Note: Descriptions are shown in the official language in which they were submitted.
CA 02249955 1998-10-13
CANADA
TO WHOM IT MAY CONCERN:
BE IT KNOWN that we GUILLET, James E., Canadian citizen
of 31 Sagebrush Lane, Don Mills, Ontario, Canada, M3A 1X4
and BURKE, Nicholas A.D., Canadian Citizen of 12 Tally Ho
Road, Dundas, Ontario, Canada, L9H 3M6, have invented
certain new and useful improvements in
GRAFT POLYMERIZATION PROCESS
of which the following is a specification.
CA 02249955 1998-10-13
- 1 -
GRAFT POLYMERIZATION PROCESS
FIELD OF THE INVENTION
This invention relates to graft copolymers, and more
particularly to processes for the preparation of graft
copolymers of controlled molecular weight.
BACKGROUND OF THE INVENTION
Graft copolymers comprise a backbone polymer having a
plurality of polymeric branches attached thereto, at
different sites along the backbone polymer. The backbone
polymer and the graft polymer may be comprised of one or
more monomers, and may be of the same or a different
chemical constitution from one another. By using different
combinations of monomers for the backbone polymer and the
graft polymer, copolymers having interesting combinations
of properties can be prepared. High impact polystyrene is
an example of a graft copolymer, in which a rubbery polymer
such as polybutadiene is used as the backbone polymer and
styrene is grafted thereon as branch chains of polystyrene.
The presence of the rubbery backbone polymer confers on the
resulting high molecular weight product a significantly
increased impact strength, as compared with homopolymeric
polystyrene, so that the resultant product has a
combination of properties derived from the individual
constituents.
Graft copolymers are commonly made by free radical
initiated, solution, suspension or bulk polymerization.
The preformed backbone, is mixed with the grafting monomer
or monomers, and subjected to the action of free radicals
which cause the development of grafting sites on the
CA 02249955 1998-10-13
- 2 -
backbone polymer chains and polymerization of the grafting
monomers onto the grafting sites. Such a process is,
however, random and uncontrolled, except within very broad
ranges. The resulting graft copolymer has a non-
homogeneous composition, with graft chains of widely
varying molecular weight and length, and including non-
grafted homopolymers and copolymers of the grafting
monomers. Whilst such a non-homogeneous polymeric product
is suitable for many applications, there are instances
where a more homogeneous product, of pre-determined
molecular weight and having the graft copolymer (branches)
thereof of generally consistent length and molecular
weight, is desirable.
It is an object of the present invention to provide a
graft polymerization process which allows control over the
length and molecular weight of the graft polymer branches
which are formed.
SUMMARY OF THE INVENTION
The process of the present invention takes advantage
of the properties of stable free radicals in effecting
graft copolymerization onto a preformed polymeric backbone.
In the process, the backbone polymer is caused to react
with molecules of a stable free radical, to attach them to
the backbone, at locations to act as potential grafting
sites. Stable free radicals have the property of existing,
in free radical form, in solution at ordinary room
temperatures for extended periods of time. They do not
react with themselves, or with other oxygen-centered free
radicals, to any significant degree, and hence can exist in
solution in relatively high concentrations. They will,
CA 02249955 1998-10-13
- 3 -
however, readily react with carbon-centered free radicals,
for example, with polymer-centered free radicals.
Thus, the creation of free radical sites on the
backbone polymer of a potential graft copolymer, in
solution or suspension, or on the surface of said polymers,
containing stable free radicals, will cause the stable free
radicals to attach to the backbone polymer at the free
radical sites. These are the potential grafting sites in
the formation of the graft copolymer.
The linkage between the backbone polymer and the
stable free radical is thermally labile. Accordingly, when
the backbone polymer-stable free radical combination is
appropriately heated, in solution or suspension containing
the graft monomer or monomers, the linkage can undergo a
reversible dissociation. Dissociation of the backbone
polymer-stable free radical linkage leaves carbon-centered
free radicals on the polymer which initiate graft
polymerization of the graft monomer or monomers onto these
free radical sites, with the free radical becoming
dispersed on the growing polymer chain end. The free
radical at the distal end of the graft chain will recombine
with the stable free radical, in a step which is the
reverse of the dissociation step. As heating continues,
the stable free radical may be dissociated again, at which
time the graft chain may be extended by the incorporation
of additional monomer units. Repetition of these three
steps (dissociation, addition of monomer, re-association of
stable free radical) during heating leads to slow and
controlled growth of the side chains. The growth of the
graft chains is an example of a stable free radical
polymerization (SFRP). By adjustment of the heating time,
along with appropriate choice of graft monomer
CA 02249955 1998-10-13
- 4 -
concentration, the length and molecular weight of the graft
polymer chain can be controlled. Moreover, substantial
uniformity between the graft copolymer chains as regards
their length and molecular weight can be achieved. The
total molecular weight of the graft copolymer will also
depend on the number of grafting sites per backbone
polymer.
