Note: Descriptions are shown in the official language in which they were submitted.
CA 02249786 1998-10-26
WO 95!01958 PCTlUS94106376
-1-
DIFLUORO STATONE ANALOGS
This invention relates to novel statone analogs, to the
processes and intermediates useful for their preparation
and to their use as anti-viral acents.
BACKGROUND OF THE PRESENT INVENTION
Retroviruses are a class of viruses which transport
their genetic material as ribonucleic acid rather than as
deoxyribonucleic acid. Retroviruses are associated with a
wide variety of diseases in man, one of which is AIDS.
Although there have been disclosures of other anti-viral
agents useful in the treatment of AIDS, for example see
patent applications EP 0 218 688, EP 0 352 000 and Inter-
national Publication No. WO 92/122123 dated June 23, 1992,
the compounds of the present invention have not been pre-
viously disclosed.
DESCRIPTION OF THE PRESENT INVENTION
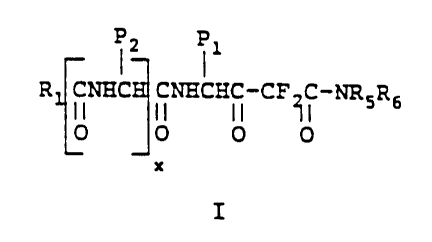
More specifically this invention relates to novel
difluoro statone analogs of Formula 1
12 I1
R CNHC CNHCHC-CF C-NRSR6
II II II X11
O O 0 O
x
I
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
_ Z _
and the stereoisomers, hydrates, isosteres and the
pharmaceutically acceptable salts thereof wherein
P1 is Q or B, wherein
B is C alk lene ~T~~ wherein
i-s y
T is ((0)b-W-R) and T' is [(O)b~-W'-R'] or H,
wherein each of W and W' are independently
Cl_6 alkylene or nothing and R and R' are each
independently -CHZCHO, hydroxy Cl_6 alkyl,
C1_6 alkoxy C1_6 alkyl, C1_6 alkyl, Q,
(R3)dor R~
provided that W is C2_6 alkylene when W is
directly attached to a nitrogen atom in R,
provided that W' is CZ_6 alkylene when W' is
directly attached to a nitrogen atom in R',
provided that W or W' are each independently
Cl_6 alkylene when R or R' are each
independently an aryl, and
provided that H is other than p-hydroxy-
benzyl or p-alkoxybenzyl;
Q is 2 w
25 tcs d - ~ o (CH2)d,;
0
P2 is Cl_6 alkyl, cyclopentyl, hydroxy C1_6 alkyl, phenyl,
benzyl or 3-tetrahydrofuryl;
Rl is benzyloxy, C1_6 alkoxy, C1_6 alkyl, phenyl, benzyl,
phenethyl, fluorenylmethylenoxy, 2-isoquinolinyl, PDL,
I I
CHIN-(CHz)3CH2, ~-(CHZ)Z-N-CH2~H2, NHSOZR4, N(R4)(benzyl),
or N(R4)(PDL),
wherein
PDL is -(CH2)a-2-,3-, or 4-pyridyl, or
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
-3-
p-substituted benzyloxy, wherein
the substitution is a vitro, OH, amino,
C1_6 alkoxy, hydroxy C1_6 alkylene, or halogeno;
R3 is Cl_6 alkenyl, Cl_6 alkoxy,
hydroxy Cl_6 alkyl, C1_6 alkyl, or OH;
R4 is H, C1_6 alkyl, phenyl or benzyl;
RS is C7_ls alkyl, C~_ls alkoxy,
CH([(CH2)d-O-CH2Jx-Re)2.
branched-chain C1_6 alkylene~ Ve~ or CH(Y)(Z)
wherei '~'n
Y is C1_ls alkyl, hydroxy C1_is alkyl or
(CHz)~ ve~ and
Z is (CH2)d-O-CHO,
C1_6 alky eve-O-(CH2)d-(O-CH2-CH2)e-O-Ci-s alkyl,
(CH2)~ ~ Vy Or (CHy)d-0(C$2)d~R7
provided that d' =2 when R~ is piperazinyl,
substituted piperazinyl, piperidyl or
morpholinyl,
wherein
V is OR4 or hydroxy C1_6 alkyl;
R6 is H or C1_3 alkyl;
R7 is piperazinyl, substituted piperazinyl, piperidyl,
morpholinyl, pyridyl, pyrazinyl, pyrimidinyl or phenyl,
wherein substituted piperazinyl is piperazinyl
substituted on one nitrogen atom thereof with CHO,
C(0)NHlt4, Cl_4 alkyl or COZR4;
Re is pyrimydyl, pyridyl, pyrazinyl or phenyl;
a is zero, 1, 2 or 3;
b and b' are each independently zero or 1;
d and d' are each independently 1 or 2;
a and e' are each independently zero, 1 or 2; and
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
- 4 -
x is zero or one.
Isosteres of the compounds of Formula I include those
wherein (a) the a-amino acid residues of the Pl and P2
substituents are in their unnatural configuration (when
there, is a natural configuration) or (b) when the normal
peptidic carbamoyl linkage is modified, such as for
example, to form
1 -CH2NH- (reduced), -C-N(CH3) (N-methylamide), -COCHy-
(keto), -CH(OH)CHZ- (hydroxy), -CH(NHZ)CHZ- (amino),
-CHZCHz- (hydrocarbon). Preferably a compound of the
invention should not be in an isosteric form. Unless
otherwise stated the a-amino acids are preferably in their
L-configuration.
A compound of the invention may be in free form, e.g.,
amphoteric form, or in salt, e.g., acid addition or anionic
salt, form. A compound in free form may be converted into a
salt form in an art-known manner and vice-versa.
The pharmaceutically acceptable salts of the peptide of
Formula I (in the form of water, or oil-soluble or
dispersible products) include the conventional non-toxic
salts or the quaternary ammonium salts of these peptides,
which are formed, e.g., from inorganic or organic acids or
bases. Examples of such acid addition salts include
acetate, adipate, alginate, aspartate, benzoate,
benzenesulfonate, bisulfate, butyrate, citrate, camphorate,
camphorsulfonate, cyclopentanepropionate, digluconate,
dodecylsulfate, ethanesulfonate, fumarate, glucoheptanoate,
glycerophosphate, hemisulfate. heptanoate, hexanoate,
hydrochloride, hydrobromide, hydroiodide, 2-hydroxyethane-
sulfonate, lactate, maleate, methanesulfonate, 2-naphthal-
enesulfonate. nicotinate, oxalate, pamoate. pectinate,
persulfate, 3-phenylpropionate. picrate, pivalate,
propionate, succinate, tartrate, thiocyanate, tosylate, and
CA 02249786 1998-10-26
WO 95101958 PCT/US94/06376
undecanoate. Base salts include ammonium salts, alkalimetal
salts such as sodium and potassium salts, alkaline earth
metal salts such as calcium and magnesium salts, salts with
organic bases such as dicyclohexylamine salts,
N-methyl-D-glucamine, and salts with amino acids such as
arginine, lysine, and so forth. Also, the basic nitrogen-
containing groups may be quaternized with such agents as
lower alkyl halides, such as methyl, ethyl, propyl, and
butyl chloride, bromides and iodides; dialkyl sulfates like
dimethyl, diethyl, dibutyl; and diamyl sulfates, long chain
halides such as decyl, lauryl, myristyl and stearyl
chlorides, bromides and iodides, aralkyl halides like
benzyl and phenethyl bromides and others.
The hydrates of the compounds of Formula I are hydrated
compounds having the partial structure
1 ~~
~ '\\~ O
HO OH
and in their end-use application are generally the active
forms .
In general, as used herein, the term "alkyl" includes
the straight, branched-chain and cyclized manifestations
thereof unless otherwise indicated, particularly such
moieties as methyl, ethyl, isopropyl, n-butyl, t-butyl,
-CHZ-t-butyl, cyclopropyl, n-propyl, pentyl, cyclopentyl,
n-hexyl, cyclohexyl and cyclohexylmethyl. The term
"aralkyl", when used, includes those aryl moieties attached
to an alkylene bridging moiety, preferably methyl or ethyl.
"Aryl" includes both carbocyclic and hetereocyclic
moieties of which phenyl, pyridyl, pyrimidinyl, pyazinyl,
indolyl, indazolyl, furyl and thienyl are of primary
interest; these moieties being inclusive of their position
isomers such as, for example, 2-, 3-, or 4-pyridyl, 2- or
CA 02249786 1998-10-26
WO 95/01958 ' PCTlUS94106376
-6-
3-furyl and thienyl, 1-, 2-, or 3-indolyl or the 1- and 3-
indazolyl, as well as the dihydro and tetrahydro analogs of
the furyl and thienyl moieties. Also included within the
term "aryl" are such fused carbocyclic moieties as
pentalenyl, indenyl, naphthalenyl, azulenyl, heptalenyl,
acenaphthylenyl, fluorenyl, phenalenyl, phenanthrenyl,
anthracenyl, acephenanthrylenyl, aceanthrylenyl,
triphenylenyl, pyrenyl, chrysenyl and naphthacenyl. Also
included within the term "aryl" are such other heterocyclic
radicals as 2- or 3-benzo[b]thienyl, 2- or 3-naphtho[2,3-
b]thienyl, 2- or 3-thianthrenyl, 2H-pyran-3-(or 4- or
5-)yl, 1-isobenzo- furanyl, 2H-chromenyl-3-yl, 2- or 3-
phenoxathiinyl, 2- or 3-pyrrolyl, 4- or 3-pyrazolyl, 2-
pyrazinyl, 2-pyrimidinyl. 3-pyridazinyl, 2-indolizinyl, 1-
isoindolyl, 4H-quinolizin-2-yl, 3-isoquinolyl, 2-quinolyl,
1-phthalazinyl, 1,8-naphthyridinyl, 2-quinoxalinyl, 2-
quinazolinyl, 3-cinnolinyl, 2-pteridinyl, 4aH-carbazol-2-
yl, 2-carbazolyl, B-carbolin-3-yl, 3-phenanthridinyl, 2-
acridinyl, 2-perimidinyl, 1-phenazinyl, 3-isothiazolyl, 2-
phenothiazinyl, 3-isoxazolyl, 2-phenoxazinyl, 3-
isochromanyl, 7-chromanyl, 2-pyrrolin-3-yl, 2-imidazol-
idinyl, 2-imidazolin-4-yl, 2-pyrazolidinyl, 3-pyrazolin-3-
yl, 2-piperidyl, 2-piperazinyl, 1-indolinyl, 1-
isoindolinyl, 3-morpholinyl, benzo[b]isoquinolinyl and
benzo[b]furanyl, including the position isomers thereof
except that the heterocyclic moieties cannot be attached
directly through their nitrogen one, two or three
substituents independently selected from C1_6 alkyl,
haloalkyl, alkoxy, thioalkoxy, aminoalkylamino,
dialkylamino, hydroxy, halo, mercapto, nitro,
carboxaldehide, carboxy, carboalkoxy and carboxamide.
Likewise the term "alkylene" includes straight or
branched-chain moieties. Some examples of branched-chain
alkylene moieties are ethylethylene, 2-methyltrimethylene,
2,2-dimethyltrimethylene, and so on. For example, C3
alkylene can mean
CA 02249786 1998-10-26
WO 95/01958 PCT/US94I06376
_ 7 _
CH3
. -CH2-CH2-CH2- or -C- or -CH2-CH- Or -CH-CHZ- .
I I I
CH3 CH3 CH3
All (C1_i5) moieties are preferably (C1_6) moieties and
all (C1_6) moieties such as C1_6 alkyl, C1_6 allenyl, Cl_6
alkoxy, and hydroxy C1_6 alkyl, are more preferably Cl_3
moieties (containing 1-3 carbon atoms instead of 1-6 carbon
atoms ) .
