Note: Descriptions are shown in the official language in which they were submitted.
CA 02248679 1998-09-10
W O 97/33678 PCT~DK~7/OOlOS
A METHOD FOR REMOVING NITROGEN OXIDES, SULFUR OXIDES, AND
OTHER ACID GASES FROM A GAS STREAM
~ 5 The present invention relates to a method for removing
nitrogen oxides, sulfur oxides, and other acid gases from
a gas stream in which
- an adsorption agent suitable for adsorbing nitrogen
oxides, sulfur oxides, and other acidic gases is
introduced and contacted with the gas stream in a
reaction zone wherein the nitrogen oxides, sulfur oxides,
and other acidic gases are adsorbed by the adsorption
agent,
- whereafter the adsorption agent is at least partially
withdrawn from the reaction zone with the gas stream as
an entrained suspension and substantially removed from
the gas stream in a separation zone,
- whereafter the exit gas from the separation zone is
discharged into the atmosphere,
- while separated adsorption agent is introduced into a
regeneration zone where the adsorbed nitrogen oxides,
sulfur oxides, and other acidic gases are substantially
removed from the adsorption agent and withdrawn from the
regeneration zone in a concentrated form,
- while the regenerated pulverous adsorption agent is
recycled to the reaction zone.
Various processes for removing nitrogen oxides and sulfur
oxides from a gas stream, e.g. from flue gas from power
plants and incinerators are known.
Most of these fall within one of the following main
groups:
(1) Wet methods, where the gas is scrubbed with
aqueous suspensions or solutions of hydroxides or
CA 02248679 1998-09-10
W097/33678 PCT~K97/00105
carbonates of alkali or alkaline-earth metals wherein the
reaction products are withdrawn as a sludge.
(2) Semi-dry methods, where the gas is brought into
contact with aqueous suspensions or solutions of
hydroxides or carbonates of alkali or alkaline-earth
metals under such conditions that the water is evaporated
and the reaction products are withdrawn as a dry powder.
(3) Dry methods, where the gas is brought into
contact and reacted with dry adsorption agents in a
reaction zone, e.g. in a fixed or a fluidized bed or in
an entrained suspension in the gas to be treated,
whereafter the reaction products are withdrawn as a dry
solid material which can be deposited as an end product
or subjected to further processing in a regeneration zone
and recycled to the reaction zone.
The methods according to the present invention may be
classified as dry methods with gas-solid contact and
regeneration of the adsorption agent.
Dry methods with regeneration of the adsorption agent
have been described in a number of articles, patents and
patent applications:
A number of patents claiming priority from US patent
application No. 659996 (priority date 12 Oct. 1984) such
as UK patent No. GB 2176771 B, US patent No. 4,755,499,
US patent No. 4,798,711, US patent No. 4,940,569, and CA
patent No. 1 261 314 describe the so-called NOXSO Process
in which a flue gas stream containing both nitrogen
oxides (NO) and sulfur oxides (SO) is passed through a
fluid bed adsorber containing suitable sorbent particles
or beads, such as for example those disclosed in the
above-mentioned US patent No. 4,755,499.
CA 02248679 1998-09-10
W097/33678 PCT~K97/00105
The various stages in the development of the NOXSO
process have been reported in a number of publications,
vide e.g. Yeh, J.T., et al., "The NOXSO Process:
Simultaneous Removal of SO2 and NO from Flue Gas", 1987
ATChE Spring National Meeting, Houston, Texas, March 29-
April 2, 1987; Yeh, J.T. et al, Integrated testing of the
NOXSO process: Simultaneous removal of SO~ and NO~ from
flue gas, Chem. Eng. Comm. 114, (1992), 65-88; Neal, L.G.
et al, Pilot Plant Test Data For The NOXSO Flue Gas
Treatment System, presented at the 4th International
Conference on Processing and Utilization of High Sulfur
Coals, Idaho Falls, Idaho, August 26-30, 1991; Ma, W.T.
et al, NOXSO SO~/NO~ flue gas treatment process,
Adsorption chemistry and kinetics, presented at the 1994
AIChE annual meeting, San Fransisco, November 1994, paper
214c; and Ma, W.T. et al, NOXSO SO2/NO~ flue gas
treatment process, Regeneration chemistry and kinetics,
presented at the 1995 AIChE Summer National meeting,
Boston, July 1995, paper 16e.
According to the NOXSO process nitrogen oxides and sulfur
oxides are adsorbed on the sorbent beads and removed from
the gas stream. The beads consist of a 840-2000 ~lm
alumina carrier activated with alkali or alkaline-earth
metal oxides.
The adsorption can take place in a single or multiple
stage fluid bed reactor.
The adsorption of SO and NO. on the sorbent proceeds in
several steps through a complex surface chemistry
mechanism which is not fully understood. A simple overall
adsorption reaction which may be used as a partial basis
for process design is:
CA 02248679 1998-09-10
W097/33678 PCT~K97/00105
4 Na~O + 3 SO + 2 NO + 3 O- = 3 Na~SO~ + 2 NaNO~
The sorbent particles having adsorbed the SO and NO
from the gas is transported to a fluid bed heater wherein
the NO. is liberated by raising the temperature of the
sorbent particles above 532~C using air as heating media.
