Note: Descriptions are shown in the official language in which they were submitted.
CA 02246l~3 l998-08-28
METHOD FOR FABRICATING SILICA GLASS
BACKGROUND OF THE II~VENTION
1. Field of the Invention
The present invention relates to a method for fabricating a silica glass
fabrication method by a sol-gel process.
2. Description of the Related Art
In general, silica glass is transparent and chemically inert, and has high
level of thermal stability and strength, and a low thermal expansion coefficient.
Because of such characteristics, the silica glass has been useful for an opticaldevice such as an optical fiber or optical lens.
Basically, the optical fiber is comprised of a core at the center thereof, and
a cladding having a refractive index different from the core such that light is totally
reflected from the core. In order to fabricate optical fibers, an optical fiber preform
including a core rod and an overcladding tube enclosing the core rod is prepared.
Then, the optical fiber preform is thermally-treated and then extended to form the
optical fibers.
The optical fiber preform is prepared by a modified chemical vapor
deposition (MCVD), vapor phase axial deposition (VAD) or outside vapor
deposition (OVD) method.
In the MCVD, the optical fiber preform is prepared from an overcladding
tube made of high purity silica glass. Here, the silica glass overcladding tube is
formed by a sol-gel process.
The sol-gel process, as a liquid phase process, can increase productivity
and freely control composition of the product. Also, because the sol-gel process is
performed at a low temperature, the sol-gel process is a very economical method.Also, since a high purity material is used as the starting material, this method is
very useful for fabricating a photomask for a semiconductor and a high purity silica
glass.
Hereinafter, a method of manufacturing an overcladding tube formed of
silica glass by the sol-gel process will be briefly described.
CA 02246l~3 l998-08-28
First, silica particles are dispersed in water to form a sol. The formed sol is
subjected to an aging treatment for a predetermined time. Then, the resultant sol
is poured into a mold for gelation. After gelation is completed, the gel is
separated from the mold and then dried.
Then, the dried gel is thermally-treated to remove organic substances from
the gel. Then, a reaction for eliminating hydroxy groups from the gel from whichthe organic substances have been removed, and a sintering process are
performed, thereby completing an overcladding tube made of silica glass.
Reactivity of the above-described sol-gel process depends on the gelation
reaction temperature, composition of the sol, pH and solvent, and it is very difficult
to maintain the reactivity within a favorable range by controlling such factors.Also, when drying the molded gel, many cracks are caused, and shrinking and
cracking occur during sintering. In order to solve these problems, a method using
a drying control chemical additive (DCCA) or a polymer binder, or redispersion
method and supercritical drying method have been developed.
The method using the DCCA minimizes a local difference of solvent's
evaporation rate from the gel, thereby minimizing the difference in the local stress
of the samples during drying. As a result, the gel becomes hard, thereby
decreasing generation of cracks.
According to the redispersion method, dried fine silica powder, i.e., fumed
silica, is dispersed in water to form a sol, and then the sol is gelated. Duringgelation, the silica particles form an agglomerate due to hydrogen bonds. After
drying the agglomerate, the dried agglomerate is subjected to a thermal process
and grinding process and then redispersed in water. The redispersed product is
gelated, molded, and then sintered.
However, the above methods are not effective in preventing cracking during
the drying of the gel, and the preparing process is complicated.
SUMMARY OF THE INVENTION
To solve the above problems, it is an objective of the present invention to
provide a method for fabricating a high density silica glass, in which cracking after
drying process is suppressed and the cracking and shrinking after sintering
process are reduced.
CA 02246l~3 l998-08-28
Accordingly, to achieve the above objective, there is provided a method for
fabricating a silica glass comprising the steps of:
(a) adding silica and a dispersant to a premix solution obtained by
dissolving a monomer for acrylic resin and a cross-linking agent in a distilled
water, and dispersing the mixed solùtion, and adjusting the pH of the mixture, to
form a sol;
(b) removing air bubbles from the sol, and then aging the sol;
~ ) adding a polymerization initiator to the aging-treated sol, and adjusting
the pH of the reaction mixture;
(d) pouring the reaction mixture into a mold, aging the mixture in an
incubator at a high temperature and then gelating the resultant;
(e) aging the obtained gel, demolding the aging-treated gel, and then drying
the demolded gel;
(f) thermally-treating the dried gel to remove organic substances from the
gel; and
(g) performing a hydroxy group elimination reaction and a sintering reaction
on the gel from which organic substances have been removed.
