Note: Descriptions are shown in the official language in which they were submitted.
CA 02251267 2004-09-10
25259-49
1
Improved medical containers
h'ield of invention
The present invention relates to improved openings for
medical containers, especially suitable for containers storing
parenterally administerable fluids which preferably should be
sterilized by steam after being finally assembled filled and sealed.
Background of the invention
For manufacturers of parenteral fluids who wish to replace
the traditional glass containers, it has been a highly demanding
problem to find a polymeric material capable of withstanding
autoclavation and yet be able to meet the often rigorous requirements
set on its oxygen barrier and water vapor barrier capacities. Especially
when sensitive fluids for use in parenteral nutrition, such as Lipid
emulsions containing long chain polyunsaturated fatty acids, amino
acid solutions and carbohydrates shall be heat sterilized and stored for
a long time, problems with oxygen induced degradation and
incompatibility with the polymeric material, might lead to the
appearance of potentially hazardous products.
When manufacturing different types of containers of
Polymeric materials for storing parenterally administerable fluids, it
has been a considerable problem to provide a suitable degree of
sterility for all the parts of the container. It would also be highly
desirable, for reasons of safety for the patients, for the convenience of
hospital personal and for economical reasons to achieve and maintain
~ a suitably high degree of sterility by means of a single steam
sterilization process (i.e. autoclavation) which is performed after- the
container has been finally assembled, filled and sealed.
Flexible containers for storage of parenteral nutrients are
conventionally provided with ports for filling and dispensing of the
nutrients. Tubular ports may be attached by means of weldin~~ when
forming side seams, as performed in the International Patent
CA 02251267 2004-09-10
25259-49
2
Application WO 95/26177 (Fresenius AG). When
manufacturing such a container two holes are pressed in the sheet for
the tubular ports, whereupon the saddle is welded to the sheet which
is folded and welded to a bag shaped container by forming two side
seams and a top seam. The container may be filled through the saddle
formed port, or preferably by one or more temporary ports in
connection to the welded seams before it is sterilized.
Conventional saddle-formed port systems normally
comprise an additive port for the introduction to the container; just
before administration, of a complementary perishable fluid, such as a
solution of vitamins to a stored parenteral nutrient. It will also
comprise a dispensing port for establishing a fluid connection between
the container and the patient in need of fluid therapy. The ports are
generally tube formed and often of a predetermined different size in
t5 order to clearly show their identity to the user .
The additive port is often sealed with a stopper made of
latex rubber fitted in mouth of the port which can be penetrated by a
needle. The dispensing port is typically formed with a membrane of
polypropylene which can be pierced with a spike connected to the
infusion device. The mouth of such a port is finally sealed before.
storage by a removable cap or a foil. These ports have a drawback in
that the small space between the stopper and the sealing cap or foil
will not be reached by sterilizing steam which constitutes a risk for
contamination in connection with the penetration. To solve this
problem, the saddle-formed port systems have either been pre-
sterilized by means of radiation before being assembled to the bags or
alternatively a water droplet has been introduced in the small space to
provide sterilizing vapor during the heat treatment. Both these
solutions are unsatisfying, since they require either an extra
sterilization routine by radiation which often might deteriorate the
quality of polymeric materials or an extra water droplet adding
routine. Whenever using this type of containers, the handling
personnel at the hospitals are instructed to, as an extra safety routine,
wipe the latex rubber with a disinfectant before piercing it with a
needle connected to an infusion tubing.
CA 02251267 2004-09-10
25259-49
3
The same problem is also present with the type of
plastic bottle formed containers made with a "blow-fill-
seal" method, as described in the Swedish patent
application 9303123A (SE 501925 C2). These type of bottles
are sealed by a resilient stopper and a cap at the top of
the bottle and finally sealed in the autoclave with a weak
seal between the stopper and the inner surface of the
container neck. The small space between the stopper and the
cap will not be properly sterilized by steam unless a water
droplet is introduced in a separate process. An incorrectly
sterilized pierceable surface means a risk for
contamination, especially when the containers are aimed for
storage of several dosages and several collections of fluid
will be made by piercing the stopper with a needle.
