immediately fluidize during the first stage of inhalation by the user. The
fluidized substance is then drawn into the hold-up chamber where it is
effectively stored in a fluidized state during the remainder of the
inhalation.
This produces three advantages.
First, the powdered substance is effectively deaggregated almost
immediately upon inhalation so as to form a relatively uniform concentration
of substance in the hold-up chamber at the commencement of inhalation. As
air is drawn through the air exit passageway by the user, a relatively
constant
concentration of substance is initially drawn into the lungs of the user. The
fluidized substance is thereafter diluted over time as more air is drawn into
the hold-up chamber. Therefore, the concentration of the first portion of the
fluidized substance initially inspired by the user will be greater than the
subsequent portions, which helps to provide a more even deposition of the
substance in the lungs of the user.
Secondly, the deaggregation of the particles by the air travelling
through the air entry passageways reduces the likelihood of large particles of
substance being present and impacting upon the throat and/or upper airways
of the user.
A third advantage is that the relatively even concentration of
substance in the hold-up chamber is formed almost immediately upon
inhalation so that even the first air drawing into the lungs of the user
contains
a diluted fluidized mass of substance. Further, as the inhalation continues,
additional air is introduced through the air entry passageways into the hold-
up chamber to mix with the remaining fluidized substance. Therefore, as the
CA 02244576 1998-08-04
inhalation continues, the substance is continuously drawn into the lungs.
Thus, the substance is drawn into a large volume of the lung.
In one embodiment, substantially all of the air entering the inhalation
device is directed at the powdered substance. In a further alternate
5 embodiment, all of the air entering the,inhalation device is directed at the
substance.
The air exit passageway is preferably sized, with a cross-sectional flow
area greater than the cross-sectional flow area of the combined air entry
. passageways, to provide the fluidized substance leaving the inhalation
device with a velocity sufficiently low for a major proportion of the
substance
not to impact on the throat and upper airways of the user but to be drawn into
the lungs and/or lower passageway of the user. The air which enters the
inhalation device at a rapid velocity through the air entry passageways may
decrease in velocity as it enters the hold-up chamber. The air may then be
drawn off from the hold-up chamber at a controlled.rate so as to provide a
velocity of the fluidized substance leaving the hold-up chamber (through the
air exit passageway) which is sufficiently low for the substance to be drawn
into the lungs of the user.
The air entry passageway and/or the lower portion of the hold-up
chamber are configured to introduce air into the hold-up chamber such that
the air entering the inhalation device will rotate, swirl or travel around the
hold-up chamber to maintain the substance in a fluidized state. In one
embodiment, the hold-up chamber has interior walls which are substantially
smooth and are of generally uniform cross-section. Accordingly, the. hold-up
chamber is preferably configured to permit cyclonic flow of air within the
hold-
up chamber and the air entry passageway and/or the lower portion of the
hold-up chamber are configured to initiate cyclonic flow of air upon
inhalation
by the user. The cyclonic flow of air may assist in further deaggregation of
the powdered substance and in maintaining the deaggregated substance in a
fluidized state.
CA 02244576 1998-08-04
6
The air exit passageway is preferably positioned in the hold-up
chamber at a position which is distal to the holding portion which receives
the
powdered substance. In one embodiment, the hold-up chamber is
cylindrically shaped and has a longitudinally extending axis around which the
air inhaled by a user rotates or swirls with the air exit passageway
positioned
on the cylindrically shaped wall of, and in flow communication with, the hold-
up chamber. In this embodiment, the hold-up chamber extends longitudinally
away from the holding portion which receives the powdered substance to the
air exit passageway. The air exit passageway preferably extends outwardly
from the hold-up chamber at an angle to the longitudinal direction of the hold-
up chamber. More preferably, this angle is about 900 (i.e. transverse to the
axis of rotation of the air in the hold-up chamber).
In another embodiment, the hold-up chamber further includes a frusto-
conically shaped portion extending from the cylindrical portion. A bottom of
the frusto-conically shaped portion communicates with the holding portion.
The shape of the frusto-conically shaped portion facilitates the cyclonic flow
of fluidized substance within the hold-up chamber.
In addition, in those embodiments _including a plurality of air entry
passageways, one or more of the plurality may be configured to direct air at
the powdered substance and one or more of the air entry passageways may
be configured to direct air so as to promote the cyclonic rotation of air in
the
hold-up chamber.
A further advantage of the instant invention is that high velocity air
may be used to extract the powdered substance from a reservoir to mix the
medicament with air and to segregate or deaggregate the substance.
Further, this extraction, mixing and deaggregation may be achieved by
inspiratory effort alone. No battery operated impellers or other mechanical
devices need be included. Further, by utilizing a plurality of air entry
passageways which, in total, have a relatively low cross-sectional area, these
high velocities can be achieved using low inspiratory flow rates.
