Note: Descriptions are shown in the official language in which they were submitted.
CA 02245894 1998-08-12
- 1 -
DESCRIPTION
HOT-DIP Zn-A1-Mg PLATED STEEL SHEET
GOOD IN CORROSION RESISTANCE AND SURFACE APPEARANCE
AND METHOD OF PRODUCING THE SAME
Technical Field
This invention relates to a hot-dip Zn-Al-Mg plated
steel sheet good in corrosion resistance and surface appearance
and a method of producing the same.
Background Art
It is known that steel sheet immersed in a hot-dip
plating bath of zinc containing an appropriate amount of A1 and
Mg to plate the steel sheet with this alloy exhibits excellent
corrosion resistance. Because of this, various avenues of
research and development have been pursued regarding this type
of Zn-Al-Mg-system. Up to now, however, no case of a plated
steel sheet of this system having achieved commercial success
as an industrial product has been seen.
The specification of U. S . Patent No . 3 , 505 , 043 , for
example, teaches a hot-dip Zn-Al-Mg plated steel sheet with
excellent corrosion resistance using a hot-dip plating bath
composed of A1: 3- l7wt . ~ , Mg : 1- 5wt . ~ and the remainder of Zn .
This was followed by proposals set out in, for example,
JPB-64-8702,. JPB-64-11112 and JPA-8-60324 for improving
CA 02245894 1998-08-12
- 2 -
corrosion resistance and productivity by incorporating various
addition elements in the basic bath composition of this type,
regulating the production conditions, and the like.
Object of the Invention
In industrial production of such hot-dip Zn-Al-Mg
plated steel sheet, while it is of course necessary for the
obtained hot-dip plated steel sheet to have excellent corrosion
resistance, it is also required to be able to produce a steel
strip product good in corrosion resistance and surface
appearance with good productivity. Specifically, it is
necessary to be able to stably produce hot-dip Zn-Al-Mg plated
steel sheet with good corrosion resistance and surface
appearance by continuously passing a steel strip through an
ordinary continuous hot-dip plating machine commonly used to
produce hot-dip galvanized steel sheet, hot-dip aluminum
plated sheet and the like. In this specification, the term
"hot-dip Zn-Al-Mg plated steel sheet" is for convenience used
also for a hot-dip Zn-Al-Mg plated steel strip produced by
passing a steel strip through a continuous hot-dip plating
machine. In other words, "plated sheet" and "plated strip" are
defined as representing the same thing.
In the equilibrium phase diagram for Zn-Al-Mg, the
ternary eutectic point at which the melting point is lowest
(melting point = 343° C) is found in the vicinity of A1 of about
4wt . ~ and Mg in the vicinity of about 3wt . ~ . In the production
CA 02245894 1998-08-12
- 3 -
of hot-dip Zn-A1-Mg plated steel sheet based on a Zn-A1-Mg
ternary alloy, therefore, it would appear at a glance to be
advantageous to make the composition close to this ternary
eutectic point.
When a bath composition in the vicinity of this
ternary eutectic point is adopted, however, a phenomenon arises
of local crystallization of a Zn11Mg2-system phase in the metal
structure of the plating, actually of an Al/Zn/Zn11Mg2 ternary
eutectic crystal matrix per se or in this matrix of a
Zn11Mg2-system phase including a [primary crystal Al phase] or
a [primary crystal A1 phase] and an (Zn single phase]. This
locally crystallized Zn11Mg2-system phase discolors more easily
than the other phase (Zn2Mg-system phase). During standing,
this portion assumes a highly conspicuous color tone and
markedly degrades the surface appearance. The value of the
plated steel sheet as a product is therefore manifestly
degraded.
Through their experience, moreover, the inventors
learned that when this Zn11Mg2-system phase locally
crystallizes there arises a phenomenon of this crystallized
portion being preferentially corroded.
An object of the invention is therefore to overcome
this problem and to provide a hot-dip Zn-Zl-Mg plated steel
sheet good in corrosion resistance and surface appearance.
The inventors further learned that when the ordinary
hot-dip plating operation of continuously
CA 02245894 2004-10-04
- 4 -
immersing/extracting a steel strip in/from a bath is
applied to a plating bath of this system, a stripe pattern
of lines running in the widthwise direction of the sheet
occurs. During production of Zn-base plated steel sheet
containing no Mg, no such line-like stripe pattern occurs
under normal conditions even if A1 should be added to the
bath, nor have cases of its occurrence been noted in
hot-dip A1 plated steel sheet. The inventors discovered
that the Mg in the bath is involved in the cause,
specifically that the stripe pattern of lines occurring at
intervals in the widthwise direction of the steel sheet is
peculiar to hot-dip galvanized steel sheet containing Mg.
The inventors believe the reason for this to be that a
Mg-containing oxide film forms on the surface of the molten
plating layer adhering to the steel strip immediately after
extraction from the bath and that owing to this formation
the surface tension and viscosity of the plating layer
surface portion are of a special nature not found in
hot-dip galvanized steel sheet, hot-dip A1 plated steel
sheet and the like. Overcoming the problem of this special
nature is indispensable for industrial production of such
plated steel.
One object of the invention is therefore to provide
such steel sheet having a good appearance without such a
pattern.
In one aspect, the present invention provides a
hot-dip Zn-A1-Mg plated steel sheet good in corrosion
resistance and surface appearance that is a hot-dip Zn-base
plated steel sheet obtained by forming on a surface of a
steel sheet a hot-dip Zn-A1-Mg plating layer composed of
A1: 4.0-lOwt.~, Mg: 1.0-4.Owt.o and the balance of Zn and
unavoidable impurities, the plating layer having a metallic
CA 02245894 2004-10-04
- 4a -
structure including a primary crystal Al phase in a matrix
of A1/Zn/Zn2Mg ternary eutectic structure.
In another aspect, the present invention provides a
hot-dip Zn-A1-Mg plated steel sheet good in
corrosion resistance and surface appearance that is a
hot-dip Zn-base plated steel sheet obtained by forming on a
surface of a steel sheet a hot-dip Zn-Al-Mg plating layer
composed of Al: 4.0-lOwt.%, Mg: 1.0-4.Owt.o and the balance
of Zn and unavoidable impurities, the plating layer having
a metallic structure including a primary crystal Al phase
and a Zn single phase in a matrix of Al/Zn/Zn2Mg ternary
eutectic structure.
In another aspect, the present invention provides a
method of producing hot-dip Zn-A1-Mg plated steel sheet
good in corrosion resistance and surface appearance that is
a method of producing a hot-dip Zn-Al-Mg plated steel sheet
using a hot-dip plating bath composed of Al: 4.0-lOwt.o,
Mq: 1.0-4.Owt.o and the balance of Zn and unavoidable
impurities, characterized in controlling a bath temperature
of the plating bath to not lower than the melting point and
lower than 470°C and a cooling rate up to completion of
plating layer solidification to not less than 10°C/s.
In another aspect, the present invention provides a
method of producing hot-dip Zn-Al-Mg plated steel sheet
good in corrosion resistance and surface appearance that is
a method of producing a hot-dip Zn-A1-Mg plated steel sheet
using a hot-dip plating bath composed of Al: 4.0-lOwt.o,
Mg: 1.0-4.Owt.o and the balance of Zn and unavoidable
impurities, characterized in controlling a bath temperature
of the plating bath to not lower than 470° C and a cooling
rate up to completion of plating layer solidification to
not less than 0.5°C/s.
CA 02245894 2004-10-04
- 4b -
In another aspect, the present invention provides a
hot-dip Zn-A1-Mg-system plated steel sheet good in
corrosion resistance and surface appearance that is a
hot-dip Zn-base plated steel sheet obtained by forming on a
surface of a steel sheet a plating layer composed of Al:
4.0-lOwt.o, Mg: 1.0-4.Owt.%, Ti: 0.002-0.lwt.o, B:
0.001-0.045wt.o and the balance of Zn and unavoidable
impurities, the plating layer having a metallic structure
including a primary crystal A1 phase in a matrix of
A1/Zn/ZnzMg ternary eutectic structure.
In another aspect, the present invention provides a
hot-dip Zn-A1-Mg-system plated steel sheet good in
corrosion resistance and surface appearance that is a
hot-dip Zn-base plated steel sheet obtained by forming on a
surface of a steel sheet a plating layer composed of A1:
4.0-lOwt.o, Mg: 1.0-4.Owt.o, Ti: 0.002-O.lwt.o, B:
0.001-0.045wt.o and the balance of Zn and unavoidable
impurities, the plating layer having a metallic structure
including a primary crystal A1 phase and a Zn single phase
in a matrix of A1/Zn/Zn2Mg ternary eutectic structure.
In another aspect, the present invention provides a
method of producing hot-dip Zn-A1-Mg plated steel sheet
good in corrosion resistance and surface appearance that is
a method of producing a hot-dip Zn-Al-Mg plated steel sheet
using a hot-dip plating bath composed of A1: 4.0-lOwt.o,
Mg: 1.0-4.Owt.o, Ti: 0.002-O.lwt.o, B: 0.001-0.045wt.% and
the balance of Zn and unavoidable impurities, characterized
in controlling a bath temperature of the plating bath to
not lower than the melting point and lower than 410°C and a
post-plating cooling rate to not less than 7°C/s.
In another aspect, the present invention provides a
method of producing hot-dip Zn-A1-Mg plated steel sheet
CA 02245894 2004-10-04
- 4c -
good in corrosion resistance and surface appearance that is
a method of producing a hot-dip Zn-Al-Mg plated steel sheet
using a hot-dip plating bath composed of A1: 4.0-lOwt.%,
Mg: 1.0-4.Owt.o, Ti: 0.002-O.lwt.o, B: 0.001-0.045wt.% and
the balance of Zn and unavoidable impurities, characterized
in controlling a bath temperature of the plating bath to
not lower than 410°C and a post-plating cooling rate to not
less than 0.5° C/s.
In a further aspect, the present invention provides a
method of producing hot-dip Zn-Al-Mg plated steel sheet
that is a method of producing a hot-dip Zn-Al-Mg plated
steel sheet by continuously immersing a steel strip in a
hot-dip plating bath composed of A1: 4.0-lOwt.% and Mg:
1.0-4.Owt. o, and the balance of Zn and unavoidable
impurities, continuously extracting the steel strip having
hot-dip plating adhered thereto from the bath and blowing
wiping gas onto the hot-dip plating layer continuously
extracted from the bath, the oxygen concentration of the
wiping gas being made not more than 3vol. o to control a
line-like stripe pattern appearing on a surface of the
plating layer.
In a further aspect, the present invention provides a
method of producing hot-dip Zn-Al-Mg plated steel sheet
that is a method of producing a hot-dip Zn-A1-Mg plated
steel sheet by continuously immersing a steel strip in a
hot-dip plating bath composed of A1: 4.0-lOwt.% and Mg:
1.0-4.Owt.o, and, as required, Ti: 0.002-O.lwt.% and B:
0.001-0.045wt.%, and the balance of Zn and unavoidable
impurities, continuously extracting the steel strip having
hot-dip plating adhered thereto from the bath into a sealed
box and in the sealed box blowing wiping gas onto the
hot-dip plating layer continuously extracted from the bath,
the oxygen concentration in the sealed box being made not
CA 02245894 2004-10-04
- 4d -
more than 8vol.% to control a line-like stripe pattern
appearing on a surface of the plating layer.
In a further aspect, the present invention provides a
Mg-containing hot-dip Zn-base plated steel sheet formed
with a plated surface whose steepness is not more than O.lo
by, during continuous extraction of a steel strip from a
hot-dip plating bath in which it is continuously immersed,
which bath is composed of A1: 4.0-l.Owt.o and Mg:
1.0-4.Owt. o, and the balance of Zn and unavoidable
impurities,
provided that the steepness (o) is a value calculated
by Equation (1) from an undulating shape curve of a unit
length of a measured undulating shape of the plating
surface in a sheet passage direction (lengthwise direction
of the strip)
Steepness (%) - 100 x Nm x (M + V)/L ~~~(1),
where:
L = Unit length (set to a value not less than 100 x
103um such as 250 x 103um) ,
Nm = Number of mountains within unit length,
M = Average mountain height within unit length (um),
V = Average valley depth within unit length (um).
In a further aspect, the present invention provides a
hot-dip Zn-base plated steel sheet obtained by applying to
a surface of a steel sheet a hot-dip Zn-A1-Mg-system
plating composed of A1: 4.0-lOwt.s, Mg: 1.0-4.Owt.%, Be:
0.001-0.05wt.o and the balance of Zn and unavoidable
impurities.
In a further aspect, the present invention provides a
hot-dip Zn-base plated steel sheet obtained by applying to
a surface of a steel sheet a hot-dip Zn-A1-Mg-system
plating composed of A1: 4.0-lOwt.°s, Mg: 1.0-4.Owt.o, Ti:
CA 02245894 2004-10-04
- 4e -
0.002-O.lwt.% and B: 0.001-0.045wt.%, Be: 0.001-0.05wt.%
and the balance of Zn and unavoidable impurities.
In a further aspect, the present invention provides a
method of controlling occurrence of a stripe pattern
appearing in a hot-dip plating layer characterized in
adding 0.001-0.05wt.% of Be to a hot-dip plating bath
composed of A1: 4.0-lOwt.% and Mg: 1.0-4.Owt.%, and the
balance of Zn and unavoidable impurities.
In yet a further aspect, the present invention
provides a method of producing hot-dip Zn-A1-Mg plated
steel sheet that is a method of producing a hot-dip
Zn-A1-Mg plated steel sheet by continuously immersing a
steel strip in a hot-dip plating bath composed of A1:
4.0-lOwt.% and Mg: 1.0-4.Owt.%, Ti: 0.002-O.lwt.%, B:0.001-
0.045wt.%, and the balance of Zn and unavoidable
impurities, continuously extracting the steel strip having
hot-dip plating adhered thereto from the bath and blowing
wiping gas onto the hot-dip plating layer continuously
extracted from the bath, the oxygen concentration of the
wiping gas being made not more than 3vol.% to control a
line-like stripe pattern appearing on a surface of the
plating layer.
