Note: Descriptions are shown in the official language in which they were submitted.
CA 02244997 1998-07-31
WO 97!27840 PCT/LTS97/00722
1
- 1 SUSTAINED DELIVERY OF AN ACTIVE AGENT USING AN
IMPLANTABLE SYSTEM
3
Technical Field
s This invention is related to the sustained delivery of a biologically
active agent. More particularly, the invention is directed to an implantable
s delivery system for the prolonged delivery of an active agent to a fluid
s environment in a natural or artificial body cavity.
11 Background of the Invention
12
13 Treatment of disease by prolonged delivery of an active agent at a
1a controlled rate has been a goal in the drug delivery field. Various
approaches
1s have been taken toward delivering the active agents.
1s One approach involves the use of implantable diffusional systems. For
1~ example, subdermal implants for contraception are described by Philip D.
1s Darney in Current Opinion in Obstetrics and Gynecology 1991, 3:470-476.
1s Norpiant~ requires the placement of 6 levonorgestrel-filled silastic
capsules
zo under the skin. Protection from conception for up to 5 years is achieved.
The
z1 implants operate by simple diffusion, that is, the active agent diffuses
through
zz the polymeric material at a rate that is controlled by the characteristics
of the
zs active agent formulation and the polymeric material. Darney further
describes
za biodegradable implants, namely CapranorT"" and norethindrone pellets.
zs These systems are designed to deliver contraceptives for about one year and
as then dissolve. The CapranorTM systems consist of poly(s-caprolactone)
z7 capsules that are filled with levonorgestrel and the pellets are 10% pure
za cholesterol with 90% norethindrone.
zs Implantable infusion pumps have also been described for delivering
so drugs by intravenous, intra-arterial, intrathecal, intraperitoneal,
intraspinal and
s1 epidural pathways. The pumps are usually surgically inserted into a
CA 02244997 2005-02-09
67696-260
2
~ subcutaneous pocket of tissue in the lower abdomen. Systems for pain
2 management, chemotherapy and insulin delivery are described in the 881.
s Newsletter, Vol. 17, No. 12, pages 209-211, December 1994. These systems
4 provide for more accurately controlled delivery than simple diffusions!
s systems.
,,
s One particularly promising approach involves osmotically driven
~ devices such as those described in U.S. Patent Nos. 3,987,790, 4,885,845,
a 5,057,318, 5,059,423, 5,112,814, 5,137,727, 5,234,892 and 5,234,693.
s These devices can be implanted
~o into an animal !o release the active agent in a controlled manner.for a
~ predetermined administratbn period. In general, these devices operate by
~z imbibing fluid from the outside environment and releasing. corresponding
~3 amounts of the active agent
,s The above-described devices have been useful for delivering alive
~s agents to a fluid environment of use. Although these devices have found
is application for human and veterinary purposes, there remains a need for
,~ devices that are capable of delivering active agents, particularly potent
~e unstable agents, reliably to a human being at a controlled rate over a
~a prolonged period of time. .
so
z~ $~~marv of the Invention
x~ Implantable osmotic systems for delivery of an active agent to an
a animal arse Hrell known. Adaptation of these systems for human use raises a
is number of difficult issues. The size of the device may need fio be
decreased
2s for human impiantaflon. The strength of the de~rice must be suffiaent to
rr ensure a robust system. Accurate and reproduabk delivery rates and
28 durations must be ensured and the.period from implantation to start-up of
Za delivery must be minimized. The active agent must return ita purity and
3o activity for extended periods of time at the elevated temperatures
s~ encountered in the body cavity. ' .
CA 02244997 2005-10-14
67696-260
2a
According to one aspect of the present invention,
there is provided a fluid-imbibing device for delivering an
active agent to a fluid environment of use, said fluid-
imbibing device comprising a water-swellable semipermeable
plug that conforms to an interior surface of an end of an
impermeable reservoir and creates a liquid-tight seal
between the water-swellable semipermeable plug and the
interior surface and an active agent to be displaced from
the fluid-imbibing device when the water-swellable
semipermeable plug swells.
According to another aspect of the present
invention, there is provided the device as described above,
further comprising a piston received within the interior
surface of the impermeable reservoir, wherein the piston
divides the reservoir into an active agent containing
chamber and a water-swellable agent containing chamber.
According to still another aspect of the present
invention, there is provided the device as described above,
further comprising: (a) a piston that divides the reservoir
into a first and a second chamber, the first and second
chambers each having an open end; (b) a water-swellable
semipermeable plug in the first chamber; and (c) the active
agent in the second chamber, wherein the water-swellable
semipermeable plug is located in the open end of the first
chamber.
According to yet another aspect of the present
invention, there is provided the device as described above
wherein the water-swellable semipermeable plug contains at
least about 64 mg NaCl.
According to a further aspect of the present
invention, there is provided the device as described above
wherein the water-swellable semipermeable plug contains
CA 02244997 2005-10-14
67696-260
2b
NaCl, a gelling osmopolymer and tabletting agents and
viscosity modifying agents.
CA 02244997 1998-07-31
WO 97/27840 PCT/US97I00722
3
- ~ Accordingly, in one aspect, the invention is a fluid-imbibing device for
2 delivering an active agent formulation to a fluid environment of use. The
3 device comprises a water-swellable, semipermeable material that is received
in sealing relationship with the interior surface at one end of an impermeable
s reservoir. The device further contains an active agent to be displaced from
s the device when the water-swellable material swells.
In another aspect, the invention is directed to an implantable device for
8 delivering an active agent to a fluid environment of use. The device
s comprises a reservoir and a back diffusion regulating outlet in a mating
relationship. The flow path of the active agent comprises a pathway formed
» between the mating surfaces of the back diffusion regulating outlet and the
reservoir.
13 In yet another aspect, the present invention is directed to a device for
storing an active agent in a fluid environment of use during a predetermined
~s administration period, the device comprising a reservoir containing an
active
1s agent. The reservoir is impermeable and formed at least in part from a
metallic material. The portion of the reservoir contacting the active agent is
~a non-reactive with the active agent, and is formed of a material selected
from
19 the group consisting of titanium and its alloys.
