Note: Descriptions are shown in the official language in which they were submitted.
CA 02251648 1998-10-15
W098/38687 PCT~S98/04071
COPOLYMERS OF VINYLIDENE FLUORIDE AND
HEXAFLUOROPROPYLENE HAVING R~L~
EXTRACTABLE CONTENT AND IMPROVED SOLUTION CLARITY
IR 3490NPl
This application claims the benefit of
provisional application serial number 60/038,346 filed
Feb. 28, 1g97
BACKGROYND OF THE I~Y~NllON
This invention relates to compositions of matter
classified in the art of chemistry as fluoropolymers,
more specifically as copolymers of vinylidene fluoride
(VDF), more specifically as copolymers of vinylidene
fluoride and hexafluoropropylene (HFP), still more
specifically as copolymers of VDF and HFP having
reduced extractable content, longer gel times and
improved solution clarity, to novel compositions of
matter and articles of manufacture containing such
copolymers, as well as to processes for the
preparation and use of the copolymers, of compositions
of manufacture containing such copolymers and of the
,
CA 022~1648 1998-10-l~i
W098/38687
PCT~S98/04071
articles of manufacture containing such copolymers.
VDF/HFP copolymers are well known and are used
for their thermoplastic engineering properties,
chemical resistance and inertness toward degradation.
They may be found in applications such as chemically
resistant piping, gasketing, plenum cable jacketing,
filtration and extraction membranes and in the
construction of lithium batteries.
The present invention provides VDF/HFP copolymers
containing up to about 24 weight ~ ~12 mole~) HFP
having among other improved properties, substantially
improved solution clarity, longer gel times and
reduced extractables as these terms are defined
hereinafter.
The process used to make the instant copolymers
requires one ratio of VDF and HFP for the initial fill
of the reactor, and a different ratio of VDF and HFP
during a subsequent continuous feed of the monomers.
Any particular desired average HFP content in the
copolymer product has corresponding partlcular initial
fill and subsequent feed ratios. The uniformity of
compositions prepared this way provide unique and
useful properties in comparison to VDF/HFP copolymers
described in the prior art.
The present invention also provides lithium
batteries fabricated from the VDF/HFP copolymers of
the present invention and lithium batteries from other
homo and copolymers more specifically described
hereinbelow prepared by known processes having
analogous structure and which the present inventors
CA 022S1648 1998- 10- lS
WO g8/38687
PCT~S98/04071
have recognized as processing properties analogous to
~ those of the VDF/HFP copolymers of the invention which
makes them uniquely suitable for lithium battery
construction.
nT~rT.oSUp~ OF P~TOR A~T
Rexford in U.S. Pat. No. 3,051,677 described
VDF/HFP copolymers of HFP content 30 to 70 wt~ ~15 to
50 mol~) which showed utility as elastomers. To make
the copolymers, a batch process with certain initial
ratios of VDF and HFP, and a continuous process with
fixed ratios of VDF and HFP throughout the process
were described. The processes described were such
that polymers lacking the improved solution clarity,
longer gel times and low extractables of the present
invention were made.
Lo in U.S. Pat. No. 3,178,399 described VDF/HFP
copolymers of HFP content of 2 to 26 wt~ (1 to 13
mol~) which showed a numerical value for the product
of the tensile strength ~psig) and percent reversible
elongation of at least 1,000,000. ~ batch process
with certain initial ratios of VDF and HFP, or,
alternately, a semicontlnuous process with fixed
ratios of V~F and HFP throughout the process were used
to make the copolymers. The processes discussed were
such that copolymers lacking the improved solution
clarity, longer gel times and low extracta~les of the
present invention copolymers were made.
Moggi, et al. in Polymer Bulletin 7, 115-122
(1982) analyzed the microstructure and crystal
structure of VDF/HFP copolymers by nuclear magnetic
CA 022S1648 1998-10-1
W O 98~8687
PCT~S98/04071
- resonance and x-ray diffraction experiments. The
copolymers of up to 31 wt~ (up to 16 mol~ HFP were
made in a batch emulsion process which was carried
only to low conversion. While the low conversion
batch process is capable of producing copolymers
having solution clarity and low extractables, no such
properties are described. It is not a practical
process for industrial use because of the low
conversions required to make the materials. In
addition, no detailed polymerization examples were
offered.
