Note: Descriptions are shown in the official language in which they were submitted.
CA 02245036 1998-08-13
1
HYDROMETALLURGICAL PROCESS FOR RECOVERY OF ZINC
The present invention relates to a
hydrometallurgical process for the recovery of zinc, and
especially to the recovery of zinc from an ore or
concentrate in which the zinc values are in the form that
may be leached in dilute sulphuric acid. In particular,
the zinc values are in the form of zinc oxide, and the
ore or concentrate is substantially free of zinc ferrite.
In embodiments, the invention relates to the process for
the recovery of zinc from the roasting of sulphide ores
or concentrates containing zinc, especially iron-bearing
zinc sulphide ores or concentrates, the zinc sulphide ore
or concentrate having been roasted so as to convert zinc
values to zinc oxide, in the absence of residual zinc
sulphide and in which the amount of zinc ferrite that is
typically formed in roasted iron-bearing zinc sulphide
concentrate is reduced or eliminated. In particular, the
present invention relates to a process for recovery of
zinc in a hydrometallurgical process in which fewer steps
and/or more economical steps may be used in the recovery
of zinc values, and especially in which a single stage
leaching step may be used to separate zinc values from
roasted ore or concentrate (calcine). The calcine may be
prepared for use in the process of the present invention
by roasting at high temperature, especially above 1050°C.
In embodiments, the calcine is treated to form magnetite,
where the ore is an iron-bearing ore, which may be
removed.
Processes for the recovery of zinc from zinc
sulphide concentrates are well known. Such processes
typically involve a fluid bed roasting. The fluid bed
roasting results in the production of gaseous sulphur
dioxide, and other volatile impurities, and a roasted
zinc calcine that contains zinc oxide, zinc ferrite, and
typically oxides of lead, cadmium, cobalt, indium and
CA 02245036 1998-08-13
2
other metals, depending on the particular composition of
the zinc concentrate subjected to the roast and the
roasting conditions that are used. The roasting is
typically carried out at controlled temperatures, to
convert zinc sulphide to zinc oxide while attempting to
limit the formation of zinc ferrite. Temperatures of
greater than 850°C are used to obtain conversion of zinc
sulphate formed in the roasting process to zinc oxide but
temperatures are maintained below 950°C as zinc ferrite
tends to be formed from the reaction of the zinc oxide
with iron oxide in the ore as the temperature increases.
As noted below, other techniques are known for reduction
of the amount of zinc ferrite that is formed.
The roasted calcine, after grinding if required, is
then subjected to a three-stage leaching step, typically
involving leaching with a neutral aqueous solution,
followed by leaching with a dilute sulphuric acid
solution and followed further by leaching with a hot
sulphuric acid solution. Liquid/solid separation steps
are used after each leach stage with the solids that are
separated in the third liquid/solid separation step being
sent to a tailings pond. The solids typically contain
lead sulphate, some calcium sulphate and other materials.
The leachate from the final leaching stage is then
treated with manganese dioxide (Mn02) and ammonium
hydroxide to effect removal of iron as jarosite. A
further liquid/solid separation step is used with the
solids being sent to the tailings pond.
The leachate solution obtained typically contains,
in particular, zinc, calcium and indium. Zinc oxide and
zinc are then added sequentially to the leachate solution
in a procedure known as cementation. A liquid/solid
separation step is used to separate solid materials,
these materials being for instance precipitates of lead,
tantalum, cobalt and indium. The liquid (leachate)
contains zinc sulphate that is sufficiently pure that
CA 02245036 1998-08-13
3
zinc may be recovered, for example by being subjected to
an electrowinning stage in sulphuric acid solution. Zinc
metal is obtained, with sulphuric acid from the
electrowinning solution being recycled to the leaching
stage on the roasted zinc calcine.
Many ores contain significant amounts of iron. In
processes involving application of heat, especially under
oxidizing conditions, complex multiple oxides of ferric
oxide are formed, known as ferrites. In particular,
processes involving dead or sulphation roasting followed
by treatment with sulphuric acid to recover zinc as
soluble sulphates, generally result in failure to recover
ferrites without subsequent severe leach conditions (high
acid concentrations and elevated temperature). For
instance, dilute acid leaching to recover zinc values
from zinc oxide formed during roasting does not recover
zinc values from zinc ferrites. The latter zinc values
must be recovered under more severe leaching conditions,
with higher acid concentration and higher temperatures.
However, under the severe leaching conditions, iron
values are not only recovered from zinc ferrite but also
from other iron oxides in the roasted ore. Consequently,
solutions containing zinc contain increased
concentrations of iron, which affects subsequent steps to
recover zinc. In addition, the higher acid
concentrations must be neutralized, thereby requiring use
of greater quantities of lime or other bases, which also
affects steps to recover metal values from tails and in
other downstream processes.
