Note: Descriptions are shown in the official language in which they were submitted.
CA 02244907 2003-02-05
1
Use of Calcium Dobesilate for the Manufacture of a Medicament for the
Treatment of
Embrr~onic Retardation
The present invention relates to a formulated product used for the prevention
and treatment
of embryonic retardation or discordance.
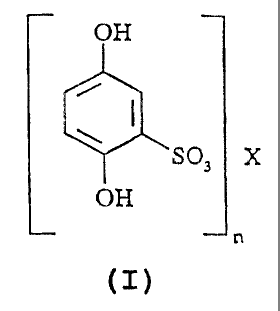
More particularly, the invention relates to the use of a compound with the
general formula:
OH
SO~ X
OH
n
wherein x represents a 1 or 2 valency metallic ion adequate from a therapeutic
point of view,
and n has a value of either 1 or 2, or a metabolite of said compound, for the
treatment or
prevention of embryonic retardation or discordance.
Formulations containing calcium dobesilate as an active ingredient have been
used in
human therapy since the 1970's, and are known to be effective in several areas
of
indication.
2 5 The active constituent has been applied in several formulations, the most
common being the
oral products (tablet, capsule) known by the trademark DOXIUMT"~, and
manufactured by
OM Laboratories Ltd. (Switzerland).
The manufacture of calcium dobesilate (calcium salt of 2,5-dihydroxybenzene
sulphonic
3 0 acid) is discussed in the US Patent 3509207 and ES Patent 335945.
Major indication areas and experiences are reviewed as follows.
Examination of 120 patients with venous dilatation (primary and secondary
varicosity) was
3 5 performed by Pietrek, G. (Z. Allg. Med. 56. /1980/ 1217-1222). Following 3
months of oral
CA 02244907 2003-02-05
2
DOXIUMT~~ treatment, Pietrek reported a decrease in patient's complaints
(parachroma,
oedema, excess weight sensation).
The treatment of patients suffering from chronic venous insufficiency by
double-blind
examination was reported by Haachen, H.J. and Lorenz, P. (Angiology 1982. July
33 (7)
480-488). As a result of treatment with DOXIUMTM, complaints of the patients
significantly
decreased and their health improved according to plethysmographic analysis.
Patients suffering from the above illnesses were examined by Balmer, A.
(Schweiz.
1 o Rundschau fur Med. Praxis, 67 (39) 1978, 1440-1443) before and after
external treatment
with DOXIVENIL_T"" gel. This product contains 2% calcium dobesilate and 2%
heparinoid
(potassium hydrodextran sulphate). Patients tolerated the treatment very well,
and the
health condition of 80-87% of them turned to favourable.
Results from a three month treatment of patients with diagnosed diabetic
retinopathy using
oral DOXIUMT"" were published by Barras, J.P. and Graf, C (VASA/1980/9(2)161-
164).
According to three different analytical methods, the viscosity of both the
plasma and whole
blood was reduced. The relative viscosity and the hematocrit value remained
unchanged.
2 o The capillary permeability of diabetic patients was examined by Sevin, R.
and Cuendet, J.F.
(Ophtalmologica 162:33-40/1971/33-40). Retinopathic patients were divided into
the
following four groups; exclusively microaneurysm, some retinal haemorrhage,
considerable
retinal haemorrhage, and extraretinal haemorrhage. According to the results of
fluorescein
angiographic analysis, oral dosage of DOXIUMT"" has a favourable effect on the
2 5 pathological permeability of the capillaries, and capillary resistance
values.
Treatment of pile haemorrhage with DOXIPROCTT"' suppository (containing 250 mg
calcium dobesilate and 40 mg lidocain active constituent) was published by
Berson, I.
(Schweizerische -.Rundschau fur Med. Praxis (1975.03.11. I 64 (10) 299-301 ).
As a result of
3 0 administering the drug twice daily for two weeks, improvement was detected
in 87% of the
cases.
From the aforementioned references, the known indications for oral application
of calcium
dobesilate can be summarized as: microangiopathy, particularly diabetic
retinopathy;
3 5 chronic venous insufficiency; primary varicosity; pregnancy varicosity;
leg ulcer; night sural
CA 02244907 2003-02-05
3
spasm; and ankle oedema. On the basis of the data presently available, it is
also applicable
as an adjuvant in the following cases: external thrombophlebitis;
postthrobotic syndrome;
oedema; stasis dermatosis; piles complaints.