CA 02244576 1998-08-04
7
A further advantage is that the dosage which is withdrawn is less
dependent on inspiratory flow rates than other known devices. Accordingly,
the dose of powdered substance, preferably a medicament, which is
withdrawn from the inhalation device is more consistent than may be
achieved with other known devices. Further, due to the low velocity of
substance as it exits the inhalation device, relatively low amounts of
substance will be deposited in the throat and upper airways of the user. Not
only does this result in more substance being drawn into the lungs of the
user, where in general it is more easily absorbed by the body, it may cause
less irritation to the throat and upper airways of the user.
Brief Description of the Drawings
These and other advantages of the instant invention will.be more fully
and completely understood in conjunction with the following drawings of the
presently preferred embodiments of the invention. Many of the features and
dimensions portrayed in the drawings have been exaggerated for the sake of
illustration and clarity.
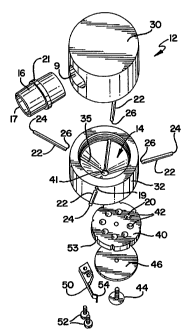
Figure 1 is a top perspective view of an inhalation device according to
the instant invention.
Figure 2 is a bottom perspective view of an inhalation device
according to the instant invention.
Figure 3 is a cross section along the line 3-3 in Figure 1.
Figure 4 is an exploded view of the inhalation device of Figure 1.
Figure 5 is a bottom view of the inhalation device of Figure 1.
Figure 6 is a perspective view of an alternative embodiment of the
inhalation device according to the instant invention.
Figure 7 is a cross section along the line 7-7 in Figure 6.
Figure 8 is a cross section along the line 8-8 in Figure 6.
Figure 9 is a cross-sectional view in the direction of line 3-3 of Figure 1
of an alternate embodiment of the inhalation device according to the instant
invention.
CA 02244576 1998-08-04
8
Figure 10 is a cross-sectional view along line 10-10 in Figure 9.
Figure 11 is a cross-sectional view along line 11-11 in Figure 9
showing the air entry passageways and the medicament cassette.
Figure 12 is a cross-sectional view along the line 3-3 in Figure 1 of a
second altemate embodiment of the inhalation device.
Figure 13 is a cross-sectional view along the line 3-3 in Figure 1 of a
third alternate embodiment of the inhalation device.
Figure 14 is a top view of an alternative embodiment of the inhalation
device.
Figure 15 is a side view of an alternative embodiment of the inhalation
device shown in Figure 14.
Figure 16 is an end view of the inhalation device shown in Fig. 14.
Figure 17 is a top view of an alternate embodiment of the inhalation
device.
Figure 18 is a side view of the inhalation device shown in Fig. 17.
Figure 19 is an end view of the inhalation device shown in Fig. 17.
Figure 20 is a graph of velocity (km/hr) and number of tubes for an
inhalation device having a total resistance to flow of 0.3 cm H20/I/min and at
a constant flow rate of 8 I/min.
Figure 21 is a graph of cross-sectional area of the air entry tubes (cm2)
versus the number of tubes for the device of Figure 19.
Figure 22 is a graph of the diameter of tubes versus the number of
tubes for an inhalation device having a total resistance to flow of 0.12 cm
HZO/i/min and at a constant flow rate of 8 I/min.
Detailed Description of the Presently Preferred Embodiments
As shown in Figures 1-4, inhaler 10 comprises a housing 12 having a
hold-up chamber 14, a mouthpiece 16 and a holding portion 20 to receive
and hold a substance, and more specifically, a powdered substance. The
powdered substance is preferably a medicament, which is generally defined
as a substance used in therapy, and more specifically as a substance used to
CA 02244576 1998-08-04
9
treat various ailments, diseases, etc. and/or to relieve pain, although it
should
be understood that the present invention would also work with other
powdered substances. Examples of dry powdered medicaments that can be
used with the present invention include, but are not limited to, antibiotics
such
as Erythromycin (for respiratory infections), Beta-Agonist (such as Ventolin
(SALBUTAMOL)), Corticosteroid (such as Flovent (FLUTICASONE)),
Cromoglycate (such as Intal (Sodium Cromoglycate)), and antihistamines
(such as Dimetene (Brompheniramine Maleate)).
Housing 12 may be of any particular shape or exterior configuration.
Accordingly, provided the hold-up chamber is of an appropriate dimension
and internal configuration, the inhaler may be shaped to suit various
aesthetic requirements. Further, housing 12 may be made from any material
which is known in the art. Preferably, housing 12 is made from a material,
such as a thermal plastic, which will prevent the build up of static
electricity
so as to minimize adherence of the substance to the internal walls of housing
12. Altemately, or in addition,.the interior walls of housing 12 may be coated
with a material, known to those of skill in the art, to reduce the adherence
of
the substance to the internal walls of housing 12. It should be understood by
those skilled in the art that other materials would also work, and that the
above-material is meant to be illustrative, rather than limiting.