It would also be highly desirable to be able to
recycle also a bag-formed container with an attached saddle-
formed port system without a laborious dismembering and
collection of different materials in separate processes, as
being made possible with the containers, according to the
mentioned Swedish patent application 9303123A
(SE 501925 C2). The frequently used resilient latex stopper
of the ports must be individually collected from used bags
before they can be recycled. The presence of any latex
stoppers will effectively spoil a recycling process of
polypropylene based bags. It would also be advantageous to
benefit from the advantage of introducing sealing weak
weldings with the final heat sterilization, as obtained
between the container body or the cap and elastomeric
sealing device in the mentioned Swedish patent application
9303123A (SE 501925 C2).
CA 02251267 2004-09-10
25259-49
3a
It would also be desirable to provide a saddle
formed port system having ports which fit to high number of
spike connections without leakage so they are compatible
with a large number of infusion sets existing on the market.
Especially dispensing ports sealed with a polypropylene
membrane, will often leak and are not sufficiently
resealable after being pierced, while latex stoppers in
addition ports have a drawback by their tendency to be
unintentionally displaced from the mouth of the port. These
type of sealing devices might also cause problems due to
particles torn off when being penetrated.
According to the present invention it is intended
to provide pierceable openings for medical containers which
can overcome the above mentioned problems both in saddle
port systems for bag formed containers and other types of
containers.
In accordance with one aspect of the present
invention there is provided a sealed container opening,
intended for fluid communication with a container for
storing medical fluids, comprising a tubular sleeve-formed
part having a resilient pierceable stopper inserted in its
mouth and a covering sealing device, wherein said container
opening contains an autoclavable polyolefinic material,
characterized in that said covering sealing device is in the
form of a peelable foil capable of being penetrated by
sterilizing steam in order to sterilize the space between
said foil and the stopper.
CA 02251267 1998-10-13
W0 97/39952 PCTISE97/00682
4
It is an object of the invention to provide a sealed opening
for a medical container which is capable of being correctly sterilized
by steam in all parts exposed to the fluid and fluid handling devices.
It is another object of the invention to provide a medical
package for parenterally administerable fluids where all parts exposed
to the fluids or to fluid transferring devices are correctly sterilized in a
single operation after it has been finally assembled and filled.
A specific object of the present invention is to provide a
flexible bag-formed container for storage of parenteral fluids having a
saddle-formed port system for introducing fluids to and dispensing
fluids from the bag which is possible to sterilize in a single operation,
and where all surfaces of the sealed opening which will be exposed to
the fluid and fluid handling devices are correctly sterilized by steam.
A further specific object of the invention is to provide such
a completely sterilizable bag-formed container with a saddle-formed
port system which has a cheap environmental friendly construction
that can be recycled in the same process without dismembering its
parts before its disposal.
A still further specific object of the invention is to provide
the saddle-formed port system of the container with openings which
are possible to attach to a high number different connecting spikes.
These objects of the invention will be attained by the
subject-matters disclosed in the appended claims. The invention as
disclosed in the following part will also provide a solution to problems
stated above.
Description of the invention
The present invention is directed to container openings for
fluid communication with a container for storing medical fluids,
especially for parenteral administration. The container opening
comprises a tubular sleeve-formed part with a resilient and pierceable
stopper inserted in its mouth and a sealing device covering said
mouth and stopper. The tubular sleeve-formed part, the stopper, alld
the sealing device contain, at least to a substantial amount, the same
polyolefinic material, so they can be recycled with same process in a
recycling plant without being dismembered and separately collected
after use. It is a characteristic feature of a sealed container opening
according to the present invention that it can be heat sterilized by
CA 02251267 1998-10-13
WO 97/39952 PCT/SE97/00682
steam in a single process while all its parts, that will be exposed to, or
come in contact with, either with the fluid directly or devices used for
handling or transferring the fluid are sterilized by means of direct
contact with steam transferred to said parts during the autoclavation.
5 According to a first embodiment of the invention, the steam
is transported to the space between a cap formed sealing device and
the stopper, otherwise unavailable for direct steam sterilization. This
space is reached with steam from a steam transporting axially directed
annular slit, formed between the peripheral surface of the stopper and
the inner peripheral surface of the tubular sleeve formed part during
the autoclavation of said container. The steam transporting slit
appears when the tubular sleeve expands more in a radial direction
than the stopper during the autoclavation. According to this
embodiment, the cap formed sealing device can be provided with a
preformed rupture line so the user can twist off the cap to expose a
sterile surface for immediate penetration with a spike or a needle.