In yet a further aspect, the present invention
provides a method of producing hot-dip Zn-A1-Mg plated
steel sheet that is a method of producing a hot-dip
Zn-A1-Mg plated steel sheet by continuously immersing a
steel strip in a hot-dip plating bath composed of Al:
4.0-lOwt.% and Mg: 1.0-4.Owt.%, Ti: 0.002-O.lwt.%, B:
0.001-0.045wt.%, and the balance of Zn and unavoidable
impurities, continuously extracting the steel strip having
hot-dip plating adhered thereto from the bath into a sealed
box and in the sealed box blowing wiping gas onto the
CA 02245894 2004-10-04
- 4f -
hot-dip plating layer continuously extracted from the bath,
the oxygen concentration in the sealed box being made not
more than 8vol.o to control a line-like stripe pattern
appearing on a surface of the plating layer.
In yet a further aspect, the present invention
provides a Mg-containing hot-dip Zn-base plated steel sheet
formed with a plated surface whose steepness is not more
than 0.1% by, during continuous extraction of a steel strip
from a hot-dip plating bath in which it is continuously
immersed, which bath is composed of A1: 4.0-l.Owt.o and Mg:
1.0-4.Owt.o, Ti: 0.002-0.lwt.o, B: 0.001-0.045wt.o, and the
balance of Zn and unavoidable impurities,
provided that the steepness (~) is a value calculated
by Equation (1) from an undulating shape curve of a unit
length of a measured undulating shape of the plating
surface in a sheet passage direction (lengthwise direction
of the strip)
Steepness (%) - 100 x Nm x (M + V)/L ~~~(1),
where:
L = Unit length (set to a value not less than 100 x
103um such as 250 x 103um),
Nm = Number of mountains within unit length,
M = Average mountain height within unit length (um),
V = Average valley depth within unit length (um).
In yet a further aspect, the present invention
provides a method of controlling occurrence of a stripe
pattern appearing in a hot-dip plating layer characterized
in adding 0.001-0.05wt.% of Be to a hot-dip plating bath
composed of A1: 4.0-lOwt.o and Mg: 1.0-4.Owt.°s, Ti: 0.002-
O.lwt.o, B: 0.001-0.045wt.o, and the balance of Zn and
unavoidable impurities.
Disclosure of the Invention
CA 02245894 1998-08-12
i _5_
This invention provides a hot-dip Zn-A1-Mg plated
steel sheet good in corrosion resistance and surface appearance
that is a hot-dip Zn-base plated steel sheet obtained by forming
on a surface of a steel sheet a hot-dip Zn-Al-Mg plating layer
composed of Al : 4 . 0- l0wt . ~ , Mg : 1. 0-4 . Owt . ~ and the balance of
Zn and unavoidable impurities, the plating layer having a
metallic structure including a [primary crystal A1 phase] or
a [ primary crystal A1 phase ] and a [ Zn single phase ] in a matrix
of [Al/Zn/Zn2Mg ternary eutectic structure].
In the metallic structure of the plating layer,
preferably the total amount of the [ primary crystal Al phase ]
and the [Al/Zn/Zn2Mg ternary eutectic structure] is not less
than 80vo1.~ and the [Zn single phase] is not greater than
15vo1.~ (including Ovol.~].
The hot-dip plated steel sheet having the plating
layer of this metallic structure can be produced by, in the
course of producing a hot-dip Zn-Al-Mg plated steel sheet using
a hot-dip plating bath composed of Al: 4.0-l0wt.~, Mg: 1.0-
4.Owt.~ and the balance of Zn and unavoidable impurities,
controlling the bath temperature of the plating bath to not
lower than the melting point and not higher than 450° C and the
cooling rate up to completion of plating layer solidification
to not less than 10° C/s or controlling the bath temperature of
the plating bath to not lower than 470° C and the post-plating
cooling rate up to completion of plating layer solidification
to not less than 0.5°C/s.
CA 02245894 1998-08-12
- 6 _
The invention further provides a hot-dip Zn-Al-
Mg-system plated steel sheet good in corrosion resistance and
surface appearance that is a hot-dip Zn-base plated steel sheet
obtained by forming on a surface of a steel sheet a plating layer
composed of Al: 4.0-l0wt.~, Mg: 1.0-4.Owt.~, Ti: 0.002-
O.lwt.~, B: 0.001-0.045wt.~ and the balance of Zn and
unavoidable impurities, the plating layer having a metallic
structure including a [primary crystal Al phase] or a [primary
crystal Al phase] and a [Zn single phase] in a matrix of
[Al/Zn/Zn2Mg ternary eutectic structure] . In the metallic
structure of this Ti/B-added plating layer, preferably the
total amount of the [primary crystal A1 phase] and the
[A1/Zn/Zn2Mg ternary eutectic structure] is not less than
80vo1.~ and the [Zn single phase] is not greater than 15vo1.~
(including Ovol.~].
In the case of this Ti/B-added hot-dip Zn-A1-Mg
plated steel sheet, a hot-dip plated steel sheet having a
metallic structure including a [primary crystal Al phase] or
a [primary crystal A1 phase] and a [Zn single phase] in a matrix
of [Al/Zn/ZnZMg ternary eutectic structure] can be produced by
using a hot-dip plating bath composed of Al: 4.0-lOwt.~, Mg:
1.0-4.Owt.~, Ti: 0.002-0.lwt.~, B: 0.001-0.045wt.~ and the
balance of Zn and unavoidable impurities and controlling the
bath temperature of the plating bath to not lower than the
melting point and lower than 410° C and the post-plating cooling
rate to not less than 7° C/s or controlling the bath temperature
CA 02245894 1998-08-12
_ 7 _
of the plating bath to not lower than 410° C and the post-plating
cooling rate to not less than 0.5°C/s.
According to the invention, in order to control the
stripe pattern of lines running in the widthwise direction of
the sheet that readily arises in a Zn-Al-Mg plated steel sheet
of this type, it was found advantageous to subject the Mg-
containing oxide film that forms on the surface layer of the
molten plating layer adhering to the surface of the steel strip
continuously extracted from the bath to morphology control
until the plating layer has solidified, more explicitly, to
regulate the oxygen concentration of the wiping gas to not
greater than 3vo1. ~ or to provide a sealed box to isolate the
steel sheet extracted from the bath from the atmosphere and make
the oxygen concentration in the sealed box not greater than
8vol.~.
Further, according to the invention, it was found
that occurrence of the stripe pattern of lines in the widthwise
direction of the sheet can be controlled by adding to the
plating bath an appropriate amount of Be, specifically,
0.001-0.05 of Be. The invention therefore also provides a
hot-dip Zn-base plated steel sheet with no stripe pattern
produced using a hot-dip plating bath obtained by adding Be:
0.001-0.05wt.~ to a hot-dip Zn-Al-Mg-system plating bath
composed of A1: 4.0-lOwt.~ and Mg: 1.0-4.Owt.~, and, as
required, Ti: 0.002-0.lwt.~ and B: 0.001-0.045wt.~, and the
balance of Zn 'and unavoidable impurities.
CA 02245894 1998-08-12
_ 8 _
Brief Description of Drawings
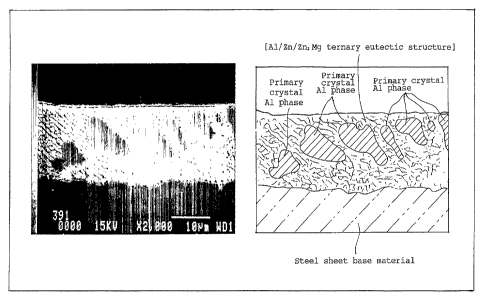
Figure 1 is an electron microscope secondary-
electron micrograph and a diagram for explaining the
micrograph, showing the cross-sectional metallic structure of
the plating layer of a hot-dip Zn-Al-Mg plated steel sheet
according to the invention.
Figure 2 is an electron microscope secondary-
electron micrograph and a diagram for explaining the
micrograph, showing an enlargement of the [A1/Zn/Zn2Mg ternary
eutectic structure] matrix portion of the metallic structure
of Figure 1.
Figure 3 is an electron microscope secondary-
electron micrograph and a diagram for explaining the
micrograph, showing the cross-sectional metallic structure of
the plating layer of a hot-dip Zn-Al-Mg plated steel sheet
according to the invention (the same structure as that in
Figure 1 except for the inclusion of Zn single phase).
Figure 4 is an electron microscope secondary-
electron micrograph and a diagram for explaining the
micrograph, showing the cross-sectional metallic structure of
the plating layer of a hot-dip Zn-A1-Mg plated steel sheet
according to the invention (the same structure as that in
Figure 1 except for the inclusion of Zn single phase; the
primary crystal Al structure being finer than in Figure 3).
CA 02245894 1998-08-12
_ g _
Figure 5 is a photograph taken of the surface of a
hot-dip Zn-Al-Mg plated steel sheet at which scattered
Zn11Mg2-system phase spots of visible size have appeared.
Figure 6 shows electron microscope secondary-
electron micrographs (2,000 magnifications) of a section cut
through a spot portion in Figure 5.
Figure 7 shows electron microscope secondary-
electron micrographs (10,000 magnifications) magnifying the
ternary eutectic portion of the structure of Figure 6.
Figure 8 shows electron microscope secondary-
electron micrographs (10,000 magnifications) of a boundary
portion of a spot in Figure 5, the upper half being the
ZnaMg-system phase matrix portion and the lower half being the
Zn11Mg2-system matrix portion of the spot portion.
Figure 9 shows x-ray diffraction charts obtained for
17mm x 17mm samples taken from the No . 3 and No . 14 plated steel
sheets in Table 3 of Example 3, the top chart in Figure 9
relating to No. 3 and the middle and bottom ones relating to
the No. 14 sample, which was taken so as to include a
Zn11Mg2-system phase spot as part of the sample area.
Figure 10 is a diagram showing the range of
conditions advantageous for production the hot-dip Zn-A1-Mg
plated steel sheet of the invention.
Figure 11 is a diagram showing the range of
conditions advantageous for production the hot-dip Zn-Al-Mg
plated steel sheet using a Ti/B-added bath.
CA 02245894 1998-08-12
- 10 _
Figure 12 is a sectional view of the essential
portion of a hot-dip plating machine showing how the applied
amount of the hot-dip plating layer is adjusted using wiping
nozzles installed in atmospheric air.
Figure 13 is a sectional view of the .essential
portion of a hot-dip plating machine showing how the applied
amount of the hot-dip plating layer is adjusted using wiping
nozzles installed in a sealed box.
Figure 14 is a chart showing an example of an
undulating curve obtained for the surface of a hot-dip Zn-Al-Mg
plated steel sheet.
Figure 15 shows a data table and a graph indicating
the relationship between the steepness and the visual stripe
pattern evaluation of the hot-dip Zn-Al-Mg plated steel sheet .
Figure 16 shows a typical example of a standard for
evaluating the stripe pattern appearing on the surface of a
hot-dip Zn-A1-Mg plated steel sheet, the stripe pattern
decreasing in order from (a) to (d).
Preferred Modes of the Invention
The hot-dip Zn-Al-Mg plated steel sheet according to
the invention is hot-dip plated using a dot-dip plating bath
composed of Al: 4.0-l0wt.~, Mg: 1.0-4.Owt.~ and the balance of
Zn and unavoidable impurities. The platipg layer obtained has
3
substantially the same composition as the plating bath.
However, the structure of the plating layer is characterized
in that it is' made into a metallic structure including a
CA 02245894 1998-08-12
- 11 -
[primary crystal A1 phase] in a matrix of [Al/Zn/Zn2Mg ternary
eutectic structure] or that it is made into a metallic structure
including a [ primary crystal Al phase ] and a [ Zn phase ] in said
matrix. By this, it simultaneously improves corrosion
resistance, surface appearance and productivity.
The [Al/Zn/ZnzMg ternary eutectic structure] here is
a ternary eutectic structure including an Al phase, a Zn phase
and an intermetallic compound Zn2Mg phase, as shown for example
by the typical example in the electron microscope
secondary-electron micrograph of Figure 2. The Al phase
forming this ternary eutectic structure actually originates
from an "Al" phase" (A1 solid solution with Zn present in solid
solution and containing a small amount of Mg) at high
temperature in the A1-Zn-Mg ternary system equilibrium phase
diagram. This A1" phase at high temperature ordinarily
manifests itself at normal room temperature as divided into a
fine A1 phase and a fine Zn phase. Moreover, the Zn phase of
the ternary eutectic structure is a Zn solid solution
containing a small amount of Al in solid solution and, in some
cases , a small amount of Mg in solid solution . The Zn2Mg phase
of the ternary eutectic structure is an intermetallic compound
phase present in the vicinity of Zn: approx. 84wt.~ in the Zn-Mg
binary equilibrium phase diagram. In this specification, the
ternary eutectic structure composed of these three phases is
represented as [A1/Zn/Zn2Mg ternary eutectic structure].
As shown for example by the typical example in the
electron microscope secondary-electron micrograph of
CA 02245894 1998-08-12
_ 12 _
Figure 1, the [primary crystal Al phase] appears as islands
with sharply defined boundaries in the ternary eutectic
structure matrix and originates from an "A1" phase" (A1 solid
solution with Zn present in solid solution and containing a
small amount of Mg) at high temperature in the Al-Zn-Mg ternary
system equilibrium phase diagram. The amount of Zn and the
amount of Mg present in solid solution in the Al" phase at high
temperature differs depending on the plating bath composition
and/or the cooling conditions. At normal room temperature,
this Al" phase at high temperature ordinarily divides into a
fine A1 phase and a fine Zn phase. In fact, when this portion
is observed further microscopically, a structure of finely
precipitated Zn can be seen but the island-like configurations
appearing with sharply defined boundaries in the ternary
eutectic structure matrix can be viewed as retaining the
skeletal form of the Al" phase at high temperature. The phase
originating from this Al" phase at high temperature (called
Al primary crystal) and shape-wise substantially retaining the
skeletal form of the Al" phase is referred to as [primary
crystal Al phase] in this specification. This [primary crystal
A1 phase] can be clearly distinguished from the A1 phase of the
ternary eutectic structure by microscopic observation.
