Note: Descriptions are shown in the official language in which they were submitted.
CA 02249616 1998-09-22
WO 97/40880 PCTIUS97/04292
1
INTRODUCER SYSTEM HAVING
KINK RESISTANT SPLITTABLE SHEATH
FIELD OF THE INVENTION
This invention relates generally to an introduces system having a kink
resistant
sheath for the insertion of catheters and other instruments into the body and
more
particularly to an introduces system having a kink resistant splittable sheath
used to
introduce pacemaker leads into the venous system.
BACKGROUND OF THE INVENTION
Generally speaking, pacing systems include an implantable pulse generator,
commonly known as a pacemaker, electrically connected to the heart by at least
one
transvenous endocardial lead. More specifically an endocardial lead provides
an
electrical pathway between the pacemaker, connected to the proximal end of the
lead,
and endocardial tissue, in contact with the distal end of the lead.
Endocardial tissue
refers to a specific layer of tissue in the interior of the heart's chambers.
In such a
manner electrical pulses emitted by the pacemaker travel through the
endocardial lead
and stimulate the heart.
Endocardial leads are often placed in contact with the endocardial tissue by
passage through a venous access, such as the subclavian vein or one of its
tributaries.
In such a manner transvenous endocardial leads offer as an advantage that they
may
be placed into contact with the heart without requiring major thoracic
surgery.
Rather, transvenous endocardial leads may be introduced into a vein and
maneuvered
therefrom into contact with the heart.
A mufti-step procedure is often used to introduce such leads within the venous
system. Generally this procedure consists of inserting a hollow needle into a
blood
vessel, such as the subclavian vein. A wire guide is then passed through the
needle
into the interior portion of the vessel. The needle is then withdrawn and an
introduces
sheath and dilator assembly is then inserted over the wire guide into the
vessel. The
assembly is advanced into a suitable position within the vessel, i.e. so that
the distal
end is well within the vessel but the proximal end is outside the patient.
Next the
CA 02249616 1998-09-22
WO 97/40880 PCT/US97104292
2 -
dilator and wire guide are removed. The introduces sheath is left in position
and
therefore offers direct access from outside the patient to the interior of the
blood
vessel. In such a fashion a lead can be passed into the vessel through the
introduces
sheath and ultimately be positioned within the heart. Finally the introduces
sheath is
removed from the body. With respect to pacemaker leads, however, which
typically
have a relatively bulky connector pin assembly at the proximal end, the
introduces
sheath is removed from the body by being split apart. In such a manner the
introduces
sheath does not have to be removed over the relatively bulky connector pin
assembly
at the proximal end of the lead.
An introduces sheath therefore, through its hollow lumen, provides access to
the interior of a vessel. A lead introduced into the blood vessel may then
moved
along the blood vessel until properly positioned within the heart.
To provide such access an introduces sheath must be flexible. Specifically,
flexibility permits the introduces sheath to bend and form to a curve
compatible with
the blood vessel. In such a manner the introduces sheath end is substantially
parallel
to the blood vessel and a lead which is introduced therethrough is properly
oriented
along the vessel interior. If the sheath did not conform to the vessel shape,
a lead
introduced would abut against the vessel wall, possibly injuring the patient
and
damaging the lead. One problem which may occur, however, due to the
flexibility
required of the introduces sheath is that the mid-portion of the sheath may
form a
kink.
Kinking along the introduces sheath may cause serious problems, especially
with respect to pacemaker leads. Generally a kink within an introduces sheath
is not
detected until a lead is attempted to be introduced therethrough. At that time
the lead,
and in particular the sensitive electrode at the distal end of the lead,
strikes the kinked
section and is blocked. Continual pushing on the lead may cause damage to the
electrode as well as damage to the helical coil and insulative sheath of the
lead body.
Because such damage may not be readily apparent, implantation of a damaged
lead
may result, in turn, creating the possibility of serious harm to the patient.
CA 02249616 1998-09-22
WO 97/40880 PCT/U897/04292
3
A further problem exists in pacemaker patients who have had multiple leads
implanted over time. Scar tissue at the site of implantation has been found to
create
difficulties with past lead introduction systems. Specifically the relatively
tough scar
. tissue hinders the introduction of a dilator and introduces sheath assembly.
