Note: Descriptions are shown in the official language in which they were submitted.
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
1
UNIFORM DRUG DELIVERY THERAPY
z
3 FIELD OF THE INVENTION
4
' s This invention pertains to a dosage form that provides a substantially
s uniform delivery of drug over an extended period of time. More particularly,
the invention concerns a dosage form that provides a known and constant
s drug release pattern for an indicated therapy. The invention relates also to
s a dosage form that provides a controlled-constant and uniform delivery of
1o a known dose of drug over time.
11
12 BACKGROUND OF THE INVENTION
13
1a A critical need exists for a dosage form for the controlled and uniform
1s administration of a drug for therapy over time. Presently, in the practice
of
1s pharmacy and medicine, a drug is administered in conventional
pharmaceutical forms, such as tablets and capsules. These conventional
1a forms deliver their drug by dumping and this leads to uneven dosing of
drug,
1s to uneven blood levels of drug characterized by peaks and valleys, and
Zo accordingly this does not provide controlled and uniform therapy over time.
i1 The prior art provided dosage forms for continuous therapy.
22 For example, in United States Pat. No. 4,327,725 issued to Cortese
z3 and Theeuwes, and in United States Pat. Nos. 4,612,008; 4,765,989;
2a and 4,783,337 issued to Wong, Barclay, Deters and Theeuwes, a dosage
is form is disclosed that provides therapy by generating an osmotic pressure
Zs inside the dosage form. The dosage form of these patents operate
27 successfully for delivering a drug for a preselected therapy. With the
delivery
is of some drugs however, these dosage forms often exhibit erratic release
29 rate patterns, such as a nonuniform variation in the drug release rate,
3o and the dosage form can stop delivering a drug, that is, the dosage form
31 can shut-down intermittently.
CA 02249637 1998-09-22
WO 97/37640 PCT/LTS97/04495
2
1 It is immediately apparent, in view of the above presentation, that an
z urgent need exists for a reliable dosage form. The need exists for a dosage
s form endowed with properties for delivering a drug at a substantial and
4 uniform rate over time. The need exists also for a dosage form substantially
s free-of-deviation in its release-rate profile, that delivers the needed dose
of
s drug with a reduced amount of drug left in the dosage form at the end of the
delivery period. It will be appreciated by those knowledgeable in the drug
a dispensing art, that is novel and unexpected dosage form is made available
s that provides a substantially uniform and known drug-release profile, free
of
1o the tribulations of the prior art, such a dosage form would represent an
11 advancement and a valuable contribution in the drug dispensing art.
1z
13 OBJECTS OF THE INVENTION
14
15 Accordingly, in view of the above presentation, it is an immediate
1s object of the invention to provide a dosage form that delivers a drug in a
substantially uniform dose to a biological drug receiving environment over
1s an extended drug-delivery therapy time.
1s Another object of the invention is to provide a novel dosage form that
zo substantially avoids administering a drug in a nonuniform and varying rate
z1 and therefore exhibits substantially the same dose-dispensing rate over
time.
zz Another object of the invention is to provide a dosage form that
23 delivers a predetermined and prescribed dose in the same mdnner over time
z4 while simultaneously lessen the amount retained or the residual drug left
in
zs and not delivered from the dosage form.
zs Another object of the invention is to provide a drug composition of
z~ matter comprising drug particles of 5 ~m to 150 Vim, micron, and
hydrophilic
za polymer particles of 5 ~.m to 250 pm, characterized by the drug particles
and
zs the hydrophilic polymer particles functioning together to provide a uniform
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
3
and nonvarying rate of release of both substantially-free of a deviation and
z substantially-free of a decrease in the rate of the release over time.
s Another object of the invention is to provide a dosage form comprising
a a membrane that surrounds a drug core comprising drug particles of 1 to
s 150 ~m and hydrophilic polymer particles of 1 to 250 pm, particles which
s are co-delivered from the dosage form through an exit formed by a process
selected from the group consisting of a drilled exit, a bioerosion exit,
a a leaching exit, a solubilizing exit, and an exit formed by rupture.
s Another object of the invention is to provide a dosage form comprising
,o a membrane comprising a semipermeable composition that surrounds a core
comprising a drug layer comprising drug particles of 1 to 150 pm and polymer
~z particles of 1 to 250 pm, and a displacement layer comprising an
,s osmopolymer-hydrogel that imbibes fluid, hydrates and increases in swelling
~a volume and thereby displaces the drug layer through an exit membrane
selected from an exit in the group consisting of an orifice, passageway, pore,
microporous channel, porous overlay, porous insert, micropore, microporous
m membrane and porepassageway.
~a Another object of the invention is to make available a process for
~s providing a substantially uniform and substantially nonvarying drug
delivery
zo program from a dosage form, wherein the process comprises the steps of
z~ selecting drug particles of 1 to 150 wm, selecting hydrophilic polymer
particles
zz of 1 to 250 pm, blending the selected particles into a drug-polymer core,
and
z3 surrounding the core with a membrane comprising means for delivering the
z4 drug from the core in a substantially-uniform and substantially-nonvarying
rate
zs of release over a period of time up to 30 hours.
zs Another object of the invention is to provide a dosage form for
z7 delivering a drug to human, wherein the dosage form comprises a drug
zs composition comprising 0.05 ng to 1.2g of drug having a particle size of
zs 1 to 150 p.m, and a hydrophilic polymer having a particle size of 1 to 250
pm,
so a push composition that imbibes fluid and expands for pushing the drug
CA 02249637 2006-10-24
67696-261
4
composition from the dosage form, a wall that surrounds
the drug and the push composition and is permeable to the
passage of fluid, an inner coat that surrounds the drug
and push compositions positioned between the inside surface
of the wall and the drug and push compositions for governing
fluid imbition into the drug and push compositions
for 30 minutes to 4 hours and 30 minutes, and at least one
exit means in the wall for delivering the drug composition
at a uniform and nonvarying rate over time.
Other objects, features, and advantages of the
invention will be more apparent to those versed in the
dispensing art comprising medicine and pharmacy from the
following detailed specification taken in conjunction with
the accompanying claims.
According to one aspect of the present invention,
there is provided a process for providing a substantially
uniform drug rate of release from a dosage form, wherein the
dosage form comprises a composition, a dose of drug in the
composition, and a hydrophilic polymer in the composition;
and wherein the process comprises (1) formulating the
composition with a drug possessing a size less
than 150 micron, and (2) formulating the composition with a
hydrophilic polymer of less than 250 micron; whereby,
through the copresence of (1) and (2) in the composition,
the drug is delivered at a substantially uniform rate of
release from the dosage form.
According to another aspect of the present
invention, there is provided a process for providing a
substantially uniform drug rate of release from a dosage
form, wherein the dosage form comprises: a drug layer
comprising a dose of drug and a hydrophilic polymer; and, a
dispensing layer comprising means for dispensing the drug
CA 02249637 2006-10-24
67696-261
4a
layer from the dosage form; and wherein the process
comprises formulating the drug layer with a drug possessing
a particle size up to 150 microns and with a hydrophilic
polymer possessing a particle size up to 250 microns; which,
through the cooperation of the drug particles and the
hydrophilic polymer particles and the dispensing layer
assisting the drug layer, the drug is delivered at a
substantially uniform rate of release from the dosage form.
According to still another aspect of the present
invention, there is provided a dosage form for the delivery
of a drug, wherein the dosage form comprises: (a) a
composition; (b) a dose of drug of less than 150 microns in
the composition; (c) a hydrophilic polymer of less
than 250 microns in the composition; (d) a wall comprising a
composition permeable to the passage of fluid that surrounds
the dose of drugs and the hydrophobic polymer; and (e) means
in the wall for delivering the drug at a substantially
uniform rate from the dosage form.
According to yet another aspect of the present
invention, there is provided a dosage form for the delivery
of a drug, wherein the dosage form comprises: (a) a drug
composition; (b) a dose of drug of less than 150 microns in
the drug composition; (c) a hydrophilic polymer of less
than 250 microns in the drug composition; (d) a coat that
surrounds the drug composition comprising means for delaying
release of drug from the drug composition; (e) a wall
comprising a composition that surrounds the coat; and, (f)
means in the dosage form for delivering the drug from the
dosage form over time.