As shown for example by the typical example in the
electron microscope secondary-electron micrograph of
Figure 3 , the [ Zn single phase ] appears as islands with sharply
defined boundaries in the ternary eutectic structure matrix
(and appears somewhat whiter than the primary crystal Al
CA 02245894 1998-08-12
- 13 -
phase). In actuality, it may have a small amount of Al and,
further, a small amount of Mg present therein in solid solution.
This [Zn single phase] can be clearly distinguished from the
Zn phase of the ternary eutectic structure by microscopic
observation.
In this specification, the metallic structure
including a [primary crystal Al phase] or a [primary crystal
Al phase] and a [Zn single phase] in the [A1/Zn/Zn2Mg ternary
eutectic structure] is sometimes called a "Zn2Mg-system
phase". Moreover, what is referred to in this, specification
as a "ZnllMgz-system phase" indicates both the metallic
structure of the [Al/Zn/ZnllMgz ternary eutectic structure]
matrix itself and the metallic structure of this matrix
including the [primary crystal A1 phase] or [primary crystal
A1 phase ] and [ Zn single phase ] . When the latter Zn11Mg2-system
phase manifests itself in spots of visible size, the surface
appearance is markedly degraded and corrosion resistance
decreases. The plating layer according to the invention is
characterized in the point that substantially no spot-like
Zn11Mg2-system phase of visible size is present.
The hot-dip Zn-Al-Mg plated steel sheet according to
this invention is thus characterized in the point of having a
specific metallic structure . The explanation will begin from
the basic plating composition of the plated steel sheet.
The A1 in the plating layer works to improve the
corrosion resistance of the plated steel sheet and the A1 in
the plating bath works to suppress generation of a dross
CA 02245894 1998-08-12
- 14 -
composed of Mg-containing oxide film on the surface of the
plating bath. At an Al content of less than 4.Owt.~, the effect
of improving the corrosion resistance of the steel sheet is
insufficient and the effect of suppressing generation of the
dross composed of Mg-containing oxide is also low. On the other
hand, when the Al content exceeds lOwt.~, growth of an Fe-Al
alloy layer at the interface between the plating layer and the
steel sheet base material becomes pronounced to degrade the
plating adherence. The preferred Al content is 4.0-9.Owt.~,
the more preferable Al content is 5.0-8.5wt.~, and the still
more preferable A1 content is 5.0-7.0 wt.~
The Mg in the plating layer works to generate a
uniform corrosion product on the plating layer surface to
markedly enhance the corrosion resistance of the plated steel
sheet. At a Mg content of less than 1.0~, the effect of uniform
generation of the corrosion product is insufficient, while when
the Mg content exceeds 4.0~, the effect of corrosion resistance
by Mg saturates and, disadvantageously, the dross composed of
Mg-containing oxide generates more readily on the plating bath.
The Mg content is therefore made 1.0-4.0~. The preferred Ma
content is 1.5-4.Owt.~, the more preferable Mg content is
2.0-3.5wt.~, and the still more preferable Mg content is
2.5-3.5wt.~.
As was pointed out earlier, it was found that when
a Zn11Mg2-system phase crystallizes in a Zn-A1-Mg ternary
composition containing such amounts of Al and Mg in Zn, the
surface appearance is degraded and the corrosion resistance is
CA 02245894 1998-08-12
- 15 -
also degraded. In contrast, it was found that when the
structure of the plating layer is made a metallic structure
including a [primary crystal Al phase] or a [primary crystal
A1 phase] and a [Zn single phase] in an [Al/Zn/Zn2Mg ternary
eutectic structure], the surface appearance is outstandingly
good and the corrosion resistance superior.
The structure of a [primary crystal Al phase]
included in an [Al/Zn/Zn2Mg ternary eutectic structure] matrix
here is a metallic structure of first-precipitated [primary
crystal A1 phase] included in an [Al/Zn/Zn2Mg ternary eutectic
structure] matrix, when the plating layer cross-section is I
observed microscopically.
Figure 1 is an electron microscope secondary-
electron micrograph (2,000 magnifications) of a cross-section
showing a metallic structure typical of this type. The
composition of the plating layer hot-dip plated on the surface
of the lower steel sheet base material steel (the somewhat
blackish portion) is 6A1-3Mg-Zn (approx. 6wt.~ Al, approx.
3wt.~ Mg, balance Zn). On the right is a diagram analyzing the
phases of the structure by sketching the structure of the
photograph in Figure 1. As shown in this diagram, [primary
crystal A1 phase] is included in the [A1/Zn/Zn2Mg ternary
eutectic structure] matrix in the state of discrete islands.
Figure 2 is an electron microscope secondary-
electron micrograph showing an enlargement of the matrix
portion of the [Al/Zn/Zn2Mg ternary eutectic structure] in
Figure 1 (10,000 magnifications). As shown in the analytical
CA 02245894 1998-08-12
- 16 -
sketch on the right , the matrix has a ternary eutectic structure
composed of Zn (white portions), Al (blackish, grain-like
portions) and Zn2Mg (rod-like portions constituting the
remainder).
The structure of a [primary crystal Al phase] and a
[Zn single phase] included in an [Al/Zn/Zn2Mg ternary eutectic
structure] matrix is a metallic structure of [primary crystal
A1 phase] and [Zn single phase] included in an [Al/Zn/ZnzMg
ternary eutectic structure] matrix, when the plating layer
cross-section is observed microscopically. In other words,
aside from the crystallization of a small amount of [ Zn single
phase] , it is no different from the former metallic structure.
Despite this crystallization of a small amount of [Zn single
phase], the corrosion resistance and appearance are
substantially as good as those of the former structure.
Figure 3 is an electron microscope secondary-
electron micrograph ( 2 , 000 magnifications ) of a cross-section
showing a metallic structure typical of this type. The
composition of the plating layer is 6A1-3Mg-Zn (approx. 6wt.~
Al , approx . 3wt . ~ Mg , balance Zn ) . As can be seen in Figure 3 ,
the structure is the same as that of Figure 1 in the point of
having discrete islands of [primary crystal A1 phase] included
in the [Al/Zn/Zn2Mg ternary eutectic structure] matrix but
further has discrete [Zn single phase] islands (gray portion
somewhat lighter in color than the primary crystal Al phase ) .
Figure 4 is an electron microscope secondarv-
electron micrograph (2,000 magnifications) of a cross-section
CA 02245894 1998-08-12
- 1~ -
of a plating layer of the structure obtained when the post-
hot-dip plating cooling rate of the same plating composition
as that of Figure 3 was made faster than that of Figure 3. In
the structure of Figure 4, the [primary crystal A1 phase] is
a little finer than that in Figure 3 and [ Zn single phase ] is
present in the vicinity thereof. There is, however, no
difference in the point that [primary crystal A1 phase] and [Zn
single phase] are included in an [Al/Zn/Zn2Mg ternary eutectic
structure] matrix.
Regarding the percentage of the whole layer
accounted for by these structures, in the former case, i.e.,
in the metallic structure having first-precipitated [primary
crystal A1 phase] scattered within an [A1/Zn/Zn2Mg ternary
eutectic structure] matrix, the total amount of [Al/Zn/ZnZMg
ternary eutectic structure] + [primary crystal Al phase] is not
less than 80vo1. ~, preferably not less than 90vo1. ~, and still
more preferably not less than 95vo1.$. The remainder may
include a small amount of Zn/Zn2Mg binary eutectic or Zn2Mg.
In the latter, i.e. , in the metallic structure having
scattered [ primary crystal A1 phase ] and also [ Zn single phase ]
crystallized within an [Al/Zn/Zn2Mg ternary eutectic
structure] matrix, the total amount of [Al/Zn/ZnZMg ternary
eutectic structure] + [primary crystal A1 phase] is not less
than 80vo1.~ and the amount of [Zn single phase] is not more
than 15vo1.$. The remainder may include a small amount of
Zn/Zn2Mg binary eutectic or Zn2Mg.
CA 02245894 1998-08-12
- 18 -
Preferably, the structures of both the former and
latter are substantially absent of Zn11Mg2-system phase. It was
found that in the composition range according to the invention,
the Zn11Mg2-system phase is likely to appear "spotwise" as a
phase of the metallic structure including [Al primary crystal]
or [Al primary crystal] and [Zn single phase] in an
[Al/Zn/ZnllMgz ternary eutectic structure] matrix.
Figure 5 is a photograph taken of the surface
appearance of a plated steel sheet (that of No.l3 in Table 3
of Example 3 set out later) wherein Zn11Mg2-system phase has
appeared spotwise . As can be seen in Figure 5 , spots of about
2-7mm radius (portions discolored blue) are visible at
scattered points in the matrix phase . The size of these spots
differs depending on the bath temperature and the cooling rate
of the hot-dip plating layer.
Figure 6 shows electron microscope secondary-
electron micrographs (2,000 magnifications) of a section cut
through a sample so as to pass through a spot portion in
Figure 5 . As can be seen in Figure 6 , the structure of the spot
portion is that of [Al primary crystal] included in an
[Al/Zn/Zn11Mg2 ternary eutectic structure] matrix. (Depending
on the sample , [Al primary crystal ] and [ Zn single phase ] may
be included in the matrix.)
Figure 7 shows electron microscope secondary-
electron micrographs of only the matrix portion of Figure 6
(portion containing no A1 primary crystal) at a higher
magnification ( 10 , 000 magnifications ) . Between the whitish Zn
CA 02245894 1998-08-12
- 19 -
stripes are clearly visible ternary eutectic structures
including Zn11Mg2 and A1 (somewhat blackish, grain-like
portions ) , i . a . , [ A1/Zn/Zn11Mg2 ternary eutectic structures ] .
Figure 8 shows electron microscope secondary-
electron micrographs (10,000 magnifications) relating to a
spot portion such as seen in Figure 5, showing a boundary
portion between the matrix phase and the spot phase. In the
photograph of Figure 8, the upper half is the matrix phase
portion and the lower half is the spot phase . The matrix phase
portion of the upper half is the same [Al/Zn/ZnzMg ternary
eutectic structure] as that of Figure 2 and the lower half shows
the same [Al/Zn/Zn11Mg2 ternary eutectic structure] as in
Figure 7.
From Figures 5 to 8 , it can be seen that the spot-like
ZnllMga-system phase is actually one having a metallic structure
of [Al primary crystal] or [Al primary crystal] and [Zn single
phase] included in an [Al/Zn/Zn11Mg2 ternary eutectic
structure] matrix and that the Zn11Mg2-system phase appears as
scattered spots of visible size in the matrix of the Zn2Mg-
system phase, i. e. , in the matrix of a metallic structure having
[primary crystal A1 phase] or [primary crystal A1 phase] and
[Zn single phase] included in an [A1/Zn/Zn2Mg ternary eutectic
structure] matrix.
Figure 9 shows examples of x-ray diffraction typical
of those providing the basis for identifying the aforesaid
metallic structures. In the drawing, the peaks marked ~ are
those of the Zn~Mg intermetallic compound and the peaks marked
CA 02245894 1998-08-12
_ 20 -
X are those of the ZnllMga intermetallic compound. Each of the
x-ray diffract ions was conducted by taking a 17mm x 17mm square
plating layer sample and exposing the surface of the square
sample to x-rays under conditions of a Cu-K ~ tube, a tube
voltage of 150Kv, and a tube current of 40mA. '
The top chart in Figure 9 relates to No . 3 in Table
3 of Example 3 and the middle and bottom charts to the No . 14
in the same Table 3. The samples of the middle and bottom charts
were taken so as to include a Zn11Mg2-system phase spot as part
of the sample area. The ratio of the spot area within the
sampled area was visually observed to be about 15~ in the middle
chart and about 70~ in the bottom chart. From these x-rav
diffractions , it is clear that the ternary eutectic structure
seen in Figure 2 is [Al/Zn/Zn2Mg ternary eutectic structure]
and that the ternary eutectic structure seen in Figure 7 is
[A1/Zn/ZnllMgz 1 .
From this metallic-structural viewpoint, in Tables
3, 5 and 6 of Examples set out later and also in Figure 10
described later, plating layers according to the invention that
have substantially no Zn11Mg2-system phase are represented as
"Zn~Mg" and those in which Zn11Mg2-system phase appears in spots
of visible size in a Zn2Mg-system phase matrix are represented
as "Zn~Mg + Zn11Mg2. " When such spot-like Zn11Mg2-system phase
appears, corrosion resistance is degraded and surface
appearance is markedly diminished. The plating layer according
to the invention is therefore preferably composed of a metallic
structure having substantially no Zn11Mg2-system phase of
CA 02245894-1998-08-12
21
visibly observable size, i.e., substantially of Zn2Mg-system
phase.
More specifically, in the plating layer of the
hot-dip Zn-Al-Mg plated steel sheet having a composition within
the aforesaid range according to the invention, [Al/Zn/ZnZMg
ternary ,eutectic structure ] matrix is present in the range of
50 to less than 100vo1.~, island-like [primary crystal Al
phase ] is present in this eutectic structure matrix in the range
of more than 0 to 50vo1. ~ , and, in some cases , island-like [ Zn
single phase] is further present therein at 0-15vo1.~. When
the surface of the plating layer is observed with the naked eye,
Zn11Mg2-system phase (phase having Al/Zn/Zn11Mg2 ternary
eutectic structure matrix) that appears in spots is not present
in visible size . In other words , the metallic structure of the
plating layer is substantially composed of [Al/Zn/Zn2Mg
ternary eutectic structure] matrix: 50 to less than 100vo1. ~,
[primary crystal A1 phase]: more than 0 to 50vo1.~, and [Zn
single phase]: 0-l5vol.$.