Thus, according to the present invention from one
aspect, there is provided a process for preparing graft
copolymers having graft chains of controlled molecular
weight, which comprises:
preparing a solution or dispersion or solid surface of
a preformed backbone polymer;
creating free radical sites on the backbone polymer;
chemically attaching stable free radicals to said free
radical sites;
effecting controlled graft polymerization at the sites
of attachment of said stable free radicals by changing the
solution conditions so as to cause dissociation of the
stable free radical attachments with formation of free
radical sites at the sites of attachment, in the presence
of a free radical polymerizable monomer;
effecting free radical graft polymerization of graft
monomer onto said free radical sites to form graft polymer
chains, with the stable free radical chemically attached to
the distal end.
CA 02249955 1998-10-13
_ 5 _
BRIEF REFERENCE TO THE DRAWINGS
Figure 1 of the accompanying drawings is a
diagrammatic representation of the scheme for preparing
graft copolymers according to the invention;
Figures 2, 3, 4 and 5 are presentations of the results
of GPC analysis of the various products prepared according
to Example 1 described herein;
Figures 6 and 7 and GPC chromatograms of products of
Example 2 below;
Figures 8 and 9 are GPC chromatograms of products of
Example 3 below;
Figure 10 is a GPC chromatogram of the product of
Example 4 below.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Suitable backbone polymers for the present invention
include substantially any polymer on which carbon-centered
free radical sites can be generated. The polymers include
hydrocarbon polymers including polyethylene, polypropylene,
polystyrene, polybutylene, other polyolefins and the like,
and unsaturated hydrocarbon polymers such as polybutadiene,
polystyrene, polyisoprene; copolymers of hydrocarbon
monomers such as ethylene-propylene copolymers and
ethylene-propylene-dime terpolymers (EPDM), polymers of
vinyl group monomers containing functional groups such as
polyacrylic acid, polymethacylic acid, polyacrylates,
polymethacrylates, copolymers thereof such as
polyethylene-vinyl acetate), carbohydrate polymers such as
CA 02249955 1998-10-13
- 6 -
celluloses, starches, nucleic acids and dextran;
polyesters; polyamides; polypeptides; and the like. One of
the advantages of the present invention lies in the fact
that it can be worked with backbone polymers containing
normally unreactive functional groups.
Free radical sites on the backbone polymers can be
prepared, according to the process of the present
invention, by a variety of different techniques. For
example, they can be prepared radio chemically - the
passage of gamma rays (from a cobalt-60 source for
instance) through a sample will create a number of radical
species. They can be prepared photochemically, e.g. by
irradiation of a suitable compound (benzophenone,
anthraquinone, polymerization photoinitiator) to produce
species (radicals, excited states) capable of H-abstraction
from the polymer chain. They can be produced chemically,
using systems such as Fenton's reagent (Fe2+/H202) and
polymerization initiators (benzoyl peroxide, AIBN, per-
sulfate) to produce radicals capable of hydrogen
abstraction.
The process of the present invention creates the free
radical sites on the backbone polymer in solution or at the
surface of a solid polymer, and chemically attaches stable
free radicals to these free radical sites. Oxygen centered
radicals are preferably used as the reactive radicals in
the chemical process for producing free radicals on the
polymer backbone chain, because stable free radicals react
rapidly with carbon-centered radicals but not with most
oxygen-centered radicals. Accordingly, radicals derived
from compounds such as hydrogen peroxide, benzoyl peroxide
and anthraquinone will be oxygen-centered and less likely
to react with the stable free radical.