Since both NO and NO2 are seen to evolve in this first
regeneration step, the overall reactions may be shown as:
2 NaNO3 = Na2O + 2 NO2 + ~O~
2 NaNO3 = Na2O + NO2 + NO + O,
The NO. is thus stripped from the sorbent particles and
carried away in the heating gas stream.
The hot sorbent particles with the NOy removed therefrom
are transferred into a two stage moving bed regenerator
where they are contacted with a suitable regenerant gas
stream in the first stage and steam in the second stage.
As regeneration gases are mentioned H , CO, H2-CO
mixtures, H2S, CH~, C3HR, Natural gas, and the before
mentioned gases mixed with CO- and/or H2O. Other gases
mentioned are all gases that will result in the formation
of H2S at regenerator process conditions, among these
are COS and CS~.
Both SO~, H2S, CO~ and H.O have been observed to evolve in
this second regeneration step. Therefore it may be
assumed that the following overall reaction ta~es place
in the regenerator:
a Na~SO~+ b CH~= c Na2O + d H?S + e SO~ + f CO~+ g H2O
SO- and H-S evolved from the second regeneration step are
converted into elemental sulfur in the Claus process:
CA 02248679 1998-09-10
W097/33678 PCT~K97/00105
SO + 2 H~S = 3 S(-) + 2 H'O
The regenerated sorbent particles are cooled in a fluid
bed cooler and recirculated to the moving bed adsorber.
A new variant of the NOXSO Process, in the following
referred to as the "ES-NOXSO Process" (entrained
suspension NOXSO Process), is disclosed in EP 549 891 A1
and EP 657 203 A2:
The sorbent bead particles (840-2000 ~m) are replaced
with sorbent powder particles of the size 30-500 ~lm. In
this process the adsorption is performed in an entrained
suspension adsorber.
The advantage of the ES-NOXSO process is found in the
fact that the adsorption rate for the comparatively large
NOXSO sorbent beads (1.23 mm) is diffusion controlled
while the adsorption rate in the ES-NOXSO process is
chemically controlled.
According to the ES-NOXSO Process as disclosed in EP 549
891 A1 NO and SO are removed from the gas in a process
comprising the following steps:
- introducing and suspending sorbent particles suitable
for adsorbing NO and SO and having a size in the range
from about 140 mesh (105 ~m) to about 70 mesh (210 ~m) in
the gas stream containing NOX and SO to be removed;
- transporting said sorbent particles through a transport
line adsorber to cause said sorbent particles to
substantially adsorb said NOj and SO from said gas;
- separating said sorbent particles from said gas to
provide a stream of substantially NO and SO free gas;
- discharging the sorbent free gas stream to the
atmosphere;
CA 02248679 1998-09-10
W O 97/33678 PCTADK~7/00105
- heating said separated sorbent particles in entrained
suspension in a multi-cyclone heater to remove NO.- from
said sorbent particle and to produce an off gas stream of
hot gas carrying away said NOy ;
- contacting said heated sorbent with a regenerant gas to
substantially remove said SOy from said sorbent
particles and to produce heated regenerated sorbent and
an off gas stream of regenerant gas carrying away said
SOx removed from said heated regenerated sorbent;
- cooling said heated regenerated sorbent in entrained
suspension in a multi-cyclone cooler to produce cooled
regenerated sorbent; and
- returning said cooled regenerated sorbent to the first
step.
According to EP 657 203 A2 this process may be modified
in the following way:
sorbent particles of an average size in the range of
about 30 ~m to about 500 ~m are used; and
the stream of separated sorbent particles is fed to a
sorbent particle splitter where it is divided into a
first stream which is recirculated directly to the first
NO and SO~ adsorption step and a second stream which is
directed to the multi-cyclone heating NO; removal step.
It is mentioned in the patents that the ES-NOXSO process,
compared to the N~XSO process, has the disadvantage that
the relatively small sorbent particles are not easily
distinguished from fly ash or other pulverous solids
suspended in the gas stream, hence requiring an efficient
particle removal both upstream and downstream of the
adsorber to prevent accumulation of fly ash or other
pulverous solids in the sorbent, and to prevent discharge
of sorbent particles into the atmosphere.
CA 02248679 l998-09-lO
WO 97/33678 PCT~DK~7/00105
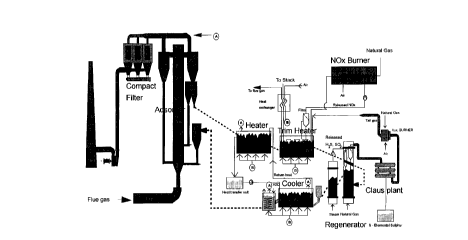
The SNAP Process.
A modification of the ES-NOXSO process, the so-called
SNAP Process is disclosed by Leif Mortensen, SK Power
~ 5 Company, Copenhagen, Denmark, Stig Bue Lading, FLS milj0
a/s, Copenhagen, Denmark, and Mark C. Woods, NOXSO
Corporation, Bethel Park, Pennsylvania in "Experiences
from a 10 MWe ~emonstration Project with an innovative
SO and NO Adsorption Process (SNAP)", EPRI DOE EPA 1995
SO, Control Symposium, Miami Florida, March 28-31 1995.
The major components of the SNAP process accomplish the
same basic operations as occur in the bead or powder
based process according to the NOXSO/ES-NOXSO concept.
The ma~or components of the SNAP process are shown
schematically in Figure 1. The major components of the
SNAP process as shown in Figure 1 are described below.
