Note: Descriptions are shown in the official language in which they were submitted.
. CA 022~1480 1998-10-26
,~
PATENT
349 1 -34-00
WASTE TREATMENT OF METAL PLATING SOLUTIONS
Field of the Invention
This invention relates to a process for the waste treatment of metal-bearing solutions
to substantially eliminate the dissolved metal content in such solutions prior to discharge to
the environment and, more particularly, to a process for the waste treatment of spent metal
plating solutions which will enable such solutions to be discharged as non-toxic waste
directly to a municipal wastewater treatment plant.
Background of the Invention
Solutions capable of plating metals onto substrates are widely used in industry. The
most commonly used metal plating solutions include electrolytic and electroless solutions.
Electroless nickel plating solutions, in particular, have come into widespread usage
in the manufacture of computer memory discs. Such solutions generally contain a nickel
metal salt, such as the sulfate, acetate, carbonate or chloride salt, for the source of
dissolved metal plating ions, a reducing agent, such as sodium hydrosulfite, sodium
hypophosphite, sodium borohydride, boranes or hyrdazines, to reduce the metal ions to
metallic form, a complexing or chelating agent, such as monocarboxylic acids, dicarboxylic
acids, hydroxycarboxylic acids, amino acids, and alkanolamines, to maintain the metal ions
in solution and prevent premature precipitation, and a pH adjuster to maintain the solution
pH typically within an acidic range of 4-5. Accelerators, stabilizers, buffers, and wetting
agents may also be included in the electroless nickel solutions.
. CA 022~1480 1998-10-26
PATENT
349 1 -34-00
These electroless nickel solutions only have a limited useful life and eventually
become depleted or spent. Typically, after a limited number of plating cycles, the
concentration is reduced of both the complexed metal in solution (by plate-out onto the
substrate) and the reducing agent (by consumption according to the chemical reaction
controlling plating) to where the plating rate of the electroless bath is slowed sufficiently
to become unsatisfactory, and the bath has to be discarded.
Disposal of such spent electroless nickel solutions, however, presents a major
problem, since a large percentage of the original complexed nickel content remains dissolved
in the spent solutions. From an environmental standpoint, it is well known that heavy metal
ions and particularly those of the metal here of interest, namely nickel, can have adverse
toxic effects on the environment if directly discharged in soluble form into effluent
wastewater streams that feed into municipal water systems or natural bodies of water.
Also, such highly chelated streams generally cannot be mixed with unchelated streams at
the wastewater treatment plant, making processing much more difficult. As a result,
discharging dissolved heavy metals, including nickel, directly into effluent wastewater
streams, in other than minute quantities, is prohibited by local, state, and federal
regulations.
A number of wastewater treatment processes have been proposed to reduce the
metal content in spent electroless solutions to low levels prior to discharge. Previously,
many users of electroless baths simply dosed their wastes with caustic soda to precipitate
the bulk of the heavy metal contaminant as insoluble hydrous oxides (metal hydroxides),
whereupon the hydrous oxide sludge was pressed into a filter cake, drummed, and disposed.
This method of metal removal, however, was known to produce a very large quantity of
metal-bearing sludge, all of which needed to be disposed of in approved hazardous landfills.
Sludge disposal is very expensive, environmentally detrimental, wasteful of natural
resources, and involves compliance with local, state and federal regulations. In addition, the
sludge producer assumes perpetual liability for the sludge deposited in the landfill site which
is undesirable. Another problem with this treatment method is that it is inefficient and
generally did not work well due to the high chelant levels in the spent solutions.
Another waste treatment method previously used for spent electroless solutions was
to simply electrolessly plate out the metal by dosing the solution at slightly alkaline pH with
reducing agents. The reducing agents typically used to convert the dissolved metal salt into
insoluble metal precipitate include sodium borohydride, sodium hydrosulfite, sodium
hypophosphite, boranes and hydrazines. An advantage of this method is that the metal-
- 2 -
CA 022~1480 1998-10-26
PATENT
349 1 -34-00
bearing sludge produced contains high levels of elemental metal which can be reclaimed by
smelting and sold at a profit. Therefore, hazardous waste disposal of the sludge is no longer
required. Plainly, this method is economical, environmentally friendly, and conserves natural
resources. One drawback, however, is that the soluble nickel content in the treated
electroless nickel baths can only be partially reduced. Generally, the nickel content can only
be lowered to about 3-70 parts per million Ippm) which no longer meets the discharge limits
in most jurisdictions.
