Note: Descriptions are shown in the official language in which they were submitted.
CA 022~1068 1998-10-22
065.6719 PATENT
SECONDARY LITHIUM ION CELL
BACKGROUND OF THE INVENTION
Field of the Invention
The invention pertains to a secondary lithium ion cell with good current loadability and a low
capacity loss after storage at high temperature.
Description of the Related Art
U.S. Patent No. 5,472,809 teaches a lithium ion cell in which a ternary organic solvent mixture
consisting of 5-40 vol.% propylene carbonate (PC), 10-20 vol.% ethylene carbonate (EC), and 50-85
vol.% dimethyl carbonate (DMC) with a conducting salt containing lithium ions dissolved in it is used as
the electrolyte. As conducting salts the following are proposed: lithium hexafluoroarsenate (LiAsF6),
lithium hexafluorophosphate (LiPF6), lithium tetrafluoroborate (LiBF4), lithium perchlorate (LiCI04),
lithium trifluoromethane sulfate (LiCF3SO3), lithium bis(trifluoromethanesulfone)imide (LiN(CF3SO2)2),
or lithium bis(trifluoromethanesulfone)methide (LiC(CF3SO2)3), or mixtures of these. The above noted
electrolytes are said to assure above all the dischargeability of lithium ion cells at low temperatures.
European Patent No. EP-A 643433 teaches that an electrolyte c~-nt:~ining LiPF6 can be produced
for lithium ion cells by reacting ammonium hexafluorophosphate (NH4PF6) in a mixture consisting of 20
vol.% PC, 30 vol.% EC, and 50vol.% diethyl carbonate (DEC) with lithium hydride.European Patent No. EP-A 312236 discloses as the electrolyte for nonaqueous cells a solvent
mixture consisting of EC, PC, and a polyethylene glycol dialkyl ether. As a reference example, a solvent
mixture consisting of 40 mol.% PC, 40 mol.% EC, and 20 mol.% DEC is reported.
With consideration of the useful capacity from cells which contain the above-mentioned
electrolyte or their loadability, however, the need exists for additional improvement. The loadability in
this case refers to the discharging of the cells with high current intensities or current densities.
SUMMARY OF THE INVENTION
The invention is directed to a secondary lithium ion cell having good high-temperature storage
stability and improved loadability. Lithium ion cells with an electrolyte according to the invention have
a lower capacity loss after storage at 60~C over a time span of 7 days. In this case, the useful capacity
from the cells produced according to the invention, as well as after storage in the charged as well as after
storage in the uncharged state, is higher than that of reference cells. In addition, the cells according to
the invention have higher loadability, which is manifested in the higher useful capacity at greater
discharge current intensities. It was also found that the lithium ion cells according to the invention,
FP 585-US - 1 - 065.6719 PATENT
CA 022~l068 l998-l0-22
despite discharging down to 0 V, can be charged and discharged more than
100 times before the useful capacity drops to a value of c 70% of the
initial capacity. The cells according to the invention therefore have a
very good deep discharge strength.
The present invention is a secondary lithium ion cell comprising a
carbon-containing anode material, a lithium-cont~;n;ng cathode material,
and a nonaqueous electrolyte comprising a solution of a lithium salt in an
organic solvent mixture which contains 20 to 40 vol.% ethylene carbonate,
15 to 30 vol.% diethyl cArhon~te, and 35 to 55 vol.% propylene carbonate.
In certain embodiments,
o The solvent mixture comprises 25 to 35 vol.% ethylene carbonate, 20 to
25 vol.% diethyl carbonate, and 40 to 50 vol.% propylene carbonate,
o The electrolyte cont~;n~ 0.9 - 2.0 mole/l LiCl04, LiPF6, LiSo3CF3,
LiBF4, LiN(CF3Sol)3, or LiC(CF3So2)3, and preferably 0.9 - 1.5 mole/l
LiCl04 or LiPF6,
o The carbon-cont~;n;ng anode material is provided with a metal conduc-
tor matrix, wherein the metallic conductor matrix is a stainless steel
band, a nickel stretched metal, or a nickel foam, and/or
o The lithium-containing cathode material is a lithiated pyrolusite or a
lithium-manganese spinel.