According to a further aspect of the present
invention, there is provided a dosage form for the delivery
of a drug, wherein the dosage form comprises: (a) a drug
CA 02249637 2006-10-24
67696-261
4b
composition comprising a drug of less than 150 micron size
and a pharmaceutically acceptable hydrophilic polymer
carrier of less than 250 micron size for this drug; (b) a
displacement composition in contact with the drug
composition comprising means for causing fluid to enter the
displacement composition whereby the displacement
composition increases in volume and displaces the drug
composition from the dosage form; (c) a wall comprising
means for permitting a fluid to enter the dosage form that
surrounds the drug composition and the displacement
composition; and (d) means in the wall for delivering the
drug at a substantially uniform rate over a dispensing time.
According to yet a further aspect of the present
invention, there is provided a dosage form for delivering a
drug orally to a patient in need of a drug, wherein the
dosage form comprises: (a) drug composition comprising a
drug having a particle size up to and including 150 microns,
and a hydrophilic polymer carrier having a particle size up
to and including 250 microns for the drug; (b) a
displacement composition in contact with the drug
composition and comprising a polymer that expands in the
presence of fluid for displacement of the drug composition
from the dosage form; (c) a coat free of drug that surrounds
the drug and the displacement composition for slowing the
passageway of fluid into the dosage form; (d) a wall that
surrounds the coat and is permeable to the passage of fluid;
and, (e) means in the dosage form for delivering the drug
from the dosage form at a substantially uniform rate over
time.
CA 02249637 2006-10-24
67696-261
4c
1s BRIEF DESCRIPTION OF THE FIGURES
14
is Figure -1 illustrates thetlrug release rate variation with a drug
~s possessing a particle size of 2 to 900 microns in the presence of a polymer
1~ possessing 25% and more of greater than 250 micron size.
~a Figure 2 illustrates the drug release rate variation from a dosage
1s form with a drug size of less than 150 micron in the presence of a polymer
2o possessing 25% and more of greater than 250 micron.
z~ Figure 3 illustrates pronounced decrease in the variation of the
zz drug release rate when the dosage form comprises a drug size of less than
23 150 micron accompanied by a polymer size of less than 250 micron.
24
zs DESCRIPTION OF THE INVENTION
is
z~ The following examples are illustrative of the invention and they
Za should not be considered as limiting the invention in any way, as these
is examples and other equivalents thereof will became apparent to those
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
versed in the dispensing art in the light of the present specification and
z the accompanying claims.
3
a EXAMPLE 1
5
s A dosage form for delivering a drug orally to the gastrointestinal tract of
the drug receiving patient in need of the drug's therapy is prepared as
follows:
s first 5 mg of 135 pm amlodipine besylate, a calcium channel blocker, is
s blended with a 5% solution of poly(vinylpyrrolidone) of 30,000 number
~o average molecular weight available from General Aniline and Film
Corporation, New York, New York, in a fluid bed processor. Then, the
~z granulated product is combined with 7.5 mg of 235 pm a poly (alkylene
oxide), a polyethylene oxide), of 175,000 number average molecular weight
,4 available from the Union Carbide Corporation, Danbury, Connecticut, 0.5 mg
~5 of sodium chloride and 0.02 mg a stearic acid, and blended to provide a
homogenous blend, by blending 35 rpm for 7 minutes. The homogenous
blend is compressed into a drug composition and surrounded with a wall
,s comprising a semipermeable composition and an exit forming agent.
,s The wall composition comprises 65 wt% cellulose acetate having an
zo acetyl content of 34% and a 30,000 number average molecular weight
z~ dissolved in acetone:water, to which 1.8 wt% triacetin and 1.5 wt% sodium
zz chloride are added with stirring constantly. The drug composition is
sprayed
zs in a fluidized bed air suspension coater to provide 10% wt wall. The dosage
z4 form is dried at 25°C for 18 hours. The dosage form releases the
amlodipine
z5 besylate in a nonvarying rate through microchannels formed by fluid
leaching
zs of the sodium chloride in the gastrointestinal fluid of the patient.
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
6
EXAMPLE 2
z
s The procedure of the above example is followed in this example,
a wherein in the present example the drug is selected from the group
consisting
s of 5 mg of lisinopril indicated as an angiotensin converting enzyme
inhibitor,
s 10 mg of buspirone hydrochloride indicated as an antianxiety drug, and 5 mg
of oxybutynin hydrochloride indicated for relief of bladder instability, and
a wherein the lubricant is magnesium stearate and the semipermeabie wall
s comprises mannitol.
~o
EXAMPLE 3
~z
~s A dosage form for the osmotically and hydrokinetically controlled
~4 release of a beneficial drug is made as follows: first, to a mixing bowl is
~s added 500 mg of the oral antibacterial ciprofloxacin hydrochloride of
125 microparticle size followed by the addition of 105 mg of sodium
carboxymethylcellulose of 22,000 number average molecular weight of
135 micron sizes and the ingredients mixed for 3 to 5 minutes to yield a
homogenous mix. Next, 10 mg of 88 microcrystalline cellulose of 11,000
zo number average molecular weight is added to the mixing bowl and 0.05 mg
z~ of drug delivery surfactant sodium lauryl sulfate added to the bowl and all
zz the ingredients mixed for 5 minutes. Then, an aqueous solution containing
z3 7.5 mg of poly(vinylpyrrolidone) of 30,000 number average molecular weight
z4 is added with mixing and the resulting mixture is passed through an
extruder
zs onta a small tray and let dry overnight. The granulation is dried for 5
hours at
zs 50°C and 0.03 mg of lubricant added with mixing for 1 minute. A
solid fluid
z7 imbibing osmotic care is prepared in tablet press with a concave punch.
za Next, an internal subcoat, drug free, is prepared comprising 94 wt%
zs hydroxyethyfcellulose of 90,000 number average molecular weight and 6 wt%
so polyethylene glycol in distilled water is coated around the drug
composition
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
7
and the subcoated drug composition is dried for 1 hour at 45°C. Then,
an
z outer coat comprising a semipermeable composition and a pore-passageway
s former is prepared by adding cellulose acetate of 39.43% acetyl content to a
a cosolvent of methylene chloride and methanol to yield a solution effected by
stirring and warming. Next, the pore-former sorbitol is added to a cosolvent
of
s water and methanol with mixing followed by adding polyethylene glycol to
produce the outer coating solution. Finally, the outer coating solution is
s coated around the subcoat in a pan coater and then dried for 18 hours at
s 45°C in a forced air oven, to yield the desired dosage form. The
dosage form,
~o in operation in the gastrointestinal fluid of a human in need of drug
therapy,
provides a uniform and nonvarying-order of drug release through exit
~z passageways of controlled porosity effected by the fluidic leaching of the
soluble pore-forming additive incorporated in the semipermeable outer coat.
~4 The cooperation of the drug particles and the hydrophilic polymer particles
provides a viscous gel that pushes the drug through the exits at the
,s given rate.
~s EXAMPLE 4
~s
zo The procedure of the above example is followed, with the proviso in
z~ this example the therapeutic member is selected from the group consisting
of
zz 40 mg of simvastatin for lowering cholesterol, 75 mg of venlafaxine
zs antidepressant, 20 mg of fluoxetine antidepressant, 20 mg of antianginal
za nifedipine, 40 mg of lovastatin indicated for lowering cholesterol, 20 mg
of
z5 enalopril maleate an angiotensin converting enzyme inhibitor, 120 mg of
zs diltiazem for managing calcium ion influx, 500 mg of ciprofloxacin
z7 hydrochloride an antibacterial, 100 mg of sertraline hydrochloride an oral
zs antidepressant, 100 mg of cyclosporin an immunosuppresant, 1 mg of
zs terazosin hydrochloride an alpha-adrenoceptor blocker, 50 mg of sumatriptan
so succinate a 5-hydroxytryptamine receptor agonist, 40 mg of pravastatin
CA 02249637 1998-09-22
WO 97/37640 PCTlUS97/04495
8
sodium a hypoiipidemic, 500 mg of an anti-HIV-proteinase inhibitor such as
z nelfinavir, saquinavir, indinavir, or ritonavir, an anti-HIV such as
zidovudine,
didanosine, or lamivudine, a reverse transcriptase inhibitor such as loviride,
a an antiviral herpes such as fumciclovir or gancidovir, 10 mg of alendronate
s sodium for treating osteoporosis, and 2.5 mg of conjugated estrogen
s indicated for the treatment of vasomotor symptoms associated with
menopause, atrophic vaginitis and osteoporosis loss of bone mass.
a
s EXAMPLE 5
~o
A dosage form for the oral uniform and nonvarying release of a drug
i2 to a biological drug receptor is manufactured as follows: first, 6000g of
,s verapamil hydrochloride, indicated for the treatment of angina and high
blood pressure, having nonuniform particle size distribution between
~s 1 micron to 900 micron, 3047g of polyethylene oxide) having a number
~s average molecular weight of 300,000 and having 25% particles greater
than 250 micron, 500g of sodium chloride and 100g of poly(vinylpyrrolidone)
~s having a number average molecular weight of 40,000 are added to a
Freund Flo-Coater's bowl, a fluid bed granulator. The bowl is attached to
2o the Flo-Coater and the granulation process is initiated. Next, the dry
powders
z, are air suspended and mixed for five minutes. Then, a solution prepared by
22 dissolving 300g of poly(vinylpyrrolidone) having a number average molecular
is weight of 40,000 in 4,500g of water is sprayed from 2 nozzles onto the
24 powder. The coating conditions are monitored during the
2s poly(vinylpyrrolidone) solution spraying as follows: a total spray rate of
Zs 240 g/min from each nozzle, an inlet temperature of 45°C, an
airflow of
1000 cfm. The coating process is computerized and automated in cycles.