Gas Suspension Adsorber (GSA).
The flue gases from a coal-fired power plant are
introduced into the GSA reactor downstream of the plant's
particulate control unit.
The GSA reactor comprises a riser with suitably designed
conical inlet, cyclones for primary gas-solid separation,
and means for solid recirculation to the bottom of the
riser. Flue gas enters the reactor through the conical
inlet at the bottom, and contacts suspended sorbent which
adsorbs SO- and NO . The GSA reactor design allows for
high flue gas velocity (3-6 m/s) and gas-solid contact
time of 2-3 seconds, at a relatively low pressure drop
(200-300 mm WG). The sorbent entrained in the flue gas is
separated in the cyclones and is partly recycled to the
bottom of the reactor, partly fed to the regeneration
unit.
CA 02248679 1998-09-10
W097/33678 PCT~K97/00105
Fine sorbent particles entrained in the gas~ exiting the
GSA cyclones are removed in a particulate control unit
and returned to the process. Clean flue gas proceeds
through a booster fan directly to the stack from where it
is released into the atmosphere.
This type of set-up necessitates, like the ES-NOXSO
process, two particulate control devices:
- one up-stream of the adsorber for removing the fly-ash,
and
- one down-stream of the adsorber for removing the
uncollected fine sorbent entrained in the flue gas.
These two particulate control devices must be suitably
designed in order to avoid accumulation of fly ash in the
system and in order to comply with the particulate
emission requirements.
Sorbent heater section
The sorbent heater section represents the first stage of
the sorbent regeneration system. A slip stream of loaded
sorbent from the GSA cyclone recycle loop and sorbent
collected in the particulate control unit are introduced
into the heater.
The first sorbent heater is a multi-stage fluidized bed
where the sorbent is heated by indirect heat transfer
from heat transfer coils immersed in the fluidized bed.
The heat transfer medium inside the heating coils is Hi
Tec heat transfer medium. In this heater the sorbent
reaches a temperature around 300~C.
The first heater is followed by a second heating step
where the sorbent is heated by indirect heat transfer
from heat transfer coils immersed in the fluidized bed.
The heat transfer medium inside these heating coils is
CA 02248679 1998-09-10
W097/33678 PCT~K~7/00105
combustion off-gases from the NOx destruction unit and
the auxiliary burner. In this heater the sorbent reaches
a temperature around 620~C.
During the second heating step all NO adsorbed on the
sorbent is released. Since the fluidized bed sorbent
heater is operated at low air velocity, the total volume
of this NO. bearing stream is relatively small. The NO
in this air stream is reduced to N~ by staged combustion
in the NO- destruction burner.
Regenerator.
The sorbent regenerator is a two-stage fluidized bed. In
the first stage the heated sorbent is contacted with a
regeneration gas, which also serves as the fluidization
medium. The sulfated sorbent reacts with regeneration
gas, and sulfur is released as SO2 and H2S. In the second
stage H S remaining on the surface is stripped off with
steam. Sodium sulfide, which may be formed during the
regeneration, is also converted into H.S by steam
hydrolysis. The paper describes that the regeneration can
be adopted to use several types of regeneration gases,
e.g. natural gas, hydrogen, fuel gas etc.
Sorbent cooler section.
The sorbent cooler section represents the last stage of
the sorbent regeneration system.
The first sorbent cooler is a multi-stage fluidized bed
where the sorbent is cooled by indirect heat transfer
from heat transfer coils immersed in the fluidized bed.
The heat transfer medium inside the coils is Hi Tec heat
transfer medium. In this cooler the sorbent reaches a
temperature around 300~C.
CA 02248679 l998-09-lO
W 097/33678 PCT~DK97/00105
The heated Hi Tec heat transfer medium is from the cooler
clrculated to the heater, which ensures that the energy
consumption in the heating process is minimized.
The first cooler is followed by a second cooling step
where the sorbent is cooled by indirect heat transfer
from heat transfer coils immersed in the fluidized bed.
The heat transfer medium inside these cooling coils is a
water/glycol solution. The temperature of the sorbent
after this final cooling step is in the range 100-300~C.
Claus unit.
The sulfur compounds from the regeneration step are fed
to a Claus unit, where they are converted into elemental
sulfur.
The tail gas from the Claus process is passed through a
burner to convert all remaining sulfur compounds into
SO,. The gas is then cooled and recycled to the flue gas
stream entering the GSA.
The problem to be solved according to the present
invention is to provide an improved version of dry
adsorption processes for removal of e.g. nitrogen oxides,
sulfur oxides and other acid gases from a gas stream with
regeneration and recirculation of the adsorption agent,
such as e.g. the SNAP process.
A major drawback of these processes is the requirement
for an efficient particulate control device upstream of
the reaction zone in order to avoid contamination of the
adsorption agent with fly ash or other particulates
contained in the inlet gas.