Bonardelli et al. in Polymer, vol. 27, 905-gog
(June 1986) studied the glass transition temperatures
of VDF/HFP copolymers having HFP content up to 62 wt.
(up to 41 mol%). The glass transition temperatures
were correlated to the overall HFP content in the
copolymers. In making the copolymers for analysis, a
semicontinuous emulsion process was used which
employed different VDF/HFP ratios for the initial fill
of the reactor and for the subsequent continuous feed
of monomers. Although reference was made to the use
of reactivity ratios to set the VDF/HFP ratio for the
initial fill, no detailed polymerization examples were
offered, and no mention of copolymers having solution
clarity, gel times and low extractables comparable to
that of the copolymers of the present invention was
made.
Pianca et al. in Polymer, vol. 28, 224-230 (Feb.
1987) examined the microstructure of VDF/HFP
copolymers by nuclear magnetic resonance, and the
CA 02251648 1998-10-lS
W098~8~7
PCT~S98/04~71
microstructure determinations were used to explain the
trend in glass transition temperatures of the
copolymers. The synthesis of the copolymers involved
a semicontinuous emulsion process which used different
VDF/HFP ratios for the initial fill of the reactor and
for the subse~uent continuous feed of monomers. No
detailed synthesis examples were provided, and there
was no discussion of copolymers having improved
solution clarity, longer gel times and low
extractables as provided by the copolymers of the
present invention.
Abusleme et al. in Eur. Pat. Appl. No. 650,982 Al
showed an emulsion process to make polymers and
copolymers of fluorinated olefins optionally with one
or more non-fluorinated olefins. The process relied
on photochemical initiation of polymerization so that
lower temperatures and pressures could be used than
those used for thermally initiated processes. While
there was general mention of the structural regularity
of the resulting polymers, the only evidence of
regularity concerned poly~vinylidene fluoride)
homopolymer, and no claims were made as to regularity
of composition. Examples of VDF/HFP copoly~erization
were given, but no discussion of the solution
extraction properties of the copolymers was given, and
there was no relation ~ade between physical properties
and the structure of the V~F/HFP copolymers.
Morgan in U.S. Pat. No. 5,543,217 disclosed
uniform tetrafluoroethylene/hexafluoropropylene
copolymers (TFE/HFP copolymers) made by a
CA 02251648 1998-10-1
W098/38687
PCT~S98/04071
semicontinuous emulsion process. Uniformity was
simply defined as there being a low proportion of
adjacent ~FP units in the polymer chains; there was no
disclosure of the disposition of TFE and HFP units
otherwise, and there was no discussion of VDF/HFP
copolymers or the properties to be expected therefrom.
None of these references teaches or suggests a
way to obtain VDF/HFP copolymers having solvent
solution clarity and fluidity, longer gel times and
low extractables comparable to the VDF/HFP copolymers
of the instant invention or that such properties are
attainable from VDF/HFP copolymers.
U.S. Patent 4,076,929 describes the synthesis of
VDF homopolymer having a uniformly distributed
relatively high defect structure in its molecular
claims.
U.S. Patent 2,752,331 describes the synthesis of
VDF-chlorotrifluoroethylene (CTFE) copolymers having a
high uniformity of comonomer distribution in its
molecular claims.
Baggett and Smith in High Polymers, Vol. XVIII,
Ham, John Wiley (1964), Chapter X, Copolymerization,
pp 587 et seq., particularly at pages 593 and 610
describe the synthesis of uniform composition
distribution copolymers of vinylidene chloride and
z5 vinyl chloride and vinyl chloride and vinyl acetate.
U. S. Patent 5,296,318 teaches that only VDF/HFP
copolymers having 8 to 25~ by weight HFP and no other
VDF homo- or copolymers are suitable for use in
fabrication of lithium battery electrodes and
CA 02251648 1998-10-15
WO 98/38687
PCT/US98/04071
~ separators.
U.S. Patent 5,348,818 mentions that among other
polymers, VDF polymer may be used in forming a solid
electrolyte for use in secondary battery ~anufacture.
No particular type of polyvinylidene fluoride is
identified and no copolymers thereof of any type are
suggested.