2s Each cycle contained 30 seconds of solution spraying followed by two
is seconds of drying and 10 seconds of filter bags with shaking to unglue
so any possible powder deposits. At the end of the solution spraying period,
CA 02249637 1998-09-22
WO 97/37640 PCT/US97l04495
9
the coated granulated particles are continued in the drying process for
z 25 minutes. The machine is turned off, and the coated granules are
s removed from the coater. The coated granules are sized using a fluid air
mill.
a The granulation is transferred to a mixer, mixed and lubricated with 50g of
magnesium stearate and mixed with 4g of butylated hydroxytoluene, to
s provide the drug composition.
Next, a push-displacement composition is prepared as follows:
a first, 73428 of polyethylene oxide) possessing a number average
s molecular weight of 7 million, 20008 of sodium chloride, 2008 of
hydroxypropylmethylceliulose of 11,200 number average molecular weight,
> > 1 OOg of black ferric oxide are added to the Freund Flo-Coater's bowl.
12 The bowl is attached to the Flo-Coater and the granulation process is
started
to effect the process. The dry powders are air suspended and mixed for
~a six minutes. Then, a solution is prepared by dissolving 3008 of
hydroxypropylmethylcellulose having a number average molecular weight of
11,200 in 4,5008 of water is sprayed from 2 nozzles onto the air suspended
powder mix. The coating conditions were monitored during the
~a hydroxypropylmethylcellulose spraying of the solution. The conditions are
,s identical to those described in the above drug granulation process, except
zo for the drying cycle of less than 25 minutes. The granulated powders are
z~ removed from the granulator and sized in a fluid air mill. The granulation
is
zz transferred to a blender, mixed and lubricated with 508 of magnesium
zs stearate and with 8 grams of butylated hydroxytoluene to yield the push-
z4 displacement composition.
zs Next, the drug composition and the push composition are compressed
zs into a bilayered core. First, 300 mg of the drug composition comprising
z7 180 mg of verapamil hydrochloride is added to the punch and tamped, then
za 100 mg of the push displacement composition is added to the punch and the
zs layers pressed under a pressure of 2200 pounds into a 13/32 inch (1.032 cm)
so diameter contacting, bilayered arrangement.
CA 02249637 1998-09-22
WO 97/37640 PCTIUS97/04495
Next, the bilayered core is coated with a subcoat. The subcoat
z comprises 95% hydroxyethylcellulose of 90,000 number average molecular
s weight and 5% polyethylene glycol of 3350 average molecular weight. The
a ingredients are dissolved in water to make a 5% solid solution. The subcoat
s forming composition is sprayed onto and around the bilayer core in a 24 inch
s Vector Hi-Coater. The dry subcoat weighed 79 mg.
Next, the hydroxyalkylcellulose, a hydroxyethylcellulose, a subcoated
s bilayered cores are over coated with a semipermeable composition. The
s overcoat membrane forming composition comprises 60% cellulose acetate
~o having an acetyl content of 39.8%, 35% hydroxypropylcellulose of 40,000
number average molecular weight and 5% polyethylene glycol of 3350 avg.
~z molecular weight is dissolved in methylene chloride:methanol (90:10 wt:wt)
cosolvent to make a 4% solid solution. The semipermeable membrane
,a forming composition is sprayed onto and around the subcoated bilayer core.
The semipermeable membrane, after drying weighed 43 mg.
Next, two 27 mil (0.686 mm) exit passageways are drilled through the
outer semipermeable membrane and the inner subcoat to connect the drug
~a layer with the exterior of the dosage form. The residual solvents are
removed
~s by drying for 96 hours at 50°C and 50% humidity. Finally, the dosage
forms
zo are dried for 2 hours at 50°C to remove any excess moisture.
z~ The dosage form manufactured by this procedure comprises a drug
zz composition with a weight of 300 mg, consisting of 180 mg of verapamil
zs hydrochloride, 91.41 mg of poly (ethylene oxide) of 300,000 molecular
weight,
z4 12 mg of poly(vinylpyrrolidone) of 40,000 molecular weight, 15 mg of sodium
zs chloride, 0.12 mg of butylated hydroxy toluene and 1.5 mg of magnesium
zs stearate. A push-displacement composition that weighs 100 mg consisting of
z7 73.5 mg of polyethylene oxide) of 7,000,000 molecular weight 20 mg of
za sodium chloride, 5 mg of hydroxypropylmethylcellulose of 11,200 molecular
zs weight, 0.92 mg of black ferric oxide, 0.08 mg of butylated hydroxytolune
and
so 0.5 mg of magnesium stearate. The dosage form subcoat weighed 78.8 mg
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
11
consisting of 74.86 mg of hydroxyethylcellulose of 90,000 molecular weight
z and 3.94 mg of polyethylene glycol of 3350 molecular weight. The outer wall
s weighed 42.6 mg consisting of 25.56 mg of cellulose acetate of 39.8% acetyl
4 content, 14.90 mg of hydroxypropylcellulose of 40,000 molecular weight, and
s 2.13 mg of polyethylene glycol of 3350 molecular weight. This dosage form
s had a (dmldt)t mean release rate of 18.6 mg/hr between the fourth and
ninth hour.
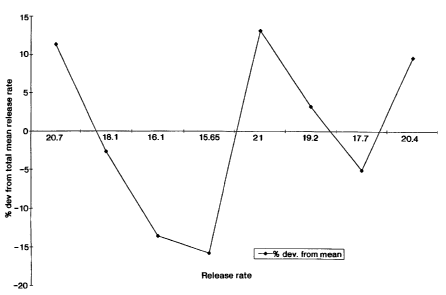
s The delivery pattern for the dosage form prepared by this example is
s illustrated in figure 1. In figure 1, the nonuniform variability release
rate is
seen over the steady portion illustrated by the line starting at zero and
extended to the right of the figure. The release rate variation is for a drug
~z having a 1 to 900 micron particle size released in the presence of a
13 hydrophilic polymer having greater than 25% particles larger then 250
micron.
The solid line depicts the % deviation from the total mean release rate.
~s The mean release rate for a given dosage form is expressed by the number
,s along the line starting at zero. In the figure No. 1 the erratic behavior
is
seen because the dosage form lacks uniform particles of a limited range.
1a The erratic behavior is characterized by a substantial deviation of
individual
system from the mean (dosage form) steady state release rate performance.