The fluorenylmethyloxy moiety is that moiety generally
called by its abbreviation FMOC, and is the fluorenyl
moiety bearing -CH20- attached to the 9-position of the
fluoroenyl moiety. Other terms defined herein are
piperazinyl
-N~ or substituted piperazinyl -N~_
the substitution (*) occurring only at one nitrogen
molecule which is not attached to the remainder of the
molecule (attachment via a nitrogen atom). The
substituents are one of CHO, C(O)NHR4, C1_4 alkyl or C02R4.
Piperidyl and morpholinyl both bind to the rest of the
-N -N
molecule via their respective nitrogen atoms while
pyrimidinyl, pyridyl and pyrazinyl bind to the rest
N~ N N
~N
of the molecule anywhere except their respective
nitrogen atoms.
More specifically, in the instance wherein PZ is either
C1_6 alkyl or hydroxy C1_6 alkyl, such moieties as -C(CH3)3,
CA 02249786 1998-10-26
WO 95/01958 PCTIUS94/06376
_ g _
-CH(CH3)2, -CH(CH3)(C2H5), -C(OH)(CH3)2 and -CH(OH)CH3 are
preferred. The "hydroxy C1_6 alkyl" moiety is illustrated in
one example by -CH2-OH, the "C1_6 alkoxy C1_6 alkyl" moiety,
is illustrated in one example by -CH2-OCH3, (although in
each instance the C1_6 alkylene may be straight or branched
and the hydroxy radical. is not limited to the terminal
carbon atom of the alkyl moiety). The ~1
~(R3)d moiety
shows a phenyl moiety which may be substituted with one or
two of the R3 moieties,(said moieties being the same or
different ) .
As it is often quite advantageous to have what is
termed an amino protecting group (Pg), the scope of those
compounds of Formula I, includes those Rl moieties which,
together with their adjacent carbonyl moiety form such
groups as acetyl (Ac), succinyl (Suc), benzoyl (Br),
t-butyloxycarbonyl (Hoc), benzyloxycarbonyl (CHZ), tosyl
(Ts), dansyl (DNS), isovaleryl (Iva), methoxysuccinyl
(MeOSuc), 1-adamantanesul hon 1 AdSO
P y ( 2), 1-adamantaneacetyl
(AdAc), phenylacetyl, t-butylacetyl (Tba), bis[(1-
naphthyl)methyl]acetyl (BNMA) and Rz wherein Rz is an aryl
group as previously described suitably substituted by 1 to
3 members selected independently from the group consisting
of fluoro, chloro, bromo, iodo, trifluoromethyl, hydroxy,
alkyl containing from 1 to 6 carbons, alkoxy containing
from 1 to 6 carbons, carboxy, alkylcarbonylamino wherein
the alkyl group contains 1 to 6 carbons. 5-tetzazolo, and
acylsulfonamido (i.e., acylaminosulfonyl and sulfonylamino-
carbonyl) containing from 1 to 15 carbons, provided that
when the acylsulfonamido contains an aryl. The aryl may be
further substituted by a member selected from fluoro,
chloro, bromo, iodo and nitro.
In those instances wherein there is an Rz moiety, it is
preferred that Rz represent acylsulfonamido, particularly
those wherein the acylsulfonamido contains an aryl moiety
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
_g_
(preferably phenyl) substituted by a halogen. The preferred
Rz moieties being 4-[(4-chlorophenyl)sulfonylaminocarbon-
yl)phenylcarbonyl, 4-[(4-bromophenyl)sulfonylamino-
carbonyl]-phenylcarbonyl and 4-[phenylsulfonylamino
carbonyl]-phenylcarbonyl (said moieties being abbreviated
as 4-C1-0-SAC-Bz, 4-Br-0-SAC-Bz and 0-SAC-Bz,
respectively).
Among the classes of amino protecting groups
contemplated are: (1) acyl type protecting groups such as
formyl, trifluoroacetyl, phthalyl, p-toluenesulfonyl
(tosyl), benzenesulfonyl, nitrophenylsulfenyl, trityl-
sulfenyl, O-nitrophenoxyacetyl, and a-chlorobutyryl; (2)
aromatic urethane type protecting groups such as benzyloxy-
carbonyl and substituted benzyloxycarbonyls such as
p-chlorobenzyloxycarbonyl, p-methoxybenzyloxycarbonyl,
p-nitrobenzyloxycarbonyl, p-bromobenzyloxycarbonyl,
1-(p-biphenylyl)-1-methylethoxycarbonyl,
a,a-dimethyl-3,5-dimethoxybenzyloxycarbonyl, and benz-
hydryloxycarbonyl; C3) aliphatic urethane protecting groups
such as tert-butyloxycarbonyl (Boc), diisopropylmethoxy-
carbonyl, isopropyloxycarbonyl, ethoxycarbonyl, and allyl-
oxycarbonyl; (4) cycloalkyl urethane type protecting groups
such as cyclopentyloxycarbonyl, adamantyloxycarbonyl, and
cyclohexyloxycarbonyl; (5) thio urethane type protecting
groups such as phenylthiocarbonyl; (6) alkyl type
protecting groups such as triphenylmethyl (trityl) and
benzyl (Hzl); (7) trialkylsilane protecting groups such as
trimethylsilane if compatible. The preferred a-amino
protecting groups are tert-butyloxycarbonyl (Boc) or
benzyloxycarbonyl (CBZ). The use of Hoc as an a-amino
protecting group for amino acids is described by Hodansky
et al. in "The Practice of Peptide Synthesis",
Springer-Verlag, Berlin (1984), p. 20.
CA 02249786 1998-10-26
WO 95/01958 PCTJUS94/06376
- 10 -
In general the compounds of this invention may be
prepared using standard chemical reactions analogously
known in the art.
In the instance wherein it is desired to prepare
compounds of the formula
12 I1
R CNHC CNHCHC-CF -C-NR R
6
O O O O
x
II
wherein R1, P2, P1, RS and R6 are as previously defined, the
process outlined by the following reaction scheme may
advantageously be utilized.
25
35
CA 02249786 1998-10-26
WO 95/01958 PCTlUS94/06376
- 11 -
REACTION SCCBEMEME A
(a)
PgNHCH-CHO Zn PgNHCA-~HCF2~OEt
BrCF2C02Et 0g II0
(3) (4)
(b)
1f I1 (c) I1
H2N-CH- iH-CF2 -iINR5R6 ~E---~ PgNH-CH-C i CF2-il-NRSR6
OH 0
OH O
(6) (5)
1
(6)
(d)/
I s _ I i ~i
R'1~-NH-CH- II NH CH- ~8-CF2-~-NR5R6 R'l~-NH-CH- C'IH-CF2-~-NRSR6
'Ii
O O OH O O
O8 O
(7) ( f) (8)
(f)
P
2 12 I 1 P1
I
R1~-NH-CH- ~-NH-CH-~- CFZ-~NRSR6 Rl~l NH CH- ~-CFZ-~RSR6
IO O O IOI O O IIO
IIA IIB
30 wherein R'1 represents optional amino protecting groups, as
herein above defined, and the R1, pi~ P2~ R5 and R6 moieties
are as previously defined.
In effecting reaction scheme A, the process is
35 initiated by conducting a Reformatsky-type reaction wherein
an aldehyde of Formula (3) is subjected to a condensation
reaction with an ester of bromodifluoroacetic acid,
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
- 12 -
preferably the ethyl ester in the presence of zinc and in
an anhydrous aprotic solvent, e.g., tetrahydrofuran, ether,
dimethoxyethane and the like under a nitrogen or argon
inert atmosphere. The reaction is gently heated to about
60°C for about 1-l2 hours or ultrasonicated to produce
compounds (4). Step (b) to obtain compounds (S) may be
effected directly or undirectly. In one instance, the
esters of Formula (4) are de-esterified using a strong base
(LiOH, KOH, NaOH and the like) in the presence of water
using a partially water miscible solvent (such as
tetrahydrofuran, dimethoxyethane, dioxane) at about room
temperature. The so-obtained de-esterified compound is then
aminated with the appropriate R5R6-substituted amine using a
peptide-like coupling procedure - i.e., using a mixed
anhydride method using DCC and hydroxybenzotriazole at room
temperature in solvents such as CH2C12, tetrahydrofuran or
dimethylformamide. Alternatively the esters (4) may be
directly subjected to a reaction with the appropriate RSR6-
substituted amine without or with a solvent (tetrahydro-
furan) at about 80°C. Following the preparation of
compounds (5), the protecting groups Pg may readily be
removed by standard procedures, e.g., hydrogenation or acid
base hydrolysis. Compounds (6) are subjected to a peptide
coupling procedure with an appropriately protected acid of
the formulae R1CONH(PZ)COOH or R1C02H, using the herein-
described procedures (or by any other coupling procedure
currently available) to produce compounds (7) and (8),
respectively. At this point, if desired, the amide formed
with R1 may be optionally deprotected, or if desired, the
amide may be replaced with another amide within the scope
of Rl. The alcohols of (7) and (8) are then oxidized to the
corresponding ketones and, if desired, the compounds may be
converted to their pharmaceutically acceptable salts.
The oxidation may be effected via the well-known Swern
oxidation procedure, or with 1,1,1-triacetoxy-2,1-benzoxiodol
CA 02249786 1998-10-26
WO 95/01958 PCT/US94106376
- 13 -
(Dess-Martin reagent). The coupling proceduzes are effected
according to standard procedures well known in the art.
"Swern oxidation" is well known in the art [see
Synthesis, (1981), 165]. For example, it may be effected by
reacting about 2 to 20 equivalents of dimethylsulfoxide
(DMSO) with about 1 to 10 equivalents of trifluoromethyl-
acetic anhydride [(CF3C0)ZO] or oxalyl chloride [(COC1)Z],
said reactants being dissolved in an inert solvent, e.g.,
methylene chloride (CHZC12), said reaction being under an
inert atmosphere (e. g., nitrogen or equivalently function-
ing gas) under anhydrous conditions at temperatures of
about -70°C to -30°C to form an in situ sulfonium adduct to
which is added about 1 equivalent of the appropriate
alcohols. i.e., compounds (7) and (8). Preferably, the
alcohols are dissolved in an inert solvent, e.g., CH2Cly,
tetrahydrofuran, or minimum amounts of DMSO, and the
reaction mixture is allowed to warm to about -50°C or -20°C
(for about 20-60 minutes) and then the reaction is
completed by adding about 3 to 30 equivalents of a tertiary
amine, e.g., triethylamine, diisopropylethylamine, N-methyl
morpholine. etc.
Another alternative process for converting the alcohols
to the desired ketones is an oxidation reaction which is
called the "Dess Martin oxidation" reaction and employs
periodane (i.e.. 1,1,1-triacetoxy-2,1-benzoxiodol), [see
Dess Martin, J. Ora. Chem., 48, 4155, (1983)]. This
oxidation is effected by contacting about 1 equivalent of
the alcohols with 1 to 5 equivalents of periodane
(preferably 1.5 equivalents), said reagent being in suspen-
sion in an inert solvent (e. g., methylene chloride) under
an inert atmosphere (preferably nitrogen) under anhydrous
conditions at 0°C to 50°C (preferably room temperature) and
allowing the reactants to interact for about 1 to 48 hours.
Optional deprotection of the amine protecting groups may be
effected as desired after the ketones have been isolated.
CA 02249786 1998-10-26
WO 95/01958 PCTIUS94/06376
- 14 -
In general, the modified Jones oxidation proceduze may
conveniently be effected by reacting the alcohols with
pyridinium dichromate by contacting the reactants together
in a water-trapping molecular sieve powder, e.g., a
grounded 3 Angstrom molecular sieve), wherein said contact
is in.the presence of glacial acetic acid at about 0°C to
50°C, preferably at room temperature followed by isolation
and then optionally removing amine protecting groups.