European Patent Application 95 120 660.6-1215
published September 4, 1996 teaches use of micro
porous (open or closed cell) VDF copolymers of about
7% to about 25~ comonomer content in lithium batteries
and the use of VDF homopolymer as a cladding material
for such batteries. Use of copolymers used by the
instant invention in solid electrolytes and the
improved properties provided thereby are not taught or
suggested. Similarly the use of the type of VDF
homopolymer used in the present invention in solid
electrolytes is not taught of suggested.
None of these references teach or suggest lithium
rocking chair batteries of the type contemplated by
the present invention and U.S. Patent 5,296,318
expressly teaches away from use of VDF homopolymer of
any type, VDF/HFP copolymers of less than 8~ by weight
HFP content or other VDF containing copolymers.
U.S. Patent 5,571,634 teaches a lithium ion
battery construction employing a VDF-CTFE copolymer
where the CTFE content in the copolymer is no less
than 8~ by weight.
CA 02251648 1998-10-1
WO 98/38687
PCT~S98/04071
ST~Y OF T~ lNV~NLlON
The invention provides in a first composition
aspect a copolymer of vinylidene fluoride and
hexafluoropropylene containing a maximum of about 24
weight percent hexafluoropropylene, having solutions
s of improved clarity and fluidity; for the copolymers
having up to about 8 weight percent nominal HFP
content, having wei~ht percent extractables within
plus or minus 1.5~ of the percent by weight
extractables calculated by an equation selected from
the group consisting of:
a) wt.% Extractable = 1.7(HFP mole%) - 3.2, and
b) wt.~ Extractable = -1.2 + l.5tHFP mole%) - 8 x 10~6(Mn),
and ~or the copolymers having greater than about 8
weight percent nominal HFP content, having a DSC
(differential scanning calorimetry) melting point at
least 2.5~C lower than the DSC melting point of
copolymers having the same nominal weight percent HFP
prepared by methods for which the prior art provides
detail.
The tangible embodiments of this first
composition aspect of the invention are straw colored
to colorless semi crystalline solids having melting
points, as determined by differential scanning
calorimetry tDSC), lower than VDF/HFP copolymers
having the same nominal HFP percentage content
prepared by processes reported in detail in the prior
art.
The tangible embodiments of this first
composition aspect of the invention also possess
CA 02251648 1998-10-15
WO 98/38687
PCT/US98/04071
- longer gelation times from solution than VDF/HFP
copolymers havin~ the same nominal HFP content
prepared by processes reported in detail in the prior
art.
The aforementioned physical characteristics taken
together with the method of synthesis positively tend
to confirm the structure and the novelty of the
compositions sought to be patented.
The tangible embodiments of the first composition
aspect of the invention have the inherent applied use
characteristics of being suitable for paint and powder
coating vehicles and as chemically resistant shaped
objects and films both supported and unsupported.
Particular mention i5 made of co~olymers of the first
composition aspect of the present invention having
from about 2 weight% HFP content to about 8 weight%
HFP, still more particularly copolymers having about 3
to 6 weight% HFP which possess the inherent applied
use characteristics of being particularly suitable as
polymeric separators and polymeric electrode matrices
for batteries, particularly lithium batteries.
The prior art, see for example U.S. 5,296,318,
has reported lithium batteries made from VDF/HFP
copolymers having from 8% to 25% by weight HFP. It is
understood that the copolymers of the present
invention having HFP content in that range are also
suitable for use in such batteries and would represent
an improvement therein. Such improved batteries are
g
CA 022~1648 1998-10-1
W098/38687
PCT~S98/04071
- also contemplated by the invention as equivalents.
Particular mention is also made of copolymers o~
the first composition aspect of the invention having
from about 7 weight percent HFP content to about 15
weight percent ~P content, more particularly
copolymers having about 10 weight percent HFP content
which possess the inherent applied use characteristic
of being suitable as flame resistant insulation for
wire and cable.
Still further mention is made of copolymers of
the first composition aspect of the invention having
greater than about 15 weight percent HFP content,
still more particularly of copolymers having about 16
by weight or greater HFP content which have the
inherent applied use characteristic as clear,
flexible, chemically resistant films.