zo This erratic behavior phenomena is attributed to the inability of the
hydrophilic
z~ polymer, the polyethylene oxide), to carry and suspend large drug
zz particles,(the verapamil hydrochloride), the difference in the hydration
time
z3 between the large and small drug particles, and the larger hydrophilic
polymer
za particles greater than 250 micron, which significantly changes the
hydration
z5 and the drug suspending properties of the drug compositional layer that
zs resulted into a large percent negative deviation in the (dmldt); from the
z7 (dmldt)~. The expression (dm/dt)t denotes the total mean release rate for
all
za dosage forms in the zero portion, (dmldt); denotes the mean release rate of
zs an individual dosage form in 4 to 9 hours, and (% dev); denotes the percent
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
12
deviation in an individual dosage form mean release rate, (4 to 9 hours) from
z the total mean release rate. The figure reports results obtained from the
s following equation:
4
(% dev) = dm/ ,~dm/dt)s
s (dm/dt)t
EXAMPLE 6
9
~o A dosage form for the delivery of a drug orally to a human is prepared
as follows: first 6000g of verapamil hydrochloride having a particle size of
~z less than 150 micron, 3047g of polyethylene oxide) possessing a number
average molecular weight of 300,000 with 25% particles larger than
250 micron, 500g of sodium chloride, 100g of poly(vinylpyrrolidone) having
15 a number average molecular weight of 40,000 are added to the bowl of a
fluid
bed granulator. The granulation is carried out for 7 to 10 minutes. Next, the
dry powders are air suspended and mixed for five minutes. Then, a solution
~a is prepared by dissolving 3008 of poly(vinylpyrrolidone) of 40,000 number
average weight in 4,500g of distilled water is sprayed from 2 nozzles onto the
zo dry powder. The coating conditions are monitored during spraying as
follows:
z~ a total spray rate of 240 g/min from each nozzle, an inlet temperature of
45°C
zz and a process airflow of 1000 cfm. The coated process is automated in
zs cycles. Each cycle consist of 30 seconds of solution spraying followed by
z4 two seconds of drying and 10 seconds of filter bags shaking to unglue and
zs possible powder deposits. At the end of the solution spraying time, the
zs coated granulated particles are continued with the drying process for
z~ 25 minutes. The machine is turned off, and the coated granules were
za removed from the coater. The coated granules are sized using a fluid air
mill,
zs the granulation is transferred to a mixer, mixed and lubricated with 50
grams
so of magnesium stearate and mixed with 4g of butylated hydroxytoluene to
s~ provide the drug composition used for forming a layer in the bilayer core.
CA 02249637 1998-09-22
WO 97/37640 PCT/US97I04495
13
Next, a push composition is prepared as follows: first, 73428 of
z poly{ethylene oxide) of 7,000,000 number average molecular weight, 20008
s of sodium chloride, 2008 of hydroxypropylmethylcellulose of 11,200 number
a average molecular weight, and 100 grams of black ferric oxide are added to
the bowl of a fluid bed granulator. The granulation process is started, and
the
s dry powders are air suspended and mixed for 6 minutes. Then, a solution is
prepared by dissolving 3008 of hydroxypropylmethylcellulose possessing a
s 11,200 number average molecular weight in 4,5008 of water that is sprayed
s onto the air suspended powder mix. The coating conditions are monitored
~o during the spraying and the physical conditions are identical as described
for the above drug granulation, except that the drying cycle was less than
25 minutes. The granulated powders are removed from the granulator.
The granules are sized in a fluid air mill, then transferred to a blender and
a4 lubricated while mixing with 508 of magnesium stearate and 8g of butylated
hydroxytoluene to yield the push composition.
~s Next, the drug composition and the push composition are pressed into
a bilayered core, with the layers in contacting arrangement. First, 400 mg of
~a the drug composition comprising 240 mg of verapamil hydrochloride is added
,s to a tablet punch and tamped, then 135 mg of the push composition is added
zo to the punch and the layers are pressed under a pressure head of 2300
pound in a 7/16 inch (1.11 cm) diameter contacting, bilayered arrangement.
ii The bilayered-core tablets are coated with a subcoat. The subcoat comprises
is 95% hydroxyalkylcellulose, a (hydroxyethylcellulose) of 90,000 'molecular
24 weight and 5% polyethylene glycol of 3350 molecular weight, dissolved in
zs water to provide a 5% solid solution. The subcoat forming composition is
2s sprayed onto the around the bilayered core in a coater. The dry subcoat
weighed 93 mg.
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
14
Next, an outer coat is applied to the dosage form. The subcoated
z bilayered-core tablets are coated with a semipermeable-membrane wall.
a The membrane forming composition comprises 60% cellulose acetate having
a a 39.8% acetyl content, 35% hydroxypropylcellulose of 40,000 molecular
s weight and 5% polyethylene glycol of 3350 molecular weight. The wall
s forming composition is dissolved in methylene chloride:methanol (90:10
wt:wt)
cosolvent to make a 4% solid solution. The semipermeable-membrane wall
s forming composition is sprayed onto and around the subcoated bilayer core
s in a coater to provide a two-coated dosage form. The semipermeable
io membrane dry weighed 51 mg.
> > Next, two 27 mil (0.686 mm) exit passageways are drilled through the
~z outer and inner coats to connect the drug layer with the exterior of the
dosage
~s form. The residual solvents are removed by drying for 96 hours at
50°C and
,4 50% humidity. Then, the osmotic dosage forms are dried for 2 hours at
50°C
15 to remove excess moisture.
The dosage form manufactured by this procedure comprises a drug
composition with a weight of 400 mg, consisting of 240 mg of verapamil
hydrochloride, 121.88 mg of polyethylene oxide of 300,000 molecular weight,
16 mg of poly(vinylpyrrolidone) of 40,000 molecular weight, 20 mg of sodium
zo chloride, 2 mg of magnesium stearate and 0.16 mg of butylated
z~ hydroxytoluene. The push composition of the dosage form weighed 135 mg
zz and consists of 99.23 mg of poly(alkylene oxide), polyethylene oxide) of
zs 7,000,000 molecular weight, 27 mg of sodium chloride, 6.75 mg of
z4 hydroxypropylmethylcellulose of 11,200 molecular weight, 1.24 mg of
z5 ferric oxide, 0.675 mg magnesium stearate, and 0.108 mg of butylated
zs hydroxytoluene. The inner subcoat weighed 93.1 mg and consists of
z7 88.45 mg of the hydroalkylcellulose, hydroxyethylcellulose of 90,000
zs molecular weight and 46.55 mg of polyethylene glycol of 3350 molecular
zs weight. The outer coat weighed 51.1 mg and consists of 30.66 mg of
so cellulose acetate of 39.8% acetyl content, 17.89 mg of
hydroxypropylcellulose
CA 02249637 1998-09-22
WO 97/37640 PCTIUS97/04495
1 of 40,000 molecular weight and 2.57 mg of polyethylene glycol of 3350
z molecular weight. The dosage form prepared by this example had a (dm/dt)t
3 mean release rate of 27 mg/hr during hours 4 to 9.
a The drug delivery pattern for the dosage form prepared by this
5 invention is seen in drawing figure 2. In figure 2, the nonuniform
variability is
s depicted for the dosage form. The erratic release behavior is characterized
by a substantial and pronounced deviation of individual dosage forms from
a the mean dosage form steady state rate performance. The figure denotes
s that larger polymer particles of from 250 micron significantly change the
,o hydration and the drug carrying ability and suspension properties of the
11 drug composition. This results in a large percent negative deviation in the
1z expression (dmldt); from the expression (dm/dt)t.
13
14 EXAMPLE 7
1s A dosage form for the delivery of a drug orally to the gastrointestinal
17 tract of a human in need of drug therapy is prepared as follows: first,
60008
1a of verapamil hydrochloride having a particle size of 150 or smaller
microns,
30478 of polyethylene oxide) of 300,000 molecular weight and having a
zo particle of 250 or smaller microns, 5008 of powdered sodium chloride, 1008
of
z1 poly(vinylpyrrolidone) having a 40,000 molecular weight are added to a
coater
Zz and granulated in air for five minutes. Next, a solution is prepared by
23 dissolving 3008 of poly(vinylpyrrolidone) of 40,000 molecular weight in
4,5008
z4 of water and sprayed onto the powder. The spray rate is 240g/min at an
inlet
i5 temperature of 45°C and an airflow of 1000 cfm. The spraying is
effected in
zs two cycles consisting of 30 seconds of solution spraying followed by two
z~ seconds of drying and 10 seconds of shaking to unglue powder deposits.
zs At the end of the solution spraying period, the coated granulated particles
are
zs dried for an additional 25 minutes. Then, the coated granules are sized in
a
3o fluid air mill. The granulation is transferred to a mixer, and lubricated
with
CA 02249637 1998-09-22
WO 97/37640 PCTlUS97/04495
16
50g of magnesium stearate and with 4g of butylated hydroxytoluene, to
z yield the drug composition.
s Next, a push displacement composition is prepared as follows:
a first, 7342g of poly(ethyiene oxide) of 7,000,000 molecular weight, 2000g
of sodium chloride, and 2000g of hydroxypropylmethylcellulose of 11,200
s molecular weight, and 100g (grams) of black ferric oxide are added to the
bowl of a fluid bed granulator. The granulation is started and the powders
a mixed for six minutes. Then, a solution is prepared by dissolving 300g of
s hydroxypropylmethylcellulose of 11,200 molecular weight in water and
sprayed onto the air suspended particles. The coating process is as
described above. The granules are sized in a fluid air mill and transferred to
~z a blender, and blended with 50g of magnesium stearate and 8g of butylated
~s hydroxytoluene, to yield the push-displacement composition.