Alternatively, 1 to 5 equivalents of a chromic
anhydride-pyridine complex (i.e., a Sarett reagent prepared
insitu) [see Fieser and Fieser "Reagents for Organic
Synthesis" Vol. 1, pp. 145 and Sarett, et al., J.A.C.S. _25.
422, (1953)) said complex being prepared insitu in an inert
solvent (e. g., CHyCl2) in an inert atmosphere under anhy-
drous conditions at 0°C to 50°C to which complex is added 1
equivalent of the alcohols allowing the reactants to inter-
act for about 1 to 15 hours, followed by isolation and
optionally removing amine protecting groups.
In certain instances, it may be prefereably to
introduce the appropriate P1 group after the R5R6 group is
attached. In those instances. P1 may comprise a protecting
group until after step (a). For example, Pl at compound
(3) may be 4-(benzyloxy)benzyl which can be hydrogenated to
the phenol, preferably by catalytic hydrogenation after
steps (a) or (b). Introduction of the remainder of the
appropriate Pl moiety can be accomplished after steps (b),
(d) or (e) by alkylation under basic conditions such as
cesium or potassium carbonate with X-C1_6 alkylene R wherein
X is an appropriate leaving group such as halogeno or
triflate, in the presence of a solvent such as dioxane,
tetrahydro-furan or dimethylformamide.
For the preparation of the necessary aldehydes of (3),
and the preparation of the acids which are to be coupled
with the amines of Formula (6), alternative alkylation
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
- 15 -
procedures are utilized depending upon whether the P1
and/or the P2 moieties are or are not residues of natural
amino acids. The preparation of these intermediates wherein
the P1 or P2 moieties are residues of natural amino acids
(or minor modifications thereof, e.g., P1 or PZ being a
methyl ether of tyrosine), the compounds are either known
or are prepared by processes and techniques well known in
the art.
To prepare the intermediates of the formula
p3
PgHN-CHC02R9
(9)
wherein Pg is an amino protecting group, P3 is either a P'1
or P'2 moiety with P'1 and P'2 being as defined for P1 and
PZ respectively, except that they are other than residues
of naturally occuring amino acids, and the R9 moiety is an
alkyl radical, preferably methyl when P3 is P'l, and ethyl
when P3 is P'2, alternative methods are available.
To prepare the intermediates of formulae
p~l P'
2
P9~-CHCHO
PgHN-CHCOOH
(10H) (10A)
the following reaction scheme may be utilized
REACTION SCHEME B
p3
PgNHCH2CO2R9
( 1 ) Hase P9~CHCOZR9
(11) (2) P3X
(12)
CA 02249786 1998-10-26
WO 95101958 PCT/US94106376
- 16 -
wherein P3 is as previously defined and X is a leaving
group, preferably halo or triflate, R9 is methyl when P3 is
P'1. and ethyl when P3 is P'Z.
In essence, the preparation of compounds (12) utilizes
the Krapcho method [Tetrahedron Letters, 26. 2205 (1976)]
for alkylation wherein compounds (11) are treated with a
base, e.g., LDA, (lithium diisopropylamide), followed by
reaction with the desired P3X in the presence of TMEDA
(i.e. tetramethylethylenediamine) in a solvent (tetrahydro-
furan) with or without AMPA (i.e. hepamethylphosphonamide)
according to the standard Krapcho conditions. Following
alkylation the compounds are then subjected to a reduction
using diisobutyl aluminum hydride (Dibal) in a mixture of
solvents, e.g., ether, toluene, hexane, tetrahydrofuran at
about -78°C for about 1 hour. Following the preparation of
the aldehydes of Fozmula (10B), the compounds are subjected
to the processes of Reaction Scheme A.
Alternatively, the compounds of (12) may be prepared by
a Malonate/Curtius type sequence of reactions, [see Yamada,
et al., J. Amer. Chem. Soc., (1972) 94, 6203] as illustrated
by the following reaction scheme
30
CA 02249786 1998-10-26
WO 95/01958 PCTIUS94/06376
- 17 -
REACTION SCHEME C p
~3
t-Bu02CCHZC02R9 t-BuOZCCH2C02R9
( 1 ) Base
(11) (2) P3X (13)
Removal of t-Bu
P3
HOZCCH2COZR9
(14)
Curtius-type
rearrangement
(12)
wherein t-Hu is t-butyl, although other selectively removal
acid protecting groups may be utilized, and P3X is as
previously defined. This reaction involves the alkylation
of the malonate ester (11) followed by selective removal of
the t-butyl protecting grou to
p produce compounds (14).
These compounds are then transformed to (12) using the
Curtius type rearrangement which entails their conversion
to the protected amine via the intermediately formed
azides, isocyanates, amines which are then protected with
standard amino protecting groups, preferentially being
protected in situ.
In the instance wherein P3 represents a P'1 moiety, the
ester is transformed to the desired aldehydes of
Formula (3) using standard Dibal reduction techniques,
particularly in this situation (wherein Pl is not a residue
of a natural amino acid). Alternatively, (as is preferred
when Pl is a residue of a natural amino acid) the ester is
de-esterified to its corresponding acid, converted to its
corresponding hydroxamate and the hydroxamate upon
treatment with lithium aluminum hydride is converted to its
aldehyde. In the instance wherein P3 represents a P'2
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
- 18 -
moiety, the ethyl ester of compounds (12) are removed and
the resulting compounds are ready for coupling as outlined
in Reaction Scheme A.
Having generically described the methods for the
preparation of the compounds of this invention, the
following specific examples illustrate the chemistry and
techniques by which the synthesis may be effected.
15
25
35
CA 02249786 1998-10-26
WO 95/01958 ~ PCT/US94/06376
- 19
EXAMPLE 1
O-(3-Pyridylmethyl)-(D)-valinol
H2N ( D )
O !~~
N
STEP A:
N-TRITYL-(D)-VALINOL
A solution of (D)-Valinol (4.95 g, 48.06 mmol),
triethylamine (7.4 ml, 52.87 mmol) and trityl chloride
(14.74 g, 52.87 mmol) in dry dichloromethane (75 ml) was
stirred for 17 hours at room temperature. The organic
solution was washed twice with water (2 x 75 ml) and dried
over sodium sulfate. After filtration and concentration in
vacuo, the resulting oil (22.4 g) was purified by flash
chromatography (silica gel, ethyl acetate/petroleum ether:
15/85) to give the title compound in 81% yield (13.5 g,
hard oil).
Rf.: 0.45 (ethyl acetate/petroleum ether: 15/85).
STEP B:
N-TRITYL-O-(3-PYRIDYLMETHYL)-(D)-VALINOL
Under nitrogen, to a suspension of sodium hydride
(1.3 g, 30 mmol, 55% dispersion in oil, previously washed
twice with pentane) in dry dimethylformamide (3 ml) was
added under stirring a solution of N-trityl-(D)-valinol
(3.45 g, 10 mmol) in dimethylformamide (23 ml). To the
reaction mixture kept for 30 minutes at room temperature
and then cooled down to 0°C, was added as a solid
tetrabutylammonium iodide (0.37 g, 1 mmol). After addition
in portions over 5 minutes of solid 3-picolyl chloride
hydrochloride (1.81 g, 11 mmol), the cooling bath was
removed and the mixture was stirred for 17 hours at room
temperature. The reaction mixture, previously cooled in an
ice bath, was hydrolyzed with water (100 ml) and extracted
twice with ethyl acetate (2 X 100 ml). The organic phases
CA 02249786 1998-10-26
WO 95!01958 PCT/US94l06376
- 20 -
were washed until neutral with water (2 X 50 ml) and the
combined organic layers were dried over sodium sulfate.
Filtration and concentration invacuo yielded a yellow oil
(4.8 g) which was purified by flash chromatography (silica
gel, dichloromethane/ethyl acetate: 9/1, Rf.: 0.42). The
title compound was obtained as an oil (3.4 g, 78% yield).
STEP C:
O-(3-PYRIDYLMETHYL)-(D)-VALINOL
A solution of N-trityl-0-(3-pyridyl)methyl-(D)-valinol
(3.63 g, 8.3 mmol) in formic acid (30 ml) was kept for
5.5 hours at room temperature. After removal of the formic
acid invacuo, the residue was dissolved in water (100 ml)
and extracted twice with ethyl acetate (100 ml, 50 ml) in
order to remove trityl alcohol. The aqueous phase was
basified with a saturated solution of sodium carbonate
(50 ml) and 4N sodium hydroxyde (3 ml) and extracted with
ethyl acetate (4 X 50 ml). After washing with brine until
neutral (2 X 50 ml), the combined organic layers were dried
over sodium sulfate. After usual work-up, the resulting
amine was used without further purification (1.32 g, 82%
yield).
Rf.: 0.12 (silica gel, dichloromethane/methanol: 8/2).
EXAMPLE 2
O-(2-Pyridylmethyl)-(D)-valinol
HZN (D)
N O
STEP A:
N-TRITYL-O-(2-PYRIDYLMETHYL)-(D)-VALINOL
The title compound was prepared in 81% yield from the
compound given in Example 1, Step A using the alkylation
CA 02249786 1998-10-26
WO 95101958 PCT/US94l06376
- 21 -
procedure described in Example 1 Step B, with 2-picolyl
chloride. hydrochloride instead of the 3-derivative.
Rf.: 5.1 (silica gel, dichloromethane/ethyl acetate: 9/1).
STEP B:
O-(2-PYRIDYLMETHYL)-(D)-VALINOL
The title amine was obtained in 80% yield from the
compound of Example 2, Step A using the formic acid
deprotection described in Example 1, Step C.
EXAMPLE 3
O-(2-(2-Methoxyethoxy)-1-ethyl]-(D)-valinol
H2N (D
O ~~OCH3
STEP A:
N-~ITYL-0-(2-(2-METHOXYETHOXY)-1-ETHYL]-(D)-VALINOL
The title derivative was prepared in 86% yield from the
compound of Example 1, Step A using 2-(2-methoxy-
ethoxy)ethyl-1-bromide as reagent in the alkylation
procedure described in Example 1, Step B.
Rf.: 0.74 (silica gel, acetone/petroleum ether: 2/8).
STEP H:
O-[2-(2-METHOXYETHOXY)-1-ETHYL]-(D)-VALINOL
A solution of N-Trityl-O-[2-(2-methoxyethoxy)-1-ethyl]-
(D)-valinol 1.0
( g, 2.28 mmol) in dry ether saturated with
HC1 gar (20 ml) was kept for 2.5 hours at room temperature.
After concentration inUacuo, the resulting solid (1.16 g)
was purified by flash chromatography (silica gel,
dichloromethane first to elute trityl alcohol and then
dichloromethane/diethylamine: 95/5, Rf.: 0.20) to give the
title free amine as a colorless oil (0.46 g, quantitative).
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
- 22
EXAMPLE 4
O-Henzyl-(D)-valinol
H2N (D)
~O I \
STEP A:
N-TERT-BUTOXYCARBONYL-(D)-VALINOL
A solution of (D)-valinol (5.1 g, 49.4 mmol) and di-tert-
butyldicarbonate (10.9 g, 50 mmol) in methanol (60 ml) was
stirred for 17 hours at room temperature. After
concentration invacuo, the residue was purified by flash
chromatography (silica gel, ethyl acetate/petroleum ether:
3/7, Rf.: 0.37) to give the title compound in quantitative
yield (10.07 g, colorless oil).
MS: MH+ = 204.