The invention provides in a second composition of
matter aspect, an improved article of manufacture
comprising an electrochemical cell having a positive
electrode, an absorber separator and a negative
electrode wherein at least either one of the
electrodes comprises a vinylidene fluoride polymer
having an electrolyte material combined therewith
and/or said absorber separator comprises a vinylidene
fluoride polymer having an electrolyte material
combined therewith wherein the improvement comprises
the polyvinylidene fluoride polymer consisting
essentially of a vinylidene fluoride polymer selected
from the group consisting of vinylidene fluoride
homopolymer having bimodal molecular weight
- 10 --
CA 02251648 1998-10-15
W O 98138687
PCT~US98/04071
- distribution, vinylidene
fluoride/chlorotrifluoroethylene copolymer having a
substantially homogeneous monomer distribution and a
vinylidene fluoride/hexafluoropropylene copolymer as
defined in the first composition aspect of the
invention.
Special mention is made of embodiments of the
second composition of the invention wherein the
VDF/HFP copolymer has a hexafluoropropylene content of
from about 2 wt % up to about 8 wt ~
hexafluoropropylene, particularly those having from
about 3 weight ~ to about 6 weight ~
hexafluoropropylene, still more particularly, those
having about 3 weight % hexafluoropropylene.
Vinylidene fluoride homopolymer having bimodal
molecular weight distribution means vinylidene
fluoride homopolymer prepared as described in U.S.
Patent 4,076,729.
Vinylidene fluoride/chlorotrifluoroethylene
copolymer having substantially homogeneous monomer
distribution means vinylidene
fluoride/chlorotrifluoroethylene copolymer prepared as
described in U.S. Patent 2,752,331.
Special mention is made of such VDF/CTFE
copolymers having from about 2 wt.~ up to about 8 wt %
CTFE content, more particularly such VDF/CTFE
copolymers having about 3 to about 6 weight percent
CTFE content.
As used herein and in the appended claims,
vinylidene fluoride polymers (or VDF polymers) of the
CA 022~1648 1998-10-1
W098/38687
PCT~S98/04071
present invention means the polyvinylidene fluoride
homopolymer having bimodal molecular weight
distribution as defined above, the VDF/CTFE copolymers
having substantially uniform monomer distribution as
defined above and/or the VDF/HFP copolymers which are
the first composition aspect of the invention.
The electrochemical cells of the type of which
the second composition of matter aspect of this
invention is an improvement are described in PCT
Application WO 95/06332, European Patent Application
95 120 660.6-1215, published as number 730,316 Al on
September 4, 1996 and U.S. Patent 5,296,318. The
disclosures of the PCT application, the European
application and the U.S. Patent are hereby
incorporated by reference.
In addition to use of solution casting techniques
for preparation of films for use in battery
constructions as described in the aforementioned
references, use of extrusion techniques to prepare
such films and the batteries fabricated therefrom are
also contemplated.
It has also been noted that batteries fabricated
from the above described VDF polymers, particularly
the VDF-HFP copolymers of the present invention, have
better adhesion o~ the polymers to metallic portions
of electrodes and higher use temperatures than
batteries fabricated from VDF-HFP copolymers of the
prior art. It has also been observed that VDF-HFP
copolymers of the present invention provide batteries
having improved electrical properties including the
CA 022S1648 1998-10-15
W O 98/38C87
PCT~S98/04071
capability of higher discharge rates than batteries
fabricated from VDF-HFP copolymers of the prior art.
It is expected by the present inventors that in
general batteries fabricated according to the present
invention will possess such higher temperature use and
higher discharge rate capabilities.
The invention provides in a third composition
aspect, a solution of a composition of the first
composition aspect of the invention having improved
solution clarity and fluidity.
Copolymers of vinylidene fluoride and
hexafluoropropylene of up to about 24 wt~
hexafluoropropylene are useful semicrystalline
thermoplastics. As the HFP content increases in the
materials, the crystallinity decreases, and,
correspondingly, the flexibility and solvent
sensitivity increase. Other properties change as
well, such as the final melting point, which decreases
with increasing HFP content. In high-purity
applications such as membrane filtration or
extraction, lithium battery construction, high-
transparency film from solution casting, and fluid
storage and transport requiring low contaminant
levels, it is desirable to have materials with low
levels of extractables, little gel formation in the
presence of solvent, and good clarity. The VDF/HFP
copolymers provided here show lower extractables,
improved solution properties, improved clarity and
fluidity, and lower melting points in comparison to
the nonuniform VDF/HFP copolymers of otherwise similar
CA 022~1648 1998-10-15
W098/38687
PCT~US98/04071
HFP content and manufacture known in the prior art.