2o In a further aspect, the invention is an implantable fluid-imbibing active
21 agent delivery system that comprises an impermeable reservoir. The
as reservoir contains a piston that divides the reservoir into an active agent
zs containing chamber and a water-swellable agent containing chamber. The
2a active agent containing chamber is provided with a back-diffusion
regulating
as outlet. The water-swellable agent containing chamber is provided with a
as semipermeable plug. Either the plug or the outlet is releasable from the
z7 reservoir at an internal pressure that is lower than the maximum osmotic
as pressure generated by the water-swellable agent.
~ 2s The invention is further directed to a fluid-imbibing implantable active
so agent delivery system where the time to start-up of delivery is less than
10%
~ of the predetermined administration period.
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
4
- ~ In another aspect, the invention is directed to a method for preparing a
2 fluid-imbibing implantable active agent delivery system. The method
3 comprises injection molding a semipermeable plug into the end of an
4 impermeable reservoir such that the plug is protected by the reservoir.
s In still another aspect, the invention is directed to an impermeable
s active agent delivery system for delivering an active agent that is
susceptible
z to degradation. The reservoir contains a piston that divides the reservoir
into
s a water-swellable agent chamber and an active agent chamber. The open
s end of the water-swellable agent chamber contains a semipermeable
~o membrane and the open end of the active agent chamber contains a back-
diffusion regulating outlet. The system effectively seals the active agent
~2 chamber and isolates it from the environment of use.
~s In a further aspect, the invention is directed to a back-diffusion
~4 regulating outlet useful in an active agent delivery system. The outlet
defines
~s a flow path wherein the length, inferior cross-sectional shape and area
~s provide for an average linear velocity of active agent that is higher than
the
linear inward flow of fluid in the environment of use.
The invention is also directed to a semipermeable plug useful in an
active agent delivery system. The plug is water-swellable and must expand
20 linearly in the delivery system to commence pumping upon insertion of the
2~ system into the fluid environment of use.
22 The invention is further directed to impiantable delivery systems useful
23 for delivering leuprolide.
24
25 Descri,~tion of the Drawinc_as
2s
27 The figures are not drawn to scale, but are set forth to illustrate various
,
2s embodiments of the invention. Like numbers refer to like structures.
2a Figs. 1 and 2 are partial cross-sectional views of two embodiments of
so the delivery device of the invention.
CA 02244997 1998-07-31
WO 97127840 PCT/US97/00722
1 Fig. 3 is an enlarged cross-sectional view of the back-diffusion
2 regulating outlet of Fig. 1.
s Fig. 4 is a graph that shows the effect of orifice diameter and length on
4 drug diffusion.
s Figs. 5, 6, 7 and 8 are enlarged cross-sectional views of further
s embodiments of the semipermeable plug end of the reservoir according to the
7 invention.
s Figs. 9, 10 and 11 are graphs of release rates for systems with
leuprolide (Fig. 9) and with blue dye and with different membranes (Figs. 10
1o and 11).
11
1z Detailed Description of the Invention
13
1a The present invention provides a device for the delivery of an active
1s agent to a fluid environment of use in which the active agent must be
1s protected from the fluid environment until it is delivered. Prolonged and
17 controlled delivery is achieved.
18
19 Definitions
a1 The term "active agent" intends the active agents) optionally in
az combination with pharmaceutically acceptable carriers and, optionally
a3 additional ingredients such as antioxidants, stabilizing agents, permeation
2a enhancers, etc.
is By a "predetermined administration period" is intended a period of
zs greater than 7 days, often between about 30 days and 2 years, preferably
a~ greater than about 1 month and usually between about 1 month and 12
2s months.
29 By the time to "start-up" of delivery is intended the lime from insertion
so into the fluid environment of use until the active agent is actually
delivered at
31 a rate not less than approximately 70% of the intended steady-state rate.
CA 02244997 1998-07-31
WO 97!27840 PCT/US97/00722
6
- ~ The term "impermeable" intends that the material is sufficiently
z impermeable to environmental fluids as well as ingredients contained within
s the dispensing device such that the migration of such materials into or out
of
the device through the impermeable device is so low as to have substantially
s no adverse impact on the function of the device during the delivery period.
s The term "semipermeable" intends that the material is permeable to
z external fluids but substantially impermeable to other ingredients contained
s within the dispensing device and the environment of use.
s As used herein, the terms "therapeutically effective amount" or
~o "therapeutically effective rate" refer to the amount or rate of the active
agent
needed to effect the desired biologic or pharmacologic effect.
12 The active agent delivery devices of the invention find use where the
~s prolonged and controlled delivery of an active agent is desired. In many
~a cases the active agent is susceptible to degradation if exposed to the
~s environment of use prior to delivery and the delivery devices protect the
agent
from such exposure.
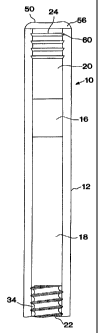
~7 Fig. 1 shows one embodiment of the device according to the invention.
In Fig. 1 a fluid-imbibing system 10 is shown that comprises an impermeable
reservoir 12. The reservoir 12 is divided into two chambers by a piston 16.
zo The first chamber 18 is adapted to contain an active agent and the second
z~ chamber 20 is adapted to contain a fluid-imbibing agent. A back-diffusion
zz regulating outlet 22 is inserted into the open end of the first compartment
18
z3 and a wafer-swellable semipermeable plug 24 is inserted into the open end
of
z4 the second chamber 20. In Fig. 1, the back-diffusion regulating outlet 22
is
zs shown as a male threaded member in a mating relationship with the smooth
zs interior surface of the reservoir 12 thereby forming therebetween helical
flow
z7 path 34. The pitch (x), the amplitude (y), and the cross-sectional area and
,
zs shape of the helical path 34 formed between the mating surfaces of the back-
zs diffusion regulating outlet 22 and the reservoir 12 as shown in Fig. 3 are
so factors that affect both the efficiency of path 34 preventing back-
diffusion of
s~ external fluid into the formulation in chamber 18 and the back pressure in
the
CA 02244997 1998-07-31
WO 97/27840 PCT/US97100722
7
~ device. The geometry of outlet 22 prevents water diffusion into the
reservoir.
z In general, it is desired that these characteristics be selected so that the
s length of the helical flow path 34 and the velocity of flow of active agent
a therethrough is sufficient to prevent back-diffusion of external fluid
through the
s flow path 34 without significantly increasing the back pressure, so that,
s following start-up, the release rate of the active agent is governed by the
osmotic pumping rate.
s Fig. 2 is a second embodiment of the device of the invention with a
s reservoir 12, piston 16 and plug 26. In this embodiment, the flow path 36 is
~o formed between a threaded back-diffusion regulating outlet 40 and threads
38
formed on the interior surface of the reservoir 12. The amplitudes of the
threaded portions of the back-diffusion regulating outlet 40 and reservoir 12
~s are different so that a flow path 36 is formed between the reservoir 12 and
the
14 back-diffusion regulating outlet 40.