Thus, according to a first aspect of the present
invention this problem is solved by a method for removing
CA 02248679 1998-09-10
W097/33678 PCT~K~7/~105
11
nitrogen oxides, sulfur oxides, and other acid gases from
a gas stream containing fly ash and/or other solid
materials in which
- an adsorption agent suitable for adsorbing nitrogen
oxides, sulfur oxides, and other acidic gases is
introduced and contacted with the gas stream in a
reaction zone wherein the nitrogen oxides, sulfur oxides,
and other acidic gases are adsorbed by the adsorption
agent,
- whereafter the adsorption agent is at least partially
withdrawn from the reaction zone with the gas stream as
an entrained suspension and substantially removed from
the gas stream in a separation zone,
- whereafter the exit gas from the separation zone is
discharged into the atmosphere,
- while separated adsorption agent is introduced into a
regeneration zone where the adsorbed nitrogen oxides,
sulfur oxides, and other acidic gases are substantially
removed from the adsorption agent and withdrawn from the
regeneration zone in a concentrated form,
- while the regenerated adsorption agent is recycled to
the reaction zone,
which is characterized in that the separation zone
comprises a first electrostatic filter and optionally an
additional filter arranged upstream of the first
electrostatic filter.
It has been discovered that the resistivity of the
adsorption agent decreases considerably during the
adsorption in the reaction zone. This phenomenon makes it
possible to obtain an extremely efficient removal of the
adsorption agent relatively to fly ash and/or other solid
materials present in an electrostatic filter downstream
of the reaction zone.
CA 02248679 1998-09-10
W O 97/33678 12 PCT~D W 7/00105
Hence, this discovery allows operation under high dust
conditions (i.e. high concentrations of fly ash and/or
other solid materials in the inlet gas) without
excessive loss or contamination of the adsorption agent
with fly ash and/or other solid materials.
It is believed that the above mentioned change of
resistivity during the adsorption process is primarily
due to adsorption of SO on the adsorption agent surface,
which is particular efficient when using the NOXSO
sorbent particles, disclosed e.g. in the above-mentioned
NOXSO patents, included by reference.
According to a preferred embodiment the adsorption agent
separated in the separation zone is partly recycled to
the reaction zone, and partly introduced into the
regeneration zone.
When the separation zone comprises a first electrostatic
filter as well as an additional filter, e.g. a system of
one or more cyclones, the solid material precipitated in
the additional filter is preferably divided into two
streams, where the first stream is recycled to the
reaction zone, whereas the second stream is directed to
the regeneration zone. The solid material precipitated in
the first electrostatic filter is divided and recycled in
the same way.
The reaction zone may be accommodated in all types of
adsorption reactors, including fluid bed reactors (e.g.
dense bed, spouted bed etc.~, moving bed reactors and
entrained suspension adsorbers. In the last mentioned
case pulverous adsorption agents are used.
According to preferred embodiments the reaction zone
comprises gas-solid contact in an entrained suspension
CA 02248679 l998-09-lO
W O 97/33678 rCT~DK~7/00105
13
adsorber, but in some applications the reaction zone
comprises a fluid bed or a moving bed. In such
- applications fines of the adsorption agent and fly ash
are elutriated from the bed and carried with the gas as
~ 5 an entrained suspension. In these cases an additional
particulate stream is withdrawn directly from the
reaction zone, introduced into a regeneration zone, and
returned to the reaction zone.
According to another preferred embodiment fly ash and/or
other solid materials contained in the gas stream
introduced into the reaction zone are partially removed
in a second filter arranged upstream of the reaction
zone.
In the present context the term "partial removal of fly
ash and/or other solid materials" is used to designate
removal to particulate concentrations in the outlet gas
from said second filter of at least 100 mg/Nm3, such as
at least 1 g/Nm3 and in particular in the range 1-10
g/Nm3.
The second filter may be a member selected from the group
consisting of cyclones, ceramic filters, bag filters,
gravimetric settling filters, and electrostatic filters.
According to another preferred embodiment fly ash and/or
other solid materials contained in the exit gas stream
from the first electrostatic filter for removal of the
adsorption agent are removed in a third filter arranged
downstream of the first electrostatic filter.
The third filter may be a member selected from the group
consisting of bag filters, ceramic filters, and
electrostatic filters.
CA 02248679 1998-09-10
W097/33678 PCT~K97100105
14
The third filter is preferably an electrostatic filter
arranged adjacent to the first electrostatic filter in a
common housing.
In still another preferred embodiment the first
electrostatic filter is used for separation of the
sorbent with respect to the sulfur content. Hence, the
operation of the electrostatic filter is adjusted so that
the particulate stream withdrawn from said first
electrostatic filter has a substantially higher sulfur
content than the particulate part of the incoming
entrained suspension.
According to another preferred embodiment the adsorption
agent consists of particles having an average particle
size within the range 20-200 um, preferably within the
range 40-80 um consisting of:
(a) a gamma alumina substrate having a surface area in
the range lO0-500 m-/g and
a pore volume in the range 0.3-0.8 ml/g,
(b) an alkali metal component,
the substrate being impregnated with the alkali metal
component, and
(c) an alumina stabilizer selected from the group
consisting of silica, lanthana, other rare earths,
titania, zirconia, clay, alkaline earths and mixtures
thereof in an amount from an effective amount up to about
30 mole-~.
The gamma alumina substrate has preferably a bimodal pore
size distribution comprising micropores and macropores,
the micropores having an average pore diameter dl in the
range 30 - 400 Angstroms and the macropores having an
average pore diameter d in the range 80 - 3000
Angstroms.
CA 02248679 l998-09-lO
W 097/33678 rCT~DK~7/00105
According to a preferred embodiment the adsorbed nitrogen
oxides, sulfur oxides, and other acidic gases are
substantially removed from the adsorption agent in the
regeneration zone comprising the following steps:
- a heating step;
- a reducing gas treatment step;
- a stripping step; and
- a cooling step,
and optionally
- an adsorption agent buffer tank.
