Note: Descriptions are shown in the official language in which they were submitted.
CA 02249~48 1998-09-11
Title: Endotoxin-Specific Membranes
Description
The invention relates to a microfiltration membrane for the
separation of endotoxins from liquid media as well as the use
of such microfiltration membranes.
Endotoxins are lipopolysaccharides from the outer cell membrane
of Gram-negative bacteria which act as pyrogens. Due to the
omnipresence of bacteria, such endotoxins are ubiquitous.
However, in contrast to bacteria they cannot be removed or
rendered harmless by standard methods such as sterile
filtration or autoclave processing (1). For this reason,
sterile cannot be equated with endotoxin-free. The presence of
endotoxins in injection or infusion solutions (paranteralia) is
particularly critical, since intervenous application can induce
fever already in an amount 1 ng per kg body weight. With
increasingly higher dosings (e.g. in high volume parenteralia)
the symptoms range up to severe shock and death (2, 3). For
this reason, apart from sterilization, almost all pharmacopeia
prescribe strict upper limits of endotoxins, e.g. 0.2 EU per mg
chloramphenicol for injection or only 0.003 EU per heparin unit
(4). The attainment of these requirements is difficult in
practice. In particular, the production of biological
medicaments cannot take place free of endotoxins in all steps.
The main sources of endotoxins include:
CA 02249~48 1998-09-11
- raw materials such as plasma or tissue, which could
already be contaminated with bacteria.
- for recombinant products the introduction of host-
specific endotoxins can be expected.
- bacterial contamination of devices, filters or additives
during the production.
The usual heat-decontamination for thermally stable agents (30
minutes at 250 ~C) is just as unsuitable for the preparations
as is the treatment with acids, alkaline solutions or strong
oxidizing agents (H2O2) (1).
When using active coal or depth filters such as ZETA PLUS,
considerable product loss is often the result, so that their
use remains limited to treatments in aqueous solution (5, 6).
The ultrafiltration as a very gentle method has achieved great
popularity in the field of endotoxin removal. Here one works
with cut-offs of 5000 or 10000 in order to also effectively
separate monomer components (MW ca. 14000), which are also
present apart from high molecular aggregates (up to a molecular
weight of several million). Despite the above, reoccuring
problems arise with low molecular break-up components which
also have pyrogenic effects (e.g. Lipid A). This especially
concerns hemodialysis. Although the dialysis buffer is
ultrafiltrated, 400000 dialysis patients annually develop
septic symptoms (7) for example in the USA. In addition, the
neccessity of lower cut-offs restricts the use of
ultrafiltration to the decontamination of low molecular
substances (8).
In the case of high molecular products such as pharmaceutical
proteins, albumin preparations or heparin, great difficulties
still exist. If endotoxin contamination occurs in these
preparations, the only remaining possibility at the moment is
CA 02249~48 1998-09-11
to perform reprocessing to meet the requirements of the FDA,
USP or EP.
To avoid this extensive procedure, but still meet product
requirements, the possibility has been investigated to
selectively decontaminate impure products with chromatographic
sorbents having endotoxin specific ligands. This procedure has
also not provided the desired result. Despite high to very high
association constants at high endotoxin starting
concentrations, the reported affinity sorbents having His, Him
or PMB as the ligands have not proven to be suitable (9). In
addition, in the presence of proteins competing protein
adsorption was observed, which led to reduced endotoxin removal
rates and in part to high protein loss (in particular for
acidic proteins such as BSA).
Literature
(1) S.K. Sharma
Endotoxin detection and elimination in biotechnology
Biotechnol. Appl. Biochem. 8 (1986), 5 - 22
(2) N. Haeffner-Cavaillon, J.M. Cavaillon, L. Szabo
Cellular receptors for endotoxin
Handbook of Endotoxins, Vol. 3: Biology of Endotoxins,
1 - 24
Elsevier Science Publishers B.V. (1985)
(3) D.C. Morrison, J.L. Ryan
Endotoxins and disease mechanisms
Ann. Rev: Med. ~8 (1987), 417 - 32
(4) USP XXII Suppl. 5 (Nov. 1991)
CA 02249~48 1998-09-11
(5) K.C. Hou, R. Zaniewski
Depyrogenation by endotoxin removal with positively
charged depth filter cartridge
J. Paranteral Sci. Tech., Vol. 44, No. 4 (1990), 204 -
209
(6) CUNO Newsletter for Pharmaceuticals (Oct. 1995), p. 3
(7) B.P. Smollich, D. Falkenhagen, J. Schneidewind,
S. Mitzner, H. Klinkmann
Importance of endotoxins in high-flux dialysis
Nephrol. Dial. Transplant 3 (Suppl.) (1991) 83 - 85
(8) E. Flindt
Pyrogenentfernung mittels Ultrafiltration
Memoscript CONCEPT-Symposium "Pyrogene II"
(June 1983), p. 54 - 60
(9) F.B. Anspach, O. Hillbeck
Removal of endotoxins by affinity sorbents
J. Chromatogr. A 711 (1995), 81 - 92
This prior art is improved according to the present invention
by a microfiltration membrane for seperation of endotoxins from
liquid media, in particular water, protein solutions or
parenteralia, where the microfiltration membrane is
characterized by covalently bonded ligands for endotoxins and
where the ligands are carried on a polymer which is applied to
the membrane.