Ts The water-swellable semipermeable plugs 24 and 26 shown in Figs. 1
~s and 2 respectively are inserted into the reservoir such that the reservoir
wall
concentrically surrounds and protects the plug. In Fig. 1, the top portion 50
of
~s the plug 24 is exposed to the environment of use and may form a flanged end
cap portion 56 overlaying the end of reservoir 12. The semipermeable plug
ao 24 is resiliently engaged with the interior surface of the reservoir 12 and
in
21 Fig. 1 is shown to have ridges 60 that serve to frictionally engage the
semipermeable plug 24 with the interior of reservoir 12. In addition, the
23 ridges 60 serve to produce redundant circumferential seals that function
24 before the semipermeable plug 24 expands due to hydration. The clearance
is between ridges 60 and the interior surface of the reservoir 12 prevents
is hydration swelling from exerting stresses on the reservoir 12 that can
result in
2~ tensile failure of the reservoir 12 or compression or shear failure of the
plug
za 24. Fig. 2 shows a second embodiment of the semipermeable plug 26 where
. zs the plug is injection molded into the top portion of the reservoir and
where the
so top of the semipermeable plug 26 is flush with the top 62 of the reservoir
12.
31 !n this embodiment, the diameter of the plug is substantially less than the
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
diameter of the reservoir 12. In both embodiments the plugs 24 and 26 will
2 swell upon exposure to the fluid in body cavity forming an even tighter seal
s with the reservoir 12.
a The novel confrgurations of the components of the above-described
s embodiments provide for implantable devices that are uniquely suited for
s implantation info humans and can provide delivery devices which are capable
z of storing unstable formulations at body temperatures for extended periods
of
s time, which devices have start-up times of less than 10% of the
administration
s period and can be designed to be highly reliable and with predictable fail
safe
~o modes.
11 Reservoir 12 must be sufficiently strong to ensure that it will not leak,
crack, break or distort so as to expel its active agent contents under
stresses
is it would be subjected to during use while being impermeable. In particular,
it
~a should be designed to withstand the maximum osmotic pressure that could be
generated by the water-swellable material in chamber 20. Reservoir 12 must
~s also be chemically inert and biocompatible, that is, it must be non-
reactive
with the active agent formulation as well as the body. Suitable materials
~a generally comprise a non-reactive polymer or a biocompatible metal or
alloy.
1s The polymers include acrylonitrile polymers such as acryionitrile-butadiene-
ao styrene terpolymer, and the like; halogenated polymers such as
2~ polytetrafluoroethylene, polychlorotrifluoroethylene, copolymer
as tetrafluoroethylene and hexafiuoropropylene; polyimide; polysulfone;
Zs polycarbonate; polyethylene; polypropylene; polyvinylchloride-acrylic
~4 copolymer; polycarbonate-acryionitriie-butadiene-styrene; polystyrene; and
as the like. The water vapor transmission rate through compositions useful for
2s forming the reservoir are reported in J. Pharm. Sci., Vol. 29, pp. 1634-37
27 (1970), Ind. Eng. Chem., Vol. 45, pp. 2296-2306 (1953); Materials ,
is Engineering, Vol. 5, pp. 38-45 (1972); Ann. Book ofASTM Stds., Voi. 8.02,
2s pp. 208-211 and pp. 584-587 (1984); and lnd. and Eng. Chem., Vol. 49, pp.
ao 1933-1936 (1957). The polymers are known in the Handbook of Common
3~ Polymers by Scott and Roff, CRC Press, Cleveland Rubber Co., Cleveland,
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
9
- ~ OH. Metallic materials useful in the invention include stainless steel,
titanium,
z platinum, tantalum, gold and their alloys as welt as gold-plated ferrous
alloys,
s platinum-plated ferrous alloys, cobalt-chromium alloys and titanium nitride
coated stainless steel. A reservoir made from titanium or a titanium alloy
s having greater than 60%, often greater than 85% titanium is particularly
s preferred for the most size-critical applications, for high payload
capability and
for long duration applications and for those applications where the
formulation
s is sensitive to body chemistry at the implantation site or where the body is
a sensitive to the formulation. Preferred systems maintain at least 70% active
~o agent after 14 months at 37°C and have a shelf stability of at least
about 9
months, or more preferably at least about two years, at 2-8°C. Most
~z preferably, systems may be stored at room temperature. In certain
~s embodiments, and for applications other than the fluid-imbibing devices
14 specifically described, where unstable formulations are in chamber 18,
particularly protein andlor peptide formulations, the metallic components to
which the formulation is exposed must be formed of titanium or ifs alloys as
17 described above.