Next, the drug composition and the push composition are compressed
~s into a bilayered tablet as follows: first, 400 mg of the drug composition
containing 240 mg of verapamil hydrochloride is added and tamped, then it
is overlayed with 135 mg of the push composition, and the two compositions
~s pressed under 2300 pounds into a 7116 inch (1.11 cm) diameter contacting,
19 bilayered arrangement.
zo Next, the compressed bilayer tablets are coated with a subcoat
z~ laminate. The subcoat comprises 95% hydroxyethylcellulose of 90,000
zz molecular weight and 5% polyethylene glycol of 3350 molecular weight
zs dissolved in distilled water to make a solid solution. The subcoat forming
z4 composition is sprayed onto and around the bilayered tablet in a coater
zs to provide an encompassing laminate. The dry subcoat weighed 93 mg.
zs Next, the subcoat is overcoated with a semipermeable wall.
z~ The semipermeable composition comprises 60% cellulose acetate having
zs an acetyl content of 39.8%, 35% hydroxypropyicellulose of 40,000 molecular
zs weight and 5% polyethylene glycol of 3350 average molecular weight.
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
17
The wall-forming composition is dissolved in a methylene-chloride:methanol
z (90:10 wt:wt) cosolvent to make a 4% solid solution. The semipermeable
s overcoat is sprayed onto and around to encase the subcoat. The
a semipermeable wall weighed 51 mg.
Next, two 27 mil (0.686 mm) exit passageway are drilled through the
s dual oats to connect the drug layer with the exterior of the dosage form.
The residual solvents are removed by drying for 96 hours at 50°C
and
s 50% humidity. Next, the osmotic, fluid imbibing dosage forms are dried
s for 2 hours at 50°C to remove excess moisture.
The dosage form prepared by this example embraces the same
composition as the example immediately above, except for the controlled
~z drug particle size and the controlled hydrophilic polymer particle size in
the
drug composition. This double particle control produces substantially uniform
dose dispensing, substantially-free of a wide variation in the dose dispensing
~s pattern. Accompanying figure 3 depicts the drug delivery pattern for this
~s example. The figure depicts a release rate of (dm/dt)t equal to 27.9 mg/hr
» during hours 4 to 9. The figure illustrates that a nonuniform variability is
not
observed for the dosage form provided by this example.
,s
zo EXAMPLE 8
z,
zz A dosage form prepared according to Example 8 wherein the drug in
zs the dosage form is a calcium channel blocking drug selected from the group
z4 consisting of isradipine, nilvadipine, flunarizine, nimodipine, diltiazem,
z5 nicardipine, nitredipine, nisoldipine, filodipine, amlodipine, cinnarizine,
zs and fendiline.
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
18
EXAMPLE 9
2
3 The procedure described in the above is repeated in this example,
a with the processing conditions as previously set forth, except that, in this
example the drug is an angiotensin converting enzyme inhibitor selected
s from the group consisting of alacipril, benazepril, cialzepril, captropril,
delapril, enalapril, fosinopril, lisinopril, moveltypril, perindopril,
quinapril,
a ramipril, spirapril, and zofenopril.
9
1o EXAMPLE 10
11
12 The procedures of the above examples are followed in this example
13 with the addition of the drug and is protected against oxidative attack and
1a oxidation by adding to the processing drug composition 0.05 ng to 7 mg of
an
1s antioxidant selected from the group consisting of d-alpha tocopherol, dl-
alpha
1s tocopherol, d-alpha tocopherol acetate, d-alpha tocopherol acid succinate,
dl-alpha tocopherol acid succinate, dl-alpha tocopherol palmitate, ascorbic
1a acid, ascorbyl oleate, ascorbyl palmitate, butylated hydroxyanisole,
butylated
hydroxytoluene, sodium ascorbate, calcium ascorbate, and propyl gallate
2o stabilizers.
21
22 EXAMPLE 11
23
24 The procedures of the above examples are followed in this example
2s with an addition to the drug composition comprising 0.05 ng to 7 mg of an
2s antioxidant stabilizer and 0.05 ng to 7.5 mg of a lubricant selected from
the
27 group consisting of magnesium stearate, calcium stearate, magnesium
2a oleate, magnesium palmitate, corn starch, potato starch, bentonite, citrus
2s pulp, and stearic acid; and, with all the ingredients in the drug
composition
3o when expressed in weight percent equal to 100 wt% weight percent.
CA 02249637 1998-09-22
WO 97137640 PCT/US97/04495
19
1 EXAMPLE 12
2
3 The procedures of the above examples are followed in this example
a with an addition to the drug composition of means protection the drug
against
daylight and ultraviolet light; wherein, the addition comprising adding to the
s drug composition 0.01 mg to 10 mg of surface-active agent selected from
anionic, cationic, amphoteric and nonionic surfactants including dialkyl
s sodium sulfosuccinate, polyoxyethylene glycerol, polyoxyethylene stearyl
s ether, propoxy-ethoxy copolymer, polyoxyethylene fatty alcohol ester,
1o polyoxyethylene fatty acid ester, ethoxylated hydrogenated castor oil, and
11 butoxylated hydrogenated castor oil; and adding to the drug composition
12 0.01 mg to 10 mg of riboflavin to stabilize the drug against light.
13
1a ADDITIONAL DISCLOSURE OF THE INVENTION
1s In the specification and in the accompanying claims, the term
17 beneficial agent also includes drugs. The term drug includes any
1s physiologically or pharmacologically active substance that produces a local
1s or a systemic effect, in animals, including warm-blooded mammals, humans
Zo and primates; avians, household, sport, and farm animals; laboratory
animals;
i1 fishes; reptiles; and zoo animals. The term "physiologically" as used
herein,
22 generically denotes the administration of a drug to produce generally
normal
23 drug levels and functions. The term "pharmacologically" denotes generally
24 variations in response to the amount of drug administered to a host. The
drug
i5 can be in various forms such as unchanged molecules, molecular complexes,
is pharmacologically acceptable salts such as hydrochloride, hydrobromide,
27 sulfate, laurate, palmitate, phosphate, nitrite, nitrate, borate, acetate,
maleate,
za tartiate, oleate, salicylate, and the like. For acidic drugs, salts of
metals,
is amines, or organic cations, for example quarternary ammonium can be used.
3o Derivatives of drugs, such as bases, ester and amide can be used. A drug
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
that is water insoluble can be used in a form that is water soluble derivative
z thereof, or as a base derivative thereof, which in either instance or in its
s delivery by the osmotic system, is converted by enzymes, hydrolyzed by the
a body pH, or by other metabolic processes to the original therapeutically
active
s form. The amount of drug in a dosage form, that is, in the drug composition
is
s 25 ng to 750 mg. The dosage form comprising the drug can be administered,
once, twice, or thrice daily.
s The active drug that can be delivered includes inorganic and organic
s compounds without limitation, including drugs that act on the peripheral
~o nerves, adrenergic receptors, cholinergic receptors, nervous system,
skeletal
~1 muscles, cardiovascular system, smooth muscles, blood circulatory system,
,z synoptic sites, neuroeffector functional sites, endocrine system, hormone
~s systems, immunological system, organ systems, reproductive system,
skeletal system, autocoid systems, alimentary and execretory systems,
~s inhibitory of autocoids and histamine systems, and physiological systems.
Is The active drug that can be delivered for acting on these animal systems
includes depressants, beta-blockers, hypnotics, sedatives, psychic
~e energizers, tranquilizers, anti-convulsants, muscle relaxants, steroids,
,s antiparkinson agents, analgesics, anti-inflammatories, polypeptides, local
zo anesthetics, muscle contractants, anti-microbials, antimalarials, hormonal
z, agents, contraceptives, sympathomimetics, diuretics, anti-parasitics,
z2 neopiastics, hypoglycemics, ophthalmics, electrolytes, diagnostic agents,
is cardiovascular drugs, calcium channel blockers, angio-tensin-converting
z4 enzyme inhibitors, and the like.