According to a second embodiment the inventive container
openings are provided with a covering sealing device in the form of
peelable foil. This foil can be penetrated by steam during in the
autoclave, so the upper pierceable surface beneath the foil and the
space between the foil and the stopper is sterilized by direct contact
with steam.
It is preferred, according to the invention, that the stopper,
besides the polyolefinic material, contains a thermoplastic elastomer.
Preferred polyolefinic materials, according to the invention are
polypropylene and/or polyethene.
The present invention is also directed to a container having
at least one of the aforedescribed openings comprising a tubular
sleeve-formed part closed with a resilient, pierceable stopper and a
sealing device, wherein all parts of the container and its orifice
essentially consist of the same polyolefin. The container can either be
in the form of a flexible bag having at least one of said orifices or in a
conventional bottle formed container of a polyolefin based material
with a sealed orifice having said features.
Detailed description of the invention
Fig. 1 shows an inventive saddle-formed port systems with two ports
having openings according to the present invention.
CA 02251267 1998-10-13
W0 97/39952 PCT/SE97/00682
6
Fig. 2 is an enlargement of the steam transporting axial slit between
the stopper and the tubular sleeve wall in one of the ports according to
Fig. 1.
Fig. 3 shows an alternative embodiment of an opening according to
the present invention.
Fig. 4 shows a saddle-formed port system having the alternative
opening of Fig. 3 attached.
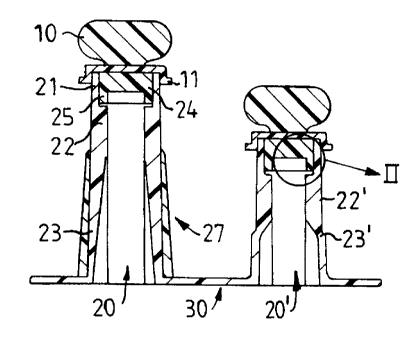
Fig. 1, shows a side view of an inventive saddle-port system
30 with two openings for fluid communication with a container (not
shown) constituted by the ports 20 and 20' which are of somewhat
different size for easily identifying the dispensing and additive port,
respectively. Both ports have sealed openings according to the present
invention and are largely identical and consist of a generally
cylindrical, tubular sleeves 22, 22' which preferably have slightly
beveled part 23, 23' of a shape designed to fit various fluid transfer
devices, such as a conventional spike-formed connections to an
infusion device. It is particularly preferred to have such a shape that
fits spikes according to the conventional ISO-standard.
A cylindrical resileint and pierceable stopper 24 is
positioned in the mouth part 21 of the orifices by means of an insert
device 25 which rests on an annular shelf 26. The stopper is made of a
resilient thermoplastic elastomer and is designed to fit snugly and
sealingly in the mouth. It is conceivable within the context of the
present invention to find other suitable designs of the insert device
and the annular shelf extended around the inner periphery of the
tubular sleeves. The ports can be provided with a fingergrip portion 27
to give the user a more comfortable stability when inserting devices
for fluid transfer into the port. The mouth of the opening is sealed
with a cap formed sealing device 10 which is provided with a flange 11
fitted over the edge of the mouth. To obtain a safe sealing against the
environment, the contact surface between the mouth and the cap
formed sealing device can be welded together by, for example
ultrasonic welding. The cap formed sealing device can also be
provided with a preformed rupture line {not shown) which preferably
is circular and will burst when it is twisted by the user to provide a
CA 02251267 1998-10-13
WO 97139952 PCT/SE97/00682
7
round aperture, through which a needle or spike can penetrate the
stopper and establish fluid communication with the container.
As best demonstrated in Fig. 2, there is a small closed space
40 extended between the cap formed sealing device and the upper face
of the stopper which might never be reached by sterilizing steam from
the inside of the container during the autoclavation process. As a
result, the surface penetrated by a needle or a connecting spike might
be contaminated due to an unsatisfying sterilization which at worst
case will waste the fluids of the container and be hazardous for the
t0 patient.