According to the literature data relating to its mechanism of action, calcium
dobesilate
effects the regulation of the pathological capillaric wall functions
(increased permeability and
reduced resistance). 1t inhibits the decomposition of collagen fibres, and
reduces
hyperviscosity of both the plasma and the blood. Indirectly, it inhibits the
lymph flow, and as
a consequence reduces the oedema. Maximum blood concentration can be reached 6
hours after oral dosing with 500 mg of calcium dobesilate, and can reach as
high as 8
Ng/ml. Between 3 and 10 hours blood levels remain constant, and after 24 hours
fall to 3
Nglml. On average, the plasma half-life of the drug is 5 hours. Calcium
dobesilate is
connected to the plasma peptide by its 20-25% rate. It can not be transferred
through the
blood-brain barrier or through the placenta barrier, although it can be
detected in small
quantity in the mother's milk. In the first 24 hours about 50% of the orally
dosed quantity is
excreted in the urine, and a similar amount is excreted in the faeces. The
majority of this
compound is eliminated without any modification.
Independent from the treatment period, the compound is well tolerable. Its
toxicity is very
2 0 low, the LD5° value tested with mice is 700 mg/kg. According to the
literature data, there are
no known drug-drug interactions.
According to our knowledge, application of calcium dobesilate during
pregnancy, except for
treatment of varicosity, has not been tested. A possible reason is that
information from the
manufacturer of the oral formulation (DOXIUMT"", OM Laboratories, Ltd.
Switzerland)
contains the following statements: "It is not recommended during the first
three months of
the pregnancy", "ln case of pregnancy the medical attendant should be
informed", and
"Medicine does not get across the barrier of the placenta".
3 o During pregnancy several clinical problems can occur, including embryonic
retardation.
Embryonic retardation is defined as retardation of development compared to the
average
development ratio in a given pregnancy phase. According to domestic and
international
statistical data the frequency of embryonic retardation is within 10-30%.
Large fluctuations
in this statistic reflect that the demand for detection and the availability
of instrumentation
3 5 were established only in the past 5-10 years. Currently, babies who are
born with a 2500 g
CA 02244907 2003-02-05
4
birth weight are not automatically regarded as retarded new-born infants, but
are classified
as premature, especially if the birth occurred before the 372'' pregnancy
week. In the case of
new-born infants who are born after this period and exceed the 2500 g birth
weight,
diagnosis of retardation is established only when a neonatal specialist is
present during the
birth, or if the new-born infant needs intensive therapy. For this reason the
statistical data
may be more favourable than the real incidence rate.
Additional problems associated with embryonic retardation are that
complications of the
illness often leads to intrauterine necrosis, and the prenatal mortality rate
and incidence of
l0 further damage are very high.
According to the literature, development of the illness may be caused by
certain
predisposing maternal factors, such as smoking, alcahofism, malnutrition,
metabolic and
endocrine illnesses, chronic kidney, liver, or lung illnesses, gravid
toxaemia, or pre-
eclampsia. The placenta can be anather possible source, wherein disorders in
uterine
development, insertion difficulties of the placenta, early aging, or
discordance during twin
pregnancy leads to the illness. However these factors have net been examined
completely
since, aside from congenital uterine disorder, after retarded pregnancy even a
normal
pregnancy can be developed.
There are two classical forms of intrauterine retardation. The first is when
retardation
occurs in the early stage of the pregnancy, and is referred to as "primary
retardation". The
second form occurs when, after a normal initial development, the development
of the
embryo becomes retarded or halted during the second or third trimester. This
is referred to
as '"secondary retardation". Diagnosis of such disorders was made possible by
the spread
of modern ultrasanographic techniques.
According to the recommendation of the European (and the Hungarian) Ultrasound
Association the examination of the following five parameters are necessary and
sufficient to
3 0 diagnose cases of embryonic retardation: diameter of the embryonic
temporal bone,
circumference of the embryonic skull, circumference of the embryonic chest,
diameter of the
embryonic chest, and the length of the embryonic femur.
By the application of the Colour Doppler ultrasonographic technique, certain
circulatory
measurements may be done as well, which can reinforce the diagnosis. As a
result of these
CA 02244907 2003-02-05
data, it has become evident that the haematocrit values of retarded embryos
are high, and
their carbohydrate and lipid reserves are depleted. In the placenta of
retarded embryos,
multifocal thrombotization and early necrosis have been found, which leads to
prematurity of
the placenta.
5
Efforts have been made since the 1950's to treat embryonic retardation. Trials
were set up
to increase maternal carbohydrate levels, sometimes along with low doses of
insulin.