zs Exemplary of drugs that can be delivered from the dosage form of
zs this invention include a drug selected from the group consisting of
amifostine,
27 prochlorperazine edisylate, ferrous sulfate, aminocaprioc acid, potassium
za chloride, mecamylamine hydrochloride, procainamide hydrochloride,
zs amphetamine sulfate, benzphetamine hydrochloride, isoproternal sulfate,
so methamphetamine hydrochloride, phenmetrazine hydrochloride, bethanechol
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
21
chloride, methacholine chloride, pilocarpine hydrochloride, antropine sulfate,
2 methascopolamine bromide, isopropamide iodide, tridihexethyl chloride,
s phenformin hydrochloride, methylphenidate hydrochloride, oxprenolol
4 hydrochloride, metroprolol tartrate, cimetidine hydrochloride, diphenidol,
s meclizine hydrochloride, prochlorperazine maleate, phenoxybenzamine,
s thiethylperzine, maleate, anisindone, diphenadione erythrityl teranitrate,
dizozin, isofurophate, reserpine, acetazolamide, methazolamide,
s bendroflumenthiazide, chlorpropamide, tolazamide, chlormadinone acetate,
s phenaglycodol, allopurinol, aluminum aspirin, methotrexate, acetyl
,o sulfisoxazle, erythromycin, progestins, estrogenic progrestational,
corticosteroids, hydrocortisone acetate, cortisone acetate, triamcinolone,
methyltesterone, 17~i-estradiol, ethinyl estradiol, ethinyl estradiol 3-methyl
is ether, prednisolone, 17-hydroxyprogesterone acetate, 19-nor-progesterone,
,a norgestrel, norethindone, norethiderone, progesterone, norgestrone,
~s orethynodrei, aspirin, indomethacin, aproxen, fenoprofen, sulidac,
diclofenac,
indoprofen, nitroglycerin, propranolol, metroprolol, vallproate, oxyprenolol,
w timolol, atenoloi, alpreholol, cimetidine, clonidine, imipramine, levodopa,
~a chloropropmazine, resperine, methyldopa, dihydroxyphenyllalanine,
pivaloyloxyethyl ester of s-methyldopa hydrochloride, theophylline, calcium
2o gluconate ferrous lactate, ketoprofen, ibuprofen, cephalexin, erythromycin,
haloperiodol, zomepirac, vincamine, diazepam, phenoxybenzamine,
ii ~-blocking agents; calcium-channel blocking drugs such as nifedipine,
Zs diltiazem, isradipine, nilvadipine verapamil, flunarizine, nimodipine,
felodipine,
2a amlodipine, cinnarizine and fendiline; angiotensin converting enzyme
Zs inhibitors selected from the group consisting of angiotensin converting
is enzyme inhibitors that are essentially free of sulfur, angiotensin
converting
27 enzyme inhibitors containing a sulfhydryl group, angiotensin converting
28 enzyme inhibitors containing a linear sulfide, angiotensin converting
enzyme
zs inhibitors containing a cyclic sulfide angiotensin converting enzyme
inhibitors
so containing a methylsulfonyl group and angiotensin enzyme inhibitors
CA 02249637 1998-09-22
WO 97/37640 PCTIUS97/04495
22
represented by a member selected from the group consisting of ramipril,
z fosinopril, altiopril, benazepril, libenzapril, alacepril, citazapril,
cilazaprilate,
a perindopril, zofenopril, enalapril, lisinopril, imidapril, spirapril,
rentrapril,
a captopril, delapril, alindapril, indolapril, and quinapril; propranolol,
naproxen,
s phenylpropanolamine, glipizide, venlafaxine, and beneficial drugs known to
s the dispensing arts in Pharmaceutical Sciences, 1990, edited by Remington
18th Edition published by Mack Publishing Co., Easton, PA; Physicians' Desk
s Reference, 50th Edition, (1996) published by Medical Economics Co.,
s Montvale, NJ, and, USP Dictionar~r, 1995, published by the United States
Pharmacopeial Convention, Inc., Rockville, Maryland.
> > The dosage form of the invention is provided with at least one exit
~z means. The exit means cooperates with the drug core for the uniform and
,3 substantially nonvarying drug-dose release from the dosage form. The exit
~a means can be provided during manufacture of the dosage form, or the exit
means can be provided during drug delivery by the dosage form in fluid
environment of use. The expression exit means, as used for the purpose of
this invention, included a member selected from the group consisting of
~s passageway, aperture, orifice, bore, pore, micropore, porous element
19 through which a drug can be pumped, diffuse, travel, or migrate, a hollow
zo fiber, capillary tube, porous insert, porous overlay, microporous member,
z~ and porous composition. The expression includes also a compound or
zz polymer that erodes, dissolves or is leached from the outer coat or wall,
zs or from the inner coat to form at least one exit, or a multiplicity of
exits.
z4 The compound or polymer includes an erodible poly (glycolic) acid or
zs poly (lactic) acid in the outer or inner coats, a gelatinous filament,
zs a water-removable poly (vinyl alcohol), a teachable compound such as
z7 a fluid removable pore-former selected from the group consisting of an
za inorganic, organic, acid, salt, oxide, and carbohydrate. An exit or a
plurality
zs of exits can be formed by leaching a member selected from the group
so consisting of sorbitol, lactose, fructose, glucose, mannose, galactose,
talose,
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
23
sodium chloride, potassium chloride, sodium citrate, and mannitol; to provide
z an uniform-release dimensioned pore-exit means. The exit means can have
s any shape such as round, triangular, square, elliptical and the like for the
a uniform-metered dose release of a drug from the dosage form. The dosage
s form can be constructed with one or more than one exits in spaced apart
s relation or one or more than one surface of the dosage form. The exit means
can be performed by drilling including mechanical and laser drilling through
s the outer, or inner or through both coats. Exits and equipment for forming
s exits are disclosed in U.S. Pat. Nos. 3,845,770 and 3,91fi,899 by Theeuwes
,o and Higuchi; in U.S. Pat. Nos. 4,063,064 by Saunders, et al; and in U.S.
Pat.
No. 4,088,864 by Theeuwes, et al. Exit means comprising dimension, sized,
shaped and adapted as a releasing-pore formed by aqueous leaching to
~s provide a drug releasing pore are disclosed in U.S. Pat. Nos. 4,200,098
,a and 4,285,987 by Ayer and Theeuwes.
,s The particles used for the purpose of this invention are produced by
comminution that produces the size of the drug and the size of the
» accompanying hydrophilic polymer used according to the mode and the
~a manner of the invention. The means for producing particles include spray
,s drying, sieving, lyophilization, sieving, crushing, grinding, jet milling
Zo micronizing and chopping to produce the intended micron particle size.
z, The process can be performed by size reduction equipment such as
22 micropulverizer mill, fluid energy grinding mill, grinding mill, roller
mill,
2s hammer mill, attrition mill, chaser mill, ball mill, vibrating ball mill,
impact
24 pulverizer mill, centrifugal pulverizer, coarse crusher and fine crusher.
2s The size of the particle can be ascertained by screening including grizzly
zs screen, flat screen, vibrating screen, revolving screen, shaking screen,
oscillating screen and reciprocating screen. The processes and the
2a equipment for preparing particles are disclosed in Pharmaceutical Sciences
Zs by Remington, 17th Ed., pg. 1585-1594, (1985}; Chemical Eng~~ineers:
so Handbook, by Perry, Sixth Edition, pg. 21-13 to 21-19 (1984); Journal of
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
24
Pharmaceutical Sciences, by Parrot, Vol. 61, No., 6, pg. 813 to 829 (1974);
z and Chemical Eng ii neer, by Hixon, pg. 94 to 103, (1990).
s In accordance with the practice of this invention, it has now been found
a the dosage can be provided with a semipermeable wall, also identified for
s the purpose of this invention as an outercoat. The semipermeable wall is
s permeable to the passage of an external fluid such as water and biological
fluids, an it is substantially impermeable to the passage of a beneficial
agent,
a as osmogent, an osmopolymer, and the like. The selectively semipermeable
s compositions used for forming the wall are essentially non-erodible and they
are insoluble in biological fluids during the life of the dosage form.
11 Representative polymers for forming the wall comprise semipermeable
homopolymers, semipermeable copolymers, and the like. In one presently
,s preferred embodiment, the compositions comprise cellulose esters, cellulose
~4 ethers, and cellulose ester-ethers. The cellulosic polymers have a degree
~s of substitution, D.S. of their anhydroglucose unit from greater than 0 up
to 3 inclusive. By degree of substitution is meant the average number
of hydroxyl groups originally present on the anhydroglucose unit that
~s are replaced by a substituting group, or converted into another group.