With respect to membrane technology and also membrane
production reference is made to Ho ~ Sirkar (Editors), Membrane
Handbook, van Norstrand Reinhold, New York, 1992.
CA 02249~48 1998-09-11
The covalently bonded ligands can include
(a) an endotoxin specific ligand, preferably histamine,
histidine, polyethylene imine, poly-L-lysine or
polymyxin B and/or
(b) a ligand which is not endotoxin speciflc per se,
preferably diaminohexane, diethylaminoethyl or
desoxycholate.
The membrane material for the microfiltration membrane
according to the invention can be regenerated cellulose,
cellulose acetate, polysulfone, polyethylene vinyl alcohol or
polyamide, preferably Nylon.
The polymer, which according to the invention is applied to the
microfiltration membrane, can include a hydrophilic polymer, in
particular dextran, polyvinyl alcohol or modified cellulose,
preferably hydroxyethylcellulose.
These hydrophilic polymers can be water soluble, swellable in
water or insoluble in water.
The polymers can be carried on the microfiltration membrane
according to the invention with the aid of a spacer. The
covalently bonded ligand can also be carried by a spacer. These
spacers can include those derived from bisoxirane,
glutardialdehyde, epihalogenhydrin or diisocyanate, optionally
after oxidative activation.
Concerning activation and immobilization chemistry, also with
respect to endotoxin specific ligands and spacers, reference is
made for example to Hermanson, Mallia & Smith, Immobilized
Affinity Ligand Techniques, Academic Press Inc., San Diego,
1992.
CA 02249~48 1998-09-11
According to the invention, microfiltration membranes are thus
provided, which have suitably modified surfaces and which
separate endotoxins from water and aqueous solutions tbuffers,
protein solutions). The surface modification can comprise the
application of a bi-functional covalently bonded spacer, which
is reacted with a hydrophilic polymer, whereby non-specific
interactions of the membrane are reduced, especially with
proteins. The covalently bonded hydrophilic polymer can be
reacted with the endotoxin specific ligand, optionally via a
further spacer. The principle of the surface modification of
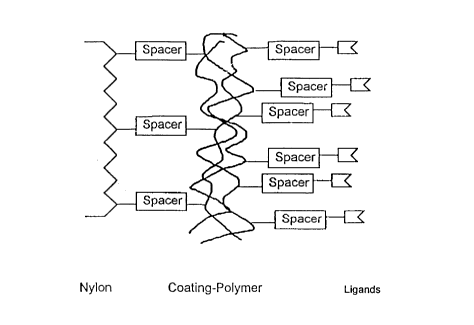
the membrane is shown in Fig. 1.
The endotoxin removal in the presence of proteins can be
dependent upon the net load of proteins. By optimizing the
conditions (pH value), acid proteins (such as BSA and mouse IgG
1) can be completely decontaminated, namely without appreciable
loss of proteins. For alkaline proteins (for example lysozyme
and bFGF) high removal rates can also be achieved.
Polymer-coated microfiltration membranes according to the
invention with covalently bonded endotoxin specific ligands can
remove endotoxins in one pass, also from highly loaded
solutions (6000 EU ml~1).
In principle, the structure of the membranes is shown in Fig.
1. Initially, a hydrophilic polymer is applied over a spacer,
which is then combined with a endotoxin specific ligand,
optionally via a spacer. Particularly suited for the membrane
material is:
- cellulose
- polysulfone
- PEVA (polyethylene vinyl alcohol)
- polyamide (especially Nylon, such as for example N66).
CA 02249~48 1998-09-11
.
Reactive bi-functional compounds are suitable as the spacer.
Particularly suited are:
- bisoxirane
- glutardialdehyde
- epihalogenhydrin
- diisocyanate.
