Note: Descriptions are shown in the official language in which they were submitted.
CA 02248226 2005-09-07
CLOT CAPTURE COIL
10 Field of the Inveg,~~~n
The present invention relates to medical devices that are useful in treating
thmmboembolic disorders and for removal of foreign bodies in the vascular
system.
Background of the vention
Thromboembolic disorders, such as stroke, pulmonary embolism, peripheral
thrombosis,
atherosclerosis, and the like, affect many people. These disorders are a major
cause of morbidity
and mortality in the United States.
Thromboembolic events are characterized by an occlusion of a blood vessel. The
occlusion is caused by a clot which is viscoelastic (jelly like) and is
comprised of platelets,
fibrinogen and other clotting proteins.
When an artery is occluded by a clot, tissue ischemia (lack of oxygen and
nutrients)
develops. The ischemia will progress to tissue infarction (cell death) if the
occlusion persists.
Infarction does not develop or is greatly limited if the flow of blood is
reestablished rapidly.
Failure to reestablish blood-flow can lead to the loss of limb, angina
pectoris, myocardial
infarction, stroke or even death.
Occlusion of the venous circulation by thrombi leads to blood stasis which can
cause
numerous problems. The majority of pulmonary embolisms are caused by emboli
that originate
in the peripheral venous system. Reestablishing blood flow and removal of the
thrombus is
highly desirable.
There are many existing techniques employed to reestablish blood flow in an
occluded
vessel. One common surgical technique, an embolectomy, involves incising a
blood vessel and
introducing a balloon-tipped device (such as the Fogarty catheter) to the
location of the
occlusion. The balloon is then inflated at a point beyond the clot and used to
translate the
obstructing material back to the point of incision. The obstructing material
is then removed by
the surgeon. While such surgical techniques have been useful, exposing a
patient to surgery may
be traumatic and best avoided when possible. Additionally, the use of a
Fogarty catheter is
problematic because of the great risk of damaging the interior lining of the
vessel as the catheter
is being withdrawn.
-1-
CA 02248226 1998-09-04
WO 97127808 PCT/US97/01807
Percutaneous methods are also utilized for reestablishing blood flow. A common
percutaneous technique is referred to as balloon angioplasty where a balloon-
tipped catheter is
introduced to a blood vessel, typically through an introducing catheter. The
balloon-tipped
catheter is then advanced to the point of the occlusion and inflated in order
to dilate the stenosis.
Balloon angioplasty is appropriate for treating vessel stenosis but is not
effective for treating
acute thromboembolisms.
Another percutaneous technique is to place a microcatheter near the clot and
infuse
streptokinase, urokinase or other thrombolytic agents to dissolve the clot.
Unfortunately,
thrombolysis typically takes hours to days to be successful. Additionally,
thrombolytic agents
can cause severe hemorrhage and in many patients the agents cannot be used at
all.
U.S. Patent Nos. 4,706,671 and 5,011,488 both describe the use of a coiled
section for the
removal of thromboembolic material. However, neither patent describes a device
that is
1 S marketed. U.S. Patent No. 4,706,671 teaches the use of a hollow flexible
elastomeric material
to form the shape of the coiled section. The coiled section is hollow to allow
for the insertion
of a liquid into the hollow center such that the coils become stiff. U.S.
Patent No. 5,011,488
teaches the use of a coiled section that is fixed on both the proximal and
distal ends such that the
operator of the device can change the shape and size of the coils. However,
this device may be
impossible to manufacture and is impossible to use in small vessels.
Another problematic area is the removal of foreign bodies. Foreign bodies
introduced into
the circulation can be fragments of catheters, pace-maker electrodes, guide
wires, and
erroneously placed embolic material such as thrombogenic coils. The only
available retrieval
devices for the removal of foreign bodies are devices which form a loop that
can ensnare the
foreign material by decreasing the size of the diameter of the loop around the
foreign body. The
use of such removal devices is difficult and sometimes unsuccessful.
Thus, there exists a need for the development of a device that can be easily
deployed into
the circulatory system for the removal of viscoelastic clots and foreign
bodies. There is also a
need for a device which could be used as a temporary arterial or venous filter
to capture and
remove thromboemboli formed during endovascular procedures.