Techniques for roasting of zinc ores or concentrates
for reduction of formation of ferrites are known. For
instance, U.S. 4 478 794 relates to the roasting of mixed
zinc sulphide-lead sulphide concentrates to provide an
oxidic feed for a smelting process, in which 2-20% by
weight of zinc oxide is mixed with the sulphide materials
and roasting is conducted at at least two temperatures,
CA 02245036 1998-08-13
4
viz. 850-950°C for a first stage and 950-1050°C for the
last stage.
U.S. 4 541 993 relates to sulphatization of non-
ferrous metal sulphides in which an alkali metal
carbonate or bi-carbonate is added to the roasting stage
to promote the conversion of the metal sulphides to metal
sulphates. Preferred roasting temperatures are from
about 550°C to 650°C.
U.S. 4 619 814 describes a method for recovering
zinc from sulphidic ores and concentrate. The material
in a subdivided form is roasted in a fluidized bed
reactor at a temperature of from 620-700°C with 20-60%
excess air to obtain a calcine containing zinc primarily
as sulphate and oxysulphate and iron primarily as
hematite. The calcine is leached with water or dilute
sulphuric acid at a temperature below 80°C, and then with
hot strong sulphuric acid at a temperature above 80°C but
below the boiling point of the solution. Most of the
zinc ferrite and unreacted sulphide of zinc is converted
to sulphates of iron and zinc, which dissolve in the
leach solution.
U.S. 4 789 529 states that in conventional zinc
recovery processes, zinc sulphide-bearing minerals are
dead roasted at temperatures in the range of 850-1050°C
such that essentially all the iron present is converted
to zinc ferrite and only the remainder of the zinc
present is in the form of zinc oxide. The patent
describes a process for the recovery of zinc from
sulphidic zinc-bearing ores and concentrates where
oxidation roasting is controlled to retain a portion of
the sulphides in the calcine, thereby retarding zinc
ferrite formation. Roasting is carried out at 700-
1050°C.
U.S. 4 889 694 describes roasting iron-bearing zinc
sulphide concentrates with a oxidizing gas containing
molecular oxygen at a temperature of at least about 900°C
CA 02245036 1998-08-13
but below the sintering temperature of the material to
effect desulphurization and to convert zinc and iron
values to the oxide form, while retaining residual
sulphide-sulphur throughout. It is stated that it is
5 necessary to minimize the formation of ferrite because
ferrites are insoluble in relatively dilute mineral
acids. In the case of zinc processing, this lack of
solubility of ferrites means that the solid residue
remaining after the leaching step contains a significant
concentration of zinc which would be lost and
significantly affect the overall efficiency of the
leaching process if no steps were taken for recovery.
Notwithstanding the variations in processes for
recovery of zinc values, especially from iron-bearing
zinc sulphide ores, hydrometallurgical processes that
provide for recovery of the zinc values under mild
leaching conditions and with less potential environmental
impact, would be beneficial. Such a process has now been
found .
Accordingly, one aspect of the present invention
provides a process for the recovery of zinc from a zinc
ore comprising the steps of:
(a) leaching material obtained from a zinc ore with
dilute sulphuric acid, essentially all of the zinc in
said material being in the form of zinc oxide; and
(b) separating the leachate so obtained for
recovery of zinc therefrom.
Another aspect of the present invention provides in
a process for the recovery of zinc from zinc sulphide
concentrates, said process comprising roasting said
concentrate, separating roasted concentrate containing
zinc oxide, zinc ferrite and lead oxide therefrom,
subjecting the separated roasted concentrate to multi-
stage leaching, said multi-stage leaching including
leaching with dilute and with hot sulphuric acid, the
improvement comprising roasting the zinc sulphide
CA 02245036 1998-08-13
6
concentrate at a temperature of greater than 1050°C,
separating roasted concentrate and subjecting it to a
single stage leach with dilute sulphuric acid.
The process of the present invention is illustrated
by the embodiment shown in the drawing, in which:
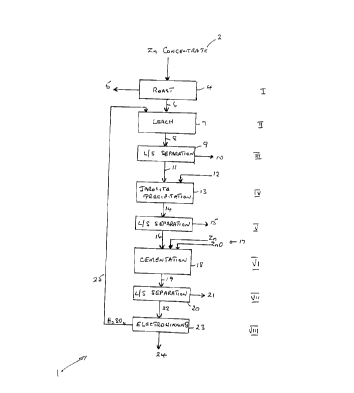
FIG. 1 is a schematic representation of a process of
the recovery of zinc.