This problem is solved with the present invnetion by the
temporary formation of an axially extended annular slit 41 between
the peripheral surface of stopper and the inner peripheral wall of the
tubular sleeve of the orifice. The slit 41 opens for transportation of
steam from the interior of the container to the closed space 40 during
the autoclavation of the container. Such an axial slit is formed when
the tubular sleeve expands more in a radial direction than the stopper
during the heat treatment in the autoclave. When the container
subsequently is cooled in the autoclave, the slit closes because of a
comparable contraction of the stopper and the sleeve and a weak seal
is formed in their contact surface.
The formation of a slit and the subsequent formation of a
weak seal in the autoclave requires a careful selection of polymeric
materials. To successfully obtain weak seals, it is important that the
stopper contains a certain amount of a thermoplastic elastomer, such
as a dispersed EPDM-rubber or SEBS (styrene-ethylene-butadien-
styrene copolymer), so the stopper can exert a balancing pressure
when tubular sleeve expands and contracts during the autoclavation
process. A high compatibility between these parts are also required,
because molecules must be exchanged in the contact surface of said
parts, in order to form a weak seal. Both the stopper and the tubular
orifice sleeve, should therefore contain the same polyolefinic material,
in order to obtain such a molecular compatibility. This requirement
that must also be set on the entire port system for enabling it to be
recycled together with the rest of the container. The port system must,
consequently, also be compatible with the material of the flexible
container, so it can successfully he attached to it by means of welding
during the assembly. Moreover, the stopper must have certain
resilience to meet the requirements to obtain a weak welding, as well
CA 02251267 1998-10-13
WO 97!39952 PCT/SE97/00682
8
as being resealable, so it can be penetrated several times and maintain
the integrity of the container. It is also a requirement that the stopper
material shall have a certain friction against the connection spike to
prevent the spike to be unintentionally displaced from the stopper and
to provide a sealing connection with a high number of different types
of connecting spikes existing on the market.
A suitable material for the stopper is a polyolefin polymer
which contains a thermoplastic elastomer. The same polyolefin must
be present, both in the remaining parts of the port system and in the
container. Suitable polyolefin materials are polyetylenes or
polypropylene, their mixtures and copolymers of various medical
grades. It has been shown in the present invention that it is
surprisingly advantageous to have a high amount of polypropylene in
the port system compatible with a polypropylene containing material
in the containers.
For the stopper it is especially preferred to select materials
of polypropylene containing a certain amount of a thermoplastic
elastomer like Dynaflex~ from GLS Corp. comprising polypropylene
and SEBS. However other polypropylene based materials having
comparable characteristics can be used in the present invention, such
as those having dispersed particles of EPDM-rubber in the matrix like
Santoprene~ from Monsanto. A stopper made of such a material will
also solve the problem with particles torn away as a result of its
penetration and it has a high resealing capacity after a penetration.
The material of the remaining saddle-formed port system
shall preferably be compatible with the material of the infusion bag in
order enable a suitable attachment, for example by means of welding.
Both materials shall preferably contain the same polyolefinic material
so they are capable of being recycled in the process and so a separate
collection procedure is avoided. A suitable material for the bag formed
infusion container is based on polyolefines, such as polyethylene or
polypropylene, their mixture and copolymers. A preferred material is
Excel~ from McGaw Inc., generally described in the European patent
0228919.
Excel~ has a multilayered structure substantially comprising:
a) an inner, sealant layer facing the medical fluid consisting of a
mixture of a polyethylene/polypropylene copolymer (FINA Dypro Z
CA 02251267 1998-10-13
WO 97/39952 PCT/SE97/00682
9
9450) and Kraton~ G 1652 from Shell (a
styrene/ethylene/butadien/styrene copolymer);
b) a middle, tie layer of pure Kraton~ 61652; and
c) an outer, release layer of Ecdel~ 9965 (or 9566 or 9967) from
Eastman Kodak & Co. which is a cycloaliphatic thermoplastic
copolyester (a copoly(ester ether), a condensation product of the traps
isomer of 1,4-dimethyl-cyclohexanedicarboxylate,
l0 of cyclohexanedimethanol and hydroxyterminated polytetramethylene
glycol).