-2-
CA 02248226 1998-09-04
WO 97/27808 PCT/US97/01807
Summary of the Invention
The present invention is a coil type device that is useful in removing clots
and foreign
S bodies in vessels. The invention comprises a catheter with at least one
Lumen. Located within
the catheter is a clot capture coil that is connected to an insertion mandril.
The clot capture coil
is made out of a solid elastic or superelastic material which has shape
memory. The elasticity
or superelasticity of the coil allows it to be deformed within the catheter
and to then reform its
original coil configuration when the coil is moved outside of the catheter
lumen.
In an alternate embodiment, the coil is made out of a biphasic material which
changes
shape upon heating or the passage of electrical current. The coil is straight
initially, and then
after passing electrical current or heat the coil changes to its coil
configuration.
Once the coil configuration has been established, the coil can be used to
ensnare and
corkscrew a clot in a vessel. The clot is extracted from the vessel by moving
the clot capture coil
and catheter proximally until the clot can be removed or released into a
different vessel that does
not perfuse a critical organ. The coil can also be used a temporary arterial
or venous filter to
capture and remove thromboemboli formed during endovascular procedures.
Foreign bodies are
captured by deploying the coil distal to the foreign body and moving the clot
capture coil
proximally until the foreign body is trapped within the coil. By removing the
device from the
body, the foreign material is also removed.
30
-3-
CA 02248226 1998-09-04
WO 97/27808 PCT/US97/01807
Brief Description of the Drawings
Embodiments of the invention will now be described with reference to the
following
drawings which:
FIG. 1 a is a schematic illustration of an occluded artery with a
microcatheter and the clot
capture coil of the present invention;
FIG. 1 b is a schematic illustration of an occluded artery with a
microcatheter and a clot
capture coil inserted through an occlusion;
FIG. 1 c is a schematic illustration of the deployment of the clot capture
coil within an
occluded artery;
FIG. 1 d is a schematic illustration of the clot capture coil of the present
invention
encountering a clot in an occluded artery;
FIG. 1e is a schematic illustration ofthe clot capture coil ensnaring the clot
in an occluded
artery;
FIG. 1 f is a schematic illustration of the clot of FIG. 1 a being moved
within an occluded
artery via the clot capture coil;
FIG. 1 g is a cross section of the artery and the catheter of FIG. 1 a along
line 1 g-1 g.
FIG. 2a is a schematic illustration of an occluded artery and an alternate
embodiment of
the clot capture coil;
FIG. 2b is a schematic illustration of a microcatheter passed through a clot
within an
occluded artery and the extended coil of the clot capture coil within the
catheter;
FIG. 2c is a schematic illustration of the deployment of the clot capture coil
in an
occluded artery;
FIG. 2d is a schematic illustration of a clot capture coil ensnaring a clot
within an
occluded artery;
FIG. 2e is a schematic illustration of the removal of a clot via a clot
capture coil
illustrating the corkscrewing and ensnaring effect of the coil within the
viscoelastic clot;
FIG. 2f is a cross section of the artery and catheter of FIG. 2d at line 2f
2f.
FIG. 3 is an alternate coil configuration;
FIG. 4 is an alternate coil configuration;
FIG. 5 is a further coil configuration;
FIG. 6 is an additional coil configuration;
FIG. 7 is a further coil configuration;
FIG. 8 is a double helix coil configuration and a single lumen catheter;
FIG. 9 is a double helix coil configuration and a double lumen catheter;
FIG. 1 Oa is a schematic illustration of the clot capture coil and an
introducer;
FIG. l Ob is a schematic illustration of a clot capture coil straightened
within the inner
-4-
CA 02248226 1998-09-04
WO 97/27808 PCT1US97/01807
lumen of the introducer of FIG. 1 Oa;
FIG. 11 is a plan view of the present invention being deployed within an
introducing
catheter with a side suction port;
FIG. 12 is a schematic view of the present invention being deployed within an
introducing
catheter such that the coil section is within the inferior vena cava of a
patient;
FIG. 13 is an alternate coil configuration that is particularly useful for
removing clots in
a surgically created arteriovenous fistula of a hemodialysis patient;
FIG. 14 is a further coil configuration; and
FIG. 15 is another coil configuration.