Still another prior waste treatment method known for reducing the dissolved metal
content of spent electroless baths to acceptable discharge levels involves organosulfur
precipitation of the metal by dosing the spent solution at a pH of 5-8 with water-soluble
sodium dithiocarbamate (DTC) precipitating agents, such as sodium dimethyldithiocarbamate
(DTC-Na). The dissolved metal salt complexes with the soluble dithiocarbamate salts to
form insoluble metal dithiocarbamate precipitates. The metal dithiocarbamate particles are
generally very fine and not conducive to settling, and typically require the aid of coagulants
and flocculants to form larger, faster settling flocs which are more capable of removal by
filtration. The dithiocarbamate method effectively removes the heavy metal content in
spent electroless baths to non-detectable levels, but undesirably produces huge quantities
of heavy metal-bearing carbamate sludge which create potentially dangerous hydrolytic
loading on the typical liquid-solid system. Furthermore, these sludges must be classified as
hazardous waste and disposed of predominantly in approved hazardous landfills. Here again,
sludge disposal is very expensive, environmentally detrimental, and wasteful of natural
resources.
Prior attempts to waste treat spent electroless nickel solutions with borohydride
reduction followed by dithiocarbamate precipitation without removing the reduction
precipitants or lowering the pH prior to dithiocarbamate precipitation have generally been
met without much success. With the combined method, no appreciable differences in the
nickel levels have been shown over straight borohydride reduction, which levels fall above
current discharge limits.
While the prior processes reduce the metal content of the spent electroless plating
solutions, the need still exists for efficient and economical methods for waste treating such
solutions which also meet current discharge limits in the low parts per million without
' CA 022~1480 1998-10-26
PATENT
349 1 -34-00
~ simultaneously generating significant amounts of hazardous waste. Moreover, as the
regulatory rules for waste stream effluent discharge limits become more stringent, such as
to certain fractional parts per million, a search for improved processes to remove the metal
content becomes mandatory.
Summary of the Invention
It is an object of this invention, therefore, to provide an efficient and economical
process for the waste treatment of metal-bearing waste solutions, particularly spent
electroless nickel plating solutions that contain dissolved nickel, sometimes other metals,
and complexing agents, to substantially remove the dissolved metal content therein, so as
to leave the treated solutions classifiable as non-polluting waste by law and suitable for
discharge directly to a municipal wastewater treatment plant or a natural body of water.
It is another object of this invention to provide a waste treatment process that is
simple in operation and employs an effective, sequential, two-step chemical treatment
method involving chemical reduction, particularly borohydride reduction, followed by
organosulfur precipitation, particularly dithiocarbamate precipitation, and that surprisingly
meets current discharge limits.
It is a yet another object of this invention to provide a waste treatment process that
generates reduced amounts of hazardous sludge, resulting in a significant reduction in
hazardous waste disposal costs.
Yet another object of this invention is to provide a waste treatment process which
conserves natural resources by generating, for the most part, a sludge containing high levels
of solid metal compounds, including elemental metal ore, which can be recovered and
reclaimed, rather than disposed of as a hazardous waste.
Still another object of this invention is to provide a waste treatment process which
reduces the dissolved metal content down to the low parts per million and, in most case,
down to fractional parts per million.
And still another object of this invention is to provide a waste treatment process
which can treat multiple metal-bearing wastewater streams, which previously required
separate treatments.
Yet another object of this invention is to provide an apparatus suitable for use in
performing such a waste treatment process.
In accordance with this invention, a process is provided for the waste treatment of
metal-bearing wastewater solutions containing dissolved chelated or non-chelated metals,
- 4 -
' CA 022~1480 1998-10-26
",~
PATENT
349 1 -34-00
particularly electroless nickel plating solutions, whereby the treated solution can be
discharged directly to a municipal wastewater treatment plant or to the environment.