Holding portion 20 is preferably sized so as,to receive therein a single
dose of powdered substance, or medicament. Holding portion 20 may be
provided at any particular location in housing 12. However, it is preferably
positioned such that, when inhaler 10 is to be used, holding portion 20 will
be
positioned at the bottom of housing 12 and will open facing upwardly into
hold-up chamber 14 as shown in Figure 3. This will assist in maintaining the
substance in holding portion 20 while inhaler 10 is in use.
Housing 12 includes at least one air entry passageway 22 which is
sized and configured to direct air entering inhaler 10 at holding portion 20
so
as to at least substantially fluidize the substance upon inhalation by the
user.
Housing 12 may have a plurality of such air entry passageways. For
CA 02244576 2006-10-04
example, housing 12 may have 1 to 8 air entry passageways and, more
preferably, from 3 to 5 air entry passageways. As shown in Figures 3 and 4,
housing 12 includes four such passageways 22. Each passageway 22 has
an entry port 24 and an exit port 26. Air entry port 24 may be positioned at
5 any point in or about housing 12. Preferably, each entry port 24 is located
adjacent exterior surface 18 of housing 12, which includes port 19 that
communicates with the entry port of the air entry passageway 22. Each exit
port 26 may be positioned and/or each passageway 22 may be configured so
as to direct air travelling through passageways 22 at the substance in holding
10 portion 20 to fluidize or assist in fluidizing the substance positioned
therein.
Preferably, the exit port 26 is positioned immediately adjacent and proximate
to the holding portion, or adjacent the edge of the holding portion, so as to
direct air to impinge upon the substance in the holding portion.
In the exemplary embodiment shown in Figures 3 and 4, each air entry
passageway 22 has a relatively uniform diameter and cross-sectional flow
area throughout its length. The air entry passageways are preferably
straight. In one suitable embodiment, the internal diameter of the air entry
passageways is about 1.75 mm, which results in a cross-sectional flow area
of approximately 2.405 mm2 for each air entry passageway, and a total
combined cross-sectional flow area of approximately 9.62 mm2 for four air
entry passageways. One of skill in the art should understand that other
diameters, cross-sectional flow areas and/or cross-sectional shapes, such as
a square, would also work.
Referring to Figure 21, various tube configurations are shown as
having total combined cross-sectional flow areas ranging from about .024 cm2
(2.4 mm2) for a single air entry passageway (tube) to about .064 CmZ
(6.4 mm2) for eight air entry passageways (tubes). As is also well understood
by those of skill in the art, the average velocity (km/hr) of the flow is
equal to
the volume flow rate (vol./hr) divided by the cross-sectional flow area.
Accordingly, for example, a volume flow rate of 8 L/min through the various
tube configurations (having cross-sectional flow areas of from about 0.024
CA 02244576 1998-08-04
11
cm2 to about 0.064 cm2) results in a flow rate (velocity) ranging from about
75
km/hr to about 210 km/hr as shown in Figure 20. Although the minimum
velocity required to fluidize the substance 20 is formulation dependent,
generally a minimum flow rate of about 45 km/hr is sufficient to fluidize the
types of medicament generally administered in dry powder form, with a more
preferred minimum of about 60 km/hr.
Because the air entry passageways 22 are positioned immediately
adjacent and proximate to and directed at the substance, the air flowing from
the exit ports 24 impinges on the substance and extracts it from the holding
portion 20 so as to thereby mix it in the air so as to produce a dust cloud in
the chamber. The fluidization of the substance includes two distinct phases.
First, the substance is deaggregated into separate respirable particles by the
impinging air flow. Deaggregation is the separation of the substance
particles, which may have a tendency to clump together. Preferably, most, if
not all, of the particles are deaggregated to a size of less than 5.8 microns.
Second, the substance particles are thereafter suspended in a stream of air
or gas in the hold-up chamber. Fluidization is distinguished from a simpie
entrainment of the substance, wherein relatively large lumps of aggregated
substance can be carried into and suspended in the air flow, either by suction
from above or by air flowing through the substance. As the substance is
fluidized, or impinged upon by the air scooping the substance out of the
holding portion, the initial concentration of the substance in the cloud
formed
in the hold-up chamber is dependent on the nominal dose of the substance
and the volume of the hold-up chamber.
As shown in Figures 3 and 4, the hold-up chamber 14 is in flow
communication with and positioned immediately above holding portion 20.
Hold-up chamber 14 is configured to maintain the substance in a fluidized
state during inhalation by the user and may also be configured to assist in
fluidizing the substance in holding portion 20. Hold-up chamber 14 is
accordingly designed to produce or assist in producing an air flow pattern
such that the substance may be readily deaggregated upon inhalation by the
CA 02244576 2006-10-04
12
user and maintained in a deaggregated condition during inhalation.