In FIG. 1, a zinc recovery process is generally
indicated by 1. Zinc recovery process 1 uses a zinc ore
or zinc concentrate 2 as a feed material. The ore or
concentrate will be a zinc sulphide ore, and typically a
zinc sulphide ore or concentrate that also contains lead
sulphide and iron-bearing materials. It is also to be
understood that zinc sulphide ore will contain other
metal values, possibly including indium, cadmium, cobalt
and tantalum, depending on the particular ore. Methods
for forming the zinc concentrate from ore, if desired,
are known. The method will generally be described herein
with reference to zinc concentrate.
Zinc concentrate 2 is transferred to a roasting step
(Step I). In Step I, zinc concentrate 2 is subjected to
a roast at a temperature of greater than 1050°C, for
instance 1050-1200°C and preferably
1100-1150°C, to obtain a calcine product that has a
reduced amount of zinc ferrite.
It has been understood, as noted herein, that
roasting temperatures should be in the range of 850 -
950°C, as the formation of ferrite increases with
increasing temperature. However, it has now been found
that at high temperatures, greater than 1050°C and
especially 1100 - 1150°C, zinc ferrite decomposes under
the conditions of roasting.
In preferred embodiments, the calcine does not
contain ferrite. In particular, the temperature of the
roast is sufficiently high that ferrite is not formed, or
CA 02245036 1998-08-13
7
if formed is dissociated into zinc oxide and ferric
oxide.
Step I results in the formation of volatile
materials, particularly SOz resulting from oxidation of
the sulphides in the concentrate, as well as volatile
metal values. In particular, depending on the
composition of the concentrate, the volatile metal values
may include indium, cadmium and particularly lead. Such
volatile material may be treated for recovery of the SO2,
in the form of sulphuric acid, and for the recovery of
the metal values.
The roasted calcined ore or concentrate 6 from Step
I is transferred to a leaching step, Step II. The
leaching step is a single stage leaching step, in which
the leaching material is dilute sulphuric acid. The
dilute sulphuric acid may have a concentration typically
in the range of 10-20 g/1. The leaching may be carried
out over a range of temperature e.g. from ambient
temperature to 95°C, and especially 50-90°C. It is
believed to be not necessary to conduct leaching using
neutral solution and/or hot concentrated sulphuric acid.
After a period of time in the leaching step, the
mixture 8 obtained is transferred to a liquid/solid
separation step (Step III). In this step, the solid
material is transferred to tailings. The leachate 11 is
then transferred to a step for removal of iron (Step IV).
Leachate 11 would typically contain dissolved zinc
compounds, iron(ferric) compounds, and optionally cobalt
compounds and other soluble compounds from the leaching
step, depending on the composition of the ore. In Step
IV, MnOz and ammonium hydroxide are added to effect
precipitation of iron as jarosite, 13. The mixture 14
from Step IV is transferred to a liquid/solid separation
step (Step V) in which solids are separated, 15, and
forwarded to a tailings pond.
The solution 16 passing from Step V, is then
CA 02245036 1998-08-13
8
subjected to a step for a removal of impurities. Such a
step may be a so-called cementation step 18, (Step VI),
in which zinc metal and zinc oxide are added. In such a
step, zinc compounds selectively remain in solution
whereas other materials separate as precipitates. The
resulting solution 19 is forwarded to a liquid/solid
separation step 20 (Step VII) in which solid materials
are separated (21). The liquid from Step VII, which is
zinc sulphate in sulphuric acid solution is then
typically sent to an electrowinning step, 23 (Step VIII).
In Step VIII, zinc is recovered as metallic zinc, as
a cathode, and the sulphuric acid may be recycled 25, to
the initial leaching step, Step II.
In Step I, volatile impurities are removed as a
result of the high roasting temperature that is used.
The gas stream from the roasting step must be treated to
separate solid calcine particles from the gas containing
the volatiles before any volatiles condense and coat the
particles. This effects removal of such metal values,
and simplifies subsequent separation steps in the
process. It is understood that the gas stream containing
the volatile compounds would typically be treated for
recovery of SOZ, as sulphuric acid, and for recovery of
metal values, in an environmentally acceptable manner.
In addition, zinc ferrite, if formed in the roasting
step, will tend to dissociate at the temperature used
into zinc oxide and ferric oxide (Fe203), both of which
may be leached in a single dilute sulphuric acid leaching
step (Step II). Ferric oxide may be converted to
magnetite (Fe304), which may be removed magnetically.
Fe304 is less readily leached than Fez03.