~s The devices of this invention provide a sealed chamber 18 which
effectively isolates the formulation from the fluid environment. The reservoir
ao 12 is made of a rigid, impermeable and strong material. The wafer-swellable
z~ semipermeable plug 24 is of a lower durometer material and will conform to
zz the shape of the reservoir to produce a liquid-tight sea( with the inferior
of
zs reservoir 12 upon wetting. The flow path 34 isolates chamber 18 from back-
za diffusion of environmental fluid. Piston 16 isolates chamber 18 from the
zs environmental fluids that are permitted to enter chamber 20 through
zs semipermeable plugs 24 and 26 such that, in use at steady-state flow,
active
z7 agent is expelled through outlet 22 at a rate corresponding to the rate at
a
za which water from the environment flows into the water-swellable material in
za chamber 20 through semipermeable plugs 24 and 26. As a result, the plug
so and the active agent formulation will be protected from damage and their
31 functionality will not be compromised even if the reservoir is deformed. In
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
addition, the use of sealants and adhesives will be avoided and the attendant
z issues of biocompatibility and ease of manufacture resolved.
s Materials from which the semipermeable plug are made are those that
a are semipermeable and that can conform to the shape of the reservoir upon
s wetting and adhere to the rigid surface of the reservoir. The semipermeable
s plug expands as it hydrates when placed in a fluid environment so that a
seal
is generated between the mating surfaces of the plug and the reservoir. The
s strength of the seals between the reservoir 12 and the outlet 22 and the
a reservoir 12 and the plugs 24 and 26 can be designed to withstand the
maximum osmotic pressure generated by the device. In a preferred
alternative, the plugs 24 and 26 may be designed to withstand at least 10X
~z the osmotic agent compartment 20 operating pressure. fn a further
~s alternative the plugs 24 and 26 may be releasable from the reservoir at an
~a internal pressure that is lower than the pressure needed to release the
back
~s diffusion regulating outlet. In this fail safe embodiment, the water-
swellable
~s agent chamber will be opened and depressurized, thus avoiding dispelling
the diffusion regulating outlet and attendant release of a large quantity of
the
~s active agent. In other cases, where a fail-safe system requires the release
of
the active agent formulation rather than the water-swellable agent
zo formulation, the semipermeable plug must be releasable at a pressure that
is
z, higher than the outlet.
zz In either case, the semipermeable plug must be tong enough to
zs sealably engage the reservoir wall under the operating conditions, that is,
it
za should have an aspect ratio of between 1:10 and 10:1 length to diameter,
zs preferably at least about 1:2 length to diameter, and often between 7:10
and
zs 2:1. The plug must be able to imbibe between about 0.1 % and 200% by
z7 weight of water. The diameter of the plug is such that it will sealingly
fit inside
zs the reservoir prior to hydration as a result of seating contact at one or
more
zs circumferential zones and will expand in place upon wetting to form an even
so tighter seal with the reservoir. The polymeric materials from which the
semipermeabfe plug may be made vary based on the pumping rates and
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
11
- ~ device configuration requirements and include but are not limited to
z plasticized cellulosic materials, enhanced polymethyimethacrylate such as
s hydroxyethylmethacrylate (HEMA) and elastomeric materials such as
a polyurethanes and pofyamides, polyether-poiyamide copolymers,
s thermoplastic copolyesters and the like.
s The piston 16 isolates the water-swetlable agent in chamber 20 from
the active agent in chamber 18 and must be capable of sealably moving
s under pressure within reservoir 12. The piston 16 is preferably made of a
a material that is of lower durometer than the reservoir 12 and that will
deform
~o to fit the lumen of the reservoir to provide a fluid-tight compression seal
with
11 the reservoir 12. The materials from which the piston are made are
12 preferably elastomeric materials that are impermeable and include but are
not
~a limited to polypropylene, rubbers such as EPDM, silicone rubber, butyl
~4 rubber, and the like, and thermoplastic elastomers such as plasticized
15 potyvinytchloride, polyurethanes, Santoprene~, C-Flexes TPE (Consolidated
~s Polymer Technologies Inc.), and the like. The piston may be of a self
loading
~7 or compression-loaded design.
is The back-diffusion regulating outlet 22 forms the delivery pathway
~s through which the active agent flows from the chamber 18 to the
implantation
2o site where absorption of the active agent takes place. The seal between the
z~ outlet 22 and the reservoir 12 can be designed to withstand the maximum
~2 osmotic pressure generated within the device or to fail-safe in the modes
is described above. In a preferred embodiment, the pressure required to
2a release back-diffusion regulating outlet 22 is at least 1 OX the pressure
is required to move piston 16 and/or at least 10X the pressure in chamber 18.
Zs The exit flow path of the active agent is the pathway 34 formed
z~ between the mating surtaces of the back-diffusion regulating outlet 22 and
the
zs reservoir 12. The pathway length, interior cross-sectional shape and area
of
. is the outlet path 34 or 36 are chosen such that the average linear velocity
of
so the exiting active agent is higher than that of the linear inward flux of
materials
s~ in the environment of use due to diffusion or osmosis, thereby attenuating
or
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
12
moderating back-diffusion and its deleterious effects of contaminating the
z interior of the pump, destabilizing, diluting, or otherwise altering the
s formulation. The release rate of active agent can be modified by modifying
4 the outlet pathway geometry, which relationship is shown below.
s The connective flow of active agent out of outlet 22 is set by the ,
s pumping rate of the system and the concentration of active agent in chamber
20 and can be represented as follows:
s
Qca = (Q) (Ca) (1 )
~o where
Qua is the connective transport of agent A in mg/day
~z Q is the overall connective transport of the agent and its
is diluents in cm3/day
a Ca is the concentration of agent A in the formulation within
T5 chamber 20 in mg/cm3
~s
m The diffusive flow of agent A through the material in the outlet 22 is a
~a function of agent concentration, cross-sectional configuration of flow path
34
~s or 36, agent diffusivity and length of flow path 34 or 36, and can be
zo represented as follows:
zt
az Qaa = D ~ r2 0 Ca/L (2)
zs where
a4 Qua is the diffusive transport of agent A in mg/day
as D is the diffusivity through the material in path 34 or 36 in
zs cmz/day
z7 r is the effective inner radius of the flow path in cm ,
zs OCa is the difference between the concentration of agent A in
zs the reservoir and in the body outside of the outlet 22 in
so mg/cm3
s~ L is the length of the flow path in cm
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
13
1
2 In general, the concentration of agent in the reservoir
is much greater
s than the concentration of agent in the body outside of the
orifice such that the
difference, ~Ca can be approximated by the concentration
of agent within the
s reservoir, Ca.
s
Qaa = D ~t r2 Ca/L (3)
s
a It is generally desirable to keep the diffusive flux of
agent at less than
10% of the connective flow. This is represented as follows:
11
Qda/Qca = D ~ ~ Ca/QCaL = D~ r2/QL 5 0.1 (4)
13
14 Equation 4 indicates that the relative diffusive flux decreases
with
1s increasing volumetric flow rate and path length and increases
with increasing
16 diffusivity and channel radius and is independent of drug
concentration.