Many
times, only through use of larger incisions than are otherwise desirable is
such an
assembly able to be inserted.
Previously many have attempted to solve the problem of introduces kinking.
For example, in U.S. Patent No. 5,409,469 Schaerf proposed fitting at least a
portion
of the introduces sheath with a series of bellows or pleats. Such a design,
however,
failed to permit the introduces to be smoothly inserted into the tissue. As
can be
appreciated, the bellows or pleats give rise to a relatively large frictional
drag of the
sheath by the tissue. U.S. Patent No. 5,380,304 of Parker disclosed the use of
a flat
wire coil to prevent kinking of an introduces. Such a design, however, did not
permit
the introduces sheath to be slit or split and thus removed from the lead
disposed
therethrough.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide an introduces system
having
a sheath which is resistant to the formation of kinks.
It is a further object of the invention to provide an introduces system having
a
kink resistant sheath for introducing an elongated object into the venous
system and
especially for introducing a cardiac pacemaker lead.
It is a further object of the invention to provide an introduces system having
a
kink resistant sheath which minimizes the frictional drag of the sheath by the
tissue as
the sheath is inserted through the tissue.
It is a further object of the invention to provide an introduces system having
a
kink resistant sheath which permit the introduces sheath to be removed from
the lead
disposed therethrough without requiring the introduces sheath to be removed
from an
end of the lead disposed therethrough, by slitting or splitting the introduces
sheath, for
example.
CA 02249616 2006-02-17
.66742-676
4
These objects are met by the present invention
which provides an introducer system featuring a sheath which
is kink resistant. Such a sheath thereby permits the sheath
to be dramatically bent while still allowing a lead to be
introduced therethrough. The kink resistance is provided by
a composite construction of the sheath. In particular the
sheath has at least one integral reinforcing fiber. In the
preferred embodiment the reinforcing fibers are provided in
a braided configuration. The sheath preferably is
constructed to be readily split using a slitter in a
longitudinal direction and thus permits the sheath to be
removed from the venous system without having to withdrawn
the sheath over an end of the pacemaker lead. In an
alternate embodiment the sheath is scored, including the
reinforcing fibers, to thereby permit the sheath to be
longitudinally split apart and removed from the venous
system without having to withdrawn the sheath over an end of
the pacemaker lead.
The invention may be summarized as an introducer
system for use with a catheter or lead comprising: a sheath
having a first end and a second end, the sheath being
compatible for insertion within a body, the first end
configured to insert the sheath within the body with the
second end extending out of the body; the sheath having a
central lumen configured to permit introduction of at least
one lead or catheter therethrough; means for permitting
removal of the sheath from the lead or catheter disposed
therethrough without requiring the sheath to be removed from
an end of the lead or catheter disposed therethrough; and a
dilator having a first end and a second end, the dilator
configured to be disposed through the central lumen of the
sheath; wherein the sheath has at least one reinforcing
fibre, and wherein the reinforcing fibre in cross-section is
CA 02249616 2006-02-17
66742-676
4a
circular or has a major dimension and a minor dimension, the
major dimension parallel to the lumen of the sheath.
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing and other aspects of the present
invention will be best appreciated with reference to the
detailed description of the invention in conjunction with
the accompanying drawings, wherein:
FIG. 1 depicts the venous positioning and
placement of transvenous endocardial leads in a patient.
FIG. 2 depicts an appropriate entry site for
implantation of a transvenous endocardial lead.
FIGS. 3-14 depict successive stages of introducing
a transvenous endocardial lead into a vein.
FIG. 15 depicts an introduces sheath used in a
body and having a kink.
FIG. 16 depicts a lead introduces system in
accordance with one embodiment of the present invention.
FIG. 17 depicts an introduces sheath of the
present invention used in a body and not having a kink.
FIG. 18 is a detailed cross-sectional view of the
wall of the kink resistant sheath used in an introduces
system of the present invention.
CA 02249616 1998-09-22
WO 97/40880 PCT/US97/04292
FIG. 19 is a detailed cross-sectional view of an alternate embodiment of the
wall of the kink resistant sheath used in an introduces system of the present
invention.
FIG. 20 is a detailed cross-sectional view of an alternate embodiment of the
wall of the kink resistant section used in an introduces system of the present
invention.