According to a preferred embodiment
- the reaction zone comprises an entrained suspension
adsorber;
- the heating step comprises heating of the adsorption
agent in a fluidized bed heater to remove NO and the
main part of the other acidic gases from the adsorption
agent, withdrawing the liberated gases and the adsorption
agent from the fluidized bed heater;
- the reducing gas treatment step comprises contacting
the exit adsorption agent from the heating step with a
reducing gas in a fluidized bed reactor to remove and
release SO~ as a mixture of SO. and H2S, withdrawing the
mixture of SO. and H~S and the adsorption agent from the
fluidized bed reactor;
- the stripping step comprises contacting the exit
adsorption agent from the reducing gas treatment step
with water vapour in a fluidized bed to remove adsorbed
H-S from the adsorption agent;
- the cooling step comprises cooling the exit adsorption
agent from the stripping step in a fluidized bed cooler;
and
- the exit adsorption agent from the cooler is recycled
to the reaction zone either directly or via an adsorption
agent buffer tank.
CA 02248679 l998-09-lO
W O 97t33678 rCT~DK~7/00105
16
The heating step/cooling step is preferably carried out
by indirect heat transfer from heating/cooling coils
immersed in a fluidized bed.
According to a preferred embodiment the reducing gas is
used as fluidization medium in the fluidized bed reactor
in the reducing gas treatment step.
According to another embodiment the reaction temperature
in the fluidized bed reactor in the reducing gas
treatment step is within the range 200-700 ~C, preferably
300-600 ~C, and in particular 300-50Q ~C.
The regeneration process is a crucial part of any
regenerable adsorption process, that constitutes a major
part of the operational costs in the heating of the
adsorption agent to the regeneration temperature, and in
the addition of reducing agent.
The regeneration temperature is known to depend on the
reducing agent. As a rule of thumb the regeneration
temperature required for pure substances may be
correlated to the spontaneous ignition temperature of the
substance. For example, the regeneration temperature
required for methane is in the range 620-640 ~C, being
close to the spontaneous ignition temperature of 632 ~C.
It would therefore be an obvious choice to carry out the
regeneration by using a reducing agent having a low
spontaneous ignition temperature (SIT). Examples of such
reducing agents are: acetylene (SIT = 305 ~C) and ethanol
(SIT = 392 ~C).
CA 02248679 1998-09-10
W097/33678 PCT~K97/00105
17
It has, however, been observed that such reducing agents
exhibit a strong tendency to soot and coke formation on
the adsorption agent, resulting in a physical blockage of
the pores impeding the regeneration process.
Tests with acetylene as well as ethanol showed a very
pronounced darkening of the adsorption agent due to the
above mentioned formation of soot and coke. For both
tests a considerable, unacceptable increase in residual
sulfur was found.
It can thus be concluded that the above mentioned
reducing agents and other coke forming compounds are
unsuitable for the regeneration process.
It is has also been discovered that mixtures of gases in
general decrease the temperature of regeneration compared
to the regeneration temperature for the pure substances.
For example, the regeneration temperature for methane is
in the range 620-640 ~C, while the regeneration
temperature for natural gas (containing approximately 90
% Methane) is in the range 560-580 ~C. This may be due to
a synergistic effect of the mixture. Natural gas may
contain up to lO ~ non-combustibles. Such non-combustible
- compounds, viz. C0~, H~0, etc., may be responsible for
synergetic effects, thus enhancing the regeneration
significantly.
CA 02248679 1998-09-10
W097/33678 18 PCT~K97/001~
Further, it has been discovered that the conditions in
the reducing agent treatment step in the regeneration
zone are able to convert sulfur- and nitrogen-containing
compounds into H S, S0 and N~. The produced sulfur
compounds may thus participate directly in a regular
downstream Claus process, while nitrogen ls a non-
pollutant.
It-is known from prior art, US Patent No. 4,323,544, that
water vapour and/or carbon dioxide may be present in the
reducing gas, that the presence of those gases will
improve the removal of sulfide from adsorption agent, and
that regeneration without these components will
necessitate a second regeneration (stripping) step where
the adsorption agent is treated with one of these gases,
as described and realized in the SNAP second regeneration
stage.
This means that regeneration with a mixture containing
substantial amounts of H20 and/or C0~ may exhibit the
advantage of eliminating at least one downstream
processing (stripping) step and thus reduce the capital
and operational costs.
Another problem to be solved by the present invention is
to provide another improved version of the above
mentioned dry adsorption processes with regeneration and
recirculation of the adsorption agent.
According to a second aspect of the present invention it
has been found that regeneration temperature as well as
CA 02248679 1998-09-10
W097/33678 PCT~K~7/00105
1 9
operational cost may be reduced by use of low grade fuel
in the reducing agent treatment step.