Broadly stated, the process comprises the sequential steps of chemical reduction, followed
by organosulfur precipitation, with an intermediate filtration step and pH adjustment
interposed there between. In a preferred embodiment, the first step comprises borohydride
reduction and the second step comprises dithiocarbamate precipitation. The heavy metal
content in the precipitated matter produced in the first stage of this process can ultimately
be recovered as metal, essentially eliminating the need for large volumes of hazardous waste
disposal .
The various objects, features and advantages of this invention will become more
apparent from the following description and appended claims.
Brief Description of the Drawings
With this description of the invention, a detailed description follows with reference
made to the accompanying drawing in which:
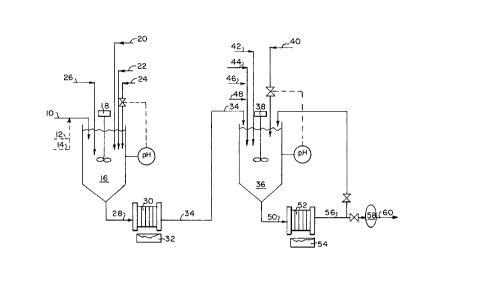
FIG. 1 represents a flow chart showing the currently preferred process and apparatus
for waste treatment of spent electroless nickel solutions in accordance with this invention.
Detailed Description of the Preferred Embodiments of the Invention
This invention provides a sequential, two-step, simple, efficient, economical, and
environmentally favorable, waste treatment process for metal-bearing wastewater solutions,
particularly spent electroless nickel plating solutions, which can lower the dissolved metal
content in such solutions to acceptable levels, generally on the order of about ca. ~ 3 ppm
and preferably ca. 0.5 ppm, so that the solutions can be discharged directly into a
wastewater treatment plant stream or a natural stream.
The metal-bearing solutions to be waste treated may come form a variety of sources
such as metal process streams from mining and metallurgical operations, electrolytic and
electroless metal plating baths, other metal recovery operations, such as ion exchange, and
chemical manufacture, and the like. Metal-bearing solutions containing heavy metals, such
as nickel, copper, cobalt, silver, gold, lead, mercury, platinum, and any other metals reduced
or precipitated by alkali metal borohydrides may be treated according to this invention.
Such metals can be found throughout the transition metals, lanthanides, actinides, and post-
transition metals. Other metal-bearing solutions that contain heavy metals, such as zinc,
which are not reduced by alkali metal borohydrides but can be precipitated by alkali
- 5 -
CA 022~1480 1998-10-26
PATENT
349 1 -34-00
thiocarbamates may also be treated according to this invention. However, the invention is
particularly effective in treating metal-bearing waste solutions containing dissolved nickel.
Thus, for convenience, the following description of this invention will be directed to the
treatment of discarded or spent electroless nickel plating solutions.
Spent electroless nickel solutions to be treated usually contain substantial quantities
of dissolved nickel. In general, the dissolved nickel content can vary within very wide limits
dependent upon the source of the specific solution treated. For purposes of illustration only,
the nickel content of a conventional spent electroless nickel solution to be treated in
accordance with this invention usually varies between about 4,000-6,000 ppm (4-6grams/liter). Consequently, a successful waste treatment process permitting direct disposal
of the solution to the environment should comprise lowering the dissolved metal content in
the solution to non-toxic discharge levels generally on the order of ca. 3 ppm Ni, and
preferably ca. 0.5 ppm.
The waste treatment process of this invention is amenable to batchwise or
continuous processing, although batchwise processing is presently preferred.