STEP H:
N- TERT-BUTOXYCARHONYL-O-HENZYL-(D)-VALINOL
To a solution of N-tent-butoxycarbonyl-(D)-valinol (10 g,
49.3 mmol) and benzylbromide (5.86 ml, 49.3 mmol) in
anhydrous DMF (50 ml) was added at -5°C and under nitrogen,
potassium-tent-butoxide (11.06 g, 98.6 mmol) as a solid,
portionwise, and in such a way that the internal
temperature does not exceed +5°C. The reaction mixture was
stirred for 2 hours at 0°C, diluted with ethyl acetate (2 X
300 ml), extracted with a 1N solution of potassium
hydrogenosulfate (50 ml) and water (250 ml) and washed
twice with water (2 X 200 ml). After drying of the organic
phase on sodium sulfate, filtration and concentration in
vacuo, the resulting oil was purified by flash
chromatography (silica gel, ethyl acetate/petroleum ether:
1/9, Rf.: 0.42) to give the title compound as a colorless
oil (9.95 g, 69% yield).
MS: MH+ = 294.
CA 02249786 1998-10-26
WO 95/01958 PCTIUS94106376
- 23 -
STEP C:
O-HENZYL-(D)-VALINOL
A solution of N-tent-Hutoxycarbonyl-D-benzyl-(D)-valinol
(9.95 g. 34 mmol) in formic acid (50 ml) Was stirred for
4 hours at room temperature. After removal of the formic
acid invacuo, the sticky residue was dissolved in water
(100 ml), neutralized with a saturated solution of sodium
bicarbonate (100 ml) and the organic material extracted
twice with ethyl acetate (2 X 200 ml). The organic phases
were washed until neutral with water (2 X 100 ml) and the
combined organic layers were dried on sodium sulfate.
Filtration and evaporation of the solvent invacuo afforded
the title amine as a slightly yellowish oil (5.20 g, 79%).
MS : MH'* = 19 4 .
ERAMPLE 5
O-2-Methogyethoxymethyl-(D)-valinol
HZN ( D ) O /~O ~OCH3
STEP A:
N-TERT-BUTOXYCARHONYL-O-(2-METHOXYETFiOXYMET$YL)-(D)-VALINOL
To a solution of N-tent-butoxycarbonyl-(D)-valinol
(2.03 g, 10 mmol) in anhydrous dimethylformamide (20 ml)
cooled under nitrogen at -10°C, was added 1-methoxy-
ethoxymethyl chloride (1.37 ml, 12 mmol) and then in two
portions potassium tent-butoxide (1.35 g, 12 mmol, rinced
with 10 ml of dimethylformamide). The cooling bath being
removed, the reaction mixture was stirred for 3.5 hours at
room temperature. After hydrolysis with water (~ 5 ml), the
~jor part of the solvent was removed with a high vacuum
pomp. The residue was taken up in slightly acidic water
CA 02249786 1998-10-26
WO 95!01958 PCTIUS94I06376
- 24 -
(potassium hydrogenosulfate), extracted twice with ethyl
acetate (2 X 100 ml) and the organic phases washed with
water until neutral (2 X 50 ml). Usual work-up afforded an
oil (2.8 g) which was purified by flash chromatography
(silica gel, petroleum ether/ethyl acetate: 7/3; Rf.: 0.43)
to give the title ether in 37% yield.
MS: MH+ = 292, MNH4+ = 309.
STEP B:
0-(2-METHOXYETHOXYMETHYL)-(D)-VALINOL
The title amine was obtained in 77% yield from the
compound of Example 5. Step A using the procedure described
in Example 4, Step C, the washings being performed with
brine to avoid the loss of this amine in the aqueous phase.
ERAN~LE 6
4-tert-Butoxycarbonylamino-2.2-difluoro-3-hydroxy-5(4-
benzyloxy)phenyl pentanoic acid, ethyl ester
BOC HN
O
STEP A:
N-TERT HUTOXYCARHONYL-L-O-BENZYLTYROSINE-N.O-DIMETHYL-
HYDROXAMATE
A mixture of N-tert-butoxycarbonyl-L-O-benzyltyrosine
(37~1 g, 100 mmol), dicyclohexylcarbodiimide (20.6 g,
100 mmol) and N-hydroxybenzotriazole, hydrate (15.3 g,
100 mmol) in anhydrous dichloromethane (350 ml) was stirred
at 0°C for 10 minutes. To that mixture were added, at 0°C,
CA 02249786 1998-10-26
WO 95/01958 PCT/US94106376
- 25 -
N,0-dimethylhydroxylamine hydrochloride (9.75 g, 100 mmol)
and N-methylmorphiline (10.1 g, 100 mmol). The temperature
was allowed to raise to room temperature while the stirring
was continued for 15 hours. The white precipitate was
filtered off, rinsed with dichloromethane. The filtrate was
evaporated to dryness. The crude mixture was purified by
flash chromatography (silica gel, ethyl
acetate/cyclohexane: 2/8). 34.3 g of the expected
hydroxamate were isolated as a white solid (83% yield).
Rf: 0.36 (ethyl acetate/cyclohexane: 1/1).
STEP H:
N-TfRT-BUTOXYCARBONYL-L-O-BENZYLTYROSINAL
To a solution of N-tert-butoxycarbonyl-L-O-benzyl-
tyrosine, N,O-dimethylhydroxamate (18.2 g, 44 mmol) in a
4:1 mixture of anhydrous dietylether and dimethoxyethane
(300 ml) was added at 0°C, portionwise, lithium aluminium
hydride (1.82 g, 48 mmol). Stirring was continued for
1.5 hours at 0°C. Hydrolysis was done by dropwise addition
of a 1 M solution of potassium hydrogeno sulfate (55 ml).
The aqueous phase was decanted and reextracted with ethyl
acetate (2 X 200 ml). The combined organic layers were
washed with 3 N hydrochloric acid (250 ml) and brine
(200 ml). The organic phase was dried over anhydrous
magnesium sulfate. Filtration and removal of the solvent in
vacuo yielded the expected aldehyde as a white solid.
Recrystallization from ethyl acetate/pentane afforded 13 g
of crystalline N-rert-butoxycarbonyl-L-O-benzyltyrosinal.
Rf: 0.51 (silica gel, ethyl acetate/cyclohexane: 1/1).
35
CA 02249786 1998-10-26
WO 95/01958 PCTlUS94106376
- 26 -
STEP C:
4-TERT-BUTOXYCARBONYLAMINO-2,2-DIFLUORO-3-HYDROXY-5-(4-
BENZYLOXY)PHENYLPENTANOIC ACID, ETHYL ESTER
To a suspension of zinc (1.95 g, 30 matg) in anhydrous
tetrahydrofuran (5 ml) was added, under nitrogen, a mixture
of ethyl bromodifluoroacetate (6.09 g, 30 mmol) and N-fert-
butoxycarbonyl-L-O-benzyltyrosinal (3.55 g, 10 mmol) in
anhydrous tetrahydrofuran (25 ml). After addition of 2 ml
of that solution, the suspension was heated at reflux with
stirring. Gentle reflux was maintained by slow addition
(dropwise) of the rest of the solution of aldehyde and
bromoester. The mixture was stirred for 4 additional hours
at room temperature after completion of the addition.
Hydrolysis was performed by addition of 1 M sulfuric acid
(20 ml) and the mixture was extracted with ethyl acetate
(3 X 50 ml). The combined organic layers were washed with
brine and dried over anhydrous magnesium sulfate.
Filtration and removal of the solvent in vacuo afforded an
oil that was purified by flash chromatography (silica gel.
gradient of ethyl acetate/cyclohexane: 1/9 to 3/7). 1.8 g
of the title compound were isolated (38% yield).
Rf: 0.55 and 0.5 (ethyl acetate/cyclohexane: 1/1).
Analysis calculated for Cy5H3iNOsF2:
C: 62.62 H: 6.52 N: 2.92
Found: C: 62.81 H: 6.67 N: 3.05
35
CA 02249786 1998-10-26
WO 95!01958 PCT/US94I06376
- 27
EXAMPLE 7
N-[4-(N-Benzyloxycarbonyl-1-valyl)amino-2,2-difluoro-1,3-
dioxo-5-(4-benzyloxy)phenyl-pentyl]-O-((3-pyridyl)methyl] -
D-valinol
O
0 O
O O NH L
NH
CF2~~~ D
° ~° °J
O O N
STEP A:
N-I4-TERT-BUTOXYCARHONYLAMINO-2,2-DIFLUORO-3-HYDROXY-1-OXO-
5-(4-BENZYLOXY)PHENYL-PENTYL]-O-[(3-PYRIDYL)METHYL]-D-
VALINOL
A solution of 1.14 g (2.38 mmol) of the ester of
Example 6, Step C and 1.32 g (6.8 mmol) of the amine of
Example 1, Step C in dry tetrahydrofuran (1.5 ml) was
heated for 2 days under reflux. After cooling, the reaction
mixture was diluted with ethyl acetate (5 ml), pentane
(10 ml) and the precipitate thus obtained was filtered off
and rinsed with pentane. The residue (1.25 g) was
recrystallized from a mixture of dichloromethane/drops of
methanol/pentane and the title compound was obtained as a
white solid (0.8 g, 54% yield).
Rf: 0.5 (silica gel, ethyl acetate).
MS: MH+ = 628.
CA 02249786 1998-10-26
WO 95/01958 PCTlUS94106376
_ 28 _
STEP B:
N-f4-AMINO-2,2-DIFLUORO-3-HYDROXY-1-OXO-5-(4-BENZYLOXY)-
PHENYL-PENTYL]-O-[(3-PYRIDYL)METHYL]-D-VALINOL
The title compound was prepared in 91% yield from the
carbamate of Example 7. Step A following the deprotection
procedure described in Example 4, Step C using sodium
carbonate instead of sodium bicarbonate.
MS: MH+ = 528.
STEP C
N-[4-(N-BENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-
HYDROXY-1-OXO-5-(4-HENZYLOXY)PHENYL-PENTYL]-O-[(3-PYRIDYL)-
METHYL]-D-VALINOL
To a solution of N-benzyloxycarbonyl-L-valine (0.101 g,
0.4 mmol) in anhydrous dimethylformamide (2 ml) were added
under nitrogen N-hydroxybenzotriazole, hydrate (0.115 g.
0.4 mmol) and 1-ethyl-3(3-dimethylaminopropyl)carbodiimide,
hydrochloride (0.085 g~ 0.44 mmol) with the help of 1 ml of
dimethylformamide. To the reaction mixture stirred for
0.5 hour at room temperature was added the amine of
Example 7, Step H (0.211 g, 0.4 mmol) with 1 ml of
dimethylformamide. The stirring was continued for 15 hours
and the reaction mixture was diluted with ethyl acetate
(80 ml) and washed twice with water (2 X 80 ml), the
aqueous phases being extracted a second time with ethyl
acetate (80 ml). The combined organic layers were dried
over sodium sulfate. After filtration and concentration in
vacuo, the solid residue (0.360 g) was purified by flash
chromatography (silica gel, dichloromethane/ethanol: 95/5.
Rf: 0.23) to give the title compound in 85% yield (0.260 g).
MS: MH+ = 761.
Analysis calculated for C42H5oNa0~FZ:
C: 66.38 H: 6.62 N: 7.36
Found: C: 66.68 H: 6.68 N: 7.40
CA 02249786 1998-10-26
WO 95/01958 PCTlUS94I06376
- 29 -
STEP D:
N-[4-(N-BENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-1,3-
DIOXO-5-(4-BENZYLOXY)PHENYL-PENTYL)-O-[(3-PYRIDYL)METHYL) -
f1-T7T T T1T~1T
To a solution of oxalyl chloride (0.23 ml, 2.63 mmol)
in anhydrous dichloromethane (1 ml) at - 60°C was added
under nitrogen, freshly distilled dimethylsulfoxide
(0.42 ml, 5.26 mmol) in 2 ml of dichloromethane. After
minutes of stirring at - 60°C, the temperature was
10 allowed to rise to -20°C. Immediately was added dropwise to
that mixture a solution of the alcohol of Example 7, Step C
(0.2 g, 0.263 mmol) in dichloromethane (7 ml) and
dimethylsulfoxide (1 ml). After stirring for 3.5 hours at
- 20°C, the reaction mixture was cooled down to - 78°C,
hydrolyzed with diisopropyl ethyl amine (1.24 ml,
8.94 mmol) and kept for 5 more minutes at - 78°C. The
cooling bath was removed and the mixture was allowed to
return to~room temperature. After dilution with
dichloromethane (25 ml), the mixture was washed twice with
water (2 X 25 ml), the aqueous layers being extracted again
with dichloromethane (25 ml). The combined organic phases
were dried on sodium sulfate. After filtration and
concentration in vacuo. the residue (0.240 g) was purified
by flash chromatography (silica gel. dichloromethane/ethyl
acetate: 30/70 followed by neutral alumina act. III,
tetrahydrofuran/dichloromethane/water: 10/20/0.1, in order
to remove residual starting material) to give the title
ketone in 37% yield (0.075 g).