The anhydroglucose unit can be partially or completely substituted with
zo groups such as acyl, alkanoyl, alkenoyl, aroyl, alkyl, alkoxy, halogen,
carboalkyl, alkylcarbamate, alkylcarbonate, alkylsulfonate, alkysulfamate,
22 semipermeable polymer forming groups, and the like.
zs The semipermeable compositions typically include a member selected
24 from the group consisting of cellulose acylate, cellulose diacylate,
cellulose
2s triacylate, cellulose triacetate, cellulose acetate, cellulose diacetate,
cellulose
Zs triacetate, mono-, di- and tri-cellulose alkanylates, mono-, di-, and tri-
27 alkenylates, mono-, di-, and tri-aroylates, and the like. Exemplary
polymers
2a include cellulose acetate have a D.S. of 1.8 to 2.3 and an acetyl content
of
Zs 32 to 39.9%; cellulose diacetate having a D.S. of 1 to 2 and an acetyl
content
so of 21 to 35%, cellulose triacetete having a D.S. of 2 to 3 and an acetyl
content
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
of 34 to 44.8%, and the like. More specific cellulosic polymers include
z cellulose propionate having a D.S. of 1.8 and a propionyl content of 38.5%;
s cellulose acetate propionate having an acetyl content of 1.5 to 7% and an
a acetyl content of 39 to 42%; cellulose acetate propionate having an acetyl
s content of 2.5 to 3%, an average propionyl content of 39.2 to 45%, and a
s hydroxyl content of 2.8 to 5.4%; cellulose acetate butyrate having a D.S.
of 1.8, an acetyl content of 13 to 15%, and a butyryl content of 34 to 39%;
a cellulose acetate butyrate having an acetyl content of 2 to 29%, a butyryl
s content of 17 to 53%, and a hydroxyl content of 0.5 to 4.7%; cellulose
triacylates having a D.S. of 2.6 to 3 such as cellulose trivalerate, cellulose
trilamate, cellulose tripalmitate, cellulose trioctanote, and cellulose
~z tripropionate; cellulose diesters having a D.S. of 2.2 to 2.6 such as
cellulose
,s disuccinate, cellulose dipalmitate, cellulose dioctanoate, cellulose
dicarpylate,
and the like; mixed cellulose esters such as cellulose acetate valerate,
,s cellulose acetate succinate, cellulose propionate succinate, cellulose
acetate
~s octanoate, cellulose valerate palmitate, cellulose acetate heptonate, and
the
like. Semipermeable polymers are known in US Pat. No. 4,077,407 and they
is can be synthesized by procedures described in Enc~rclopedia of Polymer
~s science and Technology, Vol. 3, pages 325 to 354, 1964, published by
zo Interscience Publishers, Inc., New York.
z, Additional semipermeable polymers for forming the outer wall comprise
zz cellulose acetaldehyde dimethyl acetate; cellulose acetate ethylcarbamate;
zs cellulose acetate methyl carbamate; cellulose dimethylaminoacetate;
z4 semipermeable polyamide; semipermeable polyurethanes; semipermeable
zs sulfonated polystyrenes; cross-linked selectively semipermeable polymers
zs formed by the coprecipitation of a polyanion and a polyfcation as disclosed
z7 in U.S. Pat. Nos. 3,173,876; 3,276,586; 3,541,005; 3,541,006; and
3,546,142;
za semipermeable polymers as disclosed by Loeb et al in U.S. Pat.
zs No. 3,133,132; semipermeable polystyrene derivatives; semipermeable
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
26
poly (sodium styrenesulfonate); semipermeable poly
(vinylbenzyltremethylammonium chloride); semipermeable polymers,
exhibiting a fluid permeability of 10-5 to 10-2 (cc. mil/cm hr.atm) expressed
a as per atmosphere of hydrostatic or osmotic pressure differences across a
s semipermeable wall. The polymers are known to the art in U.S. Pat.
s Nos. 3,845,770; 3,916,899; and 4,160,020; and in Handbook of Common
Polymers, by Scott, J.R., and Roff, W.J., 1971, published by CRC Press,
a Cleveland, Ohio.
s The subcoat of the invention is in contacting position with the
inner surface of the semipermeable wall, which outer semipermeable
wall surrounds and encases the inner subcoat. The inner subcoat is
0.01 mm to 3 mm thick and it comprises a member selected from
~s group consisting of hydroxyalkyl, hydroxyethylcellulose,
,a hydroxyisopropylcelluose, hydroxybutylcellulose, and
hydroxyphenylcellulose.
15 The hydroxyalkylcellulose comprises a 9,500 to 1,250,000 number average
is molecular weight.
The drug composition comprised a hydrophilic polymer for providing
1s in the drug composition a hydrophilic polymer particle that contributes to
the
uniform and nonvarying drug delivery pattern. Representatives of these
2o polymers comprise a member selected from the group consisting of a poly
(alkylene oxide) of 100,000 to 750,000 number average molecular weight
ii including poly (ethylene oxide), poly (methylene oxide), poly (butylene
oxide),
Zs and poly {hexylene oxide); and a poly (carboxymethylcellulose) of 40,000 to
24 400,000 number average molecular weight represented by poly (alkali
Zs carboxymethylcellulose), poly (sodium carboxymethylcelluose), poly
Zs (potassium carboxymethylcellulose), and poly (lithium
27 carboxymethylcellulose). The drug composition can comprise a
2s hydroxypropylalkylcellulose of 9,200 to 125,000 number average
zs molecular weight for enhancing the delivery properties of the dosage
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
27
form as represented by hydroxypropylethylcellulose,
z hydroxypropylmethylcellulose, hydroxypropylbutylcellulose, and
s hydroxypropylpentylcellulose; and a poly (vinyipyrrolidone) of
7,000 to 75,000 number average molecular weight for enhancing
s the flow properties of the dosage form.
s The push-displacement composition in contacting layered arrangement
comprised a polymer that imbibes an aqueous or biological fluid and swells to
s push the drug composition through the exit means from the dosage form.
s Representative of fluid-imbibing displacement polymers comprise a member
,o selected from the group consisting of a poly (alkylene oxide) of 1,000,000
to
~ 1 15,000,000 number average molecular weight as represented by poly
~z (ethylene oxide) and a poly (alkali carboxymethylcellulose) of 500,000 to
~s 3,500,000 number average molecular weight wherein the alkali is sodium,
potassium or lithium. Examples of further polymers for formulation, the push-
displacement composition comprise osmopolymers comprise polymers that
form hydrogels such as Carbopol~ acidic carboxypolymer, a polymer of
acrylic and cross-linked with a polyallyl sucrose, also known as
~a carboxypolymethylene and carboxyvinyl polymer having a molecular weight
,s of 250,000 to 4,000,000; Cyanamer~ polyacrylamides; cross-linked water
zo swellable indenemaleic anhydride polymers; Good-rite~ polyacrylic acid
z~ having a molecular weight of 80,000 to 200,000; Aqua-Keeps~ acrylate
zz polymer polysaccharides composed of condensed glucose units such as
zs diester cross-linked polygluran; and the like. Representative polymers that
za form hydrogels are known to the prior art in U.S. Pat. No. 3,865,108 issued
z5 to Hartop; U.S. Pat No. 4,002,173 issued to Manning; U.S. Pat. No.
zs 4,207,893 issued to Michaels; and in Handbook of Common Po~mers,
z7 by Scott and Roff, published by the Chemical Rubber Co., Cleveland, Ohio.
za The osmagent, also known as osmotic solute and as osmotically
zs effective agent, that exhibits an osmotic pressure gradient across the
outer
so wall and subcoat comprises a member selected from the group consisting of
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
28
sodium chloride, potassium chloride, lithium chloride, magnesium sulfate,
z magnesium chloride, potassium sulfate, sodium sulfate, lithium sulfate,
potassium acid phosphate, mannitol, urea, inosital, magnesium succinate,
a tartaric acid raffinore, sucrose glucose, lactose, sorbitol, inorganic
salts,
s organic salts and carbohydrates.
s Exemplary solvents suitable for manufacturing the hydroactivated layer
and the wall comprise inert inorganic solvents that do not adversely harm the
a materials, the capsule, and the final laminated wall hydro-activated layer.
s The solvents broadly include members selected from the group consisting of
,o aqueous solvents, alcohols, ketones, esters, ethers, aliphatic
hydrocarbons,
halogenated solvents, cycloaliphatic, aromatics, heterocyclic solvents and
mixtures thereof. Typical solvents include acetone, diacetone alcohol,
~s methanol, ethanol, isopropyl alcohol, butyl alcohol, methyl acetate, ethyl
,4 acetate, isopropyl acetate, n-butyl acetate, methyl isobutyl ketone, methyl
~s propyl ketone, n-hexane, n-heptane, ethylene glycol monoethyl ether,
ethylene glycol monothyl acetate, methylene dichloride, ethylene dichloride,
propylene dichloride, carbon tetrachloride nitroethane, nitropropane
tetrachloroethane, ethyl ether, isopropyl ether, cyclohexane. cyclooctane,
benzene, toluene, naphtha, 1,4-dioxane, tetrahydrofuran, diglyme, water,
Zo aqueous solvents containing inorganic salts such as sodium chloride,
2, calcium chloride, and the like, and mixture thereof such as acetone and
22 water, acetone and methanol, acetone and ethyl alcohol, methylene
23 dichloride and methanol, and ethylene dichloride and methanol:
z4 The semipermeable wall and the subcoat of the dosage form can
2s be formed in one technique using the air suspension procedure. This
2s procedure consists of suspending and tumbling the bilayer core in a current
27 of air, an inner subcoat composition and an outer semipermeabfe wall
forming
Za composition, until in either operation the subcoat and the outer wall coat
is
Zs applied to the bilayer core. The air suspension procedure is well-suited
for
so independently forming the wall of the dosage form. The air suspension
CA 02249637 1998-09-22
WO 97/37640 PCT/US97104495
29
procedure is described in U.S. Pat. No. 2,799,241; in J. Am. Pharm. Assoc.,
z Vol. 48, pp. 451 to 459, (1959); and, ibid, Vol. 49, pp. 82 to 84, (1960).