In accordance with this invention, the first step in the waste treatment of the spent
electroless nickel plating solution is removal of the dissolved metal from solution by
chemical reduction, particularly borohydride or borohydride catalyzed reduction. The spent
electroless solution to be waste treated is first contacted with a reducing agent for a
sufficient time to cause the dissolved metal salt to undergo chemical reduction, resulting in
the precipitation of metal compounds out of the solution. A preferred method foraccomplishing this is by the addition of an alkali or alkaline earth metal borohydride,
preferably sodium borohydride from an economic standpoint, to the spent electroless nickel
solution. The sodium borohydride is usually added as an aqueous solution of sodium
borohydride and sodium hydroxide (for hydrolytic stability of the borohydride ion and
reduction of hydrogen gas evolution). An example of a suitable hydrolytically stabilized
borohydride reduction solution is sold under the trademark VenMet~ by Morton
International, Inc. of Chicago, IL.
The amount of sodium borohydride solution used will vary depending upon the
particular system for which treatment is desired and will mainly be influenced by the metal
content of the waste solution and the presence of other ligands and chelant molecules.
Although stoichiometric quantities of sodium borohydride vis-a-vis the dissolved metal
content may be employed, it is generally preferred to employ a stoichiometric excess
amount of sodium borohydride to compensate for consumption during competing oxidative
- CA 022~1480 1998-10-26
"
.
PATENT
349 1 -34-00
side reactions. Normally, the sodium borohydride solution is added in a sufficient amount
to provide at least about 4 to 15 times the amount of sodium borohydride required to reduce
the waste metal in the spent electroless nickel solution. In some cases less than
stoichiometric levels of borohydride can be employed as the borohydride reduced nickel
often catalyzes additional hypophosphite reduction of the chelated nickel ions in solution.
Adjustment of the pH of the electroless nicl<el solution before the addition of sodium
borohydride is generally desired to maximize the rate of sodium borohydride reduction. For
systems in which the primary metal ion is nickel, the borohydride reduction can be carried
out in a pH range of between about 4 and 11 and preferably in a range of between 7 and
11. The pH adjustment is typically accomplished with the addition of sufficient quantities
of alkaline agents, such as sodium hydroxide, calcium hydroxide, magnesium hydroxide, and
lime, or mixtures thereof, to the electroless nickel solution before the addition of the sodium
borohydride solution. Normally between about 200 and 2,000 ppm of lime is employed and
then final pH adjustment is made to the solution.
It may also be desirable to add sufficient amounts of oxidizer scavenging agents,
such as sodium bisulfite, sodium metabisulfite, and sodium sulfite, or mixtures thereof, to
the reaction mixture in order to minimize consumption of the sodium borohydride prior to
metal reduction. This material is typically added to the spent solution at a low solution pH
of about 4-5 prior to final pH adjustment. Normally between about 200 and 2,000 ppm of
bisulfite is employed in this first treatment stage.
The treated solution is allowed to settle and the nickel-bearing precipitate which
results from such treatment is separated from the treated electroless solution by
conventional solid-liquid separation techniques, such as decantation or filtration. It should
be understood that in order to run the process of this invention in an effective manner, the
inventors have found that it is critical to separate the precipitated matter from the treated
liquid prior to the subsequent chemical treatment of the liquid. Typically, the precipitate is
allowed to settle and is then withdrawn from the bottom of the reaction vessel as a sludge.
The sludge is then passed through a filter press to separate the precipitate as a filter cake
from the treated liquid. The filter cake which contains high levels of metallic nickel
compounds is desirably transported to a smelting operation to recover elemental nickel
which can be reused in various chemical processes, instead of being disposed as hazardous
waste in a landfill.
The dissolved nickel content in the essentially precipitate-free treated solution
following borohydride reduction is typically between about ca. 3-70 ppm.
- 7 -
' CA 022~1480 1998-10-26
PATENT
349 1 -34-00
Following chemical reduction, the second step in the sequential treatment process
of this invention is the removal of substantially all of the remaining dissolved metal content
from the treated solution by organosulfur precipitation, particularly thiocarbamate
precipitation. As mentioned above, the solution prior to this treatment stage is essentially
free of precipitated matter generated from the first treatment stage. This prevents the
reduction precipitate from interfering with the subsequent thiocarbamate precipitation, and
allows for direct contact between the precipitating agent and the remaining nickel in the
treated solution. Furthermore, cross-contamination of precipitates is eliminated, which
preserves the high purity of the reduction precipitate.
