Note: Descriptions are shown in the official language in which they were submitted.
CA 02250397 1998-09-28
WO 97/35537 PCT/GB97/00838
Title: Improvements in or relating to Encapsulation
Field of Invention
This invention concerns encapsulation and relates to a method of
encapsulation,
encapsulation apparatus and the resulting capsules.
Background to the Invention
The provision of water soluble and digestible capsules containing
pharmaceutical or
cosmetic preparations is well established. Typically oils are encapsulated in
gelatin shells
designed to release their contents when subjected to immersion in water or
exposure to
digestive juices. These oils include dietary enhancement substances or, in the
case of
cosmetic preparations, fragrant oils for release into bath water. A
substantial industry has
been built up around this principle, based primarily on the use of gelatin for
the capsule
shells. This gelatin is derived from the bones and skins of animals.
With concern for the environment and animal welfare and fear of animal related
diseases
such as Bovine Spongiform Encephalopathy (BSE) increasing, the number of
individuals
adopting a serious stance on the use of animals and animal-derived products in
food
substances and cosmetic applications has risen dramatically. As a result, the
sales of such
gelatin capsules are very limited among such individuals. There exists
therefore the need
for the provision of a suitable substitute for gelatin in order to provide
water soluble or
digestible capsules which are not derived from animals. Whilst this is a
desirable aim,
few materials lend themselves to such use and the machinery currently creating
such
capsules has been specifically designed to suit the properties of gelatin. As
a result, a
change in material for the capsule shell requires a redesign of the machinery
if it is to
have the capability of efficiently producing capsules from the replacement
material. It is
a change in material and the commensurate machine requirements needed to
enable
successful processing which this invention addresses.
CA 02250397 1998-09-28
WO 97/35537 _ PCT/GB97/00838
2
Summary of the Invention .
In one aspect, the present invention provides a method of encapsulation,
characterised by
supplying to an encapsulation unit two films of like material capable of
deforming
elastically at least when partially solvated, and applying solvent to at least
one of the films
prior to encapsulation to cause partial solvation of the material surface,
such that the
partially solvated surface can adhere to and seal with the film material.
In the encapsulation unit, the substance to be encapsulated is supplied
between the films,
the films are formed, typically by a moulding process, into suitably shaped
capsule
portions which can adhere to each other as a result of the adhesive action of
the partially
solvated surface(s), and which seal together encapsulating the supplied
substance, forming
a capsule.
In a further aspect, the invention provides a method of encapsulation,
comprising
supplying two films of like material capable of deforming elastically at least
when partially
solvated; applying solvent to at least one of the films to cause partial
solvation of the
material surface; supplying substance to be encapsulated between the films;
forming the
films into suitably shaped capsule portions which can adhere to each other as
a result of
the adhesive action of the partially solvated surface(s); and sealing the
capsule portions
together, encapsulating the supplied substance, to form a capsule.
Conventional gelatin encapsulation relies upon heat as the mechanism for
sealing the two
portions of the shell together to enclose the contents. The capsules made by
this invention
do not use heat as the primary means of securing the two portions of the
capsule together,
but instead make use of the adhesive effects manifested when suitable films
are partially
solvated at their surface.
The films may be of a range of different materials. Suitable materials soluble
in water
(hot or cold) include polyvinyl alcohol (PVA), alginate, hydroxypropyl methyl
cellulose
and polyethylene oxide. In this case it is simply necessary to apply water at
a suitable
temperature to the film or films to cause partial solvation. The resulting
capsules release
CA 02250397 1998-09-28
WO 97/35537 PCT/GB97/00838
3
their contents when immersed in water or exposed to digestive juices and thus
lend
themselves to such uses as the release of fragrant -oil in a bath or the
release of dietary
supplements after ingestion. If the material is only soluble in hot water then
it is
necessary to apply water at appropriately elevated temperatures, but the
partial solvation
and the subsequent adhesive effects are still effective to seal the capsule.