For activation of the vicinal diol bond resulting from the use
of bisoxirane and epihalogenhydrin, oxidation through periodate
can be employed, where an aldehyde group results. The spacer
bonded to the membrane is further reacted with a hydrophilic
polymer. Such polymers preferably include:
- dextran
- polyvinyl alcohol (PVA)
- modified celluloses, especially hydroxyethylcellulose
(HEC).
The further reaction takes place either directly with the
endotoxin speclfic ligand or again via an intermediate spacer
as mentioned above, optionally after its oxidative activation.
The endotoxin specific ligands include (see list of
abbreviations): DAH, Him, His, PEI, PLL, PMB. In addition, the
ligands normally not specific to endotoxin, such as DEAE and
DOC, are found to be highly specific in the membrane
configuration, at the same time with a high passage of the
proteins.
The performance of the developed membranes can be taken from
the given examples. The endotoxin removal can almost always be
considered complete. It is normally below 1 EU ml~1, often
below the detection limit with the LAL test.
CA 02249~48 1998-09-11
No endotoxin depletion was found with the control membranes
(Nylon without modification and with attached hydrophilic
polymer with or without a spacer) without endotoxin specific
ligands.
The new membranes can be employed for endotoxin removal from
water and parenteralia. Good results are also achieved in the
presence of proteins. However, in the case of alkaline
proteins, it should be considered that interactions of the
proteins with the endotoxins can arise, which can lead to a~
endotoxin masking. Endotoxin bound to the protein can not be
clearly detected by the LAL test. In this conjunction it should
be mentioned that it is not finally clarified whether protein-
bonded endotoxin is still toxic.
The membranes according to the invention have numerous
applications.
Medical and pharmaceutical fields:
- hemodialysis.
- safe infusion and injection solutions (parenteralia).
- safe diagnostic materials (e.g. antibodies).
In biotechnology:
- production of pharmaceutical products
- endotoxin separation in process water and raw materials.
- decontamination of products (measures for processing are
eliminated).
Methods
1. Production of the membranes
CA 02249~48 1998-09-11
Hydrophilic polymers, in particular dextran, polyvinyl alcohol
and hydroxyethylcellulose are covalently bonded on
microfiltration membranes based on Nylon (preferably 0.45 um or
larger). In the next step, endotoxin specific ligands are
applied to the polymers. Fig. 1 illustrates the structure of
the mebranes.
1.1. Membrane coating as an example with dextran
The Nylon membranes were first activated with bisoxirane. For
this, they were shaken for 16 hours at 80 ~C in a mixture of 9
ml bisoxirane, 1 ml ethanol and 1 ml 25 mM sodium carbonate
buffer (pH 11) (Fig. 2a). After thorough washing, each membrane
was incubated with 5 ml of a 20 ~ dextran 40000 solution (pH
11) for 15 minutes at room temperature (Fig. 2b). The membranes
were then dried for 14 hours at 120 ~C. To remove non-specific
bonded dextran, the membranes were washed three times with a
0.1 M caustic soda solution and a further three times with
water.
As Fig. 3 shows, the coated membranes display a significantly
reduced non-specific interaction, which is expressed through
the adsorbed amount hemoglobin.
As also shown in Fig. 3, a single dextran coating cannot
achieve the same effect as with PVA and HEC. With a second
layer, an improved result can be achieved, while a third layer
only has a small effect. Dextran was therefore always used in a
double coating.
1.2. Immobilization of endotoxin specific ligands
The ligands PLL, PMB and PEI were immobilized either directly
on the periodate-activated coating polymers or after
incorporation of a periodate-oxidizable spacer (bisoxirane).
The procedure is illustrated in Fig. 4 by way of example. DEAE
CA 02249~48 1998-09-11
.
was coupled to the matrix directly without a spacer, the other
low molecular ligands were bonded via epibromidehydrin.
1.2.1. PEI immobilization via bisoxirane
For activation, the membranes coated with hydrophilic polymer
were incubated for 3 hours at room temperature in a mixture of
100 mg sodium borohydride, 5 ml bisoxirane and 45 ml 1 M
caustic soda solution. After hydrolysis of the free oxirane
ring (30 minutes incubation at pH 2.5) and periodate oxidation
of the resulting vicinal diol (90 minutes incubation in 0.2 M
sodium periodate), the membranes were reacted for 2 hours at
room temperature in a solution of 0.5 g PEI (MW 50000) in 0.1 M
phosphate buffer, which was held at a pH of 8, so that the
structure shown in Fig. 1 is produced. Finally, washing was
performed with 1 M sodium chloride solution and water.