BRIEF DESCRIPTION OF THE DRAWING
The above objective and advantages of the present invention will become
more apparent by describing in detail preferred embodiments thereof with
reference to the attached drawing in which:
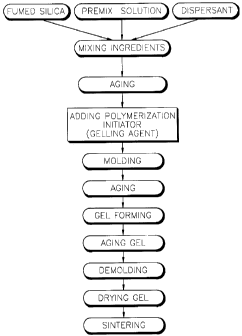
FIG. 1 is a flowchart illustrating a method for fabricating a silica glass
according to the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
In a method for fabricating a silica glass according to the present invention,
chemical gelation in which a silica is added to a premix solution obtained by
dissolving a monomer for acrylic resin and a cross-linking agent in water, and
dispersed and polymerized, and thermal gelation in which a gelling agent is added
to a composition for silica glass and pH pf the resultant is properly adjusted, are
simultaneously performed. As a result, a polymer binder having a 3-dimensional
network structure, formed from the monomer for acrylic resin and the cross-linking
CA 02246l~3 l998-08-28
agent, is evenly distributed in the whole gel, so that a conventional problem
caused from the sol-gel process, that is, a density gradient, can be suppressed.Also, the strength of the gel increases, thereby suppressing cracking during drying
and shortening the drying time.
Preferably, the content of the monomer for acrylic resin is 2~20wt% based
on the weight of the premix solution, and the content of the cross-lining agent is
0.05~1.0wt% based on the weight of the premix solution.
Preferably, the concentration of the premix solution containing the monomer
for acrylic resin and the cross-linking agent is low. If the concentration of the
premix solution is low, carbon does not remain after organic substances are
decomposed during the sol-gel process and the transparency of the silica glass is
deteriorated after sintering process. Also, if such dilute premix solution is used,
moist gel becomes smooth, and the strength of the moist gel can be improved
through thermal gelation by controlling the pH.
The monomer for acrylic resin and the cross-linking agent are not
specifically restricted. However, the monomer for acrylic resin is preferably acryl
amide or methacryl amide, and the cross-linking agent is preferably N,N'-
methylenebisacrylamide ((H2C=CHCONH)2CH2).
A method for fabricating a silica glass according to the present invention will
be described with reference to FIG. 1.
First, a monomer for acrylic resin and a cross-linking agent are dissolved in
deionized water to prepare a premix solution. A fumed silica and a dispersant are
added to the premix solution, and then dispersed, and the pH of the reaction
mixture is adjusted to 11~13, thereby forming a sol. Here, a mixing ratio of thefumed silica and the premix solution is 4:6~6:4 based on the weight. Here, the
dispersant helps the fumed silica to be evenly dispersed in the premix solution.Any dispersant may be used without specific restriction. Preferably, however,
tetramethylammonium hydroxide is preferably used as the dispersant.
Then, air bubbles are removed from the sol using a vacuum pump, and the
sol is aged for a predetermined time to stabilize silica particles of the sol.
Then, a polymerization initiator and a catalyst are added to the reaction
mixture, and a gelling agent is further added if required. Then, the pH of the
reaction mixture is adjusted to 9~11. The obtained mixture is poured into a mold
CA 02246l~3 l998-08-28
for gelation, and then left for aging in an incubator. Preferably, the aging process
is performed at 60~100~C for 30 minutes~3 hours. Then, the gel is left for agingat room temperature.
Here, ammonium persulfate is used as the polymerization initiator, and
methyl formate, methyl lactate or ethyl lactate is used as the gelling agent.
The aging-treated gel is demolded, and then dried in an incubator at
20~50~C and relative humidity (RH) of 70~95%. Then, in order to eliminate the
organic substances remaining in the dried gel, such as additive and binder, a first
thermally- treating process is performed. Here, for the first thermally- treating
process, the gel is heated to 300~700~C at a rate of 50~C per hour, and then themaintained at that temperature for 2~8 hours. Also, in order to eliminate the
remaining hydroxy groups from the first thermally-treated gel, the gel is heated to
900~1,200~C at a rate of 100~C per hour under a chlorine gas atmosphere, and
then maintained at that temperature for 1~8 hours. Then, the gel is heated to
1,100~1,500~C at a rate of 100~C per hour under a helium gas atmosphere, and
then maintained at that temperature for 1~8 hours. thereby completing a high
density and high purity silica glass.
Examples of a method for fabricating silica glass according to the present
invention will be described. However, the present invention is not limited to the
following examples.