20
30
-5-
CA 02248226 1998-09-04
WO 97/27808 PCTIUS97/01807
Description of the Preferred Embodiments
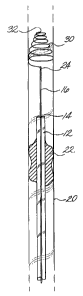
Turning now to FIGS. 1 a-1 g, a clot capture coil device 10, is generally
illustrated within
an artery 20 with a clot 22. The device comprises a catheter 12 with at least
one lumen 14, a clot
capture coil 18, and an insertion mandril 16.
The catheter 12 can be any commercially available catheter that is made out of
any
appropriate biologically compatible material. Typically, the catheter will
have a single lumen
14 and is constructed out of a flexible elastomeric materials such as
silicone, rubber,
polyvinylchloride, polyurethanes, polyesters, polytetrafluoroethylene, and the
like. The catheter
has to be flexible enough and long enough to navigate through blood vessels to
the occluded
vessel 20 where clot 22 is located. Typically the catheter will range in
length from about 20 to
about 175 cm.
The outer diameter of the catheter can also vary. Typically the outer diameter
will range
from about 2 to about 10 French (one French = 0.013 inch). The inner diameter
will range from
about 1 to about 9 French.
The insertion mandril 16 has to be relatively stiff to support the coil 18. In
the preferred
embodiment, the insertion mandril is made out of stainless steel and is a
solid wire of from about
0.006 to about 0.038 inches in diameter. Other materials could be used such as
a hard plastic,
nitinol, and the like to make the insertion mandril. The insertion mandril is
10 to 20 cm longer
than the catheter such that the operator of the device (typically a physician)
can control the
insertion mandril by gripping the proximal end which extends from the proximal
end of the
catheter.
Connected to the insertion mandril is the clot capture coil 18. In one
embodiment, the coil
is made from a flexible solid elastic or superelastic material which has shape
memory, i.e., it can
deform to a straighten position and then return to a resting coil
configuration. In a preferred
embodiment, the coil is made out of a solid nitinol wire with a diameter of
about 0.001 to about
0.038 inches. The use of nitinol in medical devices is well known in the art.
Nitinol is preferred
because of its superelasticity and its shape memory. However, other solid
materials that are also
elastic or superelastic and have shape memory could also be used such as some
synthetic plastics,
metallic alloys, and the like. To make the coil, the nitinol wire is wrapped
around a mandrel into
the coil configuration. The nitinol is then heated to an appropriate
temperature such that the
nitinol wire adopts the coil configuration as its resting shape upon cooling.
The diameter of the
coils can vary depending on the size of the vessel occluded. The diameter can
range from about
1 mm for small vessels to about 30 mm for large vessels such as the pulmonary
arteries or
inferior vena cava. The length of the coil can also vary but typically ranges
from about 3 to
about 100 mm in the proximal to distal direction. Because the nitinol coil is
superelastic, the coil
can be extended to a completely straight configuration with the use of minimal
force and then
-6-
CA 02248226 1998-09-04
WO 97/27808 PCT/US97/01807
reform to its natural resting configuration when the force is removed. In use,
the coil is extended
by using the insertion mandril to insert the coil and the mandril into the
narrow lumen of the
catheter.
In an other embodiment, the coil is made out of a solid biphasic material
which changes
shape upon heating or the passage of electric current. A presently preferred
material is biphasic
nitinol which has a straight configuration initially, and changes to a coiled
configuration upon
the passage of electric current or heating. The use of biphasic nitinol is
well known in the
medical arts for other purposes. The biphasic nitinol coil would be made using
ordinary skill in
the art such that the nitinol coil is straight initially and then forms the
appropriate coil
configuration. As would be apparent to a person skilled in the art, the
biphasic coil could also
be constructed such that the initial coil configuration is the normal shape
and that the biphasic
coil straightens upon passing electric current or heating. The coil dimensions
would be similar
to the dimension detailed above for the shape memory coil.