In the second stage, the treated electroless solution is contacted with an
organosulfur precipitating agent, such as a thiocarbamate, for a sufficient time to form an
insoluble metal organosulfur precipitate. A convenient method for accomplishing this is by
the addition of a water-soluble alkali and alkaline earth metal polythiocarbamate to the
treated electroless nickel solution. Examples of particularly useful water-soluble alkali metal
polythiocarbamates include sodium dimethyldithiocarbamate, sodium diethyldithiocarbamate,
sodium trithiocarbamate, and the like. Generally, the alkali metal dialkyldithiocarbamates
are most desirable, particularly sodium dimethyldithiocarbamate. Specific examples of
sodium dimethyldithiocarbamates are those sold under the trademark Metal PlexTM 143 by
Morton International, Inc. of Chicago, IL.
Although stoichiometric amounts of the sodium thiocarbamate vis-a-vis the metal
content may be employed, it is generally preferred to employ a stoichiometric excess of this
precipitating agent. Generally, the sodium thiocarbamate is used in an amount sufficient to
provide at least about 20 times the amount required to precipitate the waste metal as
insoluble nickel thiocarbamate complexes.
Adjustment of the solution pH before precipitation is effected is generally desired.
The pH of the electroless solution is typically lowered to between about 5-8 prior to the
addition of the thiocarbamate precipitating agent with appropriate pH adjusters, such as
nitric acid, sulfuric acid, acetic acid, or hydrochloric acid. It is also possible to adjust the
pH with spent acid process solutions which may or may not contain additional metals. It
is particularly desirable to employ spent acid solutions, such as those derived from the
plating process, in this stage of the waste treatment. This eliminates the need to treat such
solutions in a separate waste treatment process.
The metal thiocarbamate precipitates usually form a very fine floc which is not
conducive to settling at an appreciable rate and filtration. Thus, it is generally desirable to
- 8 -
CA 022~1480 1998-10-26
J
PATENT
349 1 -34-00
add both coagulation and flocculation agents to the precipitate for more efficient removal.
Suitable flocculation agents useful herein include anionic polymers, such as anionic
palyacrylamides. An example of a flocculation agent based on an anionic polyacrylamide
is sold under the trademark MetaFlocTM 495 by Morton International, Inc. of Chicago, IL.
Suitable coagulation agents include cationic polymers, such as cationic polyquarternary
amines and cationic polyacrylamides. An example of a coagulation agent based on cationic
polyquarternary amine is sold under the trademark MetaFlocTM 137 by Morton International,
Inc. of Chicago, IL. It is generally desirable to first add from about 50 to 300 ppm of the
cationic polymer to the reaction mixture to coagulate the precipitated particulates, followed
by the addition of from about 1 to 10 ppm of the anionic polymer to bridge the coagulated
particulates together to form larger, faster settling flocs. Usually, it is also desirable to add
between about 400 and 2,400 ppm of dry diatomaceous earth to the reaction mix. The
diatomite acts as a filtering aid by further building-up the size of the particulates.
The treated solution is allowed to settle and the nickel-bearing precipitate which
results from the second stage of the treatment is separated from the electroless solution by
conventional solid-liquid separation techniques, such as decantation or filtration. Typically,
the precipitated floc is allowed to settle and is then withdrawn from the bottom of the
reaction vessel as a sludge. The sludge is then transported to a filter press to separate the
precipitate as a filter cake from the treated liquid. The filter cake containing the insoluble
nickel thiocarbamate complex can either be mixed with the sludge produced from the first
step and smelted together, or discarded separately as hazardous waste in a landfill. Since
the amount of sludge produced from the second step is minimal, even if it is required to
dispose of the sludge as hazardous waste due to the presence of metal thiocarbamates, the
hazardous waste disposal costs are minimized. The two-step treated liquid can then be
pumped directly to a municipal wastewater treatment facility or a natural stream. It is
usually desirable to filter the treated liquid prior to discharge, since residual particulates may
be present in the liquid extracted from the filter press.