Rf: 0.23 (silica gel, dichloromethane/ethyl acetate:
30/70 ) .
MS: MH* = 759.
Analysis calculated for C42H48N407F2, 0.5 H20:
C: 65.70 H: 6.43 N: 7.30
Found: C: 65.49 H: 6.34 N: 7.14
CA 02249786 1998-10-26
WO 95/01958 PCTIUS94106376
- 30
EXAMPLE 8
4-(N-Benzyloxycarbonyl-L-valyl)amino-2,2-difluoro-3-oxo-5-
(4-benzyloxy)phenyl-N(1-isopropyl-2-methyl-propane)-
pentanamide
O
O
a
O NH- CF2~~~
0
L
0 0 O
O
STEP A:
4-TERT-BUTOXYCARBONYLAMINO-2,2-DIFLUORO-3-HYDROXY-5-(4-
HENZYLOXY)PHENYL-N(1-ISOPROPYL-2-METHYL-PROPANE)PENTANAMIDE
A solution of the ester of Example 6. Step C (0.50 g,
1.04 mmol) in 0.77 ml of 3-amino-2,4-dimethyl pentane
(5.2 mmol) was heated at 75°C for 90 hours. After dilution
with ethyl acetate (15 ml), extraction with 1N potassium
hydrogeno sulfate (15 ml) and washing with water (2 X
15 ml) - the aqueous phases being extracted again with
ethyl acetate (15 ml) - the combined organic layers were
dried over sodium sulfate. Filtration and concentration of
the solvent afforded a residue (0.54 g) which was purified
by flash chromatography (silica gel, petroleum ether/ethyl
acetate: 75/25, Rf: 0.34) to give the title compound in 42%
yield (0.24 g).
MS: MH+ = 549.
STEP B
4-AMINO-2,2-DIFLUORO-3-HYDROXY-5-(4-HENZYLOXY1PHENYL-N(1-
ISOPROPYL-2-METHYL-PROPANE)PENTANAMIDE
The title amine was obtained in 92% yield from the
compound of Example 8, Step A, using the deprotection
method described in Example 7, Step B.
MS: MH+ = 449.
CA 02249786 1998-10-26
WO 95/01958 PCT/US94l06376
- 3I -
STEP C:
4-(N-HENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-
HYDROXY-5-(4-BENZYLOXY)PHENYL-N(1-ISOPROPYL-2-METHYL-
PROPANE)PENTANAMIDE
To a stirred solution of N-benzyloxycarbonyl-L-valyl
anhydride (0.181 g, 0.37 mmol) and the amine described in
Example 8, Step B (0.140 g, 0.31 mmol) in anhydrous
dimethylformamide (3 ml) was added under nitrogen 0.041 ml
of N-methylmorpholine (0.37 mmol). The reaction mixture was
kept overnight at room temperature, diluted with water
(15 ml) and extracted with ethyl acetate (2 X 15 ml), the
organic layers being washed a second time with water
(15 ml) and then dried over sodim sulfate. After
filtration, removal of the solvent in vacuo and
purification of the residue (0.200 g) by flash
chromatography (silica gel, dichloromethane/ethyl acetate:
85/15. Rf: 0.16) the title compound was obtained as a white
solid (0.080 g, 38% yield).
MS: MH+ = 682.
STEP D:
4-(N-BENZYLOXYCARHONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-OXO-5-
~4-BENZYLOXY)PHENYL-N(1-ISOPROPYL-2-METHYL-PROPANE)-
PENTANAMIDE
The title compound was obtained in 51% yield from the
alcohol, of Example 8, Step C using the Swern oxidation
depicted in Example 7, Step D.
MS: MH+ = 680, MNH4+ = 697.
Analysis calculated for C39H41N306F2~
C: 64.14 H: 6.97 N: 6.18
Found: C: 64.53 H: 6.57 N: 5.75
CA 02249786 1998-10-26
WO 95101958 PCT/US94l06376
- 32 -
cwntu«r.~ O
4-[N-(3-PVridylpropionyl)-L-valyl]amino-2,2-difluoro-3-oxo-
5-(4-benzyloxy)phenyl-N(1-isopropyl-2-methyl-propane)-
~entanamide
N O NH CF2~~~
L
O 0 0 O
STEP A:
4-(N-TERT-BUTOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-
HYDROXY-5-(4-BENZYLOXY)PHENYL-N(1-ISOPROPYL-2-METHYL-
PROPANE)PENTANAMIDE
The title compound was prepared in 76% yield from the
amine of Example 8. Step H and N-tent-butoxycarbonyl-L-
valine
using the coupling procedure given in Example 7, Step C
with dichloromethane as solvent instead of dimethyl-
formamide.
Rf: 0.17 (silica gel, dichloromethane/ethyl acetate:
90/10).
MS: MH+ = 648.
STEP B
4-(L-VALYL)AMINO-2,2-DIFLUORO-3-HYDROXY-5-(4-
BENZYLOXY)PHENYL-N(1-ISOPROPYL-2-METHYL-PROPANE)PENTANAMIDE
The title amine was prepared in quantitative yield from
the compound described in Example 9. Step A, using the
procedure given in Example 7, Step B.
MS: MH+ = 548.
CA 02249786 1998-10-26
WO 95101958 PCTIUS94l06376
- 33 -
STEP C:
4-[N-(3-PYRIDYLPROPIONYL)-L-VALYL]AMINO-2,2-DIFLUORO-3-
HYDROXY-5-(4-HENZYLOXY)PHENYL-N(1-ISOPROPYL-2-METHYL-
PROPANE)PENTANAMIDE
The title compound was obtained in 84% yield from the
amine of Example 9, Step B and 3-pyridylpropionic acid
using the coupling method described in Example 7, Step C.
Rf: 0.16 (silica gel, ethyl acetate)
MS: MH+ = 681.
Analysis calculated for C38H50N405F2~
C: 67.04 H: 7.40 N: 8.23
Found: C: 67.34 H: 7.60 N: 7.74
STEP D:
4-[N-(3-PYRIDYLPROPIONYL)-L-VALYL]AMINO-2.2-DIFLUORO-3-OXO-
5-(4-BENZYLOXY)PHENYL-N(1-ISOPROPYL-2-METHYL-PROPANE)-
PENTANAMIDE
The title compound was prepared in 57% yield from the
alcohol of Example 9. Step C using the Swern oxidation
procedure described in Example 7, Step D.
Rf: 0.2 (silica gel, ethyl acetate);
Analysis calculated for C38H48N405Fz, 0.75 H20:
C: 65.92 H: 7.21 N: 8.09
Found: C: 65.93 H: 7.21 N: 7.92
EXAMPLE 10
N-[4-(N-Henzyloxycarbonyl-L-valyl)amino-2,2-difluoro-1,3-
dioxo-5-(4-benzyloxy)phenyl-pentyl]-di(O-benzyl)serinol
O
O
O NH L ~ O
CF2 NH
p ~ ~~ p O
0
CA 02249786 1998-10-26
WO 95/01958 PCTlUS94l06376
- 34 -
STEP A:
N-TERT-BUTOXYCARBONYL SERINOL
The title derivative was prepared as a white solid in
94% yield from commercially available serinol using the
protection procedure described in Example 4, Step A.
Rf: 0.33 (silica gel, ethyl acetate).
STEP B:
N-TERT-BUTOXYCARBONYL-DI(O-HENZYL)SERINOL
The title compound was prepared in 49% yield from N-
tert-butoxycarbonyl serinol using the procedure given in
Example 4, Step B, but using tetrahydrofuran as solvent,
2.4 equivalents of benzyl bromide and 2.2 equivalents of
potassium-tert-butoxide.
RF: 0.17 (silica gel, petroleum ether/ethyl acetate:
90/10).
STEP C:
DI(O-BENZYL)SERINOL
The title amine was prepared in 80% yield from the
compound depicted in Example 10, Step B, following the
deprotection method described in Example 7, Step B.
STEP D:
N-[4-TERT-BUTOXYCARHONYLAMINO-2,2-DIFLUORO-3-HYDROXY-1-OXO-
5-(4-BENZYLOXY)PHENYL-PENTYL]-DI(O-BENZYL)SERINOL
A solution of the ester of Example 6, Step C (0.256 g,
0.534 mmol) and di(O-benzyl)serinol (0.43 g, 1.6 mmol) in
dry tetrahydrofuran (2 ml) was heated under reflux during
40 hours. After removal of the solvent, the residue was
taken up in ethyl acetate (15 ml), extracted with 1N
potassium hydrogeno sulfate (15 ml) and whashed twice with
water (2 X 15 ml), the aqueous phases being extracted again
with 15 ml of ethyl acetate. After drying of the organic
layers on sodium sulfate, filtration and concentration in
vaCUO, the residue (0.52 g) was purified by flash
chromatography (silica gel, petroleum ether/ethyl acetate:
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
- 35 -
70/30. Rf: 0.35) to give the title derivative in 52~ yield
(0.33 g).
STEP E:
N-[4-AMINO-2.2-DIFLUORO-3-HYDROXY-1-OXO-5-(4-HENZYLOXY)-
PHENYL-PENTYL]-DI(O-HENZYL)SERINOL
The title amine was obtained in 92% yield from the
compound of Example 10, Step D using the deprotection
procedure given in Example 7, Step H.
STEP F:
N-[4-(N-BENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-
HYDROXY-1-OXO-5-(4-HENZYLOXY)PHENYL-PENTYL]-DI(O-
HENZYL)SERINOL
The title compound was obtained in 47% yield from the
amine given in Example 10, Step E and N-benzyloxycarbonyl-
L-valyl anhydride following the coupling procedure
described in Example 8, Step c and using dichloromethane as
solvent.
RF: 0.30 (silica gel, dichloromethane/ethyl acetate:
90/10).
MS: MH'* = 838r MNH4+ = 855.
STEP G:
N-[4-(N-HENZYLOXYCARHONYL-L-VALYL)AMINO-2,2-DIFLUORO-1.3-
DIOXO-5-(4-HENZYLOXY)PHENYL-PENTYL]-DI(O-BENZYL)SERINOL
The title derivative was obtained in 26% yield from the
alcohol given in Example 10, Step F, following the Swern
oxidation described in Example 7, Step D.
RF: 0.11 (silica gel, petroleum ether/ethyl acetate:
70/30).
MS: MH+ = 836.
Analysis calculated for C48H51N308F2~ 0.5 HZO:
C: 68.23 H: 6.20 N: 4.97
Found: C: 68.02 H: 6.16 N: 4.81
CA 02249786 1998-10-26
WO 95101958 PCTlUS94106376
- 36 -
EXAMPLE 11
_4-(N-Henzyloxycarbonyl-L-valyl)amino-2,2-difluoro-3-oxo-5-
(9-benzyloxy)phenyl-N(ar-L-methyl)benzyl pentanamide
O
O
O ~ CF _ L O
~l~ L~~
O O O O
STEP A:
4-TERT-BUTOXYCARHONYLAMINO-2,2-DIFLUORO-3-HYDROXY-5-(4-
HENZYLOXY)PHENYL-N(a-L-METHYL)HENZYL PENTANAMIDE
The title compound was prepared in 75% yield from the
ester of Example 6, Step C and a-L-methyl benzylamine
following the procedure depicted in Example 10, Step D.