Preferably, hold-up chamber 14 is configured to produce a swirling or
cyclonic air flow in hold-up chamber 14. Accordingly, in the preferred
embodiment shown in Figure 3, the housing 12 is provided with lower portion
30 and upper portion 32 that define the hold-up chamber 14. As shown in
Figure 3, the upper portion 32 is threadably secured to the lower portion 30.
It should be understood, however, that the portions could be connected in
any number of ways including, but not limited to a press-fit, a detent, an
adhesive or any type of mechanical attachment. Alternatively, the upper and
lower portions could be integrally formed as a single unit,
Lower portion 30 is provided with angled walls 38. In an exemplary
embodiment, the walls are angled at about an angle of approximately 45
degrees, although it should be understood that other configurations and
angles would also work. By angling walls 38, a lower portion of hold-up
chamber 14 has a frusto conical shape so as to encourage the cyclonic or
swirling flow of air in hold-up chamber 14. Further, the configuration and
orientation of passageways 22 may be such as to encourage the formation of
the cyclonic air flow. As shown in Figure 3, passageways 22 are preferably
spaced around lower portion 30 and are straight. It will be appreciated that
provided a cyclonic or swirling flow of air is produced, any particular
configuration may be provided to passageways 22 and the internal surfaces
of lower portion 30. For example, passageways may be curved to direct at
least a portion of the air tangentially into the hold-up chamber.
As shown in Figures 3 and 4, grooves 35 extend substantially radially
upward from opening 41 formed at the bottom of the frusto-conical shaped
lower portion. Opening 41 forms a cylindrical passageway that is in flow
communication between the holding portion 20 and hold-up chamber 14. The
air entry passageways 22, which are preferably configured as circular tube
members, are supported by the grooves 35 in the lower portion such that the
exit port 24 of the air entry passageways opens directly into the opening 41
at
the edge thereof and is directed at the holding portion. In an exemplary
CA 02244576 1998-08-04
13
embodiment, the exit port 24 is positioned a distance from the holding portion
of from about 2.5 mm to about 3.0 mm. As shown in the embodiment of
Figures 3-5 at least one of the exit ports 24 of the air entry passageways is
slightly off-set from a longitudinally extending axis 39 of the opening 41 and
the underlying holding portion 20, which is coaxial with the longitudinally
extending axis of the hold-up chamber, whereby a cyclonic air flow is created
within the chamber about the longitudinal axis 39. It should be understood by
those of skill in the art that the cyclonic flow can be initiated without the
aid of
the angled walls. The tube members can be affixed in the groove formed in
the lower portion using an adhesive or the like. Alternatively, the air entry
passageways can be integrally formed in the lower portion as shown in the
embodiment of Figure 7, wherein the passageways 122 are provided
immediately below the angled surface 138 of the lower portion 132.
As shown in Figure 3, the upper portion 32 is provided with ceiling 36.
Side walls 34 extend between angled walls 38 and ceiling 36.' The upper
portion has a cylindrical shape with a longitudinal axis 39 coaxial with the
axis of opening 41.
Upon inhalation, air travels downwardly through passageways 22 and
is directed at the substance in holding portion 20. By directing the air at
the
substance, the substance is removed from holding portion 20 and is therefore
at least partially deaggregated if not substantially deaggregated upon the
commencement of inhalation. Upon continued inhalation, the configuration
and orientation of passageways 22 and/or the configuration of lower portion
of hold-up chamber 14 causes the air entering hold-up chamber- 14 to
25 adopt a cyclonic flow path.
Side walls 34 of hold-up chamber 14 may be of any particular
configuration which does not inhibit the cyclonic or swirling flow of air in
hold-
up chamber 14. Accordingly, side walls 34 are preferably smooth and, in
addition, are preferabiy of generally circular cross section. In one
30 embodiment, side walls 34 are preferably of generally constant circular
cross
section so that hold-up chamber 14 may accordingly define a cylindrical
CA 02244576 1998-08-04
14
chamber in inhaler 10. Similarly, ceiling 36 is preferably flat but can also
be
domed, indented or otherwise configured. Accordingly, once air commences
to move in a cyclonic pattern in the lower portion of the hold-up chamber,
this
pattern will be maintained in the upper portion thereof. The continual
movement of air in the upper portion of the hold-up chamber will keep the
substance in motion so that the substance will generally not have an
opportunity to aggregate. Further, the shear forces produced during the
swirling action will assist in deaggregating those portions of the substance
which were not deaggregated when the substance was removed from holding
portion 20 upon the initial inhalation by the user.
Mouthpiece 16 is provided to draw off air from a portion of hold-up
chamber 14 wherein the substance has been substantially deaggregated.