The nickel content in the electroless nickel solution following thiocarbamate
precipitation and prior to discharge is typically about ca. < 3 ppm Ni and more typically
about ca. ~ 0.5 ppm.
In accordance with this invention, the inventors have also surprisingly found that
incorporation of other metal removal process waste streams, such as zincate waste (a zinc
caustic solution) and ion exchange regenerate waste (a nickel acidic solution), into the spent
CA 022~1480 1998-10-26
PATENT
3491 -34-00
nickel levels. The addition of both acidic and basic metal-bearing streams into the
electroless nickel does not alter the pH enough to require additional steps and/or caustic
- prior to chemical reduction. This ability to incorporate additional waste streams into the
electroless nickel treatment eliminates the need for separate treatments for each stream and
associated costs. Sludge generation is also significantly reduced as a result.
With reference to FIG. 1, the following is a preferred listing of processing steps for
waste electroless nickel solutions utilizing the process of this invention. It is understood
that the exact steps will vary depending on the nature of the waste stream and the
equipment being used in the treatment system. A waste electroless nickel solution 10,
along with optional zincate waste solution 12 and optional ion exchange regenerate waste
solution 14, is fed at room temperature to a first reaction vessel 16 equipped with a stirrer
18. Under agitation, sodium metabisulfite 20 and lime 22 to facilitate reduction and
filtration are added and the pH of the solution is then raised to the 7-11 range with the
addition of sodium hydroxide 24. Still under agitation, excess stabilized sodium borohydride
reduction solution 26 (VenMet~ solution) is added. Agitation is continued until substantial
completion of the borohydride reduction reaction. The borohydride reduction reaction can
be represented by the following equation:
8Ni+2X + NaBH4 + 2H20 ~ NaB02 + 8HX + 8Ni~,s~
where X= the anion (chloride, sulfate, carbonate, acetate). After completion, the agitation
is discontinued and the solid precipitate is allowed to settle as a slurry or sludge 28. The
sludge 28 can be drawn off the bottom of the reaction vessel 16 and fed to a filter press
30 where the treated liquid is separated from the solid precipitate. The filter cake 32 which
contains high concentrations of solid nickel compounds is drummed and set aside for
subsequent nickel recovery by a smelting operation (not shown).
Next, the treated liquid 34 containing substantially reduced levels of nickel and being
essentially free of solids is then fed at room temperature to a second reaction vessel 36
equipped with a stirrer 38. Under agitation, nitric acid 40, which may be drawn from a
spent acid stream, is added to lower the pH of the solution to within the range of 5-8. Still
under agitation, excess sodium dimethyldithiocarbamate 42 (Metal PlexTM 143) is added.
Agitation is continued until substantial completion of the organosulfur precipitation reaction.
The organosulfur precipitation reaction is represented by the following equation:
2DTC-Na + Ni+2(complexed) -~ 2DTC-Ni,s,.
After completion, still under agitation a coagulant 44 (MetaFlocTM 137) is added and mixed
until sufficient coagulation, then a flocculant 46 (MetaFlocTM 495) is added and mixed until
- 1 0 -
. CA 022~1480 1998-10-26
,.
PATENT
3491 -34-00
sufficient flocculation, and lastly sufficient dry diatomaceous earth 48 is added and mixed
until the desired build-up of the precipitate is complete. The agitation is then discontinued
and the solid organosulfur precipitate is allowed to settle as a slurry or sludge 50. The
sludge 50 can then be drawn off the bottom of the reaction vessel and fed to a filter press
52 where the treated liquid is separated from the solid precipitate. The filter cake 54
containing essentially all remaining nickel is minimal and can be combined with the filter
cake 32 that resulted from the borohydride treatment. The treated liquid 56 is then fed
through a filter 58 (0.2 micron filter) to ensure removal of any residual solid particulates
from the solution. Also, as shown in FIG. 1, at start-up the treated liquid 56 may be
returned back to the second reaction vessel 36 instead of being initially passed through the
filter 58 to prevent unwanted clogging of the filter. The filtered liquid 60 can then be safely
discharged directly into a wastewater treatment plant stream or a natural stream (not
shown) .