D~.~CRIPTION OF T~ DRAWING~
Figure 1 is a comparison of the final
differential scanning colorimeter/(DSC) melting point
of copolymers of the invention with DSC melting points
of prior art compounds whose synthesis is described in
detail.
Figure 2 shows the effect on HFP level on polymer
extractibles in dimethyl carbonate (DMC) at 40~C for
copolymers of the invention and copoly~ers of the
prior art whose synthesis is described in detail.
Figure 3 shows the relationship between HFP
content and log of gelation time from solution (20 wt~
in propylene carbonate) of copolymers of the present
invention and of copolymers of the prior art having
sufficient synthesis detail for reproduction.
Figure 4 is a cross section of an electrochemical
cell in accord with the present invention.
DETATT F~n DESC~RTPTIQN
The invention provides copolymers of vinylidene
fluoride and hexafluoropropylene having hexafluoro-
propylene content o~ up to about 24 wt% and having
improved solution clarity and fluidity and reduced
extractables. The copolymers are conveniently made by
an emulsion polymerization process, but suspension and
solution processes may also be used. In an emulsion
polymerization process a reactor is charged with
deionized water, water-soluble surfactant capable of
emulsifying the reaction mass during polymerization,
paraffin antifoulant, vinylidene fluoride,
CA 022S1648 1998-10-15
W098/38687
PCT~S98/04071
- hexafluoropropylene, chain-transfer agent to control
copolymer molecular weight, and initiator to start and
maintain the polymerization. To obtain the VDF/HFP
copolymers of the present invention, the initial
charge of VDF and HFP monomers is such that the amount
of HFP is up to 48% of the combined weight of the
monomers initially charged, and then VDF and HFP are
fed continuously throughout the reaction such that the
amount of the HFP is up to 24% of the combined weight
of the monomers fed continuously. The VDF/HFP ratios
are different in the initial charge and during the
continuous feed, and each final polymer composition
has definite and related ratios for the initial charge
and continuous feed. The process uses total amounts
of VDF and HFP monomers such that the amount of HFP
used is up to about 24% of the combined total weight
of the monomers.
The reactor is a pressurized polymerization
reactor equipped with a stirrer and heat control
means. The temperature of the polymerization can vary
depending on the characteristics of the initiator
used, but it is typically between 65~ and 105~C, and
most conveniently it is between 75~ and 95~C. The
temperature is not limited to this range, however, and
might be higher or lower if a high-temperature or low-
temperature initiator is used. The VDF/~FP ratios
used in the polymerization will be dependent on the
temperature chosen for reaction. The pressure of the
polymerization is typically between 2750 and 6900 kPa,
but it can be higher if the equipment permits
CA 022~1648 1998-10- lS
WO 98/38687
PCT~S98/04071
- operation at higher pressure. The pressure is most
conveniently between 3790 and 5860 kPa.
Surfactants used in the polymerization are water-
soluble, halogenated surfactants, especially
fluorinated surfactants such as the ammonium,
substituted ammonium, quaternary ammonium, or alkali
metal salts of perfluorinated or partially fluorinated
alkyl carboxylates, the perfluorinated or partially
fluorinated monoalkyl phosphate esters, the
perfluorinated or partially fluorinated alkyl ether or
polyether carboxylates, the perfluorinated or
partially fluorinated alkyl sulfonates, and the
perfluorinated or partially fluorinated alkyl
sulfates. Some specific, but not limiting examples
are the salts of the acids described in U.S. Pat. No.
2,559,752 of the formula X(CF2)nCOOM, wherein X is
hydrogen or fluorine, M is an alkali metal, ammonium,
substituted ammonium (e.g., alkylamine of 1 to 4
carbon atoms), or quaternary ammonium ion, and n is an
integer from 6 to 20; sulfuric acid esters of
polyfluoroalkanols of the formula X(C~2)nCH2OSO3M,
where X and M are as above; and salts of the acids of
the formula CF3(CF2)n(CX2)mSO3M, where X and M are as
above, n is an integer from 3 to 7, and m is an
integer from 0 to 2, such as in potassium
perfluorooctyl sulfonate. The surfactant charge is
from 0.05~ to 2~ by weight on the total monomer weight
used, and most preferably the surfactant charge is
from 0.1% to 0.2~ by weight.