The coil section of either the shape memory coil or the biphasic coil can have
many
different configurations. Similar reference numerals are used throughout the
figures to indicated
similar components of the embodiments. In the embodiment illustrated in FIGS.
1 a-I f, the coil
is barrel shaped such that the diameter of the coils are relatively small at
the distal and proximal
ends of the coil and is relatively large in the center of the coil. In a
typical coil configuration,
the diameters of the coils range from 2 mm at the proximal and distal ends and
expand to 10 mm
in the center. However, other sizes are also useful depending on the relative
size of the occluded
vessel. At the proximal end of the coil is a small circular loop 26. In the
preferred illustrated
embodiment, the circular loop is placed around the stainless steel wire and is
freely slidable over
the wire. The distal end of the barrel shaped coil is permanently connected to
the distal end 24
of the insertion mandril. Thus, in this embodiment the coil extends proximally
from the distal
end of the insertion mandrel. In the preferred embodiment the coil is welded
onto the distal end
of the insertion mandril. Other means of permanently connecting the coil could
also be used
such as crimping the coil, gluing the coil, screwing the coil into a screw
type mount, and the like.
A different coil configuration is illustrated in FIGS. 2a-2f. In this
embodiment, the coil
30 is connected at its proximal end to the distal end 24 of the insertion
mandril 16. Thus, the coil
extends distally from the distal end of the insertion mandril. The distal end
32 of the coil is free
floating. The coil is comically shaped with the diameter of the coils
decreasing distally to the free
end 32. Embodiments where the coil is connected to the proximal end are
preferred for use in
removing clots from small and/or tortuous vessels as will be discussed below.
The size and shape of the coils can vary and different representative
embodiments are
illustrated in the different figures. FIG. 3 illustrates an alternate
embodiment where the coil 34
is attached at its proximal end to the distal end of the insertion mandril I
6. Thus, the coil extends
CA 02248226 1998-09-04
WO 97!27808 PCT/US97/01807
distally away from the distal end of the insertion mandril. The coil is shaped
like an inverted
cone with the diameter of the coils increasing distally. This embodiment is
particularly useful
for retrieving clots from small (1-2 mm diameter) vessels in the cerebral and
coronary
circulations. The diameter of the coils in this configuration are typically
from about 1 mm to
about 3 mm, could be larger depending on the relative size of the occluded
vessel.
FIG. 4 illustrates a cone shaped coil 36 where the distal end of the coil is
connected to the
distal end of the insertion mandril. FIG. 5 is a similar embodiment to FIG. 4
except that the coil
38 is wound tighter such that there are more revolutions per inch. In both
FIGS. 4 and S the coil
section extends proximally from the distal end of the insertion mandril.
FIG. 6 illustrates a different embodiment where the proximal end of the coil
42 is
connected to the insertion mandril's distal end. The coil is shaped like an
inverted cup which has
a constant diameter until the coil reaches its most distal end where the
diameter decreases.
FIG. 7 is a similar embodiment to FIGS. la-if except that the barrel shaped
coil 40 is
connected to the distal end of the insertion mandril such that the coil
extends distally instead of
proximally.
The embodiment of FIG. 8 is a double helix coil configuration that is useful
for large clot
removal. The configuration is such that one continuous piece of wire is used
to form the double
helix configuration. Both ends of the coil 44 and 46 are connected to the
distal end of the
insertion mandril 16. In the preferred embodiment, both ends are welded onto
the insertion
mandrel 16 at weld lines 45 and 47. The coil has been heat treated such that
it forms a resting
double helix shape. The two helixes 48 and 50 intertwine and are connected at
the top of each
helix at point 52. When the double helix coil is withdrawn into the single
lumen catheter by
translating the insertion mandril, the helixes straighten until the coils are
completely withdrawn
into the catheter's lumen. By translating the insertion member in the opposite
direction, the coil
is forced out of the lumen of the catheter and then reforms the double helix
configuration.