CA 02249955 1998-10-13
-
The stable free radicals useful in the present
invention are thus those which can exist in solution for at
least 24 hours in free radical form, without recombining
with one another to any substantial extent. They are
highly reactive with carbon-centered free radicals, but
substantially unreactive with oxygen-centered free
radicals, and accordingly are derived from oxygen-centered
free radical generating compounds themselves. They are
known in the art, and representative ones of them are
commercially available. The most common type of stable
free radicals are aminoxyl radicals (also known as nitroxyl
radicals or nitroxides), examples of which include:
3-aminomethyl-PROXYL (AMP) of chemical formula:
2o NH.~
4-amino-TEMPO (AT), of chemical formula:
N
and
CA 02249955 1998-10-13
- g -
DOXYL, of chemical formula:
o'
I
0
Graftable comonomers for use in the present invention
include substantially any monomer which can be polymerized
by free radical mechanisms, in solution or suspension.
They include styrene, ethylene, propylene, butylene,
butadiene, isoprene, isobutylene, vinyl acetate, acrylic
acid, methacrylic acid, methylmethacrylate, vinyl chloride
and the like, and combinations of 2 or more such monomers.
The process of the present invention, in its preferred
embodiment, proceeds by first attaching to the backbone
polymer a stable free radical compound, by subjecting the
backbone polymer to free radical creating conditions in the
presence of the stable free radical compound. The bond
between the free radical compound and the polymer backbone
so formed is thermally labile, so that, by heating the
solution of the polymer-stable free radical complex above
a temperature of, for example, 110°C, this stable free
radical compound dissociates from the polymer backbone,
leaving carbon-centered free radical site on the polymer.
This takes place in the presence of the graft polymerizable
monomer or monomers, and upon this creation of the polymer
backbone free radical site, graft copolymerization takes
place at that site by the SFRP mechanism described
previously. In common with all other free radical
polymerization processes, the free radical element remains
CA 02249955 1998-10-13
_ g _
at the distal end of the growing graft polymer chain. The
length of the graft copolymer branches is controlled by the
length of time between the elevation in temperature to
split off the stable free radical molecule, and the
subsequent reduction in temperature to render the stable
free radical molecule inactive. By this means, control of
the polymerization time and temperature controls the
copolymer branch length, all such copolymer branches being
substantially uniform in length and molecular weight.
The graft copolymer is subsequently isolated from
excess, unpolymerized monomer by conventional means, e.g.
precipitation.
Since the side chains of the graft copolymer bear a
stable free radical group at the distal end of each chain,
it is possible to further extend the side chain, if so
desired. Treating the graft copolymer under SFRP
conditions will cause lengthening of the side chain where
the new section of the side chain may be composed of the
same monomers) used in the initial grafting process or
different ones.
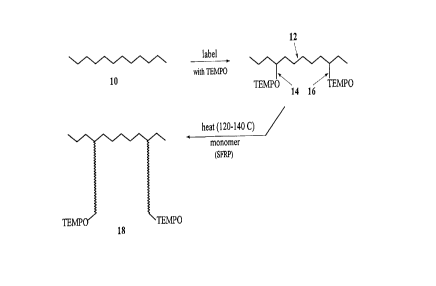
Figure 1 of the accompanying drawings shows
diagrammatically a pre-formed backbone polymer 10 which, in
a first "labelling" step, is treated to create carbon-
centred free radicals in the presence of the stable free
radical compound TEMPO. This produces a "labelled"
backbone polymer 12, with stable free radical TEMPO groups
covalently bonded thereto through thermally labile bonds
14, 16, etc. On heating to a temperature, preferably in
the 120-140°C range, these bonds dissociate creating free
radicals at the positions of dissociation on the backbone
polymer chains. This heating takes place in the presence
CA 02249955 1998-10-13
- 10 -
of monomer, which polymerizes by a free radical mechanism
from the carbon centred free radicals so formed on the
backbone polymer chain. The graft polymerization proceeds
slowly until terminated by re-attachment of the TEMPO
stable free radicals to the growing polymer chains, with
each such graft polymer chain growing substantially evenly
in length, to produce graft copolymer 18.
The process of the present invention is further
illustrated in the following specific examples.