Non-water soluble film materials may also be used, such as polycaprolactone
and
gelatinized starch based materials. In this case it is necessary to apply a
suitable solvent
such as N-methyl pyrrolidone rather than water to at least one film surface to
induce
partial solvation. The partial solvation of such films causes them to soften,
enabling them
to take up the internal dimensions of a mould used to create a capsule.
Capsules made
from films which are biodegradable but not water soluble release their
contents as a result
of microbial action instead of immersion in water, and can fmd use in
agricultural and
industrial applications.
The currently preferred film material is PVA, preferably plasticised with
glycerin.
Suitable films are commercially available in a range of different grades,
types and
thicknesses. An appropriate film can be readily selected having regard to the
intended
use, capsule contents and desired capsule properties. For example, PVA film is
available
in thicknesses ranging between 20 and 1000 microns. For cosmetic applications,
good
results have been obtained with 80 micron PVA film, eg Hi-Selon (Hi-Selon is a
Trade
Mark) cold water soluble PVA B9, obtainable from British Traders and Shippers,
429-431
Rainham Road South, Dagenham, Essex.
It is preferred to use film material that becomes more flexible when in
partially solvated
conditions as this assists capsule formation. PVA has this property.
Instead of using pre-formed films, the films may be formed during the
encapsulation
method, eg by being cast from solution.
In practising the invention it is appropriate to use two films of like
material. The films
should be chemically alike but need not be identical in terms of factors such
as grade,
CA 02250397 2005-03-08
4
thickness etc.
The solvent is selected having regard to the film material, and is
conveniently water in the
case of water soluble materials. The solvent should be applied in an
appropriate amount,
either in isolation or as pan of a formulation containing materials such as
thickening
and/or wetting agents, to cause a suitable degree of partial soIvation of the
film material
surface: this can be readily determined by experiment.
The solvent is preferably applied just prior to encapsulation at an
appropriate location to
obtain optimum speed of capsule production.
The solvent can be applied in a variety of different ways, including by
atomisation such
as in the form of a spray or jet, by dipping, electrostatic coating, roller,
air knife or
Meyer bark or with a sponge. The currently preferred technique is by means of
a gravure
or flexo printing process as this enables ready control and regulation of the
amount and
uniformity of solvent application.
Solvent may be applied to one or both films as appropriate.
A vacuum is conveniently applied during capsule portion formation to assist
deformation
of the film material.
The invention may be used to encapsulate a wide range of substance in the form
of solids,
liquids or gases. The invention may, for example, be used to encapsulate all
of the
substances currently encapsulated in gelatin, such as drugs, vitamins,
powders, oils,
cosmetic preparations, drug delivery systems, paint etc. A typical cosmetic
application
is encapsulation of bath oils to produce capsules intended to be used in the
bath, where
the capsule shell dissolves releasing the oil into the bath water.
The capsules may have a variety of different sizes and shapes, usually
determined by the
shape of the mould employed. Typically the capsules are spherical or oval, but
more
elaborate forms eg based on fruit, animal or abstract shapes may be produced,
usually for
CA 02250397 1998-09-28
WO 97/35537 _ PCT/GB97/00838
cosmetic applications.
In a further aspect the invention provides encapsulation apparatus comprising
means for
supplying two films of material to an encapsulation unit; an encapsulation
unit; and means
for supplying a solvent for the film material to at least one of the films
upstream of the
encapsulation unit.
The encapsulation unit may be based on those used in conventional apparatus
currently
used for gelatin encapsulation. In typical conventional apparatus, two
separate ribbons of
gelatin film are first produced by pouring heated liquid gelatin at a
controlled rate onto
the peripheral faces of two cylinders each rotating about a horizontal axis.
The liauid
gelatin cools on the cylinders and forms two ribbons which are fed from
opposed sides to
an encapsulation unit.
The encapsulation unit typically comprises a pair of similar moulding drums.