Rf: 0.06 (silica gel, petroleum ether/ethyl acetate:
80/20).
STEP H:
4-AMINO-2,2-DIFLUORO-3-HYDROXY-5-(4-BENZYLOXY)PHENYL-N(a-L-
METHYL)BENZYL PENTANAMIDE
The title amine was obtained in 92% yield from the
derivative described in Example 11. Step A, using the
formic acid deprotection given in Example 7, Step B.
STEP C
4-(N-BENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-OXO-5-
(4-BENZYLOXY)PHENYL-N(a-L-METFiYL)HENZYL PENTANAMIDE
The title compound was obtained in 87% yield from the
amine of Example 11, Step B and N-benzyloxycarbonyl-L-valyl
anhydride using the procedure described in Example 8,
Step C and with dichloromethane as solvent.
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
- 37 -
STEP D:
4-(N-BENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-OXO-5-
(4-BENZYLOXY)PHENYL-N(Gt-L-METHYL)BENZYL PENTANAMIDE
The title derivative was obtained in low yield from the
alcohol of Example 11, Step C using the oxidation procedure
described in Example 7. Step D (recovery of more than 50%
of starting alcohol despite of 2 successive Swern
oxidations).
Rf: 0.14 (silica gel, petroleum ether/ethyl acetate:
70/30 ) .
Analysis calculated for C39H41N306F2~ 0~5 H20:
C: 67.42 H: 6.09 N: 6.05
Found: C: 67.22 H: 5.91 N: 5.74
EXAMPLE 12
N-f4-(N-Benzyloxycarbonyl-L-valyl)amino-2.2-difluoro-1.3-
diozo-5-(4-benzyloay)phenyl-pentyl]-O-(2-methoxyethoxy-
methyl)-D-valinol
v
~ O L NH CF2 NH D O ~O~ OCH3
1~
0 0 o O
STEP A:
N-f4-(TfRT-HUTOXYCARHONYLAMINO-2.2-DIFLUORO-3-HYDROXY-1-
OXO-5-(4-BENZYLOXY)PHENYL-PENTYL]-O-(2-METHOXYETHOXY-
METHYL)-D-VALINOL
The title compound was prepared in 56% yield from the
ester of Example 6, Step C and the amine of Example 5.
Step B using the substitution procedure described in
Example 10, Step D.
CA 02249786 1998-10-26
WO 95101958 PCTIUS94/06376
- 38 -
Rf: 0.35 (silica gel, petroleum ether/ethyl acetate:
55/45).
STEP B:
N-[4-AMINO-2,2-DIFLUORO-3-HYDROXY-1-OXO-5-(4-
BENZYLOXY)PHENYL-PENTYL]-O-(2-METHOXYETHOXYMETHYL)-D-
VALINOL
The title amine was obtained in quantitative yield from
the compound of Example 12, Step A using the deprotection
method given in Example 7. Step H, the reaction temperature
being kept at 5°C instead of room temperature.
STEP C:
N-[4-(N-HENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-
HYDROXY-1-OXO-5-(4-BENZYLOXY)PHENYL-PENTYL)-O-(2-
METHOXYETHOXYMETHYL)-D-VALINOL
The title derivative was prepared in 59% yield from the
amine of Example 12, Step B and N-benzyloxycarbonyl-L-valyl
anhydride following the procedure described in Example 8,
Step C and with dichloromethane as solvent.
Rf: 0.25 (silica gel, dichloromethane/ethyl acetate:
80/20).
MS: MH* = 758. MNH4* = 775.
STEP D
N-(4-(N-BENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-1,3-
DIOXO-5-(4-HENZYLOXY)PHENYL-PENTYL]-O-(2-METHOXYETHOXY-
METHYL)-D-VALINOL
The title compound was obtained in 73% yield from the
alcohol of Example 12, Step C using the Swern oxidation
depicted in Example 7, Step D.
Rf: 0.26 (silica gel, dichloromethane/ethyl acetate:
70/30).
MS: MH* = 770.
Analysis calculated for C40H51N309F2~
C: 63.56 H: 6.80 N: 5.56
Found: C: 63.55 H: 6.78 N: 5.49
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
- 39
EXAMPLE 13
N-[4-(N-Henzyloxycarbonyl-L-valyl)amino-2,2-difluoro-1,3-
dioxo-5-(4-benzyloxy)phenyl-pentyl]-O-formyl-D-valinol
v
p N L NH CF2\ NH D
1~ ~~ ~ lI~ °
° ° ° °
A solution of the compound given in Example 12, Step D
(0.050 g, 0.066 mmol) in formic acid (5 ml) was stirred for
5 hours at room temperature. After concentration in vaCUO,
the residue (0.043 g) was purified by a micro flash
chromatography (silica gel, dichloromethane/ethyl acetate:
70/30, Rf: 0.49) to give the title compound in 44% yield.
MS: MH+ = 696.
Analysis calculated for C37H43N3°8F2~
C: 63.87 H: 6.23 N: 6.04
Found: C: 64.15 H: 6.35 N: 5.78
EXAMPLE 14
N-.[4-(N-Henzyloxycarbonyl-L-valyl)amino-2,2-difluoro-1.3-
dioxo-5-(4-benzyloxy)phenyl-pentyl]-O-[2-(2-methoxyethoxyl-
1-ethyl]-D-valinol
v
p L NH CFZ NH D °
1~ ~~ ~ ~~ °~H3
° ° ° °
CA 02249786 1998-10-26
WO 95/01958 PCT/US94l06376
- 40 -
STEP A:
_N-(4-(TERT-BUTOXYCARBONYLAMINO-2,2-DIFLUORO-3-HYDROXY-1-
OXO-5-(4-BENZYLOXY)PHENYL-PENTYL]-O-[2-(2-METHOXYETHOXY)-1-
ETHYL]-D-VALINOL
The title compound was prepared in 51% yield from the
ester of Example 6, Step C and the amine of Example 3,
Step B using the procedure depicted in Example 10, Step D.
Rf: 0.37 (silica gel. petroleum ether/ethyl acetate:
30/70).
STEP B:
N ~4-AMINO-2,2-DIFLUORO-3-HYDROXY-1-OXO-S-(4-
BENZYLOXY)PHENYL-PENTYL]-O-[2-(2-METHOXYETHOXY)-1-ETHYL]-D-
VALINOL
The title amine was obtained in 97% yield from the
compound of Example 14. Step A using the deprotection
method described in Example 7. Step B.
MS: MH+ = 539.
STEP C
N-(4-(N-HENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-
HYDROXY-1-OXO-5-(4-BENZYLOXY)PHENYL-PENTYL]-O-[2-(2-
METHOXYETHOXY1-1-ETHYL]-D-VALINOL
The title derivative was obtained in 74% yield from the
amine of Example 14, Step H and N-benzyloxycarbonyl-L-valyl
anhydride using the coupling procedure described in
Example 8, Step C and with dichloromethane as solvent.
Rf: 0.19 (major isomer at the 3-hydroxy function) and 0.13
(minor isomer) (silica gel, dichloromethane/ethyl acetate:
60/40 ) .
MS: MH+ = 772.
CA 02249786 1998-10-26
WO 95!01958 PCTlUS94106376
- 41 -
STEP D:
N-(4-(N-HENZYLOXYCARHONYL-L-VALYL)AMINO-2,2-DIFLUORO-1,3-
DIOXO-5-(4-HENZYLOXY)PHENYL-PENTYL]-O-[2-(2-METHOXY~THOXY~
1-ETHYL]-D-VALINOL
The title compound was prepared in 74% yield from the
alcohol of Example 14, Step C using the oxidation method
given in Example 7. Step D.
Rf: 0.13 (silica gel, dichloromethane/ethyl acetate:
70/30).
MS: MH+ = 770
Analysis calculated for C4iH53N309F2~
C: 63.96 H: 6.84. N: 5.46
Found: C: 63.94 H: 6.86 N: 5.38
ERAMPLE 15
4-(N-Benzyloxycarbonyl-L-valyl)amino-2,2-difluoro-3-oxo-5-
~4-benzyloxy)phenyl-N-benzydrYl pentanamide
O NH
~~ ~r L ~~
0 0
STEP A:
4-(TERT-BUTOXYCARBONYL)AMINO-2,2-DIFLUORO-3-HYDROXY-5-(4-
HENZYLOXY)PHENYL-N-BENZYDRYL PENTANAMIDE
The title compound was obtained in 45% yield from the
ester of Example 6. Step C and commercially available
benzydrylamine (distilled over potassium hydroxyde) using
the procedure described in Example 10, Step D.
Rf: 0.50 (silica gel, cyclohexane/ethyl acetate: 1/1).
MS: MH+ = 617.
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
- 42 -
STEP B:
4-AMINO-2,2-DIFLUORO-3-HYDROXY-5-(4-BENZYLOXY)PHENYL-N-
HENZYDRYL PENTANAMIDE
The title amine was obtained in 82$ yield from the
derivative of Example 15, Step A following the deprotection
method given in Example 7. Step B.
STEP C:
4-(N-HENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-
HYDROXY-5-(4-HENZYLOXY)PHENYL-N-BENZYDRYL PENTANAMIDE
The title compound was prepared in 83% yield from the
amine of Example 15. Step H and N-benzyloxycarbonyl-L-valyl
anhydride following the coupling reaction given in
Example 8. Step C using dichloromethane as solvent.
Rf: 0.49 (silica gel, cyclohexane/ethyl acetate: 1/1).
MS: MH+ = 750.
STEP D:
4-lN-HENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-OXO-5-
(4-BENZYLOXY)PHENYL-N-HENZYDRYL PENTANAMIDE
The title derivative was obtained from the alcohol of
Example 15, Step C using the Swern oxidation depicted in
Example 7. Step D.
Rf: 0.47 (silica gel, cyclohexane/ethyl acetate: 1/1).
MS: MH+ = 748. MNH4+ = 765.
Analysis calculated for C44H43N306F2~
C: 70.67 H: 5.79 N: 5.62
Found: C: 69.88 H: 5.89 N: 5.49
35
CA 02249786 1998-10-26
WO 95!01958 PCTlUS94106376
- 43
EXAMPLE 16
4-(N-Henzyloxycarbonyl-L-valyl)amino-2,2-difluoro-3-oxo-5-
(4-benzyloxy)phenyl-N(1,1-di(2-pyridyl)methyllpentanamide
O
O
v N
O NH CF NH O
°l~ L
° ° ° ° O
STEP A:
N-TERT-BUTOXYCARBONYL-1,1-DI(2-PYRIDYL)METHYL AMINE
To a solution of commercial di(2-pyridyl)ketone
(3.68 g, 20 mmol) in anhydrous methanol (60 ml) was added
ammonium acetate (15.40 g, 200 mmol) and sodium
cyanoborohydride (0.88 g, 14 mmol). After stirring at room
temperature for 24 hours, the reaction mixture was
hydrolyzed with 37% hydrochloric acid until pH - 2 and the
solvent removed in vatuo. The residue was taken off in
water (100 ml), extracted twice with diethyl ether (2 X
60 ml) and the combined organic layers were dried over
magnesium sulfate. After filtration and removal of the
solvent in vacuo, the residue was taken off in anhydrous
dichloromethane (50 ml) and di-tart-butyl dicarbonate
(2.80 g, 13 mmol) was added, the reaction mixture being
stirred for 16 hours at room temperature. The solvent was
removed invacuo and the residue was purified by flash
chromatography (silica gel, cyclohexane/ethyl acetate: 3/7)
to give the title compound in 17% yield (0.90 g).
Rf: 0.34 (silica gel, ethyl acetate).