FIG. 9 is an alternate double helix embodiment where the double helix is used
in
conjunction with a double lumen catheter 56. The lumens 58 and 60 each receive
an insertion
mandril 16 and 16'. Each insertion mandril in turn is permanently connected to
one of the ends
of the coil. At the proximal end of the insertion mandrills are optional
connecting bars 17 which
keep the relative spacial relationship of each insertion mandril constant. In
this embodiment, as
the helixes are withdrawn into the catheter, each one straightens out and is
kept separate within
the respective lumens. When the helixes are then deployed by translating the
insertion mandril,
the helixes reform the double helix configuration. The optional connecting
bars 17 are used to
ensure that each helix is being deployed by the translation of the insertion
mandrils are in unison
with each other such that the double helix configuration is always obtained
upon full
deployment.
_g_
CA 02248226 1998-09-04
WO 97!27808 PCT/US97/01807
FIG. 13 illustrates a long coil 140, ranging from about 2 cm to about 10 cm
that is
especially useful for removing clots in a surgically created arteriovenous
fistula of a
hemodialysis patient. The coil could also be used for removing long clots in
the venous system
and long clots in a surgically created by-pass graft. The arteriovenous
fistulas are normally
surgically created on the forearm of a hemodialysis patient and allows for
easy access to the
blood stream for hemodialysis treatment. Unfortunately, these fistulas often
become clogged
with long blood clots and have to be surgically repaired or a new fistula
created. The long clot
capture coil 140 is connected to the insertion mandril 16 at the coil's
proximal end.
FIGS. 14 and 15 illustrate two further coil configurations. FIG. 14 is a
cylindrical coil
150 attached to the distal end of the insertion mandril 16. FIG. 15 is a
random tangle coil
attached to the insertion mandril 16. The random tangle is manufactured by
extruding the coil
material in a random fashion. The random tangles made by such a process would
vary each time
the tangles are manufactured, and thus, the random tangle pictured in FIG. 15
is for illustration
only.
FIGS. !0a and lOb illustrate the use of an introduces 72 with lumen 74. The
introduces
is a relatively short (10 to 20 mm long) single lumen catheter that is used to
straighten the coil
section of a shape memory coil which extends distally, such as the coils in
FIGS. 2, 3, 6, and 7,
prior to insertion into the catheter 12 of the present invention. A longer
introduces would be
used for the arteriovenous fistula coil of FIG. 13. The insertion mandril is
inserted into the
introduces in a retrograde direction (indicated by the arrow in FIG. 1 Oa).
Once the introduces
reaches the shape memory coil section, the coil section straightens out almost
to a complete
straight line. In coil section that extend distally outward, the inner
diameter of the introduces and
the catheter are sized to be just slightly larger than the diameter of the
insertion mandril and the
coil section. That is, if the insertion mandril and the coil are each made
from 0.008 inch
diameter wires, then the inner diameter of the introduces is preferably 1 to 2
French. Once the
coil has been straightened out completely, the coil within the introduces is
aligned with the
catheter and then advanced in an anterograde direction into the catheter.
The above detailed description describes some of the numerous embodiments of
the
present invention. Below is a discussion of some of the numerous uses of the
invention.
In use, a patient presenting with symptoms of a thromboembolic disorder is
examined
radiographically using angiography to locate an occlusion and to confirm the
diagnosis. A large
introducing catheter 130 (see FIG. 12) is then inserted into an appropriate
vessel (usually the
3 5 femoral artery or the femoral vein). A small catheter or microcatheter 12
is then introduced into
the vessel via the introducing catheter and advanced using a guide wire or the
like into the
occluded vessel. The catheter 12 is then passed through the viscoelastic clot.
Once the catheter
is in place and through the viscoelastic clot the clot capture coil is
introduced into the catheter
-9-
CA 02248226 1998-09-04
WO 97/27808 PCT/US97/01807
using the insertion mandril and advanced to the distal tip of the catheter.
For the shape memory
clot capture coils that extend proximally from the insertion mandril (as in
FIGS. 1 a-1 f, the coil
and the insertion mandril are inserted directly into the proximal end of the
catheter and advanced
to the distal end (see FIG. 1b). For the shape memory clot capture coils that
extend distally from
the insertion mandril (as in FIGS. 2a-2e), the introducer of FIGS. 10a and lOb
is used as
described above. For the biphasic coils, the coils are introduced in the
straight configuration by
either having the straight configuration the natural configuration or by
straightening a natural
coil configuration by passing electric current or heating the coil.