The mouthpiece can be configured to be received in either the nose or the
mouth of the user, or both. As shown in Figure 3, the mouthpiece 16 includes
an entry port 15 in flow communication with the hold-up chamber, an air exit
passageway 11 and an exit port 17. The air passageway is preferably
straight, although one of skill in the art should recognize that it could be
curved, bent or otherwise configured. The mouthpiece also includes a rib 21
extending circumferentially about the outer surface of the mouthpiece. The
external diameter of the mouthpiece tapers away from the rib. The tapered
portion allows the mouthpiece to be press fit in an opening 9 provided in the
upper portion 30 which communicates with the hold-up chamber 14 formed
therein. The rib 21 limits the amount of insertion of the mouthpiece and also
functions as an indicator to inform the user that their mouth is properly
disposed about the mouthpiece. Mouthpiece 16 is preferably provided in
upper portion 32 of hold-up chamber 14 and, more preferably, adjacent
ceiling 36 of hold-up chamber 14. At this position, by the time the substance
reaches the opening of mouthpiece 16, it has traveled several times around
hold-up chamber 14 and is substantially, if not completely, deaggregated.
For example, the combined initial deaggregation obtained by fluidizing the
substance, and the subsequent further deaggregation produced by the
CA 02244576 1998-08-04
cyclonic flow, can result in substance particle sizes of less than 5.8 microns
being introduced into the mouthpiece.
It will further be appreciated that, due to the volume of hold-up
chamber 14, not all of the substance will immediately exit hold-up chamber 14
5 upon initial inhalation by the user. Instead, as the inhalation continues,
additional air will enter hold-up chamber 14 through passageways 22 and will
dilute the substance remaining in hold-up chamber 14. This dilution of the
concentration of the substance in hold-up chamber 14 is beneficial as it
allows the substance to be slowly released during inhalation by the user.
10 Therefore, the substance will be drawn in by the user during all portions
of
the inhalation and the substance will therefore travel to all regions of the
lungs of the user. In addition, the deaggregation of the substance permits the
substance particles to be drawn further into the lungs. However, if the
volume of the hold-up chamber is too large, substance can be left in the
15 device as the user's inhalation is exhausted prior to all of the fluidized
substance being extracted. In an exemplary embodiment, the cylindrical
portion of the hold-up chamber has an interior diameter of approximately 31.5
mm and the hold-up chamber has a volume of approximately 41,138 mm3.
A further advantage of hold-up chamber 14 is that it allows for differing
rates of air flow into and out of inhaler 10. - In particular, the number and
size
of passageways 22 may be adjusted so as to provide a velocity of air flow into
inhaler 10 sufficientiy rapid to deaggregate or substantially deaggregate the
substance in holding portion 20. Further, this flow rate of air is preferably
sufficient to cause the deaggregation to occur substantially upon the
commencement of inhalation by the user. This flow rate of air may also be
sufficient to cause air entering the inhaler to undergo a cyclonic or swirling
motion in the hold-up chamber or to at least assist in commencing such
cyclonic or swirling motion.
In contrast, the air exit passageway of the mouthpiece 16 is sized with
a cross-sectional flow area greater than the combined cross-sectional flow
area of the air entry passageways so as to draw air from inhaler 10 at a rate
CA 02244576 1998-08-04
16
sufficiently slow so that the substance fluidized in the air inhaled by a user
will not substantially impact upon the throat and upper airway of the user.
Thus, mouthpiece 16 will generally have a large cross-sectional area as
compared to passageways 22. Preferably the ratio of the cross-sectional
area of the air exit passageway,relative to the cross-sectional area of the
air
entry passageway is greater than 10:1 and more preferably about 25:1.
Therefore, the medicament will travel into the lungs of the user. In an
exemplary embodiment, the inner diameter of the air exit passageway formed
in the mouthpiece is about 17 mm, with a corresponding cross-sectional flow
area of about 227 mm2.
The present invention, with its fluidization and subsequent even and
continuous administration of substance, ensures a minimal loss of substance
during the administration of the substance. In particular, ratios of the
emitted
dose relative to the nominal dose between 0.7:1 and 1:1 can be obtained
(with an ideal target ratio of 1:1), where the nominal dose is defined as the
amount of substance initially carried by the holding portion and the emitted
dose is defined as the amount of substance received by the user.
As is shown in Figure 3, the cross-sectional flow area of the
mouthpiece 16, or exit passageway, is preferably less than the cross-
sectional area of the hold-up chamber, which can be defined as the cross-
sectional area substantially perpendicular to the longitudinal axis 39 of the
hold-up chamber. In the embodiment shown in Figure 3, the cross-sectional
area is measured across the cylindrical portion of the hold-up chamber. This
configuration of the hold-up chamber and exit passageway of differing
diameters helps to avoid the inhalation of a bolus, or highly concentrated or
aggregated substance. In the absence of some restriction of the exit
passageway as compared with the hold-up chamber, or some other
alternative configuration between the exit passageway and the hold-up
chamber, such as the non-parallel orientation of at least a portion of the
exit
passageway relative to the hold-up chamber and the swirling fluidized
substance contained therein, the substance can potentially be inhaled
CA 02244576 1998-08-04
17
immediately without substantial mixing such that the substance will not be
drawn as far into the lungs. For example, the orientation of the exit
passageway 11, which is preferably linear, is preferably substantially
perpendicular to the longitudinal axis 39 about which the fluidized substance
flows.