"Substantially composed" here means that other
phases, typically spot-like Zn11Mg2-system phase, are not
present in amounts that affect appearance and that even if
ZnllMga-system phase is present in such a small amount that it
cannot be distinguished by visual observation, such small
amount can be tolerated so long as it does not have an effect
on corrosion resistance and surface appearance. In other
words , since Zn11Mg2-system phase has an adverse effect on
appearance and. corrosion resistance when present in such amount
CA 02245894 1998-08-12
- 22 -
as to be observable in spots with the naked eye, such amount
falls outside the range of the invention. Moreover, presence
of Zn~Mg-system binary eutectic, Zn11Mg2-system binary eutectic
and the like is also tolerable in small amounts that cannot be
distinguished by visual observation with the naked eye.
To produce the hot-dip Zn-A1-Mg plated steel sheet
of the metallic structure according to the invention it was
found sufficient to control the bath temperature of the hot-dip
plating bath of the foregoing composition and the post-plating
cooling rate typically within the range of the hatching shown
in Figure 10.
Specifically, as can be seen in Figure 10, and as
indicated in Examples set out later, when the bath temperature
is lower than 470° C and the cooling rate is less than 10° C/s ,
the aforesaid Zn11Mg2-system phase appears in spots , making it
impossible to achieve the object of the invention. That such
a ZnllMgZ-system phase appears itself can be understood to some
degree by looking at the equilibrium phase in the vicinity of
the ternary eutectic point in the Zn-Al-Mg equilibrium phase
diagram.
It was found, however, that when the bath temperature
exceeds 450°C, more preferably rises to 470°C or higher, the
effect of the cooling rate diminishes and the Zn11Mg2-system
phase does not appear, whereby the metallic structure defined
by the invention can be obtained. It was similarly found that
even at a bath' temperature of 450° C or lower, more preferably
CA 02245894 1998-08-12
- 23 -
even at one of 470° C or lower, the metallic structure defined
by the invention can be obtained if the cooling rate is made
not less than 10°C/s, more preferably not less than 12°C/s.
This is a structure state that cannot be predicted from the
Zn-Al-Mg equilibrium phase diagram and a phenomenon that cannot
be explained by equilibrium theory.
When this phenomenon is utilized, a hot-dip Zn-Al-Mg
plated steel sheet that has a plating layer of the aforesaid
metallic structure according to the invention and is good in
corrosion resistance and surface appearance can be
industrially produced by, in a continuous hot-dip plating
machine, conducting hot-dip plating of the steel sheet surface
using a hot-dip plating bath composed of Al: 4.0-l0wt.~, Mg:
1 . 0-4 . Owt . ~ and the balance of Zn and unavoidable impurities ,
controlling the bath temperature of the plating bath to not
lower than the melting point and not higher than 450°C,
preferably lower than 470° C, and the post-plating cooling rate
to not less than 10° C/s, preferably not less than 12° C, or
conducting hot-dip plating of the steel sheet surface with the
bath temperature of the plating bath set not lower than 470° C
and the post-plating cooling rate arbitrarily set ( to not less
than 0.5°C/s, the lower limit value in an actual practical
operation).
Of note is that while it has been considered
advantageous to bring the bath composition into perfect
agreement with the ternary eutectic composition (A1 = 4wt.~,
Mg = 3wt.~ and Zn = 93wt.~ in the equilibrium phase diagram)
CA 02245894 1998-08-12
- 24 -
so as to minimize the melting point, this in actuality leads
to shrinkage of the finally solidifying portions that results
in a rough surface state of bad appearance. A perfect ternary
eutectic composition is therefore advisably avoided. As
regards the Al content , moreover, it is preferable to adopt a
content on the hypereutectic side within the aforesaid
composition range since Zn11Mg2 crystallizes out still more
readily at a composition on the hypoeutectic side.
Regarding the bath temperature, with the bath
composition of the invention, it is preferable, as indicated
in Examples set out later, to set 550° C as the upper limit of
the bath temperature and to effect the hot-dip plating at a bath
temperature not higher than this , because the plating adhesion
is degraded when the bath temperature is too high.
As pointed out earlier, within the bath composition
range defined by the invention, the bath temperature and the
post-plating cooling rate greatly influence the
generation/nongeneration behavior of Zn11Mg2 and Zn2Mg as
ternary eutectics . Although the reason for this is still not
completely clear, it is thought to be approximately as follows .
Since the rate of Zn11Mg2 crystallization decreases
with increasing bath temperature to become nil at and above
470°C, the bath temperature can be viewed as being directly
related to generation of Zn11Mg2 phase nuclei. Although a
definitive reason cannot be given for this, the physical
properties of the reaction layer (alloy layer) between the
plating layer and the steel sheet are presumed to be involved.
CA 02245894 1998-08-12
- 25 -
This is because the alloy layer is thought to be the main
solidification starting point of the plating layer.
As the post-plating cooling rate becomes more rapid,
moreover, the size of the spot-like ZnllMgz phase, i.e., the
spot-like phase including [Al primary crystal] or [Al primary
crystal] and [Zn single phase] in an [Al/Zn/Zn11Mg2 ternary
eutectic structure], gradually decreases to the point of
becoming difficult to observe visually. Then eventually at a
cooling rate of 10°C/s or higher, the size diminishes to the
point of becoming indistinguishable by visual observation. In
other words , it is considered that growth of the Zn11Mg2-system
phase is impeded with increasing cooling rate.
The inventors newly learned that generation and
growth of such a Zn11Mg2-system phase can be further controlled
by using a plating bath obtained by adding appropriate amounts
of Ti and B to the bath of the aforesaid basic composition.
According to this knowledge , even if the control ranges of the
bath temperature and the cooling rate are broadened relative
to those in the case of no Ti/Bi addition, a Zn2Mg-system phase,
i . a . , a plating layer having a metallic structure of [ primary
crystal A1 phase ] or [ primary crystal Al phase ] and [ Zn single
phase] included in an [Al/Zn/Zn2Mg ternary eutectic structure]
matrix, can be formed. A hot-dip plated steel sheet superior
in corrosion resistance and surface appearance can therefore
be more advantageously and stably produced. Since for adding
Ti and B it is~ possible to blend in an appropriate amount of
CA 02245894 1998-08-12
- 26 -
a compound of Ti and B such as TiB2, it is therefore possible
to use as additives Ti, B and/or TiB2. It is also possible to
cause TiB2 to be present in a bath added with Ti/B.
Plating layer alloy compositions obtained by adding
appropriate amounts of Ti and B to a hot-dip Zn plating layer
are set forth in, for example, JPA-59-166666 (Refinement of
Zn-Al alloy crystal grain size by addition of Ti/B), JPA-
62-23976 (Refinement of spangles), JPA-2-138451 (Suppression
of coating defoliation by impact after painting) and JPA-
62-274851 (Improvement of elongation and impact value).
However, none of these relates to a Zn-A1-Mg-system hot-dip
plating of a composition such as that to which this invention
is directed. In other words , the action and effect of Ti/B on
structure behaviors such as generation of ZnzMg-system phase
and suppression of ZnllMga-system phase have up to now been
unknown. Although JPA-2-274851 states that up to 0.2wt.~ of
Mg may be contained, it does not contemplate Mg to be contained
at not less than 1 . Owt . ~ as is contemplated by the invention .
The inventors newly discovered that in the case of the Zn-
A1-Mg-system hot-dip plating of the basic composition of the
invention described in the foregoing, when appropriate amounts
of Ti/B are added to the hot-dip plating of the basic
composition, the size of the Zn11Mg2-system phase becomes
extremely small, and that Ti and B enable stable growth of the
Zn2Mg-system phase, even at a bath temperature/cooling rate
such tends to generate Zn11Mg2-system phase.
CA 02245894 1998-08-12
- 27 -
Specifically, although Ti and B in the hot-dip
plating layer provide an action of suppressing
generation/growth of Zn11Mg2-system phase, such action and
effect are insufficient at a Ti content of less than 0 . 002wt . ~ .
On the other hand, when the Ti content exceeds 0.lwt.~, Ti-
Al-system precipitate grows in the plating layer, whereby bumps
arise in the plating layer ( called "butsu" among Japanese field
engineers) to cause undesirable degradation of appearance. The
Ti content is therefore preferably made 0.002-0.lwt.~.
Regarding the B content , at less than 0 . 001wt . ~ the action and
effect of suppressing generation/growth of Zn11Mg2 phase is
insufficient. When the B content exceeds 0.045wt.~, on the
other hand, the Ti-B or Al-B-system precipitates in the plating
layer become coarse, whereby bumps (butsu) arise in the plating
layer to cause undesirable degradation of appearance. The B
content is therefore preferably made 0.001-0.045wt.~.
It was found that when Ti and B are added to the
hot-dip Zn-Al-Mg-system plating bath, since generation/growth
of Zn11Mg2-system phase in the plating layer is impeded more than
in the case of no addition, the conditions for obtaining the
invention metallic structure composed of Zn2Mg-system phase
are eased relative to when Ti and Bi are not added, so that it
suffices to control the bath temperature of the hot-dip plating
bath and the post-plating cooling rate within the typical range
of the hatching shown in Figure 11. The relationship in
Figure 11 is broader in range than the relationship in the
CA 02245894 1998-08-12
- 28 -
earlier Figure 10. This can be viewed as the effect of Ti/B
addition.
This will be explained. In the case of Ti/B addition,
as shown in Figure 11 and indicated in Examples set forth later,
when the bath temperature is lower than 410° C and the cooling
rate is less than 7° C/s, the aforesaid Zn11Mg2-system phase
appears in spots. More specifically, it was found that the
effect of the cooling rate diminishes at bath temperatures
above 410° C so that no ZnllMgz-system phase appears and the
metallic structure defined by the invention can be obtained
even at a slow cooling rate such as 0. 5/° C. It was similarly
found that even at a bath temperature lower than 410°C, the
metallic structure defined by the invention can be obtained if
the cooling rate is made not less than 7°C/s. This is also a
structure state that cannot be predicted from the Zn-A1-Mg
equilibrium phase diagram and a phenomenon that cannot be
explained by equilibrium theory.
When this phenomenon is utilized, a hot-dip Zn-base
plated steel sheet that has a plating layer of the aforesaid
metallic structure according to the invention and is good in
corrosion resistance and surface aDnearancP ran t,A
industrially produced advantageously by, in an in-line
annealing-type continuous hot-dip plating machine, conducting
hot-dip plating of the steel sheet surface using a hot-dip
plating bath composed of Al: 4.0-l0wt.~, Mg: 1.0-4.Owt.~, Ti:
0.002-0.lwt.~, B: 0.001-0.045wt.~ and the balance of Zn and
unavoidable impurities, controlling the bath temperature of
CA 02245894 1998-08-12
- 29 -
the plating bath to not lower than the melting point and lower
than 410° C and the post-plating cooling rate to not less than
7° C/s, or setting the bath temperature of the plating bath not
lower than 410° C and the post-plating cooling rate arbitrarily
( to not less than 0 . 5° C/s . , the lower limit value in an actual
practical operation).
Regarding the bath temperature, irrespective of
addition/non-addition of Ti/B, it is preferable with the bath
composition of the invention to set 550°C as the upper limit
of the bath temperature and to effect the hot-dip plating at
a bath temperature not higher than this, because the plating
adhesion is degraded when the bath temperature is too high.
Moreover, the matters indicated regarding plating
layers not containing Ti/B explained with reference to the
photographs of Figures 1-8 and the x-ray diffraction charts of
Figure 9 substantially similarly explain the plating layers
containing Ti/B. Specifically, at small Ti/B contents such as
in this invention, Ti, B, TiB2 and the like substantially do
not appear as phases clearly observable in electron microscope
secondary-electron micrographs, while by x-ray diffraction
they appear merely as extremely small peaks. Therefore, the
metallic structure of the invention plated steel sheet
containing Ti/B can be explained similarly by the matters
explained by Figures 1-9 and falls substantially within the
same range as the metallic structure of the invention plated
steel sheet containing no Ti/B.
CA 02245894 1998-08-12
- 30 -
Next , explanation will be made regarding the stripe
pattern of lines running in the widthwise direction of the sheet
that tends to occur in the plating layer of this system and means
for suppressing occurrence thereof.
In the case of the foregoing Mg-containing hot-dip
Zn-base plated steel sheet, notwithstanding that the corrosion
resistance and surface appearance are enhanced from the aspect
of the metallic structure of the plating layer, the product
value is degraded if the line-like stripe pattern caused by Mg
oxidation occurs as mentioned earlier. Through numerous
experiments for overcoming this problem repeatedly conducted
by use of a continuous hot-dip line as the assumed production
line, the inventors discovered that the cause of the occurrence
of this peculiar Mg-induced strip pattern is in the morphology
of Mg-containing oxide film that is formed during the period
up to solidification of the plating layer on the steel strip
surface at the time the steel strip is continuously extracted
from the bath and that occurrence of the line-like stripe
pattern can be prevented by appropriately controlling the
morphology of the Mg-containing oxide film, irrespective of
other conditions.
This line-like stripe pattern is a pattern produced
by the appearance at intervals of relatively broad ribbons
extending in the widthwise direction of the sheet . Even if they
occur, they pose no problem to the industrial product so long
as they are of such a minor degree as not to be distinguishable
by visual observation. The "steepness (~)" according to
CA 02245894 1998-08-12
- 31 -
Equation (1) below was therefore adopted as an index for
quantifying the degree of the line-like stripe pattern. For
this , the undulating shape of the plating surface in the plating
direction of the obtained plated steel sheet, i.e., in the
direction of strip passage (lengthwise direction of the strip) ,
is measured and the steepness is obtained from the undulating
shape curve over a unit length (L) . When the steepness exceeds
0.1~, visually distinguishable line-like stripes appear in the
widthwise direction of the sheet.