5 FIGS. 21 and 22 depict a detailed view of the distal end of an alternate
embodiment of a lead introduces system featuring a sliding cap which may be
incorporated with the present invention.
The drawings are not necessariy to scale.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
It is to be understood, that the present invention is not limited to use only
in
introducing atrial or ventricular pacing leads, and may be employed in
introducing
many of various types of therapeutic or diagnostic devices including
transvenous
leads intended to be disposed at various places within patient 10, including,
for
example, leads intended to be disposed within the patient's coronary sinus, as
well as
various other types of electrical leads, including nerve, muscle or
defibrillation leads.
It is to be further understood, moreover, the present invention may be
employed in
introducing many of various types of therapeutic or diagnostic catheters and
is not
limited only to the introduction of electrical leads. For purposes of
illustration only,
however, the present invention is below described in the context of the
introduction of
endocardial pacing leads.
FIG. 1 depicts a typical arrangement of a pacing system implanted in a patient
10, the pacing system comprising a subcutaneously disposed pacemaker 12 and
transvenous pacing leads 14 and 16. In FIG. 1, the distal end of pacing lead
14 is
shown disposed generally in the atrial region of the patient's heart 18, while
the distal
end of pacing lead 16 is disposed generally in the ventricular region of heart
18.
The preferred prior art method of lead introduction compatible with an
introduces system in accordance with the present invention will be described
with
reference to FIGS. 2 through 14.
Referring to FIG. 2, and in accordance with common practice in the medical
arts, the entry site for a subclavian vein puncture is commonly chosen to be
just below
CA 02249616 1998-09-22
WO 97/40880 PCTILJS97/04292
6
and slightly medial to the junction of the middle and inner third of the
clavicle 20, at
an area designated generally as 22 in FIG. 2. In FIG. 2, the patient's
subclavian vein
24 and heart 18 are shown in phantom.
Turning to FIG. 3, the subclavian vein puncture is accomplished by the
physician using a disposable syringe 26 having a thin-wall needle 28
detachably
connected thereto. Aspiration is performed as the needle is advanced into the
subclavian vein, to verify proper needle placement within vessel 24. Next,
aspirating
syringe 26 is disconnected from needle 28, which remains in vessel 24 as shown
in
FIG. 4. Typically, the physician will place his or her finger over the needle
to avoid
air aspiration and excessive bleeding.
The next step in the lead implantation procedure involves insertion of a
conventional J-type guide wire 30 through needle 28, as illustrated in FIG. 5.
Typically, guide wire 30 is equipped with a tip deflector 32 for facilitating
insertion of
wire 30 into the lumen of needle 28. As shown in FIG. 6, as wire 30 is fed
through
needle 28 in the direction of arrow 34, the distal end of wire 30 exits the
tip of needle
28, and wire 30 regains its "J" shape within vessel 24. Once wire 30 has
entered
vessel 24, needle 28 is withdrawn in the direction of arrow 36 in FIG. 7,
leaving wire
30 in place. Wire 30 is advanced along vessel 24 until its distal end is
disposed
generally in the area of the patient's superior vena cava, leaving
approximately 15 to
20-cm of the proximal end of wire 30 exposed.
A small skin incision 38 is made at the guide wire entry site, parallel to
clavicle 20, as shown in FIG. 8. In the next stage of the implantation
procedure, an
introduces sheath 40 with tapered vessel dilator 42, as an assembly, are
threaded onto
the proximal end of wire 30. Sheath 40 and dilator 42 are advanced in the
direction of
arrow 44, through the subclavian fascia and into subclavian vein 24, until a
short
length (e.g., 2 to 8-cm) of sheath 40 and vessel dilator 42 remain exposed, as
shown in
FIG. 9.
Next, as shown in FIGS. 10 and 11, vessel dilator 42 is withdrawn in the
direction of arrow 46 and sheath 40 is introduced further within subclavian
vein 24,
leaving introduces sheath 40 and guide wire 30 in place with its distal end
disposed
CA 02249616 1998-09-22
WO 97140880 PCT/US97/04292
7
within subclavian vein 24. Guide wire 30 may be removed at this point as well,
although it may be left in place in case the lead needs to be repositioned or
reinserted
or an additional lead is to be implanted. As shown in FIG. 11, introduces
sheath 40
must bend to conform to the shape of subclavian vein 24 to provide an
unobstructed
conduit for lead 14 to be introduced. Through such curvature, moreover, lead
14 may
be introduced so as to be parallel to vein 24 and not abut and damage wall 25
of
subclavian vein 24.