<Example 1>
4.8wt% of acryl amide and 0.2wt% of N,N'-methylenebisacrylamide were
dissolved in 95.0wt% of deionized water to prepare a premix solution.
500ml of the premix solution, 500g of the fumed silica (Aerosil OX-50,
Degussa Co.) and 52ml of trimethylammonium hydroxide (TMAH) aqueous
solution (25.0wt% solution in water) were mixed and dispersed using a high shearmixer to prepare a sol. Then, air bubbles were removed from the sol using a
vacuum pump, and aged for 10 hours. 8ml of ammonium persulfate aqueous
solution (5.0wt% solution in water) as a polymerization initiator, and 8g of methyl
formate (1.6wt% based on the weight of the silica) were added to and mixed with
the aged sol.
CA 02246l~3 l998-08-28
The mixture was poured into a mold for gelation. Then, the mold containing
the mixture was aged in an incubator at 70~C for 1 hour, and then gelated. The
obtained moist gel was demolded, and dried in an incubator at 25~C and 75% RH
for 6 days. Then, the temperature of the gel was increased to 120~C at a rate of20~C per hour, and then maintained at that temperature for 5 hours to remove anyremaining moisture, thereby forming a dried gel.
Then, after heating the dried gel to 550~C at a rate of 50~C/hour, the dried
gel was thermally-treated at that temperature for 5 hours to remove organic
substances from the dried gel. The gel from which organic substances had been
removed was heated to 1,000~C at a rate of 100~C/hour, and maintained at that
temperature for 5 hours for glassification. Here, glassification was performed
under a chlorine gas atmosphere to remove hydroxy groups.
Lastly, the resultant was heated to 1,400~C at a rate of 100~C/hour under a
helium gas atmosphere, and the gel was sintered at that temperature for 4 hours,thereby forming a silica glass tube.
<Example 2>
A silica glass tube was manufactured by the same method as described in
Example 1, except that methyl lactate was used instead of methyl formate.
<Example 3>
A silica glass tube was manufactured by the same method as described in
Example 4, except that ethyl lactate was used instead of methyl formate.
<Example 4>
3.9wt% of methacryl amide, 0.16wt% of N,N'-methylenebisacrylamide were
dissolved in 95.94wt% of deionized water to prepare a premix solution.
500ml of the premix solution, 500g of the fumed silica (Aerosil OX-50,
Degussa Co.) and 52ml of TMAH aqueous solution (25.0wt% solution in water)
were mixed and dispersed using a high shear mixer to prepare a sol.
Then, air bubbles were removed from the sol using a vacuum pump, and
aged for 10 hours. 8ml of ammonium persulfate aqueous solution (5.0wt%
solution in water) was added to the aged sol, and the air bubbles were removed
CA 02246l~3 l998-08-28
from the reaction mixture. Then, 8g of methyl formate (1.6wt% based on the
weight of silica) was added to and mixed with the reaction mixture. Then, the
obtained mixture was poured into a mold, aged in an incubator at 70~C for 1 hour,
and then gelated into an intended shape.
Aftèr demolding the obtained moist gel, and dried in an incubator at 30~C
and 90% RH for 10 days. Then, the resultant was heated to 700~C at a rate of
50~C per hour, and then maintained at that temperature for 4 hours. Then, after
heating the dried gel to 1,100~C at a rate of 100~C/hour, the dried gel was
thermally-treated at 1,100~C for 5 hours. Here, the thermally-treating process was
performed under a chlorine gas atmosphere to remove the remaining hydroxy
groups.
Lastly, the resultant was heated to 1,500~C at a rate of 100~C/hour under a
helium gas atmosphere, and the gel was sintered at that temperature for 5 hours,thereby forming a silica glass tube.
Strength of the gels manufactured by Examples 1 through 4 was measured.
According to the result, strength of the gel was found to be increased compared
with the conventional case, so it is easy to handle the gel.
Also, it was observed whether cracks and shrinking of the silica glass tubes
manufactured by Examples 1 through 4 were generated.
As a result, in the silica glass tubes manufactured as in Examples 1 and 4,
cracking after drying process and shrinking were both decreased.
In the method for fabricating a silica glass according to the present
invention, a high purity silica glass tube, in which cracking after drying scarcely
occurs and shrinking ratio is markedly low, can be obtained. Also, a large silica
glass tube can be manufactured by this fabrication method.
The silica glass obtained by the fabrication method according to the present
invention can be applied to a silica glass for semiconductor devices, an opticallens and so on as well as for a optical fiber preform.