When using Excel~ as the material for the bag formed
container, the saddle formed port system suitable contains
polypropylene and preferably consists of a mixture of polypropylene
and Kraton~ which is weldable to the inner layer of the Excel~ film.
Suitable mixtures are in range of about 80 to 40% polypropylene and
to 60% Kraton~. The polypropylene is of homogenous
interpenetrating polymer network (IPN) quality, capable of forming
20 weak seals at about 105 to 120 ° C, preferably at about 117 °
C and a
permanent welding at about 160°C. However, the skilled person will
have no difficulty in finding appropriate compositions of
polypropylene and thermoplastic elastomer for the inventive port
system and its constituents given the provisions set out above.
An alternative embodiment of a sealed opening to a medical
container, according to the invention, is demonstrated in Fig. 3. This
opening is suitable in the previously discussed saddle-formed port
systems, has a generally cylindrical part 22A with a mouth 21 A. A
3o resilient and pierceable stopper 24A is sealingly positioned in the
mouth and rests on the annular shelf 26A formed in said sleeve 22A.
The stopper 24A is made of a resilient pierceable material, suitably a
polyolefin containing a certain amount of a thermoplastic elastomer
and preferably Dynaflex0 or a comparable material as disclosed
above, while the other parts of the orifice preferably are made of
polypropylene with mixtures of Kraton~, as also disclosed above. The
opening is sealed before sterilization by a peelable foil i 2A which is
sealingly fitted over an annular outwardly extended larourusion 2~A c>t
CA 02251267 1998-10-13
WO 97139952 PCT/SE97100682
the mouth 21 A. In order to be able to correctly steam sterilize all parts
of the orifice including the connected filled container, steam must the
transferred also to the upper surface 40A of the pierceable stopper 24A
which shall be penetrated by a needle or a spike. A transfer of steam
5 therefore must be arranged through the peelable foil in the autoclave,
while the foil also must be capable of maintaining sterile conditions
and prevent airborne or contact contamination of the surface 40A
during the subsequent storage. The material of the peelable foil must
therefore be selected among steam permeable, but heat resistant
10 materials which otherwise can form an effective sealing barrier for
contaminating agents. Suitable materials are found among spun
polyolefins, such as Tyvek~ from DuPont and among certain qualities
of lacquered papers.
As demonstrated in Fig. 4, this type of container opening is
preferably connected to the mouth part of the generally cylindrically
formed sleeves of a port of a saddle formed port system. To facilitate
the connection to the sleeves, the outer peripheral surface of the
cylindrical part 22A can be provided with an annular protrusion 29
which is intended to fit in a corresponding annular recess 29' provided
in the inner peripheral surface of the sleeve formed port. These
features will also enable the container opening to be safely fixed to the
sleeve during the handling, in order to avoid it to be unintentionally
dismembered when removing a spike or a needle penetrating the
stopper.
The openings are manufactured in a process wherein the
Dynaflex~ is injected into a pre-shaped cylindrical opening by means
of two-color mold injection machine, whereupon the foil is assembled
in a separate process.
When using such a container opening for fluid transfer, the
foil 12A will be removed by a simple peeling motion to expose the
sterile upper pierceable surface 40A of the stopper which can
immediately be pierced by a conventional connection spike or a
comparable device for establishing fluid connection without a risk for
contamination.
Besides the advantages stated above, the described sealed
container openings and the saddle-formed port systems including
them will, for many practical applications eliminate the use of a
secondary> outer pouch wrapped over the bag-formed container
during storage for standard solutions and other parenteral solutions.
CA 02251267 1998-10-13
WO 97/39952 PCT/SE97/00682
11
even if certain oxygen sensitive products like amino acids and lipid
emulsion will require additional protective measures.
It is also to be understood that the inventive container
openings, not only shall be regarded as limited in use to saddle
formed port systems connected to flexible bag-formed containers.
They are equally useful as parts of a bottle shaped more rigid polymer
container containing sensitive medical fluids which require
autoclavation before storage.
The examples given, above, are intended to illustrate
l0 functioning embodiments of the present invention and shall not be
regarded as limiting for the scope of invention, as it is presented by
the following set of claims.