Steepness (~) - 100 x Nm x (M + V)/L ~~~(1),
where:
L = Unit length ( set to a value not less than 100 x
103pm such as 250 x 103pm) ,
Nm = Number of mountains within unit length,
M = Average mountain height within unit length ( pm ) ,
V = Average valley depth within unit length (pm).
It is thought that in the state of the steel strip
being continuously extracted from the bath, generation of
non-equilibrium state solidified structure accompanying
generation of intermetallic compound progresses
simultaneously with oxidation reaction between metal
components and oxygen in the ambient atmosphere during the
period up to solidification of the hot-dip plating layer
adhering to the surface of the steel strip. When Mg is contained
at l.Owt.~ or greater, however, a Mg-containing oxide film
forms on the surface of the molten plating layer, whereby a
viscosity differential and/or a mass differential occurs
CA 02245894 1998-08-12
- 32 -
between the surface portion and the interior portion of the
plating layer and a change is produced in the surface tension
of the surface layer. When the degree of this change exceeds
a certain threshold value, a phenomenon of only the surface
portion sagging uniformly downward (slipping down) occurs
periodically. The line-like stripe pattern referred to above
is supposed to result from solidification in this state. In
actuality, when a cross-section of the outermost surface layer
of the plating layer was elementally analyzed using ESCA, the
presence of an oxide film composed of Mg, Al and O (oxygen) to
a thickness from the surface of not more than 100 was confirmed
(substantially no Zn was present) and it was found that the
amount of Mg and/or the amount of A1 in this film varied subtly
with the production conditions. This oxide film is referred
to in this specification a Mg-containing oxide film.
Taking this viewpoint, generation of the Mg-
containing oxide film should most ideally be totally avoided
up to the time that the hot-dip plating layer solidifies . In
an actual production line, however, preventing oxidation of the I
Mg, which has extremely strong oxygen affinity, up to the time
the plating layer solidifies is not easy and would require extra
equipment and expense to realize.
The inventors therefore conducted various
experiments for finding conditions enabling steepness to be
kept to or below 0.1~ even if formation of Mg-containing oxide
film is permitted. As a result, the inventors discovered that
for holding steepness to not more than 0.1~ it is helpful to
CA 02245894 1998-08-12
- 33 -
keep the oxygen concentration of the wiping gas to not more than
3vol. ~ or to provide a sealed box to isolate the hot-dip plated
steel strip extracted from the bath from the atmosphere and in
the latter case to make the oxygen concentration in the sealed
box not greater than 8vol.~.
Figure 12 schematically illustrates how a steel
strip 2 is continuously immersed through a snout 3 into a
Zn-A1-Mg-system hot-dip plating bath 1 according to the
invention, diverted in direction by an immersed roll 4, and
continuously extracted vertically from the hot-dip plating
bath 1 . Wiping gas for regulating the plating amount ( amount
applied) is blown from wiping nozzles 5 onto the surfaces of
the sheet continuously extracted from the hot-dip plating bath
1 . The wiping nozzles 5 are pipes formed with betting apertures
and installed in the widthwise direction of the steel sheet
( from the front to the back of the drawing sheet ) . By blowing
gas from these betting apertures uniformly over the full width
of the sheet being continuously extracted, the hot-dip plating
layers adhering to the sheet surfaces are reduced to a
prescribed thickness.
As explained in detail later, by conducting an
investigation of the relationship between the oxygen
concentration of the wiping gas and the steepness , it was found
that the steepness becomes 0.1~ or less without fail when the
oxygen concentration is not greater than 3vol.~. In other
words, even if up to 3vol.~ of oxygen in the wiping gas is
permitted, the line-like pattern of the Mg-containing hot-dip
CA 02245894 1998-08-12
- 34 -
Zn-base plated steel sheet can be mitigated to the point of
posing no problem in terms of appearance. When the wiping gas
is blown, a fresh surface at the plating layer interior and the
gas make contact at the blown location and the gas passes
downward and upward along the sheet surface as a film flow. When
the oxygen concentration of the wiping gas exceeds 3vo1. ~ , the
phenomenon of the surface layer portion sagging ( slipping down )
before the plating layer solidifies readily occurs to cause the
steepness to exceed 0.1~.
Figure 13 schematically illustrates the same state
as that of Figure 12 , except for the installation of a sealed
box 6 for shutting off the sheet extracted from the hot-dip
plating bath 1 from the ambient atmosphere . The edge of a skirt
portion 6a of the sealed box 6 is immersed in the hot-dip plating
bath 1 and a slit-like opening 7 is provided at the center of
the ceiling of the sealed box 6 for passage of the steel strip
2. The wiping nozzles 5 are installed inside the sealed box
6. Substantially all of the gas betted from the wiping nozzles
is discharged from the box through the opening 7. It was found
that when this type of sealed box 6 is provided, steepness can
be kept to not greater than 0.1~ even if the an oxygen
concentration within the sealed box 6 of up to 8vol.~ is
permitted. For maintaining the oxygen concentration in the box
at not greater than 8vol. ~, it suffices to set the oxygen
concentration of the gas blown from the wiping nozzles 5 in the
box at not greater than 8vo1. ~ . When the sealed box 6 is
provided as .shown in Figure 13, therefore, the oxygen
CA 02245894 1998-08-12
- 35 -
concentration of the wiping gas blown form the wiping nozzles
can be allowed be still higher than in the case of Figure 12.
By means of such regulation of the oxygen
concentration of the wiping gas or the atmosphere inside the
sealed box, the morphology of the Mg-containing oxide film of
the hot-dip plating surface layer can be made a morphology
involving no appearance of a line-like stripe pattern. It was
found, however, that occurrence of a line-like stripe pattern
can also be similarly suppressed by other means than this,
namely, by means of adding an appropriate amount of Be to the
bath.
Specifically, occurrence of a line-like stripe
pattern can be suppressed by adding an appropriate amount of
Be to the basic bath composition according to the invention.
The reason for this is conjectured to be that in the outermost
surface layer of the pre-solidified hot-dip plating that exits
the plating bath, Be oxidizes preferentially to Mg, and as a
result, oxidation of Mg is suppressed to prevent occurrence of
a Mg-containing oxide film of the nature that produces a
line-like stripe pattern.
While the pattern suppressing effect of Be addition
starts from a Be content in the bath of around O.OOlwt.~ and
strengthens with increasing content, the effect saturates at
about 0 . 05wt . ~ . Moreover, when Be is present at greater than
0 . 05wt . $ , it begins to have an adverse effect on the corrosion
resistance of the plating layer. The amount of Be addition to
CA 02245894 1998-08-12
- 36 -
the bath is therefore preferably in the range of 0.001-
0.05wt.~. (Since the line-like stripe pattern tends to become
more apparent with increasing plating amount, it is advisable
when attempting to suppress it by Be addition to regulate the
amount of Be addition within the aforesaid range based on the
plating amount.)
Although the suppression of stripe pattern by Be
addition can be effected independently of the regulation of the
oxygen concentration of the wiping gas or the atmosphere in the
sealed box, it can also be effected together with the oxygen
concentration regulation method. The effect of stripe pattern
suppression by Be addition is manifested both with respect to
a Ti/B-added bath for suppressing generation of Zn11Mg2-system
phase and with respect to a bath not added with Ti/B, without
adversely affecting generation of a Zn2Mg-system metallic
structure.
Therefore as a hot-dip plated steel sheet obtained
using a Be-added bath, the invention also provides a hot-dip
Zn-Al-Mg-system plated steel sheet with no stripe pattern and
having good corrosion resistance and surface appearance that
is a hot-dip Zn-base plated steel sheet obtained by forming on
a surface of a steel sheet a plating layer composed of Al:
4.0-l0wt.~, Mg: 1.0-4.Owt.~, Be: 0.001-0.05wt.~ and, as
required, Ti: 0.002-O.lwt.~ and B: 0.001-0.045wt.~, and the
balance of Zn and unavoidable impurities, the plating layer
having a metallic structure including a [primary crystal A1
CA 02245894 1998-08-12
- 37 -
phase ] or a [ primary crystal A1 phase ] and a [ Zn single phase ]
in a matrix of [Al/Zn/Zn2Mg ternary eutectic structure].
Examples
[Example 1]
Regarding effect of plating composition
(particularly Mg content) on corrosion resistance and
productivity.
{Processing conditions}
Processing equipment:
Sendzimir-type continuous hot-dip plating line
Processed steel sheet:
Hot-rolled steel strip (thickness: 3.2mm) of
medium-carbon steel
Maximum temperature reached by sheet in reduction furnace
within line:
600° C
Dew point of atmosphere in reduction furnace:
-40° C
Plating bath composition:
A1 = 4.0-9.2wt.~, Mg = 0-5.2wt.~, balance = Zn
Plating bath temperature:
455° C
Period of steel strip immersion in plating bath:
3s
Post-plating cooling rate: (Average value from bath
temperature to' plating layer solidification temperature; the
CA 02245894 1998-08-12
- 38 -
same in the following Examples):
3°C/s or 12°C/s by the air cooling method
Hot-dip Zn-Al-Mg plated steel strip was produced
under the foregoing conditions. The amount of oxide (dross)
generated on the bath surface at this time was observed and the
hot-dip plated steel sheet obtained was tested for corrosion
resistance. Corrosion resistance was evaluated based on
corrosion loss (g/m2) after conducting SST (saltwater spray
test according to JIS-Z-2371) for 800 hours. Amount of dross
generation was visually observed and rated X for large amount ,
O for rather large amount and ~ for small amount . The results
are shown in Table 1.
CA 02245894 1998-08-12
- 39 -
Table 1
No AI Mg Cooling SST Form Bath
rate corrosion of surface
C/s loss corrosion oxide
g/m2
1 6.0 0 12 90 Uniform O
2 6.0 0.1 12 78 Uniform O
3 6.0 0.5 12 40 Uniform
4 6.0 1.0 12 22 Uniform O
6.0 2.0 12 19 Uniform O
6 6.0 3.0 12 16 Uniform
7 6.0 4.0 12 14 Uniform
8 6.0 5.0 12 14 Uniform x
9 6.0 3.0 3 42 PreferentialO
corrosion
of
Zn"Mg2
portions
4.0 0.1 12 82 Uniform Q
1 4.0 1.2 12 25 Uniform
1
12 4.0 2.0 12 22 Uniform 0
13 4.0 3.8 12 16 Uniform O
14 4.0 5.2 12 16 Uniform x
4.0 2.0 3 48 PreferentialQ
corrosion
of
Zn"Mg2
portions
16 9.2 0.5 12 37 Uniform O
17 9.2 3.1 12 14 Uniform
18 9.2 5.0 12 14 Uniform D
19 9.2 1.5 3 40 PreferentialO
corrosion
of
Zn"Mg2
portions
From the results in Table 1 , it can be seen that the
corrosion resistance improves rapidly as the Mg content reaches
and exceeds 1~ but saturates when 4~ or more is added. It can
also be seen that at a Mg content exceeding 4~ , oxide ( dross )
on the bath surface increases even though Al is contained. At
CA 02245894 1998-08-12
- 40 -
a cooling rate of 3° C/s, Zn11Mg2-system phase crystallizes and
these portions corrode preferentially.
[Example 2]
Regarding effect of plating composition
(particularly A1 content) on corrosion resistance and
adherence.
{Processing conditions}
Processing equipment:
Sendzimir-type continuous hot-dip plating line
Processed steel sheet:
Hot-rolled steel strip (thickness: 1.6mm) of
medium-carbon steel
Maximum temperature reached by sheet in reduction furnace:
600° C
Dew point of atmosphere in reduction furnace:
-40° C
Plating bath composition:
Al = 0 . 15 -13 . Owt . ~ , Mg = 3 . Owt . ~ , balance = Zn
Plating bath temperature:
460° C
Period of immersion:
3s
Post-plating cooling rate:
12°C/s by the air cooling method
Hot-dip Zn-A1-Mg plated steel strip was produced
under the foregoing conditions. The hot-dip plated steel sheet
CA 02245894 1998-08-12
- 41 -
obtained was tested for corrosion resistance and adherence. As
in Example 1, corrosion resistance was evaluated based on
corrosion loss (g/m2) after conducting SST for 800 hours.
Adherence was evaluated by tightly bending a sample, sub jecting
the bend portion to an adhesive tape peeling test , and rating
lack of peeling as ~, less than 5~ peeling as p and 5~ or
greater peeling as X. The results are shown in Table 2.
Table 2
No AI Mg Cooling SST Form Adher-
rate corrosion of ence
C/s loss corrosion
g/m2
1 0.15 3.0 12 35 Uniform ~o
2 2.0 3.0 12 29 Uniform ~o
3 4.0 3.0 12 18 Uniform 0
4 5.5 3.0 12 17 Uniform O
7.0 3.0 12 16 Uniform O
6 9.0 3.0 12 14 Uniform
7 10.5 3.0 12 14 Uniform Q
8 13.0 3.0 12 14 Uniform x
As can be seen from the results in Table 2 , corrosion
resistance is excellent at an A1 content of not less than 4.0~
but adherence is bad at over 10~. This is caused by abnormal
development of an alloy layer (Fe-A1 alloy layer).
[Example 3]
Regarding effect of bath temperature and cooling
rate on structure and relationship between structure and
surface appearance.
{Processing conditions}
CA 02245894 1998-08-12
Processing equipment:
Sendzimir-type continuous hot-dip plating line
Processed steel sheet:
Hot-rolled steel strip of weakly killed steel
(in-line pickled; thickness: 2.3 mm)
Maximum temperature reached by sheet in reduction furnace:
580° C
Dew point of atmosphere in reduction furnace:
-30° C
Plating bath composition:
Al = 4.8-9.6wt.~, Mg = 1.1-3.9wt.~, balance =
Zn
Plating bath temperature:
390-535° C
Period of immersion:
8s or less
Post-plating cooling rate:
3-11°C/s by the air cooling method
Hot-dip plated steel strip was first produced under
the foregoing conditions using a Zn-6.2~A1-3.O~Mg bath
composition, while varying the plating bath temperature and the
post-plating cooling rate. The structure and appearance of the
plating layer of the plated steel sheet obtained were examined.