EXAMPLE 1 - PHOTOCHEMICAL TREATMENT OF BACKBONE POLYMERS TO
ATTACH STABLE FREE RADICALS THERETO
Poly(acrylic acid) (90,000 g/mol; 0.102 g), 2,6-
anthraquinone disulfonate, disodium salt (AQDS) (4.3 mg)
and amino-TEMPO (AT) (10.0 mg) were dissolved in 10 ml of
water. The solution was placed in a quartz tube,
deoxygenated by bubbling with nitrogen for 10 minutes, and
then irradiated in a Rayonet RPR-100 photoreactor (16 x 300
nm lamps). Samples (3 mL) taken before irradiation and
after 40 minutes irradiation were adjusted to pH greater
than 7 with NaOH solution or pH 10 buffer before the
addition of 0.6 ml of 0.09% (w/v) fluorescamine in
acetonitrile. The solutions were transferred to dialysis
tubing (Spectra-Por, 12-14,000 molecular weight cut off)
and exhaustively dialysed with water. The purified polymer
solutions were then analysed by UV/visible spectroscopy and
gel permeation chromatography (GPC).
For this analysis, aqueous samples were analysed with
a Waters GPC(U6K injector, 6,000 A pump) equipped with a 30
cm Shodex KB806M column (hydroxylated PMMA packing,
fractionation range 103-10'), a Waters 8401 differential
CA 02249955 1998-10-13
- 11 -
refractometer and an ABI 980 fluoroescence detector
(Applied Biosystems Inc). The fluoroescamine/amine adduct
was excited at 385 nm and a long pass filter (417 or 470
nm) was used to ensure that only emission from the
fluorescamine/amine fluorophore reached the detector.
Uv/visible spectra were measured with a Hewlett-Packard HP
8451A spectrometer.
The same general photochemical procedure was used to
generate polymer-centered radicals on several different
polymers, as follows:
polyethylene oxide, 300 k
poly(acrylic acid), 90 k
dextran, 75 k
poly(sodium 4-styrene sulfate), PSSS, 200 k and 46 k
poly(vinylpyrrolidone), PV, 44k
deoxyribonucleic acid, DNA, ca. 200 k
Following binding of the AT group to the polymer
chain, AQDS and excess AT were removed, fluorescamine was
added and then small molecule impurities were removed by
dialysis or ultrafiltration. Fluorescamine reacts with
primary amines to produce highly fluorescent adducts, so
that it acts as a convenient marker to demonstrate the
coupling of the stable free radical compound to the polymer
backbone.
Figure 2 attached hereto is the GPC chromatogram for
polyacrylic acid irradiated in the Bayonet for 0 or 40
mins. in the presence of AQDS/AT in water, and then reacted
with fluorescamine. A refractive index (RI) peak 20 and a
fluorescence peak 22 are clearly demonstrated for the
irradiated samples, showing successful binding of AT to the
polymeric backbone. In addition, the irradiated sample
CA 02249955 1998-10-13
- 12 -
displays an absorption at 385 nm in the W-visible spectrum
typical of the fluorescamine-amine adduct. This absorption
is absent from the spectrum of the unirradiated sample.
Figure 3 is a similar spectrum of the photochemically-
treated dextran(75,000 g/mol). It shows an essentially
similar result, with an RI peak 24 and a fluorescence peak
26, indicating that the stable free radical AT has
successfully attached to the polymer backbone by a free
radical mechanism, so that the polymers are now appropriate
backbone polymers for grafting according to the present
invention.
Following essentially similar features, poly(sodium
styrene sulfonate) (200,000 g/mol) has been successfully
joined to stable free radical AT, using both AQDS and
AQMTEA (2-anthraquinonylmethyl triethylammonium bromide) as
photochemical H-abstractor, and fluorescamine as
fluorescent marker. The resulting polymers were also
similarly suitable for use as a grafting backbone in the
present invention. Their spectra showed evidence of
binding of the stable free radical compound to the polymer
backbone.
In parallel experiments, various backbone polymers
(polystyrene and PMMA) were photochemically irradiated in
organic solution (benzene) in the presence of
anthraquinone (AQ) or t-butylperoxide ((t-Bu0)2) as free
radical generators, and in the presence of AT. Following
irradiation a fluorescent marker (BODIPY-FL, SE) was added
and spectral evidence of the binding of the stable free
radical compound to the backbone polymer was obtained
(Figures 4 and 5) , showing that they are appropriate for
the use as backbone polymers in graft copolymer preparation
CA 02249955 1998-10-13
- 13 -
according to the invention. Peak 28 on Figure 4 is from the
RI signal, peak 30 on Figure 4 from the fluorescence
signal. Peak 32 on Figure 5 is from the RI signal, peak 34
from the fluorescence signal. The absence of evidence for
degradation or cross-linking of the polymers in Figures 2-5
is also noteworthy.