However, it is also desirable to provide as large a cross-sectional flow
area as possible for the exit passageway 11 so as to reduce the velocity of
the fluidized substance being received by the patient so as to thereby reduce
the impaction on the back of the throat of the patient. But, it is also
necessary that the patient be able to fit the mouthpiece 16 in their mouth. As
shown in the embodiment of Figures 15 and 16, the mouthpiece has an
elliptical configuration which maximizes the cross-sectional flow area, while
still allowing the patient to insert the mouthpiece into their mouth.
Referring again to Figure 20, a chart is shown with the velocity of air
entering inhaler 10 measured against the number of tubes (with varying total
cross sections as shown in Figure 21). The total volume flow rate was
maintained at 8 litres per minute,and the resistance to flow was maintained at
0.3 cm of water per litre per minute. Resistance to flow is defined as the
pressure drop across the inhalation device divided by the volume flow rate.
As will be understood by one of skill in the art, the pressure drop is a
function
of the length and diameter of the air entry passageways. It is desirable,
therefore, to provide as high an intake velocity as is possible, so as to
ensure
a fluidization of the medicament, while providing as low a resistance as
possible so as to accommodate users capable of generating only relatively
low volume flow rates. As can be seen when viewing Figure 20, when inhaler
10 was provided with one tube having a total cross-sectional area of about
2.3 mm2 (see Figure 21), the flow rate was about 210 km per hour. When 8
tubes were provided, having a total cross-sectional area of about 6.4 mm2
(see Figure 21), the flow rate was reduced to about 75 km per hour.
Accordingly, as the number of tubes, and the cross-sectional flow area
increases, the flow rate of the air entering inhaler 10 through passageways
CA 02244576 1998-08-04
18
22 decreases. However, even with a large number of tubes, and the
corresponding increase in cross-sectional flow area, the air flow rate may
still
be maintained at a velocity which would create substantial shear forces in
inhaler 10 and which would cause the substance in holding portion 20 to be
quickly fluidized.
Figure 21 is a chart showing the total cross-sectional area of
passageways 22 as the number of tubes decreases. As can be seen, the
cross-sectional area of the tubes increases with the number of tubes which
are provided. If the cross-sectional area increases too much with an
increasing number of tubes, the diameter of some or all of the tubes may be
decreased. By adjusting the diameter of passageways 22, and the number of
passageways 22, the velocity of air entering inhaler 10 (for a given range of
flow rates) may be maintained.
Figure 22 is a chart showing the diameter of passageways 22 plotted
against the number of tubes 22 in inhaler 10. As the number of tubes is
increased, the diameter of the tubes is decreased. As the number of tubes
(and their diameter) decrease, with a corresponding overall increase in cross-
sectional flow area, the velocity of the air travelling through the tubes
decreases from about 135 km per hour to about 48 km per hour. If the
diameter of the tubes were decreased more, then a higher velocity may be
maintained. Alternately, if a larger diameter in the tubes (and corresponding
larger cross-sectional flow area) was provided, then the velocity would
decrease.
Typically, users generate volume flow rates that can vary from
relatively low flow rates (e.g., 8 liters per minute) to relative high flow
rates
(e.g., 120 liters per minute). As will be appreciated from these charts, even
at a low flow rate (e.g., 8 liters per minute), a person with a breathing
disability may still generate substantial velocities in passageways 22. These
velocities are sufficient to deaggregate the substance 20. Conversely, due to
the resistance of flow in passageways 22, a user without any breathing
difficulties would be limited in the velocity which they could achieve in air
CA 02244576 1998-08-04
19
travelling through passageways 22. For example, the present invention is
capable of -maintaining flow rates on the order of 10 liters per minute to 30
liters per minute, regardless of the lung capacity of the user. Accordingly,
the
inhaler 10 is adapted to be used by users who can generate low flow rates or
high flow rates.
Similarly, a mouthpiece with a certain diameter may be employed for
users having different lung capacities and flow rates. As mouthpiece 16 has
a relatively large diameter as compared to passageways 22, the air exiting
inhaler 14 will travel at a rate sufficiently slow so as to maintain, or at
least
substantially maintain, the substance in the air as the air travels through
the
mouth and upper airways of the user. Due to the large relative diameter of
mouthpiece 16, a user would not generate a flow rate sufficiently fast so as
to
cause substantial quantities of substance to impact upon the throat and upper
airways of the user.