Once the catheter and the clot capture coil have transversed the clot, the
insertion mandril
is translated distally relative to the catheter. With a shape memory coil, the
coil deploys and
reform its natural configuration outside the distal end of the catheter. By
comparing FIGS. 1 c
and 2c it is apparent that the shape memory coils which extend distally from
the insertion
mandril immediately start to form the coil configuration once part of the coil
is freed from the
confines of the lumen of the catheter. These embodiments are particularly
useful for clot
removal in vessels that are small and/or tortuous where there is not much room
for the
advancement of the insertion mandril and the coil. In the embodiments where
the shape memory
coil extends proximally from the distal end of the insertion mandril, the
entire length of the coil
needs to be freed from the confines of the lumen of the catheter before it
reforms the coil
configuration. These embodiments are useful for the removal of large clots in
large vessels
because the coil is better supported and the coils can collapse upon each
other. For example,
as illustrated in FIGS. 1 c-1 f the proximal end of the coil which is a
slidable loop 26 mounted
around the insertion mandril will encounter the clot material first. The
slidable loop then slides
distally until the coils form a double inverted cone shaped configuration. The
coils will overlap
and thus give more support for the removal of large clots.
The biphasic coils are deployed similarly except that electric current or heat
is used to
form the coil configurations if the straight configuration is the natural
shape. If the coil
configuration is the natural shape, then the user stops applying electric
current or heat and the
coil configuration will reform.
The clot is then retrieved by translating the insertion mandril along with the
catheter
proximally. When the clot capture coil is pulled proximally the clot becomes
ensnared.
Additionally, while pulling proximally on the insertion mandril, the coil is
rotated by rotating
the insertion mandril to transfix the clot by corkscrewing the clot into the
coils. The viscoelastic
properties of the clot allow the clot to be captured within the side coils and
to be pulled down
using the most distal coils as a capture cup. The clot can then be completely
removed or released
into a vessel that does not perfuse a critical organ such as an external
carotid artery.
A particularly useful introducing catheter is illustrated in FIG. 11. The
introducing
-10-
CA 02248226 1998-09-04
WO 97/27808 PCT/US97/01807
catheter 110 is hollow with a single lumen and has a Y junction towards its
proximal end. The
introducing catheter is a standard commercially available introducing
catheter. The introducing
catheter has two ports, 112 and 114. Port 112 is in straight communication
with the longitudinal
axis of the introducing catheter and is useful for the insertion of the
catheter 12, coil 30 and
insertion mandril 16 of the present invention. The other port which is angled
away form the
longitudinal axis of the insertion catheter is for the attachment to a suction
line from a vacuum
source. Located at the distal end 116 of the introducing catheter is a marker
band 118 that can
be located via radiographic means while the introducing catheter is being
used.
In practice, the introducing catheter 110 is inserted through a large vessel
and through the
vascular system to a position near a clot in an occluded artery under
fluoroscopic guidance. The
catheter 12, is then inserted through port 1 I2 and through the introducing
catheter such that the
distal end of the catheter 12 has passed the distal end 116 of the introducing
catheter. The
catheter 12 is then translated across the clot. The coil 30 and insertion
mandril I6 are then
inserted into the catheter 12. The insertion mandril is then translated
through the catheter 12
until the coil 30 is deployed in the vessel. The insertion mandril is then
translated proximally
to ensnare the clot within the coil and then the catheter, coil and clot are
translated toward the
distal end 116 of the introducing catheter 110. Once the clot and the coil are
at the distal end
116, suction is applied via port 114 to suck part of the clot into the distal
end 116. The suction
helps to keep the clot within the coil. Then the introducing catheter 110, the
catheter 12, the clot
and the coil 30 are removed from the patient.