In addition, polypropylene membranes immersed in
benzene solutions of AT and either 2-methylanthraquinone or
t-butyl peroxide were irradiated (300 nm). The membranes
were washed thoroughly, reacted with BODIPY-FL, SE (4,4-
difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-
propionic acid, succinimidyl ester; (Molecular Probes), and
then washed again. Fluorescence spectroscopy (SLM 4800S
spectrofluorimeter) revealed BODIPY-FL emission (AeX = 495
nm; ?1em - 512 nm) from the surface of the irradiated
membranes but not from control samples. This demonstrates
that AT was bound to the surface of the membranes and that
it is possible to prepare solid polymer surfaces so that
they are suitable for grafting. Since this is done
photochemically it leads to the possibility of making graft
polymer images on plastic films. These could be doped to
provide electrical conductivity and other electro-optical
properties to the photoinduced patterns.
EXAMPLE 2 - RADIOCHEMICAL TREATMENT OF BACKBONE POLYMERS TO
ATTACH STABLE FREE RADICALS THERETO
Polystyrene (280,000 g/mol; 0.169 g) and AT (0.256 g)
were dissolved in 25 mL benzene. 5 mL of this solution was
transferred to each of four Pyrex tubes. The samples were
degassed by three freeze/pump/thaw cycles before the tubes
were sealed. The samples were exposed to 0, 0.5, 1 or 2
Mrads of Y-rays from a 6°Co source. The polymers were
CA 02249955 1998-10-13
- 14 -
precipitated in methanol, filtered, washed with methanol
and then air dried. Each polymer sample was dissolved in
benzene (1 mL) before the addition of 1 drop of pH 10
buffer and 100 JCL of 12.8 mM BODIPY-FL, SE in DMSO. The
solutions were mixed on a vortex mixer and then stored at
room temperature for 1 hour. The polymer was precipitated
in methanol (50 mL), filtered, washed with methanol and
then purified by a second precipitation from benzene into
methanol. The polymer samples were dried in a vacuum oven
(45°C for 2 hours) and then dissolved in THF prior to
analysis by GPC.
The organic soluble samples were analysed using a
Waters GPC (U6K injector, 510 pump) equipped with two 30-cm
Zorbax PSM columns (60S and 10005), a Waters 8401
differential refractometer and an ABI 980 fluorescence
detector. BODIPY-FL was excited at 500 nm and a 515 nm
long pass filter was used to filter the emission.
The exposure of polymers to gamma rays results in the
creation of polymer-centered radicals, as is well known.
Using GPC analysis, it was shown (Figure 6) that the
polystyrene had been successfully attached to AT, following
exposure to gamma rays. Similar experiments were conducted
using as starting polymers poly(N-isopropylacrylamide)
P(NIPAM), polyacrylic acid and DNA. Figure 7 appended
hereto is the GPC chromatogram for the polyacrylic acid
experiment described above, the different traces
representing different exposure to gamma rays. The Figure
shows that the polymer bears flurophores indicative of
attachment of AT and that the degree of attachment is
proportional to the dose of radiation.
CA 02249955 1998-10-13
- 15 -
EXAMPLE 3 - THERMAL TREATMENT OF BACKBONE POLYMERS TO
ATTACH STABLE FREE RADICALS THERETO
In these experiments, chemical radical generating
systems are used, such as peroxy compounds, for example
hydrogen peroxide, persulfate, benzoyl peroxide, peroxide
nitrite, which decompose thermally to produce radical
species capable of creating radicals on the polymer chain
by H-abstraction or addition. Polymer-centered radicals so
formed, are efficiently scavenged by the spin trap provided
by the stable free radical compound.
Three aqueous solutions were prepared by combining 20
,uL of a 1.02% DNA solution (Sigma, single-stranded,
denatured, co-migrates with 587-831 base pair marker
fragments) and 400 E.cL of a 39.6 mM amino-TEMPO solution.
The solutions were bubbled with nitrogen for 10 minutes and
then 80 ,uL of hydrogen peroxide (0.75%, 3%, or 6%) was
added with vigorous stirring. The samples were maintained
at room temperature for 80 minutes.
The samples were purified by ultrafiltration in a
microcentrifuge. The samples were transferred to
centrifuge tubes equipped with ultrafiltration inserts
(Gelman NanoSpin Plus, 30000 Molecular V~leight Cut-Off).