In the final stages of the lead implantation procedure, illustrated in FIGS.
12
through 14, pacing lead 14 is inserted into the proximal end of introduces
sheath 40 in
the direction of arrow 48, and advanced into the desired position within
patient 10
through vessel 24. Lastly, introduces sheath 40 is removed. Removal of
introduces
sheath 40 rnay be accomplished in one of several known ways, depending upon
the
particular type of introduces sheath 40. For example, as disclosed in the
above-noted
Osborne '562 patent, sheath 40 may be longitudinally split by pulling tabs 50
and 52.
Other sheaths are known which are severable by means of a special slitter
device or
the like.
As shown in FIG. l, pacemaker 12 may operate in conjunction with two
pacing leads. In that case, as with single-lead implants, it may be necessary
to keep
guide wire 30 in place until after the first lead has been implanted. Thus, as
previously noted with reference to FIGS. 10 and 11, guide wire 30 may be left
in
place when dilator 42 is withdrawn. The first lead, if it is sufficiently
small, may be
introduced into subclavian vein 24 alongside guide wire 30, and then the first
introduces sheath is removed leaving guide wire 30 in place. Then, a second
introduces sheath and vessel dilator can be guided along guide wire 30 in the
same
manner as the first, before guide wire 30 is finally removed.
As depicted in FIG. 15 one problem associated with lead introduction systems
and particularly with the sheath used in previous lead introduction systems is
the
formation of a kink 54. As seen a kink 54 in sheath 56 prevents lead 14 from
being
introduced therethrough. As mentioned such kinks may be undetected so that a
lead
inserted into the sheath is blocked, possibly resulting in damage to the lead.
CA 02249616 1998-09-22
WO 97/40880 PCTlfJS97/04292
8
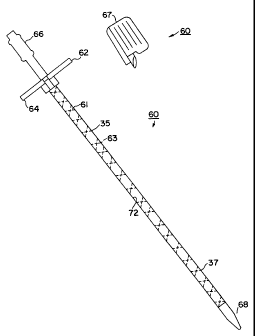
Turning now to FIG. 16, an introduces system 60 in accordance with one
embodiment of the present invention is illustrated. Introduces system 60
comprises an
introduces sheath 61 in which a vessel dilator 66 is inserted. A tapered end
68 of
vessel dilator 66 facilitates the introduction of sheath 60 into the
subclavian vessel 24.
Thereafter, guide wire 30 and vessel dilator 66 are withdrawn from the patient
and
central lumen 65 within sheath 61 provides access to the vessel 24.
As seen introduces system 60 features a sheath 61 which is kink resistant.
Kink resistance is important because it permits the sheath to be dramatically
bent
while still allowing a lead to be introduced therethrough. The kink resistance
is
provided by the composite construction of the sheath. That is, sheath 61 has a
two-
part construction with a tubular portion 71 having at least one reinforcing
fiber 72
integral therewith. In the preferred embodiment reinforcing fibers 72 are
provided in
a braided configuration with more than one fiber in parallel wound together,
the fibers
being wound between 20-45 pics per inch, with 32 pics per inch preferred.
Fibers 72
are preferably a high strength polymer fiber, such as nylon. Tubular portion
of sheath
is made of a biocompatible plastic, such as low density polyethylene. A
suitable
composite sheath 61 having a tubular portion 71 with reinforcing fibers 72
integral
therewith is available from TFX Medical, Limerick, Ireland.
One important benefit of the sheath of the present invention is that it
permits
either a thinner walled sheath to be utilized which would have the same kink
resistance as compared to a same sized sheath without the reinforcing fibers,
or an
overall more flexible material to be used for the sheath as compared to a same
sized
sheath without the reinforcing fibers. The use of a thinner walled sheath is
of benefit
because it reduces the total sheath diameter and thus reduces the incision
into the
patient's venous system. The use of a more flexible material is of benefit
because it
provides for a sheath which is less likely to jab into and damage to venous
system of
the patient.