Therefore the present invention does also relate to a
method for removing nitrogen oxides, sulfur oxides, and
other acid gases from a gas stream, in particular
according to any of claims 1-14, in which
- an adsorption agent suitable for adsorbing nitrogen
oxides, sulfur oxides, and other acidic gases is
introduced and contacted with the gas stream in a
reaction zone wherein the nitrogen oxides, sulfur oxides
and other acidic gases are adsorbed by the adsorption
agent,
- whereafter the adsorption agent is at least partially
withdrawn from the reaction zone with the gas stream as
an entrained suspension and substantially removed from
the gas stream in a separation zone,
- whereafter the exit gas from the separation zone is
discharged into the atmosphere,
- while separated adsorption agent is introduced into a
regeneration zone where the adsorbed nitrogen oxides,
sulfur oxides, and other acidic gases are substantially
removed from the adsorption agent and withdrawn from the
regeneration zone in a concentrated form, said
regeneration zone containing a reducing agent treatment
step,
- while the regenerated adsorption agent is recycled to
the reaction zone,
which is characterized in that the reducing agent used in
the reducing gas treatment step is a low grade fuel.
CA 02248679 1998-09-10
W097/33678 PCT~K97/00105
In the present context the term "low grade fuel" is
intended to designate fuels containing impurities in an
amount necessitating further processing in order to
provide a generally acceptable fuel, and/or having a low
heating value.
Low grade fuel is usually processed before it is used in
other processes, due to the general necessity of using
pollutant free fuels. These pollutants are usually sulfur
containing compounds, nitrogen containing compounds or
non-combustibles, such as nitrogen, water vapour, sulfur
oxides and carbon oxides. By means using a low grade fuel
it is thus obtained that the synergistic effect of the
gas mixture is utilized for performing the regeneration
at a relatively lower temperature and that the low grade
fuel is used without any prior processing, both
improvements resulting in lower operational costs.
As low grade fuel, the following gases may i.a. be used
according to the present invention:
- unprocessed, sulfur containing effluents from the
following processes: coal gasification, coal
liquefaction, oil shale processing, tar sands processing,
petroleum processing, mineral processing, and geothermal
energy utilization;
- various unprocessed gas streams from refineries, e.g.
flare gasi
- various gas streams in the petroleum processing;
- sour water stripper off-gas from refineries; and
CA 02248679 l998-09-lO
W 097/33678 PCTADK~7/00105
- biogas.
According to a preferred embodiment the low grade fuel
contain at least one non-combustible component, e.g. CO ,
H2O, SO., NO, NO , N~, O-, etc.
The low grade fuel may contain non-combustible compounds
in an amount up to 95, preferably up to 50, in particular
up to 2 5 ~ by volume.
According to a preferred embodiment the low grade fuel
contains non-combustible compounds in an amount of at
least 10, preferably at least 15, in particular at least
20 % by volume.
According to another preferred embodiment the low grade
fuel is a waste fuel.
The low grade fuel may in particular contain sulfur
containing compounds, such as sulfides, mercaptans,
thioethers, thioaldehydes, and thioketones.
The low grade fuel may in particular also contain
nitrogen containing compounds, such as ammonia, urea,
urethanes, amines, amides, imides, and CN-containing
compounds as well as mixed sulfur and nitrogen containing
compounds.
CA 02248679 l998-09-lO
W 097/33678 PCT~DKg7/00105
22
According to a preferred embodiment of the present
invention the adsorption agent consists of particles
having an average particle size within the range 20-200
um, preferably within the range 40-80 ~m consisting of:
(a) a gamma alumina substrate having a surface area in
the range 100-500 m-/g and
a pore volume in the range 0.3-0.8 ml/g,
(b) an alkali metal component,
the substrate being impregnated with the alkali metal
component, and
(c) an alumina stabilizer selected from the group
consisting of silica, lanthana, other rare earths,
titania, zirconia, clay, alkaline earths and mixtures
thereof in an amount from an effective amount up to about
30 mole-~.
According to another preferred embodiment of the present
invention the gamma alumina substrate has a bimodal pore
size distribution comprising micropores and macropores,
the micropores having an average pore diameter d. in the
range 30 - 400 Angstroms, and the macropores having an
average pore diameter d in the range 80 - 3000
Angstroms.
According to yet another preferred embodiment of the
present invention the adsorbed nitrogen oxides, sulfur
oxides, and other acidic gases are substantially removed
from the adsorption agent in the regeneration zone
comprising the following steps:
- a heating step;
- a reducing gas treatment step;
- a stripping step; and
- a cooling step,
and optionally
- an adsorption agent buffer tank.
CA 02248679 1998-09-10
W097/33678 PCT~K~7/00105
23
In a further embodiment of the present invention
- the reaction zone comprises an entrained suspension
adsorber;
- the heating step comprises heating of the adsorption
~ 5 agent in a fluidized bed heater to remove NO and the
main part of the other acidic gases from the adsorption
agent, withdrawing the liberated gases and the
adsorption agent from the fluidized bed heater;
- the reducing gas treatment step comprises contacting
the exit adsorption agent from the heating step with a
reducing gas in a fluidized bed reactor to remove and
release SO~ as a mixture of SO~ and H,S, withdrawing the
mixture of SO and H~S and the adsorption agent from the
fluidized bed reactor;
- the stripping step comprises contacting the exit
adsorption agent from the reducing gas treatment step
with water vapour in a fluidized bed to remove adsorbed
H,S from the adsorption agent; and
- the cooling step comprises cooling the exit adsorption
agent from the stripping step in a fluidized bed cooler.
In a further embodiment of the present invention the
adsorbed nitrogen oxides, sulfur oxides, and other acidic
gases are substantially removed from the adsorption agent
in the regeneration zone comprising the following steps:
- a heating step;
- a reducing gas treatment step; and
- a cooling step,
and optionally
- an adsorption agent buffer tank.