FIG. 12 illustrates the invention being used as a filter in the inferior vena
cava of a patient
with a venous thrombus in a lower limb. A commercially available introducing
catheter 130 is
advanced into a femoral vein I22 and into the inferior vena cava 128 below the
heart 126. A
catheter 12 is then advanced through the introducing catheter. The coil 120
and insertion
mandril 16 are then advanced through the catheter 12 and the coil I20 is
deployed within the
inferior vena cava. The coil 120 has a large diameter, around 20 mm to 30 mm,
such that when
deployed it fits snugly within the inferior vena cava. The coil acts as a
filter wherein pieces of
the thrombus become trapped in the coil instead of being transported to the
lungs. The thrombic
material can then be removed from the patient.
Foreign bodies are removed as described above except that the foreign body
becomes
ensnared in the clot capture coil instead of a clot. Due to the numerous
coils, it is much easier
to ensnare a foreign body than using a loop type device.
The following examples illustrate some of the uses of the invention. The
examples are
provided for illustration purposes and are not meant to limit the invention to
the specific
examples.
-11-
CA 02248226 1998-09-04
WO 97/27808 PCT/US97/01807
EXAMPLE 1
The clot capture coil was clinically tested in pigs. In the f rst study a
pig's femoral artery
was isolated and a large commercially available introducing catheter was
inserted into the
femoral artery. Arterial blood was then withdrawn and allowed to clot in
vitro.
An arterial catheter was then inserted through the introducing catheter and
into the carotid
artery. The coagulated arterial blood was then released into the carotid
artery branches via the
arterial catheter resulting in the formation of numerous emboli.
Angiography was used to locate the emboli. While preforming angiography a
microcatheter (outer diameter of 3 French and inner diameter of 1 French) was
inserted into an
occluded carotid artery using a guide wire for placement and standard
microcatheter placement
techniques. The microcatheter was advanced distally past the clot. The guide
wire was then
withdrawn from the microcatheter.
A shape memory clot capture coil connected to an insertion mandril was then
introduced
into the microcatheter using a small introduces. The coil configuration was
the type illustrated
in FIG. 2a. Because the coil extends distally from the insertion mandril a
small introducing
catheter had to be used to introduce the clot capture coil into the
microcatheter. The insertion
mandril and the clot capture coil was inserted in a retrograde direction into
the introducing
catheter. The inner diameter of the introducing catheter was identical to the
microcatheter. The
clot capture coil became straight due to the superelastic properties of the
coil and the small inner
diameter of the introduces. Once the coil was completely within the
introduces, the introduces
was aligned with the microcatheter and the coil was inserted into the
microcatheter in an
anterograde direction.
The clot capture coil was slowly advanced to the distal end of the
microcatheter by
translating the insertion mandril. As the insertion mandril was advanced, the
coil began to be
expressed from the distal end of the microcatheter. As more and more of the
coil was expressed,
the coil deployed and returned to its natural resting coiled shape as in FIG.
2c.
The clot capture coil was then pulled proximally to ensnare the clot. While
pulling
proximally, the coil was rotated by rotating the insertion mandril to transfix
the clot by
corkscrewing the clot into the coils. The clot was then completely removed
from the pig by
removing the microcatheter, insertion mandril, and the clot within the clot
capture coil from the
pig's femoral artery.
EXAMPLE 2
The procedure of example 1 was repeated using a shape memory clot capture coil
configuration illustrated in FIG. 3. The clot was successfully corkscrewed and
ensnared and
removed from the pig's occluded cerebral artery.
-12-
CA 02248226 1998-09-04
WO 97/27808 PCT/US97/01807
EXAMPLE 3
The procedure of example 1 was repeated using a shape memory clot capture coil
as
illustrated in FIG. 4. Because this embodiment has the coil extending
proximally from the distal
end of the insertion mandril, the clot capture coil was directly inserted into
the microcatheter
without the use of a small introducer. A clot in an occluded carotid artery
was ensnared in the
coil and completely removed.
Thus, a clot capture coil is disclosed which allows for the removal of
thromboembolic
material and foreign bodies from a blood vessel. While embodiments and
applications of this
invention have been shown and described, it would be apparent to those skilled
in the art that
many more modifications are possible without departing from the inventive
concepts herein. The
invention, therefore, is not to be restricted except in the spirit of the
appended claims.
20
30
-13-
. ~ ~5! '-: :1.. ":