1~ Equation 4 is plotted in Figure 4 as a function of length
(L) and diameter (d)
1s for D = 2 x 10-s cm2/sec and Q = 0.36 .!/day.
1s The diffusive flux of water where the orifice opens into
chamber 18
Zo can be approximated as:
z1
22 Qwa (res) = CQe ~-owW,a~ (5)
i3 where
24 C is the concentration profile of water in mg/cm3
zs Q is the mass flow rate in mg/day
2s L is the length of the flow path in cm
a~ DW is the diffusivity of water through the material in the
flow path in
2s cm2/day
as A is the cross-sectional area of the flow path in cm2
CA 02244997 1998-07-31
WO 97/27840 PCT1US97/00722
14
1 The hydrodynamic pressure drop across the orifice can be calculated
z as follows:
3
4 oP = (6)
~r4
s
7 Simultaneously solving equations (4), (5) and (6) gives the values
s shown in Table 1 where:
s
1o Q = 0.38 wl/day
11 Ca = 0.4 mg/Etl
12 L =5cm
13 Da = 2.00 E-06 cm2/sec
14 ~, =5.00E+02cp
CwD = 0 mg/~i,l
1s Dw = 6.00 E + 06 cm2/sec
17
18
1a Table 1
21
Dru Diffusionmping Water ntrusionPressure
& Pu i Drap
Effective Pump DiffusionDiffJConv
rate
OrificeCross QCs QDe QDe/QC, QD Qdw delta
dia Sec P
(mil) area m /day mg/day m !day m J si
(mm2) ear
l 0.000510.152 0.0001 0.0005 0 0 1.55800
2 0.002030.152 0.0003 0.0018 1.14E-794.16E-770.09738
3 0.004560.152 0.0006 0.0041 4.79E-381.75E-330.01923
4 0.008110.152 0.0011 0.0074 8.89E-213.25E-180.00609
5 0.012670.152 0.0018 0.0115 1.04E-1133.79E-110.00249
6 0.018240.152 0.0025 0.0166 7.16E-102.61 0.00120
E-07
7 0.024830.952 0.0034 0.0226 1.48E-075.4E-050.00065
8 0.032430.152 0.0045 0.0295 4.7E-06 0.0017150.00038
9 0.041050.152 0.0057 0.0373 5.04E-050.0183810.00024
10 0.050680.152 0.0070 0.0461 0.0002750.1002630.00016
11 0.061320.152 0.0085 0.0558 0.0009640.3517710.00011
12 0.072980.152 0.0101 0.0654 0.0025040.9138390.00008
-
13 0.085640.152 0.0118 0.0779 0.0052631.9210270.00005
14 0.099330.152 0.0137 0.0903 0.00949 3.4638360.00004
15 0.114020.152 0.0158 0.1037 0.0152695.5731950.00003
16 0.129730.152 0.0179 0.1180 0.0225358.2252240.00002
17 0.146460.152 0.0202 0.1332 .03 11.356560.00002
0
1114
18 0.164190.152 0.0227 0.1493 _ 14.881660.00001
_
0.040772
19 0.182950.152 0.0253 0.1664 0.05125318.707280.00001 '
20 0.202710.152 0.0280 0.1844 0.06230922.74270.00001
22
CA 02244997 2005-02-09
67696-260
The calculations indicate that an orifice diameter of between about 3
2 and 10 mil and a length of 2 to 7 cm is optimal for a device with the
operating
3 conditions described. In a preferred embodiment, the pressure drop across
4 the orifice is less than 10% of the pressure required to release the back-
s diffusion regulating outlet 22.
s The back-diffusion regulating outlet 22 preferably forms a llelica!
~ pathway 34 or 36 incorporating a long flow path with a means of mechanically
. a attaching the outlet into the reservoir without using adhesives or other
s sealants. The back-diffusion regulating outlet is made of an inert and
~o biocompatible material selected from but not limited to metals induding but
not limited to titanium, stainless steel, platinum and their alloys and coba8
,2 chromium alloys and the like, and polymers induding but not limited to
polyethylene, polypropylene, polycarbonate and polymethylmethacrylate and
the like. The flow path is usually between about 0.5 and 20 cm long,
.~s preferably between about 1 and 10 cm long and between about 0.001 and
~s 0.020 inches in diameter, preferably between about 0.003 and 0.015 inches
to allow for a flow of between about 0.02 and 50 ~,I/day, usually 0.2 to 10
~e ~Uday and often 0.2 to 2.0 pUday. Addiflonally, a catheter or other system
~s may be attached to the end of the back-diffusion regulating outlet to
provide.
zo for delivery of the active agent formulation at a site removed from the
implant.
z~ Such systems are known in the art and are described, for example, in U.S.
r~ Patent Nos. 3,732,865 and X4,340,054.
a~ Further, the flow path design may be useful in systems other than
z4 the fluid-imbibing devices specifically described herein.
The inventive device configurations described above also allow for a
~s ~ minimal period of delay from start-up to steady-state flow rate. This is
r accomplished in part as a result of the conflgurat<on of the semipermeabie
isplug 24 or 26. As water is imbibed ~by the semipermeable plug, it swells. .
is Radial expansion is limited by the rigid reservoir 12, thus the expansion
must
~o occur linearly, thereby pushing against the water-swellable agent in
chamber
s, 18, whicfi in tum pushes against the piston 16. This allows pumping to
CA 02244997 2005-02-09
(7696-260
16
commence prior to the time that water reaches the water-swellable agent
z which otherwise would be required before pumping could commence. ~ To
3 facilitate reliable start-up, the flow path 34 can be precharged with the
active
4 agent in chamber 18. Further, the geometry of the outlet 22 allows for
initial
s delivery that is influenced by the concentration gradient of dnrg along the
s length of the outlet. The start-up period is less than about 25% of the
~ predetermined delivery period and is often less than about 10% and usually
a less than about 5% of the predetermined delivery period. In a prefen~ed
s embodiment for a one year system, at least 70% of the steady-state flow rate
~o is achieved by day 14.