STEP B:
1,1-DI(2-PYRIDYL)METHYL AMINE
To a solution of the compound of Example 16, Step A
(0.85 g, 3 mmol) in anhydrous diethyl ether (10 ml) was
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
- 44 -
added at 0°C 40 ml of a saturated solution of hydrogen
chloride gas in anhydrous diethyl ether. The reaction
mixture was stirred at 0°C and then the temperature was
allowed to rise to room temperature overnight. The solvent
was removed in vacuo and the residue was taken off in ethyl
acetate (100 ml), washed three times with a saturated
solution of sodium carbonate (3 X 30 ml) and the organic
layer dried over magnesium sulfate. Removal of the solvent
invacuo afforded the title compound in 55% yield (0.30 g).
STEP C:
4-(TERT-BUTOXYCARHONYL)AMINO-2,2-DIFLUORO-3-HYDROXY-5-(4-
BENZYLOXY)PHENYL-N-[1.1-DI(2-PYRIDYL)METHYL]PENTANAMIDE
The title compound was prepared in 43% yield from the
ester of Example 6. Step C and the amine of Example 16.
Step H following the procedure described in Example 10,
Step D.
Rf: 0.40 (silica gel, ethyl acetate)
MS: MH+ = 619.
STEP D:
4-AMINO-2,2-DIFLUORO-3-HYDROXY-5-(4-HENZYLOXY)PHENYL-N-
[1,1-DI(2-PYRIDYL)METHYL]PENTANAMIDE
The title amine was obtained in 77% yield from the
compound of Example 16, Step C using the deprotection
method described in Example 7, Step B.
STEP E:
4-(N-BENZYLOXYCARHONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-
HYDROXY-5-(4-HENZYLOXY)PHENYL-N-[1,1-DI(2-PYRIDYL)-
METHYL]PENTANAMIDE
The title compound was obtained in 70% yield from the
amine of Example 16. Step D and N-benzyloxycarbonyl-L-valyl
anhydride using the coupling method depicted in Example 8,
Step C using dichloromethane as solvent.
Rf: 0.49 (silica gel, ethyl acetate).
MS: MH+ = 752.
CA 02249786 1998-10-26
' PCT 1US94106376
WO 95!01958
- 45 -
STE- P F:
4- N-BENZYLOXYCARHONYL-L-VALYL AMINO-2 2-DIFLUORO-3-0X0-5
(4 BENZYLOXY)PHENYL N [1.1-DI(2-PYRIDYL)METHYL]PENTANAMIDE
The title compound was prepared in 42% yield from the
alcohol of Example 16, Step E using the oxidation procedure
described in Example 7. Step D.
Rf: 0.39 (silica gel. ethyl acetate).
MS: MH+ = 750.
Analysis calculated for C42H41N506F2~ 0.5 H20:
C: 66.48 H: 5.58 N: 9.23
Found: C: 66.37 H: 5.55 N: 8.91
EXAMPLE 17
N [4 (N Henzyloxycarbonyl-L-valyl)amino-2.2-difluoro-1.3-
dioxo-5 (4 {2 N morpholyl~ethyloxy)Phenyl-pentvll-0-[(3-
pyridyl)methyl]-D-valinol
O
O
O O ~ CF 2~~~ D
l~ ~~ ~ b
5 O O p O N
STEP A:
_4 TERT BUTOXYCARBONYLAMINO-2.2-DIFLUORO-3-HYDROXY-5-(4-
HYDROXY)PHENYL PENTANOIC ACID. ETHYL ESTER
A solution of compound of Example 6, Step C (0.719 g.
1.5 mmol) in ethanol (50 ml) was kept for 7.5 hours under
an hydrogen atmosphere in the presence of 10% palladium on
charcoal (0.074 g). The hydrogen atmosphere was exchanged
by a nitrogen atmosphere, the suspension was filtered off
and the solution concentrated in vacuo. The title
CA 02249786 1998-10-26
PCTlUS94l06376
WO 95101958
- 46 -
thus obtained was used as such in the next step (0.500 g.
83% yield).
Rf: 0.51 (silica gel. petroleum ether/ethyl acetate: 1/1).
STEP H:
N- 4-TERT-BUTOXYCARBONYLAMINO-2 2-DIFLUORO-3-HYDROXY-1-OXO
5 (4 HYDROXY)PHENYL PENTYL)-O-[(3-PYRIDYL)METHYL)-D-VALINOL
The title compound was obtained in 82% yield from the
ester of Example 17. Step A and the amine of Example 1,
Step C, following the procedure described in Example 10,
Step D.
Rf: 0.47 (silica gel, ethyl acetate).
STEP C:
N- 4-TERT-BUTOXYCARBONYLAMINO-2 2-DIFLUORO-3-HYDROXY-1-OXO-
5 (4 {2 N MORPHOLYL~ETHYLOXY)PHENYL-PENTYL)-O-[(3-PYRIDYL~ -
METHYL)-D-VALINOL
A solution of the phenolic derivative described in
Example 17, Step H (0.081 g, 0.15 mmol) and 4-(2-chloro-
ethyl)morpholine, hydrochloride (0.039 g, 0.21 mmol) in dry
dimethylformamide (3 ml) was stirred under nitrogen for
66 hours at room temperature in the presence of cesium
carbonate (0.166 g, 0.51 mmol) and potassium iodide
(0.0035 mg, 0.021 mmol). The reaction mixture was diluted
with ethyl acetate (15 ml) and washed twice with water
(2 X 15 ml), the aqueous phases being extracted again with
ethyl acetate (15 ml). After drying of the combined organic
layers on sodium sulfate, filtration and concentration in
vacuo, the residue (0.117 g) was purified by flash
chromatography (silica gel, ethyl acetate/methanol: 90/10.
Rf: 0.27) to give the title compound in 51% yield
(0.050 g).
MS: MH+ = 651.
CA 02249786 1998-10-26
WO 95/01958
- 47 -
PCTlUS94106376
STEP D:
N [4 AMINO 2,2-DIFLUORO-3-HYDROXY-1-OXO-5-(4-{2-N-
MORPHOLYL~ETHYLOXY)PHENYL-PENTYL]-O-[(3-PYRIDYL)METHYL]-D-
V_ALINOL
title amine was prepared in quantitative yield from
the derivative of Example 17. Step C following the
deprotection procedure described in Example 7, Step B.
MS: MH+ = 551.
STEP E
N [4 (N BENZYLOXYCARHONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-
HYDROXY 1 OXO 5 (4 {2-N-MORPHOLYL}ETHYLOXY)PHENYL-PENTYL]-
O_-[(3-PYRIDYL)METHYL]-D-VALINOL
The title compound was obtained in 53% yield from the
amine of Example 17. Step D and N-benzyloxycarbonyl-L-
valine following the coupling method given in Example 7.
Step C.
Rf: 0.17 (silica gel, dichloromethane/ethanol: 95/5).
MS: MH+ = 784.
Analysis calculated for C41H55N50sF2,0~25H2o:
C: 62.46 H: 7.10 N: 8.88
Found: C: 62.41 H: 6.94 N: 8.69
STEP F:
N [4 (N HENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-1,3-
DIOXO 5 (4-{2-N-MORPHOLYL}ETHYLOXY)PHENYL-PENTYL]-O-[(3-
PYRIDYL)METHYL]-D-VALINOL
The title compound was prepared in 25% yield from the
alcohol described in Example 17. Step E using the Swern
oxidation depicted in Example 7, Step D.
Rf: 0.06 (silica gel, ethyl acetate/acetone: 8/2).
Analysis calculated for C41H53N50sF2'H2~'
C: 61.56 H: 6.93 N: 8.76
Found: C: 61.56 H: 6.80 N: 8.26
CA 02249786 2002-11-29
- 48 -
Alternative procedure;
To a solution of the alcohol of example 17, step E (0.244
g, 0.31 mmol) in freshly distilled dichloromethane (10 ml)
was added the Dess-Martin reagent (0.528 g, 1.24 mmol) and
tent-butanol (0.06 ml, 0.62 mmol). After stirring for 10
minutes at room temperature, the reaction mixture was
quenched with 2-propanol (1 ml) and concentrated in vatuo.
The white solid residue was suspended in dichloromethane (4
ml, then 2 ml for rinsing) and the solid part was removed
by filtratioai over a FluorcyporeTM filter. Co~centratioo is
vacuo afforded a residue which was purified by flash
chromatography (silica gel, dichloromethane/ methanol: 98/2
for removing the by-products of the Dess-Martin reagent,
then dichloromethane/ methanol: 96/4 and finally
dichloromethane/ methanol: 90/10 to eluate the desired
product). The title ketone was obtained as a white solid in
61% yield (0.148 g)
Analysis calculated for C41HS3NsCeFZ,0.5H=o:
C: 62.26 H: 6.88 N: 8.86
Found: C: 62.49 H: 5.98 N: 8.97.
EXAMPLE 18
N-j4-(N-Henzyloxycarbonyl-L-valyl)amino-2.2-difluoro-1.3-
dioxo-5-(4-t2-N-morpholyl~ethyloxy)phenyl-pentyl]-O-((2-
pyridyl)methvl]-D-valinol
O ~ 0
O C L NH CFZ ~ D
O
N
CA 02249786 1998-10-26
- ~ PCTlUS94106376
WO 95/01958
- 49 -
STEP A:
N- 4.-TERT-BUTOXYCARBONYLAMINO-2 2-DIFLUORO-3-HYDROXY-1-OXO-
(4 t2 N MORPHOLYL}ETHYLOXY)PHENYL-PENTYL]-O-[(2-PYRIDYL -
M_ETHYL]-D-VALINOL
5 The title compound was prepared in 67% yield from the
ester of Example 17. Step A and the amine of Example 2,
Step a using the procedure described in Example 10, Step D.
Rf: 0.29 (silica gel, dichloromethane/ethyl acetate: 3/7).
STEP H
N_ (4 AMINO 2.2 DIFLUORO-3-HYDROXY-1-OXO-5-(4-HYDROXY
PHENYL PENTYL]-O-[(2-PYRIDYL)METHYL]-D-VALINOL
The title amine was obtained in 96% yield from the
derivative of Example 18, Step A following the deprotection
method given in Example 7.. Step B.
MS: MH+ = 438.
STEP C:
N [4 (N HENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-
HYDROXY 1 OXO 5 (4 HENZYLOXY)PHENYL-PENTYL]-O-((2-PYRIDYL)-
M_ETHYL]-D-VALINOL
The title derivative was obtained in 47% yield from the
amine of Example 18. Step B and N-benzyloxycarbonyl-L-
valine using the coupling procedure described in Example 7.
Step C.
Rf: 0.31 (silica gel, dichloromethane/ethyl acetate: 2/8).
MS: MH+ = 671.
STEP D:
N [4 N HENZYLOXYCARHONYL-L-VALYL)AMINO-2,2-DIFLUORO-3-
H_YDROXY 1 OXO 5 (4-{2-N-MORPHOLYL}ETHYLOXY)PHENYL-PENTYL]-
O-[(2-PYRIDYL)METHYL]-D-VALINOL
The title compound is obtained from the phenol
derivative of Example 18. Step C using the alkylation
procedure described in Example 17, Step C.
CA 02249786 1998-10-26
WO 95101958
- 50 -
PCTIUS94J06376
STEP E:
N (4 N BENZYLOXYCARBONYL-L-VALYL)AMINO-2,2-DIFLUORO-1.3-
_DIOXO 5 (4 {2 N MORPHOLYL~ETHYLOXY)PHENYL-PENTYL]-O-((2-
PYRIDYL)METHYL]-D-VALINOL
The title derivative is obtained from the compound
given in Example 18. Step D using the oxidation method
described in Example 7. Step D.