The results are shown in Table 3.
Among the plating layer structures in Table 3 , that
represented by [ Zn~Mg] is the metallic structure defined by the
invention, i.e., a metallic structure of [primary crystal Al
CA 02245894 1998-08-12
- 43 -
phase ] or [ primary crystal A1 phase ] and [ Zn single phase ] in
an [Al/Zn/Zn2Mg ternary eutectic structure] matrix, wherein
actually the total of [primary crystal A1 phase] and
[A1/Zn/Zn2Mg ternary eutectic structure] is not lass than
80vo1.~ and the total of [Zn single phase] is not more than
15vo1.~.
Further, [Zn2Mg + Zn11Mg2} in Table 3 represents a
structure of spot-like Zn11Mg2-system phase of visibly
distinguishable size, like that shown in Figure 5, in the
Zn~Mg-system structure. As shown in Figure 6, this spot-like
ZnllMg~-system phase is a spot-like phase of [Al primary
crystal] or [Al primary crystal] and [Zn single phase] included
in an [Al/Zn/Zn11Mg2 ternary eutectic structure] matrix. As the
spot-like Zn11Mg2-system phase is shiner than the surrounding
phase, it forms a noticeable pattern. When left to stand
indoors for about 24 hours , this portion oxidizes ahead of the
other portions and discolors to light brown, making it stand
out even more. The evaluation of appearance in Table 3 was
therefore made by visually observing the surface immediately
after plating and 24 hours after plating. Depending on whether
or not Zn11Mg2-system phase crystallized, the appearance was
rated uneven if spots were visually observed and even if no
spots were visually observed.
CA 02245894 1998-08-12
- 44 -
Table 3
No Bath Plating Cooling Intermetallic Appear-
I
Composi- Bath Rate Compound in ance
tion Temp. Plating layer
Wt.~ C C/s Structure
Ternary eutectic
AI Mg
1 6.2 3.0 390 11 ZnZMg Even
2 6.2 3.0 410 11 Zn2Mg Even
3 6.2 3.0 430 11 Zn2Mg Even
4 6.2 3.0 450 11 Zn2Mg Even
6.2 3.0 470 3 Zn2Mg Even
6 6.2 3.0 470 5 Zn2Mg Even
7 6.2 3.0 470 9 Zn2Mg Even
8 6.2 3.0 470 11 Zn2Mg Even
9 6.2 3.0 535 3 Zn2Mg Even
6.2 3.0 535 5 Zn2Mg Even
11 6.2 3.0 535 9 Zn2Mg Even
12 6.2 3.0 535 11 ZnZMg Even
13 6.2 3.0 390 3 ZnZMg + Zn,~Mg2Uneven
14 6.2 3.0 390 6 Zn2Mg + Zn"Mg2 Uneven
6.2 3.0 390 9 Zn2Mg + Zn"Mg2 Uneven
~, 6.2 3.0 460 3 Zn2Mg + Zn"Mg2 Uneven
16
I 6.2 3.0 460 6 Zn2Mg + Zn,~Mg2Uneven
17
18 6.2 3.0 460 9 Zn2Mg + Zn"Mg2 Uneven
From the results in Table 3, it can be seen that when
the bath temperature is below 470° C and the cooling rate is low
(below 10° C/s) , Zn11Mg2-system phase appears and makes the
appearance uneven. On the other hand, even when the bath
temperature is below 470° C, substantially [primary crystal Al
phase] and [Al/Zn/Zn2Mg ternary eutectic structure] are
obtained and an even appearance is exhibited if the cooling rate
is high (not less than 10°C/s). Similarly. at a bath
temperature of 470° C or higher, substantially [primary crystal
CA 02245894 1998-08-12
- 45 -
Al phase] and [Al/Zn/Zn2Mg ternary eutectic structure] are
obtained and an even appearance exhibited even if the cooling
rate is low.
Further, hot-dip plated steel strip was similarly
produced, except for changing the bath composition to Zn-
4.3~A1-1.2~Mg, Zn-4.3~A1-2.6~Mg or Zn-4.3~A1-3.8~Mg, while
varying the plating bath temperature and the post-plating
cooling rate in the manner of Table 3. The structure and
appearance of the plating layer of the plated steel sheet
obtained were similarly examined. Exactly the same results as
shown in Table 3 were obtained. Hot-dip plated steel strip was
also similarly produced, except for changing the bath
composition to Zn-6.2~A1-1.5~Mg or Zn-6.2~A1-3.8~Mg, while
varying the plating bath temperature and the post-plating
cooling rate in the manner of Table 3. The structure and
appearance of the plating layer of the plated steel sheet
obtained were examined as in the preceding examples. Exactly
the same results as shown in Table 3 were obtained. Hot-dip
plated steel strip was also similarly produced, except for
changing the bath composition to Zn-9.6~A1-l.l~Mg, Zn-
9.6~A1-3.O~Mg or Zn-9.6~A1-3.9~Mg, while varying the plating
bath temperature and the post-plating cooling rate in the
manner of Table 3. The structure and appearance of the plating
layer of the plated steel sheet obtained were examined as in
the preceding examples . Exactly the same results as shown in
Table 3 were obtained. These results are consolidated in
CA 02245894 1998-08-12
- 46 -
Figure 10. If a bath temperature and cooling rate in the
hatched region shown in Figure 10 are adopted, then, by the
basic bath composition according to the invention, there is
obtained a plating layer of a metallic structure composed
substantially of [primary crystal A1 phase] and [Al/Zn/Zn2Mg
ternary eutectic structure ] or of these plus a small amount of
[ Zn single phase ] . As a result , there can be obtained a hot-dip
Zn-Al-Mg plated steel sheet having a plating layer excellent
in corrosion resistance and surface appearance.
[Example 4]
Regarding effect of bath temperature and cooling
rate on plating adherence.
{Processing conditions}
Processing equipment:
NOF-type continuous hot-dip plating line
Processed steel sheet:
Cold-rolled steel strip ( thickness : 0 . 8mm) of
weakly killed steel
Maximum temperature reached by sheet in reduction furnace:
780° C
Dew point of atmosphere in reduction furnace:
-25° C
Plating bath composition:
Al = 4.5-9.5wt.$, Mg = 1.5-3.9wt.~, balance =
Zn
CA 02245894 1998-08-12
- 47 -
Plating bath temperature:
400-590° C
Period of immersion:
3s
Post-plating cooling rate:
3°C/s or 12°C/s by the air cooling method
Hot-dip plated steel strip was produced under the
foregoing conditions and the plating adherence of the plated
steel sheet obtained was examined. The results are shown in
Table 4. Plating adherence was evaluated as in Example 2.
CA 02245894 1998-08-12
'~ - 48 -
Table 4
No AI Mg Bath temp. Cooling rate Adherence
C/s C/s
1 6.0 2.5 400 1 2 Q
2 6.0 2.5 450 12 0
3 6.0 2.5 540 3 Q
4 6.0 2.5 540 12 Q
6.0 2.5 560 3 x
6 6.0 2.5 560 12 D
7 6.0 2.5 590 3 x
8 6.0 2.5 590 12 x
9 4.5 1 .5 430 1 2 Q
1 4.5 1 .5 450 1 2 Q
0
1 4.5 1 .5 540 3 Q
1
12 4.5 1 .5 540 12 Q
13 4.5 1.5 560 3 x
14 4.5 1.5 560 12 D
4.5 1.5 590 3 x
1 4.5 1 .5 590 12 x
6
17 4.5 3.9 430 12 Q
18 4.5 3.9 450 12 Q
19 4.5 3.9 540 3 Q
4.5 3.9 540 12 Q
21 4.5 3.9 560 3 x
22 4.5 3.9 560 12 D
23 4.5 3.9 590 3 x
24 4.5 3.9 590 12 x
9.5 3.8 450 12 Q
26 9.5 3.8 540 3 Q
I 9.5 3.8 540_ 12 Q
27
28 9.5 3.8 560 3 x
29 9.5 3.8 560 12 x
9.5 3.8 590 3 x
31 9.5 3.8 590 12 x
From the results in Table 4 , it can be seen that in
the bath composition range of the invention the plating
adherence is poor irrespective of the cooling rate when the bath
temperature is higher than 550°C.
CA 02245894 1998-08-12
- 49 -
[Example 5]
Regarding effect of plating composition
(particularly Ti/B contents) on corrosion resistance and
adherence.
{Processing conditions}
Processing equipment:
Sendzimir-type continuous hot-dip plating line
Processed steel sheet:
Hot-rolled steel strip of weakly killed steel I
(in-line pickled), thickness: 2.3mm
Maximum temperature reached by sheet in reduction furnace:
580° C
Dew point of atmosphere in reduction furnace:
_30° C
Plating bath composition:
A1 = 6.2wt.~
Mg = 3.Owt.~
Ti = 0-0.135wt.~
B = 0-0.081wt.~
Balance = Zn
Plating bath temperature:
450° C
Period of immersion:
4s or less
Post-plating cooling rate:
4°C/s by the air cooling method
CA 02245894 1998-08-12
- 50 -
Hot-dip Zn-Al-Mg (Ti/B) plated steel sheet was
produced under the foregoing conditions. The structure and
surface appearance of the plating layer of the plated steel
sheet obtained was investigated. The results are shown in Table
5.
Table 5
No Bath Composition Plating Appearance
wt.~ Composition
AI Mg Ti B Spot Bump
1 6.2 3.0 None None Zn2Mg+Zn"Mg2 YES NO
2 6.2 3.0 0.001 0.0005 ZnZMg+Zn"Mg2 YES NO
3 6.2 3.0 0.001 0.003 Zn2Mg+Zn"Mg2 YES NO
4 6.2 3.0 0.001 0.045 Zn2Mg+Zn"Mg2 YES NO
6.2 3.0 0.001 0.081 Zn2Mg+Zn"Mg2 YES YES
6 6.2 3.0 0.002 0.0005 Zn2Mg+Zn"Mg2 YES NO
7 6.2 3.0 0.002 0.001 Zn2Mg NO NO
8 6.2 3.0 0.002 0.043 Zn2Mg NO NO
9 6.2 3.0 0.002 0.051 ZnZMg NO YES
6.2 3.0 0.010 0.0006 Zn2Mg+Zn"Mg2 YES NO
12 6.2 3.0 0.010 0.002 Zn2Mg NO NO
13 6.2 3.0 0.010 0.030 Zn2Mg NO NO
14 6.2 3.0 0.010 0.049 Zn2Mg NO YES
6.2 3.0 0.040 0.0008 Zn2Mg+Zn"Mg2 YES NO
16 6.2 3.0 0.040 0.004 Zn2Mg NO NO
17 6.2 3.0 0.040 0.015 Zn2Mg NO NO
18 6.2 3.0 0.040 0.045 Zn2Mg NO NO
19 6.2 3.0 0.040 0.061 Zn2Mg NO YES
6.2 3.0 0.080 0.008 ZnZMg+Zn"Mg2 YES NO
21 6.2 3.0 0.080 0.002 Zn2Mg NO NO
22 6.2 3.0 0.080 0.035 Zn2Mg NO NO
23 6.2 3.0 0.080 0.055 Zn2Mg NO YES
24 6.2 3.0 0.100 0.0007 ZnZMg+Zn"Mg2 YES NO
6.2 3.0 0.100 0.002 Zn2Mg NO NO
26 6.2 3.0 0.100 0.030 ZnZMg NO NO
27 6.2 3.0 0.100 0.051 Zn2Mg NO YES
28 6.2 3.0 0.135 0.0008 Zn2Mg+Zn"Mg2 YES YES
29 6.2 3.0 0.135 0.015 ZnzMg NO YES
6.2 3.0 0.135 0.055 Zn2Mg NO YES
CA 02245894 1998-08-12
- 51 -
Among the plating layer structures shown in Table 5,
those represented as [ Zn2Mg ] are composed of [ primary crystal
Al phase] and [Al/Zn/Zn2Mg ternary eutectic structure] in a
total of not less than 80vo1. ~ and [ Zn single phase ] in an amount
of not more than 15vo1.~. The ones represented as [Zn2Mg +
Zn11Mg2] are those in which spot-like Zn11Mg2-system phase
appeared in the structure having Zn~Mg-system phase at a
visibly distinguishable size. As the spot-like ZnllMgZ-system
phase is shiner than the surrounding phase,' it forms a
noticeable pattern. When left to stand indoors for about 24
hours, this portion oxidizes ahead of the other portions and
discolors to light brown, making it stand out even more. In
the evaluation of appearance in Figure 5 , Spot [ YES ] and Spot
[NO] indicate those in which Zn11Mg2-system phase spots were and
were not found upon visual observation of the surface
immediately after plating and 24 hours after plating. Bump
(YES) indicates those in which irregularities formed in the
plating layer owing to precipitates growing to large size in
the plating layer.
From the results in Table 5 , it can be seen that Ti/B
addition impedes crystallization of Zn11Mg2-system phase spots
to provide a good surface condition. Of particular note is that '
this effect is slight by B alone and that the effect is manifest
by combined addition of Ti and B. However, bumps occur to
degrade the surface condition when the Ti/B content is above
the range prescribed by the invention.