In the embodiment of FIG. 16, sheath 61 includes means for permitting
removal of sheath 61 from a lead disposed therethrough without requiring
sheath 61 to
be removed from an end of the lead. Specifically sheath 61 may be removed from
a
CA 02249616 2006-02-17
66742-676
9
pacing lead by being longitudinally slit apart using a
sheath slitter 67. As seen a pair of separable tabs 62, 64
are mounted to the proximal end of sheath 61 to facilitate
both the introduction of the sheath as well as the removal
of the sheath from about the lead.
As depicted in FIG. l7 the lead introduction
system of the present invention and particularly kink
resistant sheath 61 may be dramatically bent without a kink
being formed. As mentioned above, such kinks may be
undetected so that a lead inserted into the sheath is
blocked by the kink and cannot be inserted any further.
Such a situation may result in damage to the lead.
FIG. 18 is a detailed cross-sectional view of the
wall of the kink resistant sheath used in an introducer
system of the present invention. As seen sheath 61 has
reinforcing fibers 72 disposed in the approximate middle
portion of the sheath wall.
FIG. 19 is a detailed cross-sectional view of an
alternate embodiment of the wall of the kink resistant
sheath used in an introducer system of the present
invention. As seen sheath 61 has reinforcing fibers 72
disposed in the inner portion of the sheath wall closer to
the sheath lumen 59. This embodiment further features a
score line 63 cut into both the sheath wall and the
reinforcing fibers 72. This permits sheath 61 to be
longitudinally split apart along. score line 63 by grasping
and pulling apart tabs 62 and 64 as sheath 61 is being
withdrawn from the lead introduction site. Various other
equivalent means may also be used to accomplish splitting
sheath 61 along line 63, these include by providing a line
of weakened wall, as shown in Vegoe et al. U.S. Patent
No. 5,180,372 or the entire wall could be constructed using
CA 02249616 2006-02-17
'66742-676
material having the physical property of molecular
orientation whereby a tear in the material runs readily only
in a longitudinal direction along the length of sheath 61,
as is well known in the art.
5 FIG. 20 is a detailed cross-sectional view of an
alternate embodiment of the wall of the kink resistant
sheath used in an introduces system of the present
invention. As seen sheath 61 has non symmetrical
reinforcing fibers 73 disposed in the inner portion of the
10 sheath wall closer to the sheath.lumen 59. As shown, the
non symmetrical reinforcing fibers 73 are rectangular in
cross section. This shape causes the ratio between axial
torque and radial bending stiffness to be modified so that
the surface moment of inertia along or parallel to the coil
axis is less than the surface moment of inertia along a
radial axis in the same coil. Of course other shapes may
also be used, such as elliptical, trapezoidal or oval so
long as the dimension of the fiber measured parallel to the
fiber axis is larger than the dimension measured radially.
This embodiment further features a score line 63 cut into
both the sheath wall and the reinforcing fibers 72 to permit
sheath 61 to be longitudinally split apart along score
line 63 by grasping and pulling apart tabs 62 and 64 as
sheath 61 is being withdrawn from the lead.
In the preferred embodiment, the introduces system
of the present invention is sterilized using ethylene oxide
and packaged as a kit with a sterilized percutaneous
needle 28, a guide wire 30, syringe 26 and a dilator 42 in a
hermetically sealed plastic bag (not shown).
FIGS. 21 and 22 depict a still further alternate
embodiment of the present invention. Specifically this
embodiment features a device to inhibit blood flow through
CA 02249616 2006-02-17
66742-676
11
the sheath when a lead is not positioned within the lumen.
As seen cap 78 is positioned within recess 80 so as to slide
across the proximal end of sheath 61, and specifically
between tabs 62 and 64, and cover the lumen with sheath 61
present when dilator 66 is withdrawn. Further details
concerning the construction of such a device may be seen in
the U.S. Patent No. 5,441,504 of Pohndorf.
Although the invention has been described in
detail with particular reference to a preferred embodiment
and alternate embodiments thereof, it will be understood
variations and modifications can be effected within the
scope of the following claims. Such modifications may
include substituting elements or components which perform
substantially the same function in substantially the same
way to achieve substantially the same result for those
described herein.