1.2.2. Histidine immobilization
Histadine was immobilized over DAH on a coated membrane
activated with epibromidehydrin. Epibromidehydrin activation
was carried out as described for bisoxirane. Immobilized DAH
was activated through reaction for 8 minutes with a mixture of
5 ml epibromidehydrin and 5 ml 4 M caustic soda solution at 90
~C and immediately reacted with L-histidine at 80 ~C (0.5 g L-
histidine in 20 ml water, pH 12). The finished membrane was
washed with 1 M sodium chloride solution and water.
The corresponding procedures were used for the coating with
other polymers and the covalent bonding with the other
endotoxin specific ligands.
2. Separation Experiments
CA 02249~48 1998-09-11
All investigations on the adsorption behaviour of endotoxins on
the membranes were carried out at room temperature in the dead-
end mode.
Each individual membrane piece was fixed on the floor of an
ultrafiltration cell (membrane surface of 13.4 cm2) and washed
with a 30 % ethanol 0.1 M caustic soda solution, 1.5 M sodium
chloride solution and pyrogen-free water to remove traces of
endotoxin. After equilibration of the membrane, 20 ml of
contaminated solution was filtered through each membrane at a
flow rate of 2 ml/min. The filtrate was collected and examined
in the LAL test.
3. Endotoxin Test
To quantify the endoxin in the starting solution and in the
filtrate, a chromogenic Limulus Amebozyte Lysate test (LAL
test) was used. The test is based on the fact that the
endotoxin induces the release of the chromogen p-nitroaniline,
whereby a linear relationship exists between the released
amount of p-nitroaniline and the given endotoxin concentration
in the range of 0 to 1.2 EU/ml. With the photometric
determination of p-nitroaniline, the endotoxin concentration in
the sample can be derived with the aid of a calibration line
(standard endotoxin E. coli Olll:B4).
The LAL test was introduced in Europe in 1995 from the European
Pharmaceutical Handbook Commission for detection of endotoxins
and since 1989 has also replaced the rabbit test in the
monograph entitled "Wasser fur Injektionszwecke".
Sample Applications
CA 02249~48 1998-09-11
12
1. (Fig. 5) Separation from highly-loaded buffer solutions.
Feed: 20 ml 20 mM phosphate buffer (pH 7) with 6000
EU/ml added thereto.
The membranes marked with -d represent membranes not
incorporating a spacer.
2. (Fig. 6 to 7) Separation from endotoxin-enriched BSA
solutions
Feed: 20 ml 20 mM phosphate buffer (pH 4.66) with
1 mg/ml BSA and 6610 EU/ml added thereto.
Protein recovery: BSA
3. (Fig. 8) Separation from commercial BSA
Feed: 20 ml 20 mM phosphate buffer (pH 4.66) with
1 mg/ml BSA
Endotoxin concentration 65 EU/ml
9. (Fig. 9 to 10) Separation from commercial lysozyme
Feed: 20 ml 20 mM phosphate buffer (pH 7) with 1 mg/ml
lysozyme
Endotoxin concentration 134 EU/ml
Protein recovery: lysozyme
5. (Fig. 11 to 12) Separation from MAX 16 H 5
Feed: 20 ml 20 mM phosphate buffer (pH 5.5) with 3 mg/ml
protein
Endotoxin concentration 62.5 EU/ml
Protein recovery: IgG
CA 02249~48 1998-09-11
13
-
6. Separation from previously purified bFGF
Feed: 5 ml bFGF containing 9 EU/ml
The separation was studied with a PEI membrane. In the
filtrate, 0.202 EU/ml was still detectable.
7. Separation from milli-Q water containing endotoxin
Feed: 1 l water containing 270 EU/ml
A PEI and a DAHHis membrane were used for separation.
PEI filtrate: < 0.015 EU/ml
DAHHis filtrate: 0.07 EU/ml
CA 02249~48 1998-09-11
14
Abbreviations
BSA bovine serum albumin
bFGF alkaline fibroplast growth factor
DAH diaminohexane
DEAE diethylaminoethyl
DEX dextran
DEX/2 a membrane coated twice successively with dextran
DEX/3 a membrane coated three times successively with
dextran
DOC desoxycholate
EP European Pharmacopeia
EU endotoxin unit
FDA Food and Drug Administration
HEC hydroxyethylcellulose
Him histamine
His histidine
MW molecular weight
N66 untreated Nylon membrane
PEI polyethylene imine
PLL poly-L-lysine
PMB polymyxin B
PVA polyvinyl alcohol
USP US Pharmacopeia