CA 02245894 1998-08-12
- 52 -
Production was repeated under the same conditions as
those of Example 5 except that the plating bath composition was
changed to the following (1)-(5), namely:
(1) Al = 4.Owt.~
Mg = l.2wt.~
Ti = 0-0.135wt.~
B = 0-0.081wt.~
Balance = Zn
(2) Al = 4.2wt.~
Mg = 3.2wt.~
Ti = 0-0.135wt.~
B = 0-0.081wt.~
Balance = Zn
(3) A1 = 6.2wt.~
Mg = l.lwt.~
Ti = 0-0.135wt.~
B = 0-0.081wt.~
Balance = Zn
(4) Al = 6.lwt.~
Mg = 3.9wt.~
Ti = 0-0.135wt.~
B = 0-0.081wt.~
Balance = Zn
(5) A1 = 9.5wt.~
Mg = 3.8wt.~
Ti = 0-0.135wt.~
CA 02245894 1998-08-12
- 53 -
B = 0-0.081wt.~
Balance = Zn
As a result, platings of exactly the same plating
structure and appearance evaluation as those with the Ti
contents/B contents shown in Table 5 were also obtained when
the A1 content and Mg content were varied in the manner of
(1)-(5). In other words, it was found that the result of Ti
and B addition is manifested in the range of A1 and Mg addition
defined by the invention irrespective of the amount of Al and
the amount of Mg.
[Example 6]
Regarding effect of Ti/B addition/non-addition,
bath temperature and cooling rate on structure and surface
appearance of plating layer.
{Processing conditions}
Processing equipment:
Sendzimir-type continuous hot-dip plating line
Processed steel sheet:
Hot-rolled steel strip of weakly killed steel
(in-line pickled), thickness: 2.3mm
Maximum temperature reached by sheet in reduction furnace:
580° C
Dew point of atmosphere in reduction furnace:
-30° C
Plating bath composition:
CA 02245894 1998-08-12
- 54 -
Al = 6.2wt.~
Mg = 3.Owt.~
Ti = 0 or 0.030wt.~
B = 0 or 0.015wt.~
Balance = Zn
Plating bath temperature:
390-500° C
Period of immersion:
5s or less
Post-plating cooling rate:
0.5-10°C/s by the air cooling method
Hot-dip plated steel sheet was produced under the
foregoing conditions, while varying the bath temperature and
the post-plating cooling rate. The structure and surface
appearance of the plating of the plated steel sheet obtained
was investigated. The results are shown in Table 6. The
designation of plating structure and the presence/absence of
spots in the appearance evaluation in Table 6 are the same as
those explained regarding Table 5.
CA 02245894 1998-08-12
- 55 -
Table 6
No Bath composition Bath Cool Plating Appearance
layer
wt.96 temp. -ing compositionevaluation
C rate Presence
AI Mg Ti B C/s of spots
1 6.2 3.0 0.030 0.015 390 0.5 Zn2Mg+Zn"Mg2YES
2 6.2 3.0 0.030 0.015 390 4 Zn2Mg+Zn"MgzYES
3 6.2 3.0 0.030 0.015 390 7 Zn2Mg NO
4 6.2 3.0 0.030 0.015 390 10 Zn2Mg NO
6.2 3.0 0.030 0.015 410 0.5 ZnzMg NO
6 6.2 3.0 0.030 0.015 410 4 Zn2Mg NO
7 6.2 3.0 0.030 0.015 410 7 ZnZMg NO
8 6.2 3.0 0.030 0.015 430 0.5 Zn2Mg NO
9 6.2 3.0 0.030 0.015 430 4 Zn2Mg NO
6.2 3.0 0.030 0.015 430 7 Zn2Mg NO
11 6.2 3.0 0.030 0.015 460 0.5 Zn2Mg NO
12 6.2 3.0 0.030 0.015 460 4 Zn2Mg NO
13 6.2 3.0 0.030 0.015 460 7 Zn2Mg NO
14 6.2 3.0 0.030 0.015 500 0.5 Zn2Mg NO
6.2 3.0 0.030 0.015 500 4 ZnZMg NO
16 6.2 3.0 0.030 0.015 500 7 Zn2Mg NO
17 6.2 3.0 None None 410 0.5 Zn2Mg+Zn"Mg2YES
18 6.2 3.0 None None 410 4 Zn2Mg+Zn"Mg2YES
19 6.2 3.0 None None 410 7 Zn2Mg+Zn"Mg2YES
6.2 3.0 None None 430 0.5 Zn2Mg+Zn"Mg2YES
21 6.2 3.0 None None 430 4 Zn2Mg+Zn"Mg2YES
i 6.2 3.0 None None 430 7 Zn2Mg+Zn"Mg2YES
22
23 6.2 3.0 None None 460 0.5 ZnZMg+Zn"Mg2YES
24 6.2 3.0 None None 460 4 Zn2Mg+Zn"Mg2YES
6.2 3.0 None None 460 7 Zn2Mg+Zn"Mg2YES
From the results in Table 6, it can be seen that,
compared with platings not added with Ti/B, those added with
Ti/B do not experience Zn11Mg2-system phase spots even a low bath
temperature/slow cooling rate. Specifically, if hot-dip
plating treatment is effected at a bath temperature and a
cooling rate in the hatched region shown in Figure 11, those
CA 02245894 1998-08-12
56 _
added with Ti/B substantially become [primary crystal A1 phase]
and [Al/Zn/Zn2Mg ternary eutectic structure], thereby
providing a product exhibiting uniform appearance without
Zn11Mg2-system spots . In contrast , in the case of no Ti/B
addition, Zn11Mg2-system phase spots appear unless, as shown in
Figure 11, the bath temperature is made, preferably, not less
than 470°C or, at under 470°C, if the cooling rate is made
10°C/sec or greater.
[Example 7]
Regarding effect of plating composition
(particularly Al content in case of Ti/B addition) on corrosion
resistance and adherence.
{Processing conditions}
Processing equipment:
Sendzimir-type continuous hot-dip plating line
Processed steel sheet:
Hot-rolled steel strip (thickness: 1.6mm) of
medium-carbon steel
Maximum temperature reached by sheet in reduction furnace:
600° C
Dew point of atmosphere in reduction furnace:
-40° C
Plating bath composition:
A1 = 0.15-l3.Owt.~
Mg = 3.Owt.~
Ti = 0.05wt.~
CA 02245894 1998-08-12
- 57 -
B = 0.025wt.~
Balance = Zn
Plating bath temperature:
440° C
Period of immersion:
3s
Post-plating cooling rate:
4°C/s by the air cooling method
Hot-dip Zn-Al-Mg (Ti/B) plated steel strip was
produced under the foregoing conditions. The hot-dip plated
steel sheet obtained was tested for corrosion resistance and
adherence in the same manner as in Example 2. The results are
shown in Table 7.
Table 7
No Plating SST Adherence
bath corrosion
composition loss
(wt.96)
AI Mg Ti B g/m2
1 0.15 3.0 0.05 0.025 35 Qo
2 2.0 3.0 0.05 0.025 29 Qo
3 4.0 3.0 0.05 0.025 18 Oo
4 5.5 3.0 0.05 0.025 17 Qo
7.0 3.0 0.05 0.025 16 ~o
6 9.0 3.0 0.05 0.025 14 Qo
7 10.5 3.0 0.05 0.025 14 D
8 13.5 3.0 0.05 0.025 14 x
As can be seen from the results in Table 7, corrosion
resistance is excellent at an Al content of not less than 4.0~
but adherence is bad at over 10~. This can be viewed as being
CA 02245894 1998-08-12
_ 5g _
caused by abnormal development of an alloy layer ( Fe-A1 alloy
layer).
[Example 8]
Regarding line-like stripe pattern on plating layer
surface and suppression thereof . This example relates to a case
in which a mixed gas of nitrogen gas and air was used as a wiping
gas, without a sealed box.
Hot-dip Zn-A1-Mg plated steel sheet was produced
under the following conditions and the steepness of the surface
of the hot-dip plated steel sheet obtained was calculated in
accordance with Equation (1).
{Plating conditions}
Processing equipment:
All radiant tube-type continuous hot-dip
plating line
Processed steel sheet:
Hot-rolled steel strip (thickness: 1.6mm) of
medium-carbon aluminum-killed steel
Maximum temperature reached by sheet in reduction furnace:
600° C
Dew point of atmosphere in reduction furnace:
-30° C
Plating bath temperature:
400° C
Period of immersion:
4s
CA 02245894 1998-08-12
- 59 -
Wiping gas:
Nitrogen gas + air (oxygen adjusted to 0.1-
l2vol.~)
Post-plating cooling rate:
8°C/s by the air cooling method
Plating amount:
50, 100, 150 or 200g/mz
Plating bath composition:
Al = 6.2wt.~
Mg = 3.5wt.~
Ti = O.Olwt.~
B = 0.002wt.~
Balance = Zn
Table 8 shows for each of the plating amounts set out
above the measured steepness of various plated steel sheets
obtained by varying the mixing ratio of the nitrogen and air
(varying the oxygen concentration) of the wiping gas. The
evaluation of the line-like stripe pattern in the table rates
the visually observed degree of the pattern in three levels:
absolutely no pattern observed or extremely slight pattern
causing no problem whatsoever regarding appearance is
indicated by ~ marks , pattern observed but not so large by D
marks, and pattern clearly observed by X marks.
CA 02245894 1998-08-12
- 60 -
Table 8
Plating amountOxygen Evaluation
(per side) concentration Steepness of line-like
of wiping gas stripe
(g/mz) (Vol.~) (96) pattern
50 0.1 0.04 O
50 1.0 0.05 O
50 3.0 0.07 O
50 5.0 0.08 O
50 8.0 0.1 1 D
50 12.0 0.13 D '
100 0.1 0.05 O
100 1 .0 0.06 O
100 3.0 0.08 O
100 5.0 0.1 1 D
100 8.0 0.12 D
100 12.0 0.18 x
150 0.1 0.05 O
150 1 .0 0.06 O
150 3.0 0.09 O
1 50 5.0 0.1 2 O
1 50 8.0 0.14 D
150 1 2.0 0.25 x
200 0.1 0.06 O
200 1 .0 0.08 O
200 3.0 0.10 O
200 5.0 0.12 D
200 8.0 0.1 6 x
200 12.0 0.32 x
As can be seen from the results in Table 8 , steepness
was not more than 0.1~ and a plated steel sheet with no
appearance problem was obtained at all plating amounts insofar
as the oxygen concentration of the wiping gas was made not more
than 3vol.~. The case of a plating amount of 50 g/m2 was,
however, a special case in which an oxygen concentration of the
wiping gas up, to 5vol.~ was allowable.
CA 02245894 1998-08-12
- 61 -
[Example 9]
Regarding line-like stripe pattern on plating layer
surface and suppression thereof . This example relates to a case
in which waste gas of combustion was used as wiping gas, without
a sealed box.
Hot-dip Zn-A1-Mg plated steel sheet was produced
under the following conditions and the steepness of the surface
of the hot-dip plated steel sheet obtained was calculated in
accordance with Equation (1).
{Plating conditions}
Processing equipment:
NOF-type continuous hot-dip plating line
Processed steel sheet:
Cold-rolled steel strip ( thickness : 0 . 8mm) of
low-carbon aluminum-killed steel
Maximum temperature reached by sheet in reduction furnace:
780° C
Dew point of atmosphere in reduction furnace:
-25° C
Plating bath temperature:
450° C
Period of immersion:
3s
Wiping gas:
Waste combustion gas from nonoxidization
furnace (varied in oxygen concentration)
CA 02245894 1998-08-12
- 62 -
Post-plating cooling rate:
12°C/s by the air cooling method
Plating amount:
50, 100, 150 or 200g/m2
Plating bath composition:
Al = 9.lwt.~
Mg = 2.Owt.~
Ti = 0.02wt.~
B = 0.004wt.~
Balance = Zn
Table 9 shows for each of the plating amounts set out
above the measured steepness of various plated steel sheets
obtained by varying the oxygen concentration of the waste
combustion gas used as the wiping gas. (The oxygen
concentration of the waste combustion gas was varied as denoted
by combining variation of nonoxidization furnace air-fuel
ratio with afterburning of the waste combustion gas.) The
evaluation of line-like stripe pattern in the table is the same
as that in Example 8.
Owing to the variation of the nonoxidization furnace
air/fuel ratio and the variation of the waste combustion gas
afterburing conditions, the carbon dioxide concentration and
the steam concentration of the waste gas also varied. The
variation ranges were as follows:
Oxygen concentration: 0.1-l2vol.~
Carbon dioxide concentration: 0.3-lOvol.~
Steam concentration: 1.5-5.3vo1.~
CA 02245894 1998-08-12
- 63 -
Table 9
Plating amountOxygen Evaluation
(per side) concentration Steepness of line-like
of wiping gas stripe
(g/m2) (Vol.%) (96) pattern
50 0.1 0.04 O
50 1.0 0.05 O
50 3.0 0.07 O
50 5.0 0.08 O
50 8.0 0.12 D
50 12.0 0.15 D
100 0.1 0.05 O
1 00 1 .0 0.06 O
1 00 3.0 0.09 O
100 5.0 0.12 D
100 8.0 0.14 D
100 12.0 0.18 x
150 0.1 0.05 O
150 1 .0 0.07 O
150 3.0 0.09 O
150 5.0 0.12 D
150 8.0 0.15 D
1 50 1 2.0 0.26 x
200 0.1 0.07 O
200 1.0 0.09 O
200 3.0 0.10 O
200 5.0 0.13 D
200 8.0 0.18 x
200 12.0 0.35 x
As can be seen from the results in Table 9 , steepness
was not more than 0.1~ and a plated steel sheet with no
appearance problem was obtained at all plating amounts even
when waste combustion gas containing carbon dioxide and steam
was used as the wiping gas , insofar as the oxygen concentration
of the gas was made not more than 3vol . ~ . From this it is obvious
that what affects the morphology of the Mg-containing oxide
film that influences the steepness is free oxygen, so that if
CA 02245894 1998-08-12
- 64 -
not the oxygen in the COz and/or the oxygen in the H20 but the
free oxygen concentration is kept from exceeding 3vol.~, the
steepness can be kept to not greater than 0.1~. The case of
a plating amount of 50 g/m2 was, however, a special case in which
an oxygen concentration of the wiping gas up to 5vol.~ was
allowable.