DIAPHYLL1NT"~ has been administered in order to increase maternal circulation.
In addition,
the application of a vacuum chamber placed on the maternal abdominal wall has
been
performed in order to reduce atmospheric pressure. Furthermore, salicylate
containing
medicines have been administered in order to reduce maternal blood viscosity
(or
aggregation). Administration of acetyl salicylate was studied and published by
Shen J. et al
(Br.J.CIin.Pharmacol. 35., 1993., No.S. 564-57). This study involved
administering acetyl
salicylate to pregnant women who were endangered by pregnancy convulsions (pre-
eclampsia) and showed retarded embryonic development. It was established in
each case
that acetyl salicylate gets into the embryo, with lithe difference between
maternal and
embryonic blood concentrations. However, there was no effect reported in
connection with
embryonic retardation. Uterine arteries were examined using Doppler
ultrasonographic
methods in similar clinical cases to those described above by S. Campbell (
The Sixth World
2 0 Congress of Ultrasound in Obstetrics and Gynecology; Rotterdam, Oct. 27-
31, 1996. Book
of Abstracts p. 256, article no. 517) This study did not report any effects in
connection with
embryonic retardation when the prophylactic ASPIR1NT"" was administered. These
studies
support what is known by experts, that none of the above listed treatments
results in the
onset of weight gain in the retarded embryo. Therefore, none of these
treatments is used.
In summary, it can be concluded that the predisposing factors of embryonic
retardation are
known, that means of diagnosis of the illness are available, but khat
effective therapeutic
methods are not available.
3 o The objective of the present invention is to provide a medical treatment
which is suitable for
the treatment of a retarded embryo, the prevention of embryonic retardation in
cases where
predisposing factors exist, and improvement of the condition of a retarded
embryo in the
case of a discordance (multiple pregnancy with different development rate).
During experimentation, we unexpectedly observed that calcium dobesilate is
effective for
CA 02244907 2004-05-17
6
the above medical treatment. The effectiveness of calcium dobesilate is
surprising since it
does not cross the placenta, and cannot be detected in the embryo. This is in
contrast to
common approaches, wherein scientists search for agents Which are available to
influence
the state of the embryo directly. In addition, this result was surprising
considering other
directions of the research work, where the increase of the maternal "offer
side" was
considered as primary importance, wherein carbohydrate or glucose was
administered into
the pregnant patient. However these attempts did not produce results.
Accordingly, one aspect of the present invention provides for the use of
calcium dobesilate
1 o for the treatment or prevention of embryonic retardation and for the
treatment or prevention
of discordance.
Another aspect of the present invention relates to the use of calcium
dobesilate for the
manufacture of a medicament used for the treatment or prevention of embryonic
retardation
and for the treatment or prevention of discordance.
Accordingly, the above active constituent is suitable for treatment of
embryonic retardation
in its early stage or for its prevention. If certain predisposing factors are
established early in
pregnancy, the development of the illness is more probable. In such cases, if
retardation
2 o happened only in one pregnancy, it should not be presumed that embryonic
retardation will
occur in subsequent pregnancies. However after two or more pregnancies
resulting in
retarded new-born infants, the probability of further occurrences is high.
A possible mechanism of action for the active constituent is that the blood
supply of the
placenta on the maternal and embryonal border is increased. Consequently the
oxygen and
nutrient supply towards the embryonic side is intensified. In the area of the
villus of the
placenta, blood congestion and formation of the microthrombus is halted.
Although the
maternal and embryonal blood vessels do not mix, the improvement of maternal
blood
3 0 volume and blood pressure causes the flow of blood volume and blood
pressure towards the
embryo to be intensified.
According to our studies in cases of discordance, administering the active
constituent has a
favourable effect on the development of the gemini-B or -C, having previous
retardation of
development. In these cases, the positive results are most likely due to
improvement of the
CA 02244907 2004-05-17
7
oxygen and nutrient supply in the placenta, which allows for development of
the gemini-B or
-C.
This mechanism of the active constituent cannot be concluded from its
mechanism of action
in the other indication areas described above. This is supported by the fact
that, after the
examination of patients suffering from pre-eclampsia (which is accompanied by
maternal
oedema, albuminuria and high blood pressure) the authors stated that calcium
dobesilate
did not decrease the maternal albuminuria or hypertension, moreover, the
transformation of
the blood platelets was favourable. In this and other similar studies, changes
in embryonic
1 o retardation were not discussed (Lege Artis Medicine April 07, 1994 page
902-910).