15
25
35
CA 02249786 1998-10-26
. - PCT/US94106376
WO 95101958
- 51 -
The compounds of the present invention are useful as
inhibitors of retroviral proteases required for
replication. particularly the HIV-1 and HIV-2 viral
proteases, the prevention or treatment of infection by the
human immunodeficiency virus (HIV), and the treatment of
consequent pathological conditions such as the acquired
immunodeficiency syndrome (AIDS) in mammals capable of
being infected with HIV virus. Treating AIDS, preventing
infection by HIV or treating infection by HIV, is defined
as including. but not limited to, treating a wide range of
states of HIV infection: AIDS, ARC (AIDS related complex),
both symptomatic and asymptomatic, and actual or potential
exposure to HIV. For example, the compounds of this
invention are useful in preventing infection by HIV after
suspected past exposure to HIV by, e.g., blood transfusion,
accidental needle stick, or exposure to patient blood
during surgery.
The term "stereoisomers" is a general term for all
isomers of individuals molecules that differ only in the
orientation of their atoms in space. It includes mirror
image isomers (enantiomers), geometric (cis/trans) isomers,
and isomers of compounds with more than one chiral center
that are notmirror images of one another (diastereoisomers).
For amino-acids, the designations L/D, or R/S can be used
as described in IUPAC-IUB Joint Commission on Biochemichal
Nomenclature , Eur. J. Biochem . 138: 9-37 ( 1984 ) .
For these purposes, the compounds of the present
invention may be administered orally, parenterally
(including subcutaneous injections. intravenous, intra-
muscular, transdermal, intrasternal injection or infusion
techniques), by inhalation spray, or rectally, in dosage
unit formulations containing convention non-toxic pharma-
ceutically acceptable carriers, adjuvants and vehicles.
CA 02249786 1998-10-26
WO 95101958
PCT/US94106376
- 52 -
Thus, in accordance with the present invention there is
further provided a method of treating and a pharmaceutical
composition for treating HIV infection and AIDS. The treat-
ment involves administering to a patient in need of such
treatment a pharmaceutical composition comprising a pharma-
ceutical carrier and a therapeutically effective amount of
a compound of the present invention, or a pharmaceutically
acceptable salt thereof.
These pharmaceutical compositions may be in the form of
orally-administrable suspensions or tablets: nasal sprays:
steriel injectable preparations, for example, as sterile
injectable aqueous or oleagenous suspensions or
suppositories) or they may be administered transdermally.
When administered orally as a suspension, these
compositions are prepared according to techniques well
known in the art of pharmaceutical formulation and may
contain ~~_..rocrystalline cellulose for imparting bulk,
alginic acid or sodium alginate as a suspending agent,
methylczllulose as a viscosity enhancer, and
sweetener/flavoring agents known in the art. As immediate
release tablets, these compositions may contain
microcrystalline cellulose, dicalcium phosphate, starch,
magnesium stearate and lactose and/or other excipients,
binders, extenders. disintegrants, diluents and lubricants
known in the art.
When administered by nasal aerosol or inhalation, these
compositions are prepared according to techniques well
known in the art of pharmaceutical formulation and may be
prepared as solutions in saline, employing benzyl alcohol
or other suitable preservatives, absorption promoters to
enhance bioavailability, fluorocarbons, and/or other
solubilizing or dispersing agents known in the art.
CA 02249786 1998-10-26
PCTIUS94l06376
WO 95101958
- 53 -
The injectable solutions or suspensions may be
formulated according to known art. using suitable non-
toxic. parenterally acceptable diluents or solvents, such
as mannitol, 1,3-butanediol, water. Ringer's solution or
isotonic sodium chloride solution, or suitable dispersing
or wetting and suspending agents, such as sterile, bland.
fixed oils. including synthetic mono- or diglycerides, and
fatty acids, including oleic acid.
When rectally administered in the form of
suppositories. these compositions may be prepared by mixing
the drug with a suitable non-irritating excipient, such as
cocoa butter, synthetic glyceride esters or polyethylene
glycols, which are solid at ordinary temperatures. but
liquidize and/or dissolve in the rectal cavity to release
the drug.
Dosage levels of the order of 0.02 to 5.0 or 10.0 grams
per day are useful in the treatment or prevention of the
above-indicated conditions, with oral doses being higher.
For example, infection by HIV is effectively treated by the
administration of from 1 to 50 milligrams of the compound
per kilogram of body weight from one to three times per
day. It will be understood, however, that the specific dose
level and frequency of dosage for any particular patient
may be varied and will depend upon a variety of factors
including the activity of the specific compound employed,
the metabolic stability and length of action of that
compound, the age, body weight, general health, sex, diet,
mode and time of administration, rate of excretion, drug
combination the severity of the particular condition. and
the host undergoing therapy.
CA 02249786 1998-10-26
PCTIUS94106376
WO 95101958
- 54 -
The present invention is also directed to combinations
of the HIV protease-inhibitory compounds with one or more
agents useful in the treatment of AIDS, such as, for
example, with known antiviral agents suitable for treating
HIV 1 and HIV 2 viral infections. e.g.. AZT, with or
without a PNPase inhibitor. or in conjunctive therapy with
DDI and a PNPase inhibitor.
The compounds of this invention may be assayed for
their HIV-protease inhibition using the following published
techniques.
Preparation of Retroviral Enzyme
and
Assay for Inhibition of the Protease
A) preparation of Retroviral Enzyme
To prepare the recombinant protease, the HIV protease
was expressed via E.Coli by the published work of
C. Gu~net, et al.. in European Journal of Pharmacology.
Molecular Pharmacology Section, 172 (1989) 443-451.
H) Assay for Inhibition of Recombinant Viral Protease
Inhibition of the reaction of the protease with a
peptide substrate [Ser-Gln-Asn-Tyr-pro-Ile-Val-NH2.
Km . 1 mM were in 50 mM Na acetate. 10% glycerol. 5%
ethyleneglycol, pH 5.5. at 37°C for 1 hour. Various
concentrations of inhibitor in 10 u1 DMSO were added to
80 u1 of assay solution and the reaction initiated by
the addition of 10 u1 (1.6 ug) of recombinant
protease. The reaction was quenched with 16 u1 of 4 M
perchloric acid. Products of the reaction were
separated by HPLC (VYDAC wide pore 5 cm C-18 reverse
phase. acetonitrile gradient. 0.1% trifluoroacetic
acid). The extent of inhibition of the reaction was
determined from the peak heights of the products. HPLC
of the products. independently synthesized, provided
CA 02249786 1998-10-26
' PCTlUS94106376
WO 95/01958
- 55 -
quantitation standards and confirmation of the product
composition.
By following the techniques referenced above, as well
as by utilization of other known techniques. as well as by
comparison with compounds known to be useful for treatment
of the above-mentioned disease states, it is believed that
adequate material is available to enable one of ordinary
skill in the art to practice the invention.
As is true for most classes of compounds found to be
useful in the pharmaceutical industry, certain subgeneric
groups and certain specific compounds are more preferred
such as those exemplified and shown in the following chart.
Within the concepts of this invention, it is to be found
that the preferred compounds are those wherein R5 is
CH(Y)(Z) and P1 is B. especially when T' is H.
25
35
CA 02249786 1998-10-26
PCTlUS94106376
WO 95!01958
- 56 -
z
z
V V
v
v Q
O
..
N
C
N N ~ x
4l
~ L
_
x ~ O E
o ~ _.
N N
N G '13
d
w
1 ~, O4
1
c~
v
1
1.~ ~ ~ d
C1
Q7
x
x x x o
0 0
N
N N ~ G
C
.a
CA 02249786 1998-10-26
WO 95101958 - 5 7 -
z z
Oz Oz
0 0 0 0
n _
.a ~-
C~r ~ O G
r~
?~ ?~ N N
N
C
x x p o
o r,
>, ~, t t
s
E y E
E
E
~!3 ,C ~C
w
>. a a
a a
N
v
.r
.r r.r I 1
V' Q' d' Q'
r-1
"' p, CL
C' a Q O
O O ~ d
p O
O O ~ Ql
x x x x
p p p O
N
N
C ~ ~ y
C1
PCTIUS94106376
CA 02249786 1998-10-26
PCTIUS94l06376
WO 95101958 - 5 8 -
z z
Oz 0 O Oz
u,
0
., O O O
Cd
I~ ~ O
v
i r
'1
?~ ?~ N N
N N C O
x x O O
>' t t
, t t y
E
.,., .~ y, .
N
( N a
t f'~1
N V ...
v 'r .r
,.r
r r
d' ~'
a a y
O O o 0
w ~ t
O ~ i~
..,
ri
t y ',, >,
O x x
a ~ .. 0 0
'O N
.'1 C
r 1r
I
I
C1
CA 02249786 1998-10-26
PCT/US94106376
WO 95101958 _
59 -
s z s
U Q
v1
p O O O
0~r O O O O O
N N
N C C
C O
O ~ .a
N ~ x
x
C O O O
d
t S
y
p ~ y
~ d
E E
N
C ,~ ~p 't7
Cl .".~ ..1 '.v
j7 y 4r 1r
V
N
v ...
V
V
~1
O
O
x x x x
O
0
04 ~. a,
N N N
C C C
O ~ 4J
CA 02249786 1998-10-26
PCTIUS94106376
WO 95101958 - 60 -
m Ox
,a
N
N
C
41 .C
~ ~
>,
?~ N x
x O d o
,.
O i
.u x
W E ~ '-~ E
,. --
r1 N
'C d ...
w
1~1 v
'1 ~ 1
01 A1 ~'
1
M
N
0 ~ o
L L'~ ~ a
0 C C N
d1 ~ ...1
.~i
s s
~ ~ o
x ~
., o ~ .o C
.-~ ~
N Lr 1~
d a
1 1
M M
V V
CA 02249786 1998-10-26
PCTIUS94106376
WO 95101958 - 6 1 -
o ~ o
0 0
C~
O O O O
p O
v
r-1
N N .-~ r,
C C ?~ ?i
°' °' x x
s~ 0 0
x
O ~ y y
o _v
t y ~ ..
o O
_B E O O
..-i .-~ t t
d a
..., ..~ O E
w ''' 6 I
IG~. a Z ~ Z .-1
cat 1 ?. I ?~
!V N N N
,_, ..r .r C ~ C
1 1 1 Ill 1 07
a~ .G er L7
d a CL
O
o ° ~ a
a, o, a, o,
O~ ~ aol i~
.,.~ .,~ ...
'' x
x o
x o
O ~ ~ r,
N N N N
C G G C
O d 4l 4l
.a
CA 02249786 1998-10-26
WO 95/01958 PCT/US94/06376
- 62 -
z
oG 0 Q " z 0
z
o ° 0
m ~ ,~ _""
0 0
N N N N
C G C C
4l N 4l 4J
I x I x I x x
Z~ Z~ Z~ I
Z~
..rI ~'' I ~' I ?r
C~N y N ~ c~ y N ~
I ~ I ~ I ~ I
er .1 ~ ~
t .0C .0G
a L1~ CL CL
4. 1r ar
E E E E
a, .-, ~.,
O y
O u
N
.
u~ m y
., ...,
x O
O O
O
.- ~ ~, >,
~
N N N
N C C C
C G1 ~ y
.D
. ~ CA 02249786 1998-10-26
WO 95!01958 _ 6 3 _ PCT/US94/06376
z zz
t~ 0 0 ~ z
w
m
O ~ o 0
D p D
.M..
~i ~i
N N N N
C C G
a!
,..., ,~ ~ .Q
x x
1
z ,~ z ,~ . z o
z
C4 N ~ I ~ L 1 ~''
_ .t~ N JJ N N .~
~
Cl
~ ri
'C 'O
.~1 ~i -rl .~1
!.a 4 Ir
4l
d a d
~i
CL
s.. ~", ' O
1r
I
iJ
.,i ~"~ G1
x a' >'
x o x x
O .-.i O O
.i
N D N N
C ~ D C
.a al CJ
.a .p