. The water-swellabie agent formulation in chamber 20 is preferably a
~2 tissue tolerable formulation whose high osmotic pressure and high
solubility
~3 propels the active agent over a long period of time while remaining in v
saturated solution in the water admitted by the semipermeable membrane.
~s The water-swellable agent is preferably selected for tolerability by
,s subcutaneous tissue, at least at pumping rates and hypothetically resulting
~ concentrations to allow inadvertent dispensing from implanted devices left
in
,e the patient for a tonger than labeled period. In preferred embodiments, the
s water swellable agent should not diffuse or permeate through the
zo semipermeable plug 24 or 28 to any appreciable amount (e.g., less than 8%)
z~ under normal operating conditions.. Osmotic agents, such as NaCI with
appropriate tabletflng agents (lubricants and binders) and viscosity modifying
z3 agents, such as sodium carboxymethylCeliulose or sodium potyacrylate are
z4 preferred water-swellable agents. Other osmotic agents useful as the water
zs. swellabie agent include osmopolymers and osmagents and are described, for
zs example, in U.S. Patent No. 5,413,572.
z~ The water-swellable agent formulation can be a slurry, a tablet, a
is molded or extruded material or other form known in the art. A liquid or gel
is additive or filler may be added to chamber 20 to exclude air from spaces
3o around the osmotic engine. Exclusion of air from the devices should mean
CA 02244997 1998-07-31
WO 97!27840 PCT/US97/00722
17
- ~ that delivery rates will be less affected by nominal external pressure
changes
z (e.g., t7 p.s.i._(t5 a.t.m.}).
s The devices of the invention are useful to deliver a wide variety of
a active agents. These agents include but are not limited to pharmacologically
s active peptides and proteins, genes and gene products, other gene therapy
s agents, and other small molecules. The polypeptides may include but are not
limited to growth hormone, somatotropin analogues, somatomedin-C,
a Gonadotropic releasing hormone, follicle stimulating hormone, luteinizing
9 hormone, LHRH, LHRH analogues such as leuprolide, nafarelin and
~o goserelin, LHRH agonists and antagonists, growth hormone releasing factor,
calcitonin, colchicine, gonadotropins such as chorionic gonadotropin,
~z oxytocin, octreotide, somatotropin plus an amino acid, vasopressin,
~s adrenocorticotrophic hormone, epidermal growth factor, prolactin,
~a somatostatin, somatotropin plus a protein, cosyntropin, lypressin,
15 polypeptides such as thyrotropin releasing hormone, thyroid stimulation
~s hormone, secretin, pancreozymin, enkephalin, glucagon, endocrine agents
secreted internally and distributed by way of the bloodstream, and the like.
~a Further agents that may be delivered include a~antitrypsin, factor Vlll,
factor
19 IX and other coagulation factors, insulin and other peptide hormones,
adrenal
ao cortical stimulating hormone, thyroid stimulating hormone and other
pituitary
z~ hormones, interferon a, (3, and 8, erythropoietin, growth factors such as
Zz GCSF, GMCSF, insulin-like growth factor 1, tissue plasminogen activator,
23 CD4, dDAVP, interleukin-1 receptor antagonist, tumor necrosis factor,
24 pancreatic enzymes, lactase, cytokines, interleukin-1 receptor antagonist,
zs interleukin-2, tumor necrosis factor receptor, tumor suppresser proteins,
2s cytotoxic proteins, and recombinant antibodies and antibody fragments, and
2~ the like.
as The above agents are useful for the treatment of a variety of conditions
Zs including but not limited to hemophilia and other blood disorders, growth
so disorders, diabetes, leukemia, hepatitis, renal failure, HIV infection,
hereditary
31 diseases such as cerbrosidase deficiency and adenosine deaminase
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
18
deficiency, hypertension, septic shock, autoimmune diseases such as
2 multiple sclerosis, Graves disease, systemic lupus erythematosus and
s rheumatoid arthritis, shock and wasting disorders, cystic fibrosis, lactose
a intolerance, Crohn's diseases, inflammatory bowel disease, gastrointestinal
s and other cancers.
s The active agents may be anhydrous or aqueous solutions,
suspensions or complexes with pharmaceutically acceptable vehicles or
s carriers such that a flowable formulation is produced that may be stored for
s long periods on the shelf or under refrigeration, as well as stored in an
~o implanted delivery system. The formulations may include pharmaceutically
acceptable carriers and additional inert ingredients. The active agents may
12 be in various forms, such as uncharged molecules, components of molecular
~3 complexes or pharmacologically acceptable salts. Also, simple derivatives
of
~a the agents (such as prodrugs, ethers, esters, amides, etc.) which are
easily
~s hydrolyzed by body pH, enzymes, etc., can be employed.
It is to be understood that more than one active agent may be
~~ incorporated info the active agent formulation in a device of this
invention and
~s that the use of the term "agent" in no way excludes the use of two or more
~s such agents. The dispensing devices of the invention find use, for example,
ao in humans or other animals. The environment of use is a fluid environment
2~ and can comprise any subcutaneous position or body cavity, such as the
22 peritoneum or uterus, and may or may not be equivalent to the point of
as ultimate delivery of the active agent formulation. A single dispensing
device
2a or several dispensing devices can be administered to a subject during a
as therapeutic program. The devices are designed to remain implanted during a
as predetermined administration period. If the devices are not removed
following
2~ the administration, they may be designed to withstand the maximum osmotic
as pressure of the water-swellable agent or they may be designed with a bypass
2s to release the pressure generated within the device.
so The devices of the present invention are preferably rendered sterile
31 prior to use, especially when such use is implantation. This may be
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
19
~ accomplished by separately sterilizing each component, e.g., by gamma
radiation, steam sterilization or sterile filtration, then aseptically
assembling
s the final system. Alternatively, the devices may be assembled, then
4 terminally sterilized using any appropriate method.
s Preparation of the Devices of the Invention
s Reservoir 12 is prepared preferably by machining a metal rod or by
s extrusion or injection molding a polymer. The top portion of the reservoir
may
~o be open as shown in Fig. 1 or may contain a cavity as shown in Fig. 2.