The dosage form also can be coated with a Wurster~ air suspension coater,
a using for example, methylene dichloride methanol as a cosolvent.
An Aeromatic~ air suspension coater can be used employing a cosolvent.
s Other coating techniques, such as pan coating, can be used for providing
the dosage form. In the pan coating system, the subcoat on the wall forming
s compositions are deposited by successive spraying of the respective
s compensation on the bilayered core accompanied by tumbling in a rotating
pan. A pan coater is used because of its availability at commercial scale.
Other techniques can be used for coating the drug core. Finally, the wall or
iz coated dosage form are dried in a forced air oven at 40°C. for a
week, or in
~s a temperature and humidity controlled oven for 24 hours at 40°C. and
50%
,4 relative humidity to free the dosage form of solvent.
The dosage form of the invention is manufactured by standard
~s techniques. Fro example, in one manufacture, the beneficial drug and other
ingredients comprising the first layer facing the exit means are blended and
~s pressed into a solid layer. The layer possesses dimensions that correspond
ss to the internal dimensions of the area the layer is to occupying the dosage
zo form and it also possesses dimensions corresponding to the second layer for
z~ forming a contacting arrangement therewith. The drug and other ingredients
z2 can be blended also with a solvent and mixed into a solid or semisolid form
by
Zs conventional methods, such as ballmilling, calendering, stirring or
rollmilling,
z4 and then pressed into a preselected shape. Next, a layer of osmopolymer
z5 composition is placed in contact with the layer of drug in a like manner.
is The layering of the drug formulation and the osmopolymer layer can be
z7 fabricated by conventional two-layer press techniques. The two contacted
z$ layers are first coated with a subcoat and an outer semipermeable wall.
zs The air suspensions and air tumbling procedures comprises in suspending
CA 02249637 1998-09-22
WO 97!37640 PCT/US97/04495
and tumbling the pressed, contacting first and second layers in a current of
z air containing the delayed-forming composition until the first and second
s layers are surrounded by the wall composition.
a In another manufacture, the dosage form is manufactured by the wet
5 granulation technique. In the wet granulation technique, the drug and the
s ingredients comprising the first layer or drug composition, are blended
using
an organic solvent, such as denature anhydrous ethanol, as the granulation
s fluid. The ingredients forming the first layer or drug composition are
s individually passed through a preselected screen and then thoroughly
blended in a mixer. Next, other ingredients comprising the first layer can
> > be dissolved in a portion of the granulation fluid, the solvent described
above.
~z Then, the tatter prepared wet blend is slowly added to the drug blend with
~s continual mixing in the blender. The granulating fluid is added until a wet
,a blend is produced, which wet mass blend is then forced through a
predetermined screen onto oven trays. The blend is dried for 18 to 24 hours
at 24°C. to 35°C. in a forced air oven. The dried granules are
then sized.
Next, magnesium stearate is added to the drug granulation, it is then put
into milling jars and mixed on a jar mill for 10 minutes. The composition is
,s pressed into a layer, for example, in a Manesty~ press. The speed of the
zo press is set at 20 rpm and the maximum load set at 2 tons. The first layer
z, is pressed against the composition forming the second layer and the bilayer
zz tablets are fed to the Kilian~ dry Coata press and surrounded with the drug-
zs free coat, followed by the exterior wall solvent coating.
z4 Another manufacturing process that can be used for providing
25 the compartment-forming composition comprises blending the powdered
zs ingredients in a fluid bed granulator. After the powdered ingredients
are dry blended in the granulator, a granulating fluid, for example,
zs poly(vinylpyrrolidone) in water, is sprayed onto the powders. The coated
zs powders are then dried in the granulator. This process granulates all the
so ingredients present therein while adding the granulating fluid. After the
CA 02249637 1998-09-22
WO 97/37640 PCTlUS97/04495
31
granules are dried, a lubricant such as stearic acid or magnesium stearate is
z mixed into the granulation, using a V-blender. The granules are then pressed
s in the manner described above.
a
s METHOD OF PRACTICING THE INVENTION
s
The invention provides a process for the substantially uniform and
a substantially nonvarying rate of release of a drug from a dosage form,
herein
s the dosage form comprises a composition, a dose of drug in the composition,
,o and a hydrophilic polymer in the composition, and wherein the process
comprises (1 ) formulating the composition with a drug possession, a particle
~z size up to and including 150 microns, and (2) formulating the composition
~s with a hydrophilic polymer possessing a particle size up to and including
~4 250 microns, hereby, through the copresence of (1 ) and (2) in the
,s composition, the drug is delivered as the substantially uniform and
~s nonvarying rate of release from the dosage form.
The invention provides also a process for substantially uniform
~s and substantially nonvarying rate of release of a drug from a dosage form,
wherein the dosage form comprises a composition, a dose of drug in the
zo composition, a hydrophilic polymer in the composition, and a composition
z, for displacing the drug composition from the dosage form, and wherein the
zz process comprises (1) formulating the composition with a drug possessing
zs a particle size up to and including 150 micron, (2) formulating the
za composition with a hydrophilic polymer possessing a particle size up to
zs and including 150 microns, whereby through the copresence of (1) and
zs (2) in combination with the composition for displacing the drug composition
z7 imbibing fluid, expanding and displacing the drug composition from the
za dosage form. The drug is delivered at a substantially uniform and
nonvarying
zs rate of release over time.
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
32
The invention comprises also a method for delivering a drug to a
2 patient, wherein the method comprises: (A) admitting orally into the patient
a
s dosage form comprising: (1 ) a semipermeable wall that surrounds and forms
a a compartment; (2) a drug composition in the compartment; (3) a dose of
drug particles up to 150 micron in the drug composition; (4) a hydrophilic
s polymer of up to 250 micron in the drug composition; (5) an exit in the
z semipermeable wall; (B) imbibing fluid through the semipermeable wall
s into the drug composition whereby through the coaction of (2) and
s (3) a dispensable drug composition is formed in the dosage form; and
,o (C) delivering the drug composition through the exit to a patient at a
substantially uniform and nonvarying dose over time.
The invention comprises further a method for providing a drug-free
~s interval by placing a subcoat in the dosage form in contact with the inside
surface of the semipermeable wall and surrounding the drug composition,
~s or surrounding both a drug composition and a push composition, which
drug-free interval is followed in 2 to 5 hours by a drug delivery period of
1 to 15 hours. The latter method is indicated for the treatment of
~s hypertension and angina as it provides a drug-free interval when a patient
1s is less active, thus, at rest or when asleep, and the inventive method then
2o provides drug during the rising and waking hours mainly during the time
when activity reaches a maximum during the daytime hours.
22 The method of the invention pertains also to the management of
z3 blood pressure, the management of the systemic physiology, and to the
24 management of chronotherapy, that is timetherapy by administering a
Zs drug according to the mode and the manner of the invention.
2s The novel dosage form of this invention uses dual means for the
attainment of precise release rate of drugs that are difficult to deliver in
the
is environment of use, while simultaneously maintaining the integrity and the
zs character of the system. While there has been described and pointed out
CA 02249637 1998-09-22
WO 97/37640 PCT/US97/04495
33
features and advantages of the invention, as applied to the presently
z preferred embodiments, those skilled in the dispensing art will appreciate
that various modifications, changes, additions, and omissions in the system
a illustrated and described can be made without departing from the spirit of
the invention.