Step II has the advantage that it is a single stage
step using dilute sulphuric acid, rather than three
stages using a variety of concentrations, and in
particular hot concentrated sulphuric acid. It is not
necessary to use hot concentrated sulphuric acid in the
CA 02245036 1998-08-13
9
process of the present invention. A single-stage
leaching step has the advantage of lower acid
consumption, and the use of more dilute acid. In
addition, only one liquid/solid separation stage should
be required, as opposed to three such stages that are
typically required. The single stage leaching step may
be conducted at or about ambient temperature up to 95°C.
It is not necessary to heat the leaching solution.
The roasted ore or concentrate obtained from Step I
may need to be ground, crushed or otherwise broken into
smaller particulate matter to make the leaching step,
Step II, more effective and efficient.
Notwithstanding the description of the embodiment of
FIG. 1, it is to be understood that any method of
conversion of zinc sulphide ore, including zinc sulphide
concentrate, to calcine in which the zinc is in the form
of zinc oxide and with the substantial absence of zinc
ferrite may be used. Roasting at a temperature greater
than 1050°C is one such method.
The calcine obtained from the ore, or concentrate,
has zinc in the form of zinc oxide. The calcine should
be substantially free of zinc ferrite. In preferred
embodiments, the calcine has less than 2% of the zinc in
the form of zinc ferrite, especially less than 1% and
particularly less than 0.5% in the form of zinc ferrite.
In order to minimize iron leaching without
compromising zinc leaching efficiency, it has been found
that the calcine obtained from roasting at about 1050-
1200°C can be transferred to a subsequent reduction
pretreatment step which utilizes the enthalpy of the
calcine. This can be done by maintaining mildly reducing
conditions e.g. partial combustion of fuel to retain some
CO and Hz in the gas. The reducing condition should be
such that Fe203 is reduced to FeO. Under these conditions
the zinc remains as oxide but any ferrite formed
dissociates. On cooling the calcine, any iron in the
CA 02245036 1998-08-13
form of FeO, which is not stable at room temperature,
reverts to Fe304 and there is essentially no effect on the
subsequent leaching steps, except no zinc is tied up as
ferrite and the zinc extraction in the leaching is
5 maximized. The reduction is carried out at 700 to 900°C
and advantageously at about 800°C. An additive e.g. lime
or sodium-containing compounds e.g. Na20,can be injected
into the reactor to facilitate formation of more stable
calcium or sodium ferrites. In addition, such additives
10 will tend to effect decomposition of any residual zinc
sulphide.
A further advantage of the reduced amount of acid
used in Step II of the process of the present invention
is that in Step IV i.e. jarosite precipitation, less
neutralizing agent is required to neutralize acid and
obtain the precipitate. Typical neutralizing agents are
NH3 and Na2C03. In addition, there is a lower volume of
precipitate and a lower volume of liquid for storage.
Similarly, it is to be expected that the solid separated
in Step VI should have a reduced volume, which would
reduce the size of the tailings pond.
It is further expected that Step VII would be a
single stage, compared with two or three stages used in
other processes as zinc values are predominantly present
as zinc oxide, and less iron is present in the solution.
In preferred embodiments, iron oxide is present in
the calcine in the form of magnetite (Fe304), which may be
removed magnetically prior to the initial leaching of the
dilute sulphuric acid or, if present, tends to be less
readily leached than Fe0 under the leaching conditions
that are used. Consequently, a reduced amount of iron is
present in the leachate, which reduces the amount of
jarosite that is precipitated. Precipitated jarosite
tends to be voluminous. In addition, if impurities are
removed in a roasting stage, there is a reduced
requirement for removal subsequently in the process e.g.
CA 02245036 1998-08-13
11
in step VII.
While a preferred embodiment has been described with
respect to FIG. 1, it is to be understood that Steps IV-
VIII could be varied, depending on the impurities in the
solution and other techniques known to persons skilled in
the art.
As discussed herein, the use of higher operating
temperatures for the roast, >1050°C, can assist in the
dissociating of the ferrite and reducing the ferrite
content in the calcine.
The use of calcine having zinc in the form of zinc
oxide, with the substantial absence of zinc ferrite, in
the leaching step simplifies subsequent treatment steps
in the process, and tends to utilize less chemicals,
particularly sulphuric acid, for separation of metallic
zinc. Thus, the process of the invention offers the
advantages of a single stage dilute acid leaching step,
one liquid/solid separation step, followed by steps for
removal of impurities and recovery of zinc by
electrowinning. Such a process would offer the
advantages of lower acid consumption, lower amounts of
neutralization reagents, lower precipitation/storage
volumes, a lesser number of liquid/solid separation
stages, milder leach conditions and consequently less
demand on the materials of construction, and a single
stage impurity removal stage subsequent to separation of
iron.