[Example 10]
Regarding line-like stripe pattern on plating layer
surface and suppression thereof . This example relates to a case
in which a sealed box was installed and waste gas of combustion
was blown from the wiping nozzles inside the sealed box.
The sealed box 6 was installed to house the wiping
nozzles 5 therein as shown in Figure 13 and the oxygen
concentration of the waste combustion gas blown from the wiping
gas nozzles 5 was varied as in the case of Example 9. It was
confirmed by gas analysis measurement that the oxygen
concentration of the wiping gas and the oxygen concentration
of sealed box have a very close correlation. It can therefore
be assumed that during operation the interior of the sealed box
is maintained at a gas atmosphere of the same composition as
the wiping gas.
The plating conditions and bath composition were
made substantially the same as in the case of Example 9 and the
steepness was measured at each plating amount for plated steel
sheets obtained by varying the oxygen concentration of the
wiping gas . The results of Table 10 were obtained. In Table 10 ,
CA 02245894 1998-08-12
- 65 -
"Oxygen concentration in sealed box" is shown as the measured
value of the oxygen concentration of the wiping gas . Owing to
the variation of the nonoxidization furnace air/fuel ratio and
waste combustion gas afterburing conditions, the carbon
dioxide concentration and the steam concentration of the waste
gas also varied. The variation ranges were the same as those
in the case of Example 9.
Table 10
Plating amountOxygen Evaluation
(per side) concentration Steepness of line-like
of sealed box stripe
(g/m2) (Vol.~) (96) pattern
50 0.1 0.03 O
50 1.0 0.04 O
50 3.0 0.04 O
50 5.0 0.06 O
50 8.0 0.07 O
50 12.0 0.1 1 D
100 0.1 0.04 O
100 1.0 0.04 O
100 3.0 0.06 O
100 5.0 0.06 O
100 8.0 0.08 O
100 12.0 0.12 D
150 0.1 0.05 O
150 1.0 0.05 O
150 3.0 0.06 O
150 5.0 0.07 O
150 8.0 0.09 O
150 1 2.0 0.14 D
200 0.1 0.05 O
200 1.0 0.06 O
200 3.0 0.06 O
200 5.0 0.08 O
200 8.0 0.10 O
200 12.0 0.15 D
CA 02245894 1998-08-12
- 66 -
As can be seen from the results in Table 10, steepness
was not more than 0 . 1 and a plated steel sheet with no appearance
problem was obtained at all plating amounts even when waste
combustion gas containing carbon dioxide and steam was used as
the wiping gas, insofar as the oxygen concentration of the
wiping gas and, accordingly, the oxygen concentration in the
sealed box was made not more than 8vol.~. From this it is
obvious that what affects the morphology of the Mg-containing
oxide film that influences the steepness is free oxygen, so that
if not the oxygen in the C02 and/or the oxygen in the H20 but
the free oxygen concentration is kept from exceeding 3vol.~,
the steepness can be kept to not greater than 0.1.
[Example 11]
This Example is a steepness measurement example.
Although the steepness measurements of Tables 8-10 were
conducted as explained in the text, an actual measurement
example will be set out in the following.
Figure 14 shows an example of a measured undulating
curve of a plated steel sheet surface . The measurement for this
chart was made in the direction of sheet passage (lengthwise
direction of the steel strip) with a tracer type surface
roughness shape measuring instrument. The reference length (L)
was taken as 250 x 103um ( 250mm) .
A center line was drawn through the undulating curve,
and
Height of each mountain to the center line = ml
CA 02245894 1998-08-12
- 67 -
Number of mountains within L = Nm
Depth of each valley to the center line = V1
Number of valleys within L = Vm
were obtained. From these were calculated
Average mountain height M = E ml/Nm
Average valley depth V = E V1/Vm
Average pitch = L/Nm.
From these was calculated the Average elevation
differential = [M + V] . The Average elevation differential was
divided by the Average pitch and the result was represented as ~
to obtain the Steepness. When simplified, this operation
becomes: Steepness (~) - 100 x Nm x (M + V)/L.
Taking a specific instance, in the case of the plated
steel sheet of Table 8 obtained with a plating amount = 150g/m2
and wiping gas oxygen concentration = 5.Ovol.~:
At L = 250 x 103pm, Eml = 172pm,
Nm = 25,
EV1 = 137pm,
Vm = 25 was calculated,
Average elevation differential (M + V) - 12.4pm,
And average pitch = 10 x 103um.
Hence, Steepness = 0.12 was calculated.
Figure 15 shows the correlation between the
steepness determined in the foregoing manner and the visual
evaluation of the line-like stripe pattern. At the top of
Figure 15 is shown the relationship between the value of the
steepness ( and also the average elevation differential and the
CA 02245894 1998-08-12
- 68 -
average pitch ) and the visual evaluation explained in Example
8. This is illustrated graphically at the bottom of Figure 15.
From Figure 15 it can be seen that a plated steel sheet with
a steepness of not greater than 0.10 is an industrial product
with no line-like stripe pattern.
[Example 12]
Regarding line-like stripe pattern on plating layer
surface and suppression thereof. This example shows the
relationship between amount of Be addition and the stripe
pattern.
Hot-dip Zn-Al-Mg plated steel sheet was produced
under the following conditions and the degree of the stripe
pattern that appeared on the surface of the hot-dip Zn-Al-Mg
plated steel sheet obtained was visually rated in four levels .
The evaluation standard was as follows:
Strong stripe pattern (typical example shown in
Figure 16, photograph (a)) ~~~ Denoted by X marks
Medium stripe pattern (typical example shown in
Figure 16, photograph (b)) ~~~ Denoted by D marks
Weak stripe pattern (typical example shown in
Figure 16, photograph (c)) ~~~ Denoted b
y ~ marks
No stripe pattern (typical example shown in
Figure 16, photograph (d)) ~~~ Denoted by ~ marks
The photographs of 16(a)-(d) are all reduced 65~
relative to the actual articles (6.5mm in the photographs is
actually 10mm) and were photographed with the illumination
CA 02245894 1998-08-12
- 69 -
directed at right angles to the line-like stripe patterns
(plating direction = lengthwise direction of the steel strips)
so that the stripe patterns would photograph well.
{Plating conditions}
Processing equipment:
Continuous hot-dip plating simulator
Processed steel sheet:
Weakly killed steel sheet (thickness: 0.8mm)
Pass velocity:
50m/min.
Plating bath temperature:
400° C
Period of immersion:
3s
Wiping gas:
Oxygen concentration of 5vol.~, balance of
nitrogen and nitrogen-system gases
Wiping nozzle position:
100mm above bath
Plating bath composition:
A1 = 5.8wt.~
Mg = 3.lwt.~
Be = 0, 0.0006, 0.001, 0.015 or 0.05wt.~
Balance = Zn
With respect to each of the plating baths varied in
Be content as shown in Figure 11, the plating amount was
controlled by regulating the pressure of the betted wiping gas.
CA 02245894 1998-08-12
- 70 -
The stripe patterns appearing on the plated steel sheets are
rated under Surface appearance evaluation in Table 11.
Table 11
No Plating amountBe content Surface
per side (wt.96) appearance
(g/m2) evaluation
1 50 0 O
2 50 0.0006 O
3 50 0.001 Oo
4 50 0.015 OO
50 0.05 OO
6 100 0 O
7 100 0.0006 D
8 100 0.001 Oo
9 100 0.015 Oo
100 0.05 Qo '
11 150 0 x
12 1 50 0.0006 x
13 1 50 0.001 OO
14 1 50 0.015 OO
1 50 0.05 Oo
16 200 0 x
17 200 0.0006 x
18 200 0.001 Q
19 200 0.015 O
200 0.05 O
As can be seen from the results in Table 11, the
greater was the plating amount, the more the stripe pattern
stood out . At every plating amount , however, the stripe pattern
was decreased by Be addition. It can be seen that this effect
appears at a Be content of around 0 . OOlwt . ~ and that evaluation
rank rises with increasing Be addition but the effect
substantially, saturates at about 0.05wt.~.
CA 02245894 1998-08-12
- 71 -
Example 12 was repeated except that the plating bath
composition was changed to the following ( 1 ) - ( 7 ) . The result
was that exactly the same surface appearance evaluations as in
Table 11 were obtained for all of the bath compositions.
(1) A1 = 5.8wt.~
Mg = l.5wt.~
Be = 0, 0.0006, 0.001, 0.015 or 0.05wt.~
Balance = Zn
(2) Al = 9.5wt.~
Mg = 3.6wt.~
Be = 0, 0.0006, 0.001, 0.015 or 0.05wt.~
Balance = Zn
(3) Al = 9.5wt.~
Mg = l.2wt.~
Be = 0, 0.0006, 0.001, 0.015 or 0.05wt.~
Balance = Zn
(4) Al = 5.8wt.~
Mg = 3.lwt.~
Ti = 0.03wt.~
B = 0.006wt.~
Be = 0.0006, 0.001, 0.015 or 0.05wt.~
Balance = Zn
(5) Al = 5.8wt.~
Mg = l.5wt.~
Ti = 0.03wt.~
B = 0.006wt.~
. Be = 0, 0.0006, 0.001, 0.015 or 0.05wt.~
CA 02245894 1998-08-12
- 72 -
Balance = Zn
(6) Al = 9.5wt.~
Mg = 3.6wt.$
Ti = 0.01wt.~
B = 0.002wt.~
Be = 0, 0.0006, 0.001, 0.015 or 0.05wt.~
Balance = Zn
(7) Al = 9.5wt.~
Mg = l.2wt.~
Ti = 0.01wt.~
B = 0.002wt.~
Be = 0, 0.0006, 0.001, 0.015 or 0.05wt.~
Balance = Zn
[Example 13]
Example 12 was repeated except that the plating
conditions were changed as follows. The stripe patterns
appearing on the plated steel sheets were evaluated by the same
method as in Example 12. The results are shown in Table 12.
{Plating conditions}
Processing equipment:
Continuous hot-dip plating simulator
Processed steel sheet:
Weakly killed steel sheet (thickness: 0.5mm)
Pass velocity:
100m/min.
Plating bath temperature:
CA 02245894 1998-08-12
- 73 -
420° C
Period of immersion
2s
Wiping gas:
Air
Wiping nozzle position:
150mm above bath
Plating bath composition:
Al = 6.5wt.~
Mg = l.lwt.~
Be = 0, 0.0006, 0.001, 0.015 or 0.05.wt.~
Balance = Zn
CA 02245894 1998-08-12
- 74 -
Table 12
No Plating amountBe content Surface
per side (wt.96) appearance
(g/m2) evaluation
1 50 0 O
2 50 0.0006 O
3 50 0.001 Oo
4 50 0.015 Oo
50 0.05 Oo
6 100 0 x
7 100 0.0006 D
8 100 0.001 O
9 1 00 0.01 5 Q
100 0.05 Q
11 150 0 x
12 150 0.0006 x
13 150 0.001 O
14 150 0.015 O
150 0.05 O
16 200 0 x
17 200 0.0006 x
1 200 0.001 O
8
1 200 0.015 O
9
200 0.05 O
As can be seen from the results in Table 12, the
greater was the plating amount, the more the stripe pattern
stood out . At every plating amount , however, the stripe pattern
was decreased by Be addition. It can be seen that this effect
appears at a Be content of around O.OOlwt.~.
Example 13 was repeated except that the plating bath
composition was changed to the following (1)-(3). The result
was that exactly the same surface appearance evaluations as in
Table 12 were obtained for all of the bath compositions.
(1) Al = 6.5wt.~
Mg = 2.6wt.~
CA 02245894 1998-08-12
- 75 -
Be = 0, 0.0006, 0.001, 0.015 or 0.05wt.~
Balance = Zn
(2) A1 = 6.5wt.~
Mg = 2.6wt.~
Ti = 0.02wt.~
B = 0.004wt.~
Be = 0.0006, 0.001, 0.015 or 0.05wt.~
Balance = Zn
(3) A1 = 6.5wt.~
Mg = l.lwt.~
Ti = 0.02wt.~
B = 0.004wt.~
Be = 0, 0.0006, 0.001, 0.015 or 0.05wt.~
Balance = Zn
[Example 14]
This example shows the corrosion resistance of
plated steel sheets using a Be-added bath.
Hot-dip Zn-A1-Mg plated steel sheet was produced
under the following conditions. The corrosion resistance of
the hot-dip plated steel sheet was examined. Corrosion
resistance was evaluated based on corrosion loss (g/m2) after
conducting SST (saltwater spray test according to JIS-Z-2371)
for 800 hours. The results are shown in Table 13.
{Plating conditions}
Processing equipment:
Continuous hot-dip plating simulator
CA 02245894 1998-08-12
- 76 -
Processed steel sheet:
Weakly killed steel sheet (thickness: 0.8mm)
Pass velocity:
70m/min.
Plating bath temperature:
400° C
Period of immersion:
3s
Wiping gas:
5vo1. X02 + Balance of N2
Wiping nozzle position:
100mm above bath
Plating amount per side:
150g/m2
Plating bath composition:
A1 = 6.2wt.~
Mg = 2.8wt.~
Ti = 0.01wt.~
B = 0.002wt.~
Be = 0, 0.001, 0.02, 0.04, 0.06 or 0.08wt.~
Balance = Zn
CA 02245894 1998-08-12
- 77 -
Table 13
No Be content (wt.96)Corrosion loss
1 0 17
2 0.001 17
3 0.02 17
4 0.04 18
0.06 25
6 0.08 28
As can be seen from Table 13, addition of Be up to
0.05wt.~ has no effect on corrosion resistance.
As explained in the foregoing, the present invention
provides a hot-dip Zn-Al-Mg plated steel sheet excellent in
corrosion resistance and surface appearance and an '
advantageous method of producing the same. Owing to this
excellent corrosion resistance, the invention enables
expansion into new fields of application not achievable by
conventional hot-dip Zn-base plated steel sheet.