As shown in Figures 2-5, inhaler 10 may be provided with a source of
multiple doses of substance. For example, inhaler 10 may be provided with a
container for holding bulk medicament and metering means for providing
individual doses of medicament into holding portion 20 (not shown).
Altemately, inhaler 10 may be provided with a plurality of individual doses of
medicament. In yet another alternative, the inhaler can be configured to
support a capsule containing a powdered substance. As shown in Figures
2-5, inhaler 10 includes a cassette 40 having provided therein a plurality of
recesses 42, with each functioning as the holding portion 20 when it is moved
to be in flow communication with the hold-up chamber. Each recess 42 is
preferably sized to receive an individual dose of substance. Recesses 42 are
preferably spaced in a circular pattern around cassette 40. Cassette 40 may
be rotatably mounted to the bottom of inhaler 10 by means of bolt 44 and
washer 46. Bolt 44 may pass through washer 46 and cassette 40 into lower
portion 30 of inhaler 10. In this way, as cassette 40 is rotated, a different
recess 42 is positioned below opening 41 of inhaler 10.
CA 02244576 1998-08-04
In order to maintain a particular recess 42 in alignment with
opening 41, inhaler 10 may be further provided with a locking means. It
should be understood that many types- of mechanical devices are available
for releasably locking the cassette relative to the housing, including,.but
not
5 limited to, various detent mechanisms, set screws, ratchet mechanisms, etc.
For example, the bottom of inhaler 10 may be provided with steel spring 50,
which is secured to the bottom of inhaler 10 by means of bolts 52. As shown
in Figures 4 and 5, the spring is a cantilever spring, although it should be
understood that other types of springs, including, but not limited to,
tension,
10 compression and leaf springs would also work. Steel spring 50 is provided
with a detent 54 which is adapted to engage a mating recess 53 which is
provided on cassette 40. Accordingly, when a user needs to take a dose of
substance, they may rotate cassette 40 to expose a new dose of substance.
This dose of substance will be maintained in position by means of detent 54
15 of steel spring 50 and the mating recess on cassette 40. It will be
appreciated by those skilled in the art that any substance holder or
dispensing means may be adapted for use with inhaler 10. Further, if
required, the substance may be sealed in individual doses and an opening
means may be provided to open the individual substance container prior to
20 inhalation by the user (not shown).
For the sake, of simplicity, parts and features in various alternate
embodiments that are substantially similar to parts and features previously
referred to are identified with the same reference numbers.
Figures 6-8 show an alternative embodiment of the inhalation device
similar to the device illustrated in Figures 1-5, but with some differences.
For
example, in this embodiment, the lower portion 131 includes a lower frusto-
conical surface 138 mating with an upper cylindrical shaped interior
surface 139. 'The lower portion is secured to the upper portion 132, which
also preferably inciudes a cylindrical interior surface 134, so as to form a
uniform cross-section for the hold-up chamber. The lower portion is secured
to the upper portion in a snap-fit configuration with a detent mechanism 133,
CA 02244576 1998-08-04
21
or similar securing mechanism. One of skill in the art should also understand
that the upper and lower portions 132, 130 can be integrally formed. The
frusto-conical portion opens directly into the holding portion 20. The
mouthpiece shown in Figure 7 opens into the hold-up chamber 114
immediately adjacent the ceiling 136 of the hold-up chamber and includes a
relatively long air exit passageway 111 having entry and exit ports 115, 117.
In addition, a spring 150 extends across the bottom of the housing and
includes a detent 154 in the middle of the spring. The spring is secured at
its
ends with bolts 152 so as to function as a simply supported beam or leaf
spring, rather than a cantilever spring as shown in the embodiment of
Figures 1-5.
Figures 9-11 show an alternate embodiment of the inhaler. In this
particular embodiment, inhaler 210 is again provided with four.passageways
22. The four passageways are positioned at the four corners of a square so
that there are opposed pairs of passageways 22.
In addition, hold-up chamber 214 is of a different construction. In this
case, the lower portion-of the hold-up chamber 214 is provided with an
angled Wall 238. However, sidewall 234 is concave. The lower portion of
sidewall 234 which is opposed to angled wall 238 assists in creating a
cyclonic flow of air about longitudinal axis 239 in hold-up chamber 214. In
this embodiment, the hold-up chamber is configured so as to be positioned
over the holding portion 20 nearest or proximate the mouthpiece. Also in this
embodiment, the upper and lower portions of the hold-up chamber are formed
substantially integrally in a single member. The holding portion 20 is formed
in cassette 240 that is rotatably mounted to the housing with a carriage
member 250. The carriage member 250 includes an opening 241 having an
angled portion 258 mating with wall 238 and a concave portion 254 mating
with wall 234. The mouthpiece 216 includes an elliptically shaped air exit
passageway.