The outer
cylindrical face of each drum is formed with a plurality of indentations of
desired form,
eg hemispherical, arranged in a series of axially extending rows with, say, S
or 6
indentations in each row. The drums are supported in side by side
relationship, with a
small gap there between, and are arranged for coordinated rotation in opposed
directions
(the left hand drum clockwise, and the right hand drum anticlockwise). A
similar
arrangement may be used in the present invention. Means for applying a vacuum
inside
the drums are conveniently included, to help pull the partially solvated films
into the
indentations and so assist capsule portion formation.
The encapsulation unit typically also comprises a reservoir of the substance
to be
encapsulated, eg bath oil, and an associated supply arrangement adapted
simultaneously
to supply a plurality of metered doses (one for each indentation in a row on
the moulding
drums) of the substance to the moulding drums at predefined time intervals.
The
arrangement may employ syringe pumps or the like. Again, a similar arrangement
may
be used in the present invention.
The metered doses are initially supplied to a heated injection segment located
above the
CA 02250397 1998-09-28
WO 97/35537 _ PCT/GB97/00838
6
nip between the moulding drums, and including a row of a plurality of
injectors aligned
with the rows of indentations in the drums. A similar arrangement may again be
used in
the present invention although there is no need for the injection segment to
be heated.
In use in conventional encapsulation, two gelatin ribbons are formed and fed
over
appropriate guide rollers etc to pass below the injection segment and into the
nip between
the counter-rotating rollers. Metered doses of the substance to be
encapsulated are
injected into the nip in synchronism with the drum rotation. The heating
segment also acts
to heat the gelatin filins, which has the effect of making the filins capable
of sealing to
each other and also makes the films more elastic. As the doses of substance
are injected
between the heated films, the films deform to Iine the indentations, forming
series of pairs
of opposed capsule halves containing the substance. The pairs of capsule
halves are
brought together, sealed and cut from the gelatin ribbons on continued
rotation of the
drums, thus forming capsules containing the substance. A typical production
rate is one
row of capsules every 2 seconds. Instead of cutting the capsules from the
ribbons, they
may be left integral with the ribbons. The resulting capsules are collected
below. The
capsules are then typically tumbled in a hot air dryer and then kept in a
controlled
humidity environment for about 2 days to stabilise the capsules. The capsules
are then
ready for use or sale.
As noted above, the present invention may use an encapsulation unit generally
as
described. It is not necessary for the injection segment to be heated, as the
present
invention does not rely on heating for sealing, as in the prior art, so
processing costs may
be reduced somewhat and faster processing may be possible. However, the films
of the
present invention may optionally be heated: in some cases this may enhance
film elasticity
and sealing.
Conventional encapsulation apparatus would, of course, also need modification
by removal
of the gelatin ribbon formation equipment and substitution of equipment for
supplying (and
possibly also forming) the films of material used in the present invention. In
a simple case
this could just be a pair of spindles each for receiving a roll of the film,
to be fed to the
encapsulation unit in known manner.
CA 02250397 1998-09-28
WO 97/35537 PCT/GB97/00838
7
A further necessary modification is addition of mean for applying solvent to
one or both
of the films, preferably located just upstream of the encapsulation unit. As
noted above
these means could be a spray or jet arrangement, a bath for dipping, an
electrostatic
coating unit, a roller, an air knife, a Meyer bar, a sponge etc. Preferably,
however, a
gravure or flexo printing unit is used.
Means for applying a vacuum within the moulding drums are conveniently also
incorporated.
The invention also covers capsules formed in accordance with a method or by
use of
apparatus in accordance with the invention.
The invention also includes within its scope a capsule having a shell
comprising material
capable of adhering to and sealing with itself when in partially solvated
condition.
In a preferred aspect the invention covers a capsule having a shell comprising
polyvinyl
alcohol.