The following non-limiting examples will now illustrate the effectiveness of theprocess of this invention in rendering metal-bearing solutions safe for discharge.
EXAMPLE 1
Waste Treatment of Spent Electroless Nickel Solution
500 mL of spent electroless nickel solution was placed in a beaker with a magnetic
stir bug. 250 ppm Na2S2O5 was added to the electroless nickel solution. The pH of the
solution was then raised from pH 5 to 10.5 with 500 ppm of lime and 50% NaOH solution.
6 mL of VenMet~ reduction solution (12 mL/L) was placed in a small beaker and diluted
approximately 2: 1 with water. The diluted VenMet~ solution was poured directly into the
pH adjusted electroless nickel solution. The small beal<er was rinsed once with water and
the rinse was also poured into the electroless nickel solution. Using moderate agitation the
solution was mixed for 4 hours. After the 4 hours the electroless nickel solution was
removed from agitation and filtered through a #4 Whatman~3 filter paper to remove the
solids. The pH of the solution was then lowered to pH 7 with 35% nitric acid solution. 4
mL of Metal PlexTM 143 (20 mL/L) was added to the reduced and filtered solution. This was
allowed to mix for 1 hour using moderate agitation. After the 1 hour 100 ppm of
MetaFlocTM 137 was added to the solution and allowed to mix for 10 minutes. Then 2 ppm
CA 022~1480 1998-10-26
PATENT
3491 -34-00
of MetaFlocTM 495 was added and allowed to mix for 10 minutes. Lastly, 2 mL (dry)
diatomaceous earth (10 mLlL) was added and allowed to mix for 10 minutes. The treated
solution was then filtered through a 0.2 ~m syringe filter for analysis. The final nickel
content in the treated solution was ca. <0.1 ppm.
EXAMPLE 2
Waste Treatment of Combined Spent Electroless Nickel and Zincate Waste Solutions450 mL of spent electroless nickel solution was placed in a beaker with a magnetic
stir bug. 50 mL of waste zincate solution was also placed into the beaker. The pH of the
solution was then raised from pH 5.6 to 10.5 with 50% NaOH solution. 6 mL of VenMet0
reduction solution (12mL/L) was placed in a small beaker and diluted approximately 2:1 with
water. The diluted VenMet0 solution was poured directly into the pH adjusted electroless
nickel solution. The small beaker was rinsed once with water and the rinse was also poured
into the electroless nickel solution. Using moderate agitation the solution was mixed for 4
hours. After the 4 hours the solution was removed from agitation and filtered through a #4
Whatman3 filter paper to remove the solids. The pH of the solution was then lowered to
pH 8 with 35% nitric acid solution. 10 mL of Metal PlexTM 143 (50 mL/L) was added to the
reduced and filtered solution and allowed to mix for 1 hour using moderate agitation. After
the 1 hour 100 ppm of MetaFlocTM 137 was added to the solution and mixed for 10
minutes. Then 2 ppm of MetaFlocTM 495 was added and mixed for 10 minutes. Lastly, 2
mL (dry) diatomaceous earth (10 mLlL) was added and mixed for 10 minutes. The treated
solution was then filtered through a 0.2 ~m syringe filter for analysis. The final nickel
content in the treated solution was ca. <0.1 ppm and the final zinc content in solution was
ca. 0.16 ppm.
The overall efficiency, simplicity, economic and ecological gains, and legal benefits
which contribute to making the process of this invention most desirable for removing
dissolved heavy metals from metal-bearing solutions should now be apparent to persons
skilled in the art.
' CA 022~1480 1998-10-26
,.,
PATENT
349 1 -34-00
From the foregoing it will be seen that this invention is one well adapted to attain
all ends and objects hereinabove set forth together with the other advantages which are
apparent and inherent. Since many possible variations may be made of the invention
without departing from the scope thereof, the invention is not intended to be limited to the
embodiments and examples disclosed, which are considered to be purely exemplary.Accordingly, reference should be made to the appended claims to assess the true spirit and
scope of the invention, in which exclusive rights are claimed.
- 1 3 -
, _