The paraffin antifoulant is conventional, and any
CA 02251648 1998-10-15
W O 98/38687
PCT~US98/04071
long-chain, saturated, hydrocarbon wax or oil may be
used. Reactor loadings of the paraffin are from 0.01
to 0.3% by weight on the total monomer weight used.
After the reactor has been charged with deionized
water, surfactant, and paraffin antifoulant, the
reactor is either purged with nitrogen or evacuated to
remove oxygen. The reactor is brought to temperature,
and chain-transfer agent may optionally be added. The
reactor is then pressurized with a mixture of
vinylidene fluoride and hexafluoropropylene.
Chain-transfer agents which may be used are well-
known in the polymerization of fluorinated monomers.
Alcohols, carbonates, ketones, esters, and ethers are
oxygenated compounds which serve as chain-transfer
agents. Specific, but not limiting examples, are
isopropyl alcohol, such as described in U.S. Pat. No.
4,360,652, acetone, such as described in U.S. Pat. No.
3,857,827, and ethyl acetate, as described in the
Published Unexamined Application ~Kokai) JP 58065711.
Other classes of compounds which serve as chain-
transfer agents in the polymerization of fluorinated
monomers are halocarbons and hydrohalocarbons such as
chlorocarbons, hydrochlorocarbons,
chlorofluorocarbons, and hydrochlorofluoro-carbons;
speci~ic, but not limiting examples are
trichlorofluoromethane, such as described in U.S. Pat.
No. 4,569,978, and 1,1-dichloro-2,2,2-trifluoroethane.
Chain-transfer agents may be added all at once at the
beginning of the reaction, in portions throughout the
reaction, or continuously as the reaction progresses.
_ _ _ _ _ _ _
CA 022~1648 1998-10-15
W O 98/38687
PCT/US98/04071
- The amount of chain-transfer agent and mode of
addition which is used depends on the activity of the
agent and the desired molecular weight characteristics
of the product. The amount of chain-transfer agent
used is from 0.05% to 5~ by weight on the total
monomer weight used, and preferably it is from 0.1 to
2~ by weight.
The reactor is pressurized by adding vinylidene
fluoride and hexafluoropropylene monomers in a
definite ratio (first effective ratio) such that the
hexafluoropropylene ranges up to 48~ of the combined
weight of the monomers initially charged. The first
effective ratio used will depend on the relative
reactivity of the two monomers at the polymerization
temperature chosen. The reactivity of vinylidene
fluoride and hexafluoropropylene has been reported in
Bonardelli et al., Polymer, vol. 27, 905-909 (June
1986). The relative reactivity is such that to obtain
a particular uniform copolymer composition, more
hexafluoropropylene has to be charged to the reactor
in the initial fill than will be incorporated into the
copolymer. At the convenient polymerization
temperature range of this invention, about twice as
much hexafluoropropylene has to be charged to the
reactor in the initial fill as will appear in the
polymer.
The reaction can be started and maintained by the
addition of any suita~le initiator known for the
polymerization of fluorinated monomers including
inorganic peroxides, "redox" combinations of oxidizing
- 18 -
CA 02251648 1998-10- lS
WO 98/38687
PCT/US98/04071
- and reducing agents, and organic peroxides. ~xamples
of typical inorganic peroxides are the ammonium or
alkali metal salts of persulfates, which have useful
acti~ity in the 65~C to 105~C temperature range.
~Redox" systems can operate at even lower temperatures
and examples include combinations of oxidants such as
hydrogen peroxide, t-butyl hydroperoxide, cumene
hydroperoxide, or persulfate, and reductants such as
reduced metal salts, iron (II) salts being a
particular example, optionally combined with
activators such as sodium formaldehyde sulfoxylate or
ascor~ic acid. Among the organic peroxides which can
be used for the polymerization are the classes of
dialkyl peroxides, peroxyesters, and peroxydi-
carbonates. Exemplary of dialkyl peroxides is di-t-
lS butyl peroxide, of peroxyesters are t-butyl
peroxypivalate and t-amyl peroxypivalate, and of
peroxydicarbonates are di(n-propyl) peroxydicarbonate,
diisopropyl peroxydicarbonate, di(sec-butyl)
peroxydicarbonate, and di(2-ethylhexyl) peroxydi-
car~onate. The use of diisopropyl peroxydicarbonate
for vinylidene fluoride polymerization and
copoly~erization with other fluorinated monomers is
taught in U.S. Pat. No. 3,475,396, and its use in
making vinylidene fluoride/hexafluoropropylene
copolymers is further illustrated in U.S. Pat. No.