11 Where the reservoir 12 is open as shown in Fig. 1, a water-swellable
semipermeable plug 24 is inserted mechanically from the outside of the
~s reservoir without using an adhesive before or after insertion of the piston
and
~a water-swellable agent formulation. Reservoir 12 may be provided with
grooves or threads which engage ribs or threads on plug 24.
~s Where the reservoir 12 contains a cavity as shown in Fig. 2, the cavity
may be cylindrical in shape, as shown in Fig. 5, it may be stepped, as shown
~s in Fig. 6, it may be helical, as shown in Fig. 7 or it may be in a spaced
19 configuration, as shown in Fig. 8. The semipermeable plug 26 is then
Zo injected, inserted, or otherwise assembled into the cavity so that it forms
a
2~ seal with the reservoir wall.
z2 Following insertion of the plug 26 either mechanically, by welding or by
as injection, the water-swellable agent is assembled into the reservoir
followed
z4 by insertion of the piston, with appropriate steps taken to vent entrapped
air.
z5 The active agent is flied into the device using a syringe or a precision
is dispensing pump. The diffusion moderator is inserted into the device,
usually
z7 by a rotating or helical action, or by axial pressing.
zs The following examples are illustrative of the present invention. They
- zs are not to be construed as limiting the scope of the invention.
Variations and
so equivalents of these examples will be apparent to those of skill in the art
in
31 light of the present disclosure, the drawings and claims herein.
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
_ 1
a Examples
3
a Example 1 - Preparation of a Device with an HDPE Reservoir
s
s A system containing leuprolide acetate for the treatment of prostate
cancer was assembled from the following components:
s Reservoir (HDPE) (5 mm outside diameter, 3 mm inside diameter)
a Piston (Santoprene~)
1o Lubricant (silicone medical fluid)
11 Compressed osmotic engine (60% NaCI, 40% sodium carboxymethyl
1z cellulose)
1s Membrane plug (Hytrel polyether-ester block copolymer, injection
1a molded to desired shape)
1s Back diffusion Regulating Outlet {pofycarbonate)
1s Active agent (0.78g of 60% propylene glycol and 40% leuprolide
17 acetate)
18 Assembfv
1a The piston and inner diameter of the reservoir were Lightly lubricated
zo with silicon medical fluid. The piston 16 was inserted into the open end of
a1 chamber 20. Two osmotic engine tablets (40 mg each) were then inserted on
zz top of piston 16. After insertion, the osmotic engine was flush with the
end of
as the reservoir. The membrane plug 24 was inserted by lining up the plug with
a4 the reservoir and pushing gently until the plug was fully engaged in the
zs reservoir. Active agent was loaded into a syringe which was then used to
fill
as chamber 18 from its open end by injecting the material into the open tube
until
z~ the formulation was ~3 mm from the end. The filled reservoir was
centrifuged
zs (outlet end "up") to remove any air bubbles that have been trapped in the
zs formulation during filling. The outlet 22 was screwed info the open end of
the
so reservoir until completely engaged. As the outlet was screwed in, excess
s1 formulation exited out of the orifice ensuring a uniform fill.
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
21
1
z .Example 2 - Insertion of the Device of Example 1
3
Insertion of the device of Example 1 is done under aseptic conditions
s using a trocar similar to that used in the implantation of Norp(ant~
s contraceptive implants to position the device under the skin. The insertion
area is typically in the inside of the upper arm, 8 to 10 cm above the elbow.
s The area is anesthetized and an incision is made through the skin.
s The incision is approximately 4 mm long. The trocar is inserted into the
1o incision until the tip of the trocar is at a distance of 4 to 6 cm from the
incision.
11 The obturator is then removed from the trocar and the device of Example 1
1z inserted into the trocar. The device is then advanced to the open end of
the
13 trocar using the obturator. The obturator is then held in position, thus
14 immobilizing the device of Example 1 while the trocar is withdrawn over
both
1s the device and the obturator. The obturator is then removed, leaving the
1s implant behind in a well-controlled position. The edges of the incision are
17 then secured with a skin closure. The area is covered and kept dry for 2 to
3
1s days.
19
2o Example 3 - Removal of the Device of Example 1
21
z2 The device of Example 1 is removed as follows: The device is located
23 by fingertip palpation of the upper arm area. The area at one end of the
24 implant is then anesthetized and an approximately 4 mm, perpendicular
zs incision is made through the skin and any fibrous capsule tissue
surrounding
is the implant area. The end of the device opposite the incision is pushed so
- z7 that the device end proximal to the incision is urged out of the
incision. Any
2s further fibrotic tissue is cut with a scalpel. Following removal, the
procedure
- zs of Example 2 can be followed to insert a new device.
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
22
1 Example 4 - Delivery Rate of the Device of Example 1
2
s Glass test tubes were filled with 35 ml distilled water and then placed
a in a 37°C water bath. A single device as described in Example 1 was
placed
s in each test tube and the test tubes were changed periodically. The delivery
s rate profile from the system is shown in Fig. 9. The system does not have
any start-up time because the system exhibits a period of initial high release
s followed by a lower steady state release for a period of 200 days.
s
1o Example 5 - Delivery Rate Profiles
11
12 Glass test tubes were filled with 35 ml distilled wafer which were then
1a placed in a 37°C water bath. After the test tubes had come up to
14 temperature, a single device as described in Example 1, but with membrane
1s materials described below and containing 1 % FD&C blue dye in wafer as the
1s drug formulation, was placed in each tube. Water from the test tube
17 permeated through the membrane causing the system to pump formulation
1s (blue dye) into the surrounding water in the test tube. At regular
intervals,
1s systems were switched to fresh test tubes. The amount of dye released was
zo determined by measuring the concentration of blue dye in each test tube
z1 using a spectrophotometer. The pumping rate was calculated from the total
dye released, the volume of water in the tube, the initial concentration of
dye
2s and the interval over which the system was in the test tube. Results for
two
24 different tests are shown in Figures 10 and 11. Figure 10 shows 3 different
as systems with different plug materials (Hytrel~ 2, 3 and 12 month systems)
and
is Figure 11 shows 4 systems with different plug materials. These materials
are:
a7 Membrane Material
as 1 month Pebax 25 (Polyamide)
29 2 month Pebax 22 (Polyamide)
so 3 month Polyurethane (HP60D)
31 12 month Pebax 24 (Polyamide)
CA 02244997 1998-07-31
WO 97/27840 PCT/LTS97/00722
23
The systems were capable of delivering for a period of from 2 to 12
z months, depending on the membrane used.