In the following the invention will be further described
with reference to the drawings, in which
CA 02248679 1998-09-10
W097/33678 PCT~K97/~105
24
fig. l is a diagrammatic illustration of the SNAP process
described in the introductory part of the description,
fig. 2 schematically illustrates a process according to
the present invention for removal of nitrogen oxides,
sulfur oxides and other acid gases from a gas stream,
fig. 3 shows a preferred embodiment of the above process
having a particle stream withdrawn from the reaction zone
and sent to the regeneration zone,
fig. 4 shows further details of the regeneration process,
according to a preferred embodiment, and
fig. 5 shows an example of an preferred embodiment that
serves as a process diagram for example 4.
As shown in fig. 2, the gas stream tlO) is passed through
a reaction zone (l) where nitrogen oxides, sulfur oxides
and other acid gases are adsorbed on an adsorption agent.
The adsorption agent is withdrawn from the reaction zone
(l) with the gas stream as an entrained suspension (ll)
and substantially removed from the gas in a separation
zone (2) consisting of a first electrostatic filter (4)
and an optional filter (5) upstream of the first
electrostatic filter (4). Separated adsorption agent (13)
is introduced into a regeneration zone (3) or may be
partly recycled (14) to the reaction zone (l). In the
regeneration zone (3~ nitrogen oxides, sulfur oxides and
other acid gases adsorbed on the adsorption agent are
substantially removed from the adsorption agent and
withdrawn from the regeneration zone (3) in a
concentrated form (17). Regenerated adsorption agent (15)
is recycled to the reaction zone (l). An optional second
filter (6) may be placed upstream of the reaction zone
(l) for partial removal of fly ash and/or other solid
CA 02248679 1998-09-10
WO 97/33678 PCT~DK~7/00105
materials from the inlet gas stream (16). The gas stream
(12) from the separation zone (2) may be further
processed for the removal of fly ash andfor other solid
materials in an optional third filter (7) before the exit
gas stream (18) is discharged into the atmosphere. In the
case of choosing an electrostatic filter as the third
filter (7), it will be possible to arrange the first
electrostatic filter (4) and the third electrostatic
filter (7) in the same housing creating a filter where
adsorption agent containing particulate stream can be
withdrawn from the one end of the filter, and an
adsorption agent free particulate stream can be withdrawn
from the other end of the filter.
Figure 3 illustrates a process similar to the process
illustrated in figure 2 with a stream of particles (19)
withdrawn from the reaction zone (1) and sent to the
regeneration zone (3).
Figure 4 illustrates a process similar to the process
illustrated in figure 2 with a regeneration zone (3)
consisting of
-a heating step (20);
-a reducing gas treatment step (21);
-a stripping step (22);
-a cooling step (23); and
- an optional adsorption agent buffer tan~ (31).
Figure 5 show a process diagram for a process according
to the present invention, in which a flue gas from a
power generation station containing SO , NOx and fly ash
(10) is passed through a reaction zone (1) where
nitrogen oxides, sulfur oxides, and other acid gases are
adsorbed on an adsorption agent. The pulverous adsorption
agent is withdrawn from the reaction zone (1) with the
gas stream as an entrained suspension (11) and
CA 02248679 1998-09-10
WO 97/33678 PCT/DK97/OOlOS
26
substantially removed from the gas in a separation zone
(2) consisting of a first electrostatic filter (4) and a
cyclone (5) upstream of the electrostatic filter.
Separated adsorption agent (13) is partly introduced into
5 a regeneration zone (3), and partly recycled (14) to the
reaction zone (1). The regeneration zone consists of
-a fluidized bed heater (20) using air (24) as the
fluidizing media;
10 -a reducing gas treatment step (21) using natural gas
(26) as the fluidizing media;
-a stripping step (22) using steam (27) as the fluidizing
media;
-a cooling step (23) using air (29) as the fluidizing
15 media; and
-an adsorption agent buffer tank (31).
In this example the regeneration zone produces three
outlet streams,
20 -a stream (25) from the fluidized bed heater (20)
containing NOx in a concentrated form,
-a stream (28) from the reducing gas treatment step (21)
and the stripping step (22) containing H S and SO in a
concentrated form, and
25 - a stream (30) from the fluidized bed cooler (23) that
can be discharged to the atmosphere.
Regenerated adsorption agent (15) is recycled to the
reaction zone (1). The gas stream (12) from the
30 separation zone (2) is further processed for the removal
of fly ash and/or other solid materials in an
electrostatic filter (7) before the exit gas stream (18)
is discharged into the atmosphere. It will be possible to
arrange the first electrostatic filter (4) and the third
35 electrostatic filter (7) in the same housing creating a
filter where adsorption agent containing particulate
CA 02248679 l998-09-l0
W O 97/33678 PCTADK~7/OOlOS 27
stream is withdrawn from the one end of the~ filter, and
an adsorption agent free particulate stream (32) is
withdrawn from the other end of the filter.
The in~ention is further illustrated by way of the
following non-limiting examples:
Example 1: Sour water stripper off-gas from a refinery as
the reducing agent in the SNAP.