The invention will further be described, by way of illustration, in the
following Example
and with reference to the accompanying drawing, in which:
Figure 1 is a schematic illustration of one embodiment of apparatus in
accordance with the
invention.
Detailed Description of the Embodiment
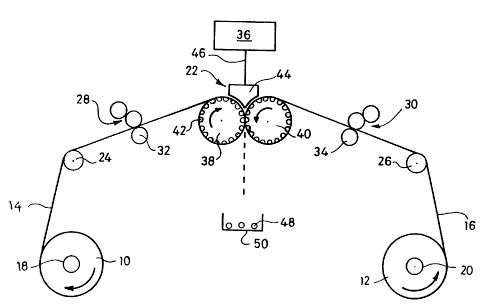
The apparatus illustrated in Figure 1 comprises two rolls 10, 12 of film 14,
16 of like
material rotatably supported on spindles 18, 20, with associated means (not
shown) for
feeding film from the rolls to an encapsulation unit 22. The films first pass
over respective
support rollers 24, 26 and then through respective flexographic printing units
28, 30 with
associated backing rollers 32, 34 for supply to a surface of the film, in an
adjustable
manner, of accurately metered quantities of solvent for the film material. In
an
CA 02250397 1998-09-28
WO 97/35537 PCT/GB97/00838
8
experimental apparatus, laboratory scale narrow flexographic heads from RK
Print Coat
Instruments Limited, Litlington, Royston, U.K. were used for this purpose.
The encapsulation unit 22 is based on the encapsulation unit of conventional
apparatus, as
discussed above, and comprises a reservoir containing the substance to be
encapsulated and
an associated supply arrangement for supplying metered doses of the substance.
The
reservoir and supply arrangement are represented schematically at 36.
The encapsulation unit further comprises a pair of similar moulding drums 38,
40. The
outer cylindrical face of each drum is formed with a plurality of
hemispherical indentations
42 arranged in a series of axially extending rows with 6 indentations in each
row.
Vacuum means (not shown) may optionally be included for applying a vacuum
inside the
drums to assist deformation of the film material. The drums are supported in
side by side
relationship with a small gap therebetween, and are arranged for coordinated
rotation in
opposed directions (the left hand drum 38 clockwise, and the right hand drum
40
anticlockwise). An injection segment 44 is located above the nip between the
moulding
drums to receive substance from reservoir and supply arrangement 36, as
illustrated
schematically by line 46. Injection segment 44 includes an array of 6
injectors (not
shown) aligned with the rows of indentations in the drums.
In use, film 14, I6 is supplied at an appropriate rate to the encapsulation
unit 22, passing
over support rollers 24, 26 and through printing units 28, 30 where solvent is
applied to
the film surface in appropriate amount. The films then pass below the
injection segment
44 and into the nip between drums 38, 40 which are counter-rotating at an
appropriate
speed. Metered doses of the substance to be encapsulated are injected into the
nip from
injection segment 44 in synchronism with the drum rotation. As the doses of
substance
are injected between the films, the films deform to line the indentations 42
of one row in
each of the drums, possibly assisted by application of a vacuum, forming a
series of 6
pairs of opposed capsule halves containing the substance. On continued
rotation of the
drums the pairs of capsule halves are brought together and seal because of the
adhesive
effect caused by partial solvation of the film surface, producing a row of
surface-
containing capsules which are cut from the films. One row of 6 capsules is
produced
CA 02250397 1998-09-28
WO 97/35537 PCT/GB97/00838 _
9
approximately every 2 seconds. The resulting capsules 48 are collected in a
tray 50, and
the waste film remaining is disposed of. The capsules are dried and stabilised
in generally
conventional manner.
Example
Using the apparatus of Figure 1 encapsulation of a typical bath oil cosmetic
product was
carried out using Hi-Selon cold water soluble plasticised polyvinyl alcohol
(B9) flm, 80
micron thick, with partial solvation carried out by application of water. This
resulted in
production of good quality capsules, suitable for cosmetic use.