3
a Example 6 - Preparation of a Delivery Device with a Titanium Reservoir
s
s A system containing leuprolide acetate for the treatment of prostate
cancer was assembled from the following components:
s Reservoir (Titanium, Ti6A14V alloy ) (4 mm outside diameter, 3 mm
s inside diameter)
~o Piston (C-Flex~)
Lubricant (silicone medical fluid) .
12 Compressed osmotic engine (76.4% NaCI, 15.5% sodium
carboxymethyl cellulose, 6% povidone, 0.5% Mg Stearate, 1.6%
water)
15 PEG 400 (8 mg added to osmotic engine to fill air spaces)
Membrane plug (polyurethane polymer, injection molded to desired
shape)
Back diffusion Regulating Outlet (polyethylene)
19 Drug formulation (0.150g of 60% water and 40% leuprolide acetate)
Zo g~semblv
ii The piston and inner diameter of the reservoir were lightly lubricated.
2a The piston was inserted ~0.5 cm into the reservoir at the membrane end.
2s PEG 400 was added into the reservoir. Two osmotic engine tablets (40 mg
24 each) were then inserted into the reservoir from the membrane end. After
Zs insertion, the osmotic engine was flush with the end of the reservoir. The
2s membrane plug was inserted by lining up the plug with the reservoir and
z~ pushing gently until the retaining features of the plug were fully engaged
in
2s the reservoir. Formulation was loaded into a syringe which was then used to
29 fill the reservoir from the outlet end by injecting formulation into the
open tube
so until the formulation was ~3 mm from the end. The filed reservoir was
~ centrifuged (outlet end "up") to remove any air bubbles that have been
CA 02244997 1998-07-31
WO 97/27840 PCT/IJS97/00722
24
- ~ trapped in the formulation during filling. The outlet was screwed into the
open
2 end of the reservoir until completely engaged. As the outlet was screwed in,
a excess formulation exited out of the orifice ensuring a uniform fill.
4
Example 7 - Preparation of a Leuprolide Acetate Delivery Device with a
s Titanium Reservoir
a A system containing leuprolide acetate for the treatment of prostate
s cancer was assembled from the following components:
Reservoir (Titanium Ti6A14V alloy) {4 mm outside diameter, 3 mm
inside diameter, 4.5 cm length)
~2 Piston (C-Flexes TPE efastomer, available from Consolidated Polymer
~s Technologies, Inc.)
~a Lubricant (silicone medical fluid 360)
Compressed osmotic engine tablet (76.4% NaCI, 15.5% sodium
~s carboxymethyl cellulose, 6% povidone, 0.5% Mg Stearate, 1.5%
i7 water, 50 mg total)
is PEG 400 (8 mg added to osmotic engine to fill air spaces)
~s Membrane plug (polyurethane polymer 20% water uptake, injection
2o molded to desired shape 3 mm diameter X 4 mm length)
2~ Back-diffusion Regulating Outlet (polyethylene, with 6 mil X 5 cm
22 channel)
23 Drug formulation (leuprolide acetate dissolved in DMSO to a measured
2a content of 65 mg leuprolide)
Ass~mblv
2s Systems were assembled as in Example 6, using aseptic procedures
27 to assemble y-irradiated subassemblies and filled aseptically with sterile
2s altered leuproiide DMSO formulation.
29 Release Rate
so These systems delivered about 0.35 p.L/day leuprolide formulation
~ containing on average 150 ~cg leuprolide in the amount delivered per day
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
- ~ They provide delivery ofi leuprolide at this rate for at least one year.
The
2 systems achieved approximately 70% steady-state delivery by day 14.
s ~mt~lantation and Removal
a Systems will be implanted under local anesthetic and by means of an
s incision and trocar as in Example 2 to patient suffering from advanced
s prostatic cancer.
7 After one year, systems will be removed under local anesthetic as
$ described in Example 3. New systems may be inserted at that time.
s
1o Example 8 - Treatment of Prostatic Cancer
11
12 Leuprolide acetate, an LHRH agonist, acts as a potent inhibitor of
1s gonadotropin secretion when given continuously and in therapeutic doses.
14 Animat and human studies indicate that following an initial stimulation,
chronic
1s administration of leuprolide acetate results in suppression of testicular
1s steroidogenesis. This effect is reversible upon discontinuation of drug
17 therapy. Administration of leuprolide acetate has resulted in inhibition of
the
1a growth of certain hormone-dependent tumors (prostatic tumors in Noble and
19 Dunning male rats and DMBA-induced mammary tumors in female rats) as
ao well as atrophy ofi the reproductive organs. In humans, administration of
z1 leuprolide acetate results in an initial increase in circulating levels of
z2 futeinizing hormone (LH) and follicle stimulating hormone (FSH), leading to
a
is transient increase in levels of the gonadal steroids (testosterone and
z4 dihydrotestosterone in males). However, continuous administration of
zs leuproiide acetate results in decreased level of LH and FSH. in males,
zs testosterone is reduced to castrate levels. These decreases occur within
two
z7 to six weefcs after initiation of treatment, and castrate levels of
testosterone in
Za prostatic cancer patients have been demonstrated for multiyear periods.
is Leuprolide acetate is not active when given orally.
CA 02244997 1998-07-31
WO 97/27840 PCT/US97/00722
26
- ~ Systems will be prepared as in Example 7, then inserted as described.
z The continuous administration of leuprolide for one year using these systems
will reduce testosterone to castrate levels.
.a The above description has been given for ease of understanding only.
s No unnecessary (imitations should be understood therefrom, as modifications
s will be obvious to those skilled in the art.