A slip stream from the process of petroleum refining is
the sour water stripper off-gas. This stream has a
composition approximately as follows:
H7S 45
H2O 30%
NH3 25%
This gas with the above composition is a typical example
of a low grade fuel that is not generally acceptable as a
fuel. The mixture contains two combustible components
(H S and NH3) and a non combustible component (H'O). The
sour water stripper off-gas is today burned in the flare.
The sour water stripper off-gas can be used as the
reducing agent used in the regeneration zone for the
SNAP, thus lowering the operational cost for the reducing
agent treatment step considerably, and simultaneously
reducing the emission of sulfur-oxides and nitrogen
oxides to the atmosphere from the refinery flare.
Example 2: Combustible gas mixture from a refinery as the
reducing agent in the SNAP.
A by-product stream from the process of petroleum
refining is a gas stream containing a mixture of
CA 02248679 1998-09-10
W097/33678 28 PCT~K~7/~105
hydrocarbon, hydrogen sulphide and hydrogen; This stream
has a composition approximately as follows:
H.S 0.2 %
H- 13.1
CH~ 33.8 %
C H~ and C~H~ 30.3
C~HR and C~H, 17.1
C~-compounds (3 different) 5.5 ~
This gas is a typical example of a low grade fuel due to
the complex composition of the mixture and the high
content of hydrogen sulfide. The gas mixture is today
used in the refinery for heat generation.
The above mentioned gas mixture can be used as the
reducing agent used in the regeneration zone for the
SNAP, thus lowering the operational cost for the reducing
agent treatment step considerably.
Example 3: Mixture of sour water stripper off-gas and a
combustible gas mixture from a refinery as a reducing
agent in the SNAP.
A mixture of the sour water stripper off-gas mentioned in
example 1 and the gas mixture mentioned in example 2 will
contain a variety of hydrocarbon, hydrogen, hydrogen
sulfide, ammonia and non-combustible water vapour and
must thus be classified as a low grade fuel.
This gas mixture can be used as the reducing agent used
in the regeneration zone for the SNAP, thus lowering the
operational cost for the reducing agent treatment step
considerably.
Example 4: Using an electrostatic filter in the
separation of SNAP adsorption agent and fly ash.
CA 02248679 1998-09-10
W097/33678 PCT~K97/00105
29
The process disclosed in this example corresponds to the
diagram shown in figure 5.
lO0000 Nm'/h at 120 ~C flue gas (lO) from a fossil fired
power station is to be cleaned.
The flue gas contains
600 ppmv SO~
200 ppmv NO~
lO g/Nm~' fly ash
The gas is led to the reaction zone (l) comprising a gas
suspension adsorber (GSA) with a diameter of 2.8 m. In
the GSA the flue gas is mixed with adsorption agent
consisting of a r-alumina carrier impregnated with sodium
oxide with a particle size of dE~ = ~~ ~lm.
SO~ and NOx are adsorbed on the adsorption agent in the
reaction zone, and the clean gas leaves the reaction zone
with loaded adsorption agent (ll).
The gas stream (ll) here contains
SO2 30 ppm
NOx 25 ppm
Fly ash 60 g/Nm~
Adsorption agent 500 g/Nm'
The flue gas enters the separation zone (2) that
comprises a cyclone (5) and an electrostatic filter (4).
Part of the solid material is removed from the gas stream
in the separation zone (2) whereafter the outgoing stream
(12) contains:
lO g/Nm Fly ash
<5 mg/Nm-' adsorption agent
CA 02248679 1998-09-10
W097t33678 PCT~K97/00105
The remaining part of the solid material is efficiently
removed down to a total concentration of 50 mg/Nm in the
second part of the electrostatic filter (7), whereafter
the gas stream is led to the atmosphere (18). The
particles collected in this second electrostatic filter
are discharged as the stream (32).
In this example the two electrostatic filters (4) and (7)
are placed in the same housing, and actually constitute
one electrostatic filter containing two outlets for
collected particles.
The solid material collected (13) in the separation zone
(2) corresponds to a total amount of 54995 kg/h, of which
49495 kg/h are recirculated via (14) to the reaction zone
(1), and 5500 kg/h are sent to the regeneration zone (3).
In the regeneration zone (3) the adsorption agent is led
to a fluidized bed heater (20) that is using air as the
fluidizing medium (24).
The adsorption agent is heated indirectly to 600'C, by
which NOx is liberated into the fluidization gas in a
concentration of 9 ~c~ and leaves the system through (25).
The hot adsorption agent is led to the reducing agent
treatment step where it is treated in a fluidized bed
reactor (21) with a diameter of 1.2 m with natural gas
(26) 43 Nm'/h in 45 minutes, and then led to the steam
treatment step, where it is treated in a fluidized bed
reactor (22) with a diameter of 0.6 m with steam (27) 7
Nm~/h for 10 minutes.
The gas stream leaving the steam treatment and reducing
agent treatment step is mixed (28) and contains 74.6 kg/h
CA 02248679 1998-09-10
W097/33678 PCT~K97/~105
31
sulfur as H,S and S0: in concentrations of 30~;' and 2~,
respectively.
The adsorption agent is afterwards led to the fluid bed
sorbent cooler (23) that is using air as a fluidizing
medium (29) where it is indirectly cooled to 125'C. The
gas leaving the sorbent cooler does not contain any
pollutants and is discharged into the atmosphere.
The cooled adsorption agent particles are recycled (15)
to the reaction zone (1) via an adsorption agent buffer
tank (31).