Figure 12 shows an alternate inhaler. In this particular example, cover
60 is provided for cassette 340. In addition, detents 62 are provided to
assist
CA 02244576 1998-08-04
22
in maintaining recesses 42, or holding portions 20, in alignment with the
opening 64. Cover 60 is a plate (which may be made of metal or plastic or
the like) which is used to seal recesses 42. Cover 60 is provided with an
opening 64, having a frusto-conical shape, which is positioned in alignment
with portion 20. Accordingly, as cassette 340 is rotated, a-different recess
42
may be positioned in alignment with opening 64 and holding portion 20 so
that a new dose of substance is available for inhalation. Cover 60 may be
required if the substance in recesses 42 is particularly sensitive to moisture
(e.g. it will deteriorate upon exposure to moisture or its rate of aggregation
may increase). Detents 62 may be protrusions provided on the lower surface
of inhaler 10 to engage recesses provided in cassette 40. In the embodiment
of Figure 13, the holding portion 20 is exposed in a rear portion of the hold-
up
chamber distal from the air exit passageway 311 and has a longitudinal axis
341 extending parallel to but not coaxial with the longitudinal axis 339.
Preferably, the hold-up chamber 314 has a circular cross-section so as to
promote a cyclonic flow about longitudinal axis 339 therein.
In the embodiment of Figure 13, the inhaler is provided with a second
set of one or more air entry passageways 66. Air entry passageways 66 are
not aimed at the medicament in recess 42. Instead, passageways 66 are
directed to provide a cross flow that assists in creating a cyclonic flow of
air in
hold-up chamber 414 about longitudinal axis 439 and further deaggregates
the medicament as it swirls in the hold-up chamber. Accordingly, a portion of
the air entering inhaler may be directed through air entry passageways 22 at
the medicament and the remainder.of the air entering inhaler 410 may pass
through air entry passageways 66 so as to assist in creating, or to create, a
cyclonic or swirling flow of air in hold-up chamber 414. The hold-up chamber
414 is preferably circular so as to promote the cyclonic flow therein.
Therefore, although the mouthpiece 416 is shown as having a depth
substantially the same as the hold-up chamber, the cross-sectional area of
the air exit passageway is less than the cross-sectional area of the hold-up
chamber, which extends longitudinally along axis 439. The air exit
CA 02244576 1998-08-04
23
passageway also communicates with the hold-up chamber distal from the
holding portion 20, or approximate the opposite end of the hold-up charnber.
The holding portion 20 has a longitudinal axis 441 extending parallel to but
not coaxial with longitudinal axis 439 of the hold-up chamber.
Similarly, as shown in the embodiment of Figures 14-16, the hold-up
chamber 514 has a generally circular cross-section and includes a generally
cylindrically shaped lower portion and a domed or concave shaped
ceiling 536. Two air entry passageways 522, 566 are provided. One
passageway 522 has an exit port 526 positioned immediately adjacent and
proximate to and directed at the holding portion so as to direct air to
fluidize
the medicament in the holding portion. A second passageway 566 is
arranged substantially perpendicular to the longitudinal axis of the hold-up
chamber 514 and tangential to the interior surface thereof such that air
coming through the passageway promotes a cyclonic flow in the hold-up
chamber about longitudinal axis 539, along which the hold-up chamber
extends. In this embodiment, the holding portions 520, which are arranged
circumferentially around the periphery of the carriage, are radially elongated
with at least one of the holding portions aligned with the first air .
passageway 520 such that the air exiting the air passageway impinges upon
the elongated holding portion to fluidize the substance and scoop the
substance out of the holding portion directly into the path of the air exiting
the
second passageway 566 which further shears the substance so as to further
deaggregate it and which also creates a cyclonic flow in the hold-up chamber.
The holding portion 520 has a longitudinally extending axis 541 parallel to
but
not coaxial with the longitudinal axis 539 of the hold-up chamber. The
mouthpiece 516 has an elliptically shaped exit port.
In yet another embodiment, shown in Figures 17-19 the exit port 726 of
the second air entry passageway 766 is flared, or has an elliptical cross-
section that provides a greater area of directed air flow to further impact on
the fluidized substance and to initiate the cyclonic flow in the hold-up
chamber as illustrated by the flow directional arrows.
CA 02244576 1998-08-04
24
Although the present invention has been described with reference to
preferred embodiments, those skilled in the art will recognize that changes
may be made in form and detail without departing from the spirit and scope of
the invention. As such, it is intended that the foregoing detailed description
be regarded as illustrative rather than limiting and that it is the appended
claims, including all equivalents thereof, which are intended to define the
scope of the invention. Therefore, it will be appreciated by those skilled in
the
art that various modifications and changes may be made to the instant inhaler
without altering the nature of the invention.