BRIEF DESCRIPTION OF THE DRAWINGS
Other aspects, features, and advantages of the present invention will
become more fully apparent from the following detailed description, the
appended claims, and the accompanying drawings in which:
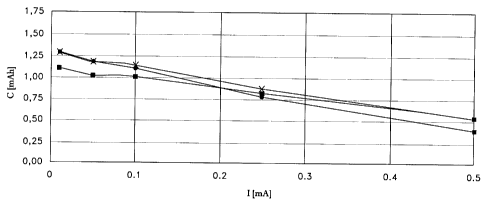
Fig. 1 compares the useful capacities from the cells according to
Example 1(-), Example 2(x), and Reference Example 1(~) as a function of the
discharge current intensity;
Fig. 2 shows the capacities in cells according to Example 1 charged
(-) and discharged as a function of the cycle number (z), where the broken
curve (~) represents the discharged capacity up to a final discharge
voltage of 1.8 V and the dotted curve (x) shows the additionally discharged
capacity for a discharge down to 0 V;
Fig. 3 shows the charged and discharged capacities for cells according
to Example 2;
Fig. 4 shows the discharge capacity of the cells according to Example
3 (x) and Example 4 (-) as a function of the cycle number (z); and
Fig. 5 shows an exemplary button cell for the present invention.
DETAILED DESCRIPTION
Example 1
A graphite anode pasted in nickel foam (diameter 4.20 mm, height 0.71
mm), a 200-~m thick polypropylene separator, and a lithium oxide-cont~;n;ng
cathode are installed in a button cell with an outer diameter of 6.8 mm and
a height of 2.1 mm. 18 ~l of a 1-molar solution of LiCl04 in an organic
CA 022~1068 1998-10-22
solvent mixture consisting of 30 vol.% EC, 20 vol.% DEC, and 50 vol.% PC is added as the electrolyte.
The cells are closed and tested for their capacity before and after high-temperature storage, their
loadability, and their deep dischargeability.
Example 2
Button cells are produced according to Example 1 with the difference that LiPF6 is used as the
conducting salt.
Reference Example 1
Button cells produced as in Example 1 contain as the electrolyte a 1-molar solution of LiCI04 in
a solvent mixture consisting of 25 vol.% EC, 10 vol.% DEC, and 65 vol.% ethyl methyl carbonate
(EMC).
Example 3
lS A round of lithium foil 0.31 mm thick and a graphite anode pasted in nickel foam are inserted in
a button cell housing with a diameter of 6.8 mm and a height of 2.1 mm. A 200-11m thick polypropylene
separator is used as the separator, and a lithium spinel-containing electrode is used as the cathode. The
electrolyte consists of a 1-molar solution of LiPF6 in an organic solvent mixture consisting of 30 vol.%
EC, 20 vol.% DEC, and 50 vol.% PC.
Example 4
Button cells are produced as in Example 3 except that a 0.25-mm thick lithium foil is used.
Reference Example 2
Button cells are produced as in Example 4, but a 1-molar solution of LiPF6 in 15 vol.% EC, 50
vol.% DMC, and 35 vol.% PC is used as the electrolyte.
As Fig. 1 shows, the cells produced according to the invention have a higher useful capacity even
at greater discharge current intensities. Figs. 2 and 3 show the surprising deep discharge strength of the
cells according to the invention. In Table 1, the useful capacities according to the invention and the
reference examples are contained, where n.d. = not determined. A comparison of the relative capacity
loss after a high-temperature storage shows that the cells according to the invention suffer lower
irreversible capacity loss.
FP 585-US -3- 065.6719 PATENT
CA 022~1068 1998-10-22
Table 1. Discharge capacity C in [mAh]
Trialwithout HT after HT direct HT relative
storage storagestorage C
No. 1st 2nd 1st 2nd 1st 2nd loss [%]
cycle cycle cycle cycle cycle cycle
Example 11.142 1.136 0.906 0.898 0.891 0.881 20.95
S Example 21.161 1.154 0.992 0.997 1.032 1.008 13.60
Ref. ex. 1 1.194 1.192 0.470 0.171 0.470 0.102 85.65
Example 31.247 1.202 0.757 0.822 0.891 0.916 31.61
Example 41.112 1.122 0.636 0.732 0.663 0.732 39.76
Ref. ex. 2 1.410 1.402 0.364 0.493 n.d. n.d. 64.84
It will be further understood that various changes in the details, materials, and arrangements of
the parts which have been described and illustrated in order to explain the nature of this invention may
be made by those skilled in the art without departing from the principle and scope of the invention as
expressed in the following claims.
FP 585-US -4- 065.6719 PATEI~IT