The samples were spun in a microcentrifuge (IEC MiniMax) at
10,000 rpm until the sample volume was reduced to about 20
~L. The retenate (which includes the polymer) was washed
twice by adding water (500 ,uL) and spinning in the
microcentrifuge until the retentate volume was about 20,uL.
To the retentate in each ultrafiltration insert was added
100 ,uL of water, 75 /..iL of pH 10 buffer and 60 E.cL of 11.5 mM
fluorescamine in acetonitrile. After 10 minutes the
solution volume was reduced to about 20 ~L by spinning in
CA 02249955 1998-10-13
- 16 -
the microcentrifuge. The retentate was washed 2 times with
water. The final retentates were diluted with water to
give a total volume of about 400 ~L. The solutions were
analysed by GPC as described in Example 1. The GPC data,
shown in Figure 8, demonstrates the occurrence of efficient
binding of AT to DNA which is proportional to the amount of
hydrogen peroxide used, and that binding is achieved
without degradation or cross-linking of the DNA. On Figure
8, curves 36 are from RI, curves 38 from fluorescence
detectors.
Radical generating systems used include hydrogen
peroxide (H202) , Fenton's reagent (H202/Fe2+) ,
persulfate/heat, persulfate/tetramethyl-ethylenediamine and
t-butylperoxide/heat. Specific individual polymers treated
in this way with one or more of these systems were
polyacrylic acid, poly(sodium styrene sulfonate), dextran,
poly(vinylpyrrolidine), poly(2-vinylnaphthalene), and
polyethylene oxide. Upon analysis as described, they
exhibited fluorescence indicating successful attachment of
the stable free radical compound to the polymer, indicating
that the resultant polymer is suitable for use as a
backbone polymer in the graft polymerization process of the
present invention. For instance, Figure 9 shows the GPC
chromatogram for poly(2-vinylnaphthalene) (100,000 g/mol)
after heating (130°C, 2.5 h) in the presence of t-
butylperoxide/AT in t-butylbenzene, and then reaction with
BODIPY-FL, SE. The treated polymer displays a strong
fluorescence peak 40 which is absent from the untreated
polymer. Curves 42 are from RI detection.
CA 02249955 1998-10-13
- 17 -
EXAMPLE 4 - GRAFT COPOLYMERIZATION
PSSS (46,000 g/mL, MW standard) attached to stable
free radical compound AT through the action of AQMTEA/AT/hv
and purified by centrifugal ultrafiltration, as described
in Example 3 above, was mixed in the amount of 10 mg with
sodium styrene sulfonate (950 mg) and 80°s ethylene glycol
(4 mL) in a small two-necked flask. The solution was
deoxygenated by bubbling with argon for 30 minutes and then
heated in an oil bath at 135°C for 5 hours under an argon
atmosphere. The polymer was precipitated in
acetone/methanol (3:1) and then further purified by
centrifugal ultrafiltration. The polymer was reacted with
fluorescamine as described above and then purified by
centrifugal ultrafiltration prior to analysis by GPC.
This experimental process is illustrated
diagrammatically in Figure 1 of the accompanying drawings.
The purified graft copolymer from this Example was
subjected to GPC analysis, using both fluorescence (F) and
refractive index detectors, and the results are shown in
Figure 10 of the accompanying drawings. Curves 44 (chain
dots, consistent with earlier Figures) are from
fluorescence detectors, curves 46 (solid lines, consistent
with earlier Figures) are from RI detectors. The polymer
gives a fairly sharp RI peak and elutes 1.5 minutes earlier
than the ungrafted PSSS sample. Comparison with a
calibration curve based on PSSS standards indicates a
molecular weight increase from 46,000 to about 230,000
g/mol. GPC analysis of the ungrafted PSSS showed that each
chain bore, on average, four AT groups, and thus, the
molecular weight increase upon grafting corresponds to
about 46,000 g/mol per graft on average. A strong
CA 02249955 1998-10-13
- 18 -
fluorescent peak which coincides with the RI peak is also
observed. This shows that the higher molecular weight
polymer bears AT groups and that the high molecular weight
polymer is the result of grafting and not SSS auto-
s polymerization. Each grafted chain is believed to be
capped with an AT group which might be used as a site to
further extend the side chains with SSS or another monomer.