In order to support its use in the new indication area, calcium dobesilate was
tested in
pregnancies divided into three groups. In the first group, embryonic
retardation was
diagnosed by ultrasound analytical testing according to measurements of the
diameter of
the embryonic temporal bone, circumference of the embryonic skull,
circumference of the
embryonic chest, diameter of the embryonic chest, length of the embryonic
femur and
embryonal cardiovascular examinations. These cardiovascular examinations
included;
analysis of the area "below the phonic graph" of artery blood flow of the
umbilical cord and
measuring the resistance index of the same artery, measurement of conditions
of the
2 0 downflow branch of the embryonic aorta, and analysis of the area "below
the phonic graph"
of embryonal artery cerebri media blood flow and measuring the resistance
index of the
same artery (these analysis were applied from the 28~" week of the pregnancy).
The second group included patients with twin pregnancies. The diagnosis was
performed
2 5 using the same methods as group 1. In the third group, because of the
occurrence of
predisposing factors, embryonic retardation was expected. This group contained
both
normal and twin pregnancies.
Group 1 - diagnosed embryonic retardation
3 o Using the diagnosis procedure described above, 144 pregnant patients were
placed in this
group. Patients received 3 times daily one DOXIUMT"' capsule (500 mg calcium
dobesilate
per capsule). The minimal treatment period was two weeks, the optimal 6-10
weeks.
Treatments were started in the second to third trimester of the pregnancy. In
addition to the
embryonal analysis and the determination of the cardiovascular conditions
described, once
3 5 therapy commenced the quantity of amniotic fluid was measured and the
maturity degree of
CA 02244907 2004-05-17
8
the placenta was determined on a weekly basis. Based on the above parameters,
if the
degree of embryonal development and weight of the embryo reached the
characteristic
value of a gestational age, we started the maintenance treatment. The
maintenance dose
was twice daily with one DOXIUMT"" capsule.
According to our results, based on both the embryonal stature and the
functional analysis
(non-stress-test, cardiovascular parameters), continuation of pregnancy became
possible in
those cases where the functional state of the embryo would have caused
termination of the
pregnancy. The effect of DOXIUMT"" was the most pronounced, resulting in
mature weights
of new-born infants in the range of 10-90 per cent, allowing continuation of
pregnancy to full
term or nearly full term, if the treatment started before the 32"d to 34'"
gestational week and
the embryonal retardation was not associated with the illness of the mother
and /or embryo
retardation in development and/or dry labour.
Group 2 - discordance embryonal twin pre nc~ancy
In this group, 23 pregnant patients were examined. Diagnosis and testing of
the patients
was performed using the same method as used for group 1.
The initial drug dose was three times daily with two DOXIUMT"" tablets (250 mg
calcium
dobesilate per tablet), and the maintenance dose was twice daily with 2
DOXIUMT"'' tablets.
The results were similar to those found in group 1. In both groups the new-
born infants
2 5 were not haemoconcentrated, rise in thrombocyte aggregation was not
characteristic,
viscosity of blood was normal, saturation of the oxygen was physiological, and
carbohydrate, lipid and peptide metabolism did not need any correction in
cases when the
treatment lasted only 8-10 weeks.
Group 3 ~ro9nosed embryonal retardation
For this study, 93 pregnant patients were selected whereby predisposing
factors were
found. Testing of the patients was performed using the same method used for
group 1.
Administration of DOXIUMT"" twice daily with 2 DOXIUMT"" tablets (250 mg
calcium
CA 02244907 2004-05-17
9
dobesilate per tablet) was started in the 14'" week of pregnancy, and was
stopped 2 weeks
before birth.
According to our study, embryonic retardation was not found in these test
cases.
In summary, these studies showed that calcium dobesilate is effective for the
treatment or
the prevention of the above illnesses, that on the basis of the treated group
no
contraindication of illness became known, and no drug-drug interactions were
observed in
the cases of the treated patients.
to
In addition, if the dosing period is not more than 2 Weeks, the recommended
minimum daily
calcium dobesilate dose is 500 mg and the maximum dose is 3000 mg.
The present invention is advantageous considering that the active constituent
is applied to a
new indication area, and has been in human use for more than 20 years, current
formulations have no or very limited side effects, the active constituent does
not cross the
placenta, so it will not enter the embryo, it provides good therapeutic
results in those areas
where previous treatments had only limited success, and treatment can be
started during
the 2"d or 3'~ trimester, even for prevention.