4,360,652. The use of di(n-propyl~ peroxydicarbonate
in ~inylidene fluoride polymerizations is described in
the Published Unexamined Application (Kokai) JP
58065711. The quantity of an initiator required for a
- 19 -
,,
CA 022~1648 lsss-lo-
W098/38687
PCT~S98/04071
- polymerization is related to its activity and the
temperature used for the polymerization. The total
amount of initiator used is generally between 0.05% to
2.5% by weight on the total monomer weight used.
Typically, sufficient initiator is added at the
beginning to start the reaction and then additional
initiator may be optionally added to maintain the
polymerization at a convenient rate. The initiator
may be added in pure form, in solution, in suspension,
or in emulsion, depending upon the initiator chosen.
As a particular example, peroxydicarbonates are
conveniently added in the form of an aqueous emulsion.
As the reaction progresses, a mixture of
vinylidene fluoride and hexafluoropropylene monomers
is fed in a definite ratio ( second effective ratio)
so as to maintain reaction pressure. The second
effective ratio used corresponds to the monomer unit
ratio desired in the final composition of the
copolymer, and it can range up to 24% of the com~ined
weight of the monomers being fed continuously
throughout the reaction. The feed of vinylidene
fluoride, hexafluoropropylene, and optionally
initiator and chain-transfer agent is continued until
the desired reactor fill is obtained.
Upon reaching the desired reactor fill, the
monomer feeds are terminated. To achieve the
copolymer havin~ optimum solution clarity and minimal
extractables, all other feeds are stopped at the same
time as the monomer feeds, and the reactor is vented
as soon as is practicable. Alternatively, to achieve
- 20 -
- CA 022~1648 1998-10-1
W098/38687
PCT~S98/04071
- highest yield at the expense of solution clarity and
extractables, a react-out period to consume residual
monomer is used with optional continuation of
initiator feed. For react-out, the reaction
temperature and agitation are maintained for a period
S of 20 to 30 minutes, but a longer period can be used
if required in order to consume monomer to the point
where the reactor pressure is no longer falling to any
significant degree. A settling period of typically 10
to 40 minutes may be used following the react-out
period. During the settling period, temperature is
maintained but no initiator feed is used. The reactor
is then cooled and vented.
The product is recovered as a latex. To obtain
dry resin, the latex is coagulated, the coagulum is
separated and the separated coagulum may be washed.
To provide powder, the coagulum is dried.
For the coagulation step, several well-known
methods can be used including freezing, the addition
of acids or salts, or mechanical shear with optional
heating. The powder, if desired, can be further
processed into pellets or other convenient resin
forms.
The electrochemical cells of the present
invention are based on a positive electrode, an
absorber-separator sometimes referred as a solid
electrolyte and negative electrode operatively
associated with one another wherein at least one of
the electrodes or the absorber-separator, and
preferably both electrodes and the absorber-separator
CA 022~1648 1998-10-1
W098/38687
P~T~S98/04071
comprise a vinylidene fluoride polymer of the present
invention and wherein the vinylidene fluoride polymer
of the present invention electrodes have an electrode
material combined therewith and the vinylidene
fluoride polymer of the present invention absorber-
separator has an electrolyte ~aterial combined
therewith. A plurality of electrodes and absorber-
separator elements can be used in the cell structure
in order to increase the voltage, and/or amperage of
the combined elements in a manner well known to the
art.
Vinylidene fluoride polymer of the present
invention is not required to have an open or closed
porous structure for operability. It provides
enhanced electrolyte mobility in combination with the
intrinsic ionic conductivity effects of the polymer
regardless of its initial porous or non porous state.
Vinylidene fluoride electrode or separator-absorber
combined with electrode or electrolyte materials at
the surface of the pores of the porous polymer was
previously believed to make the utilization of the
active material, whether electrode material or
electrolyte material more efficient and provide a
method for the easy manufacture of the efficient
electrodes and separator-absorber structures. There
are, however, other advantages to use of porous
polymer structures even for the polyvinylidene
fluoride polymers of the present invention.
It is also believed that the segregation of the
active materials on the surface of active pores will
- 22 -
CA 022~1648 1998-10-1
WO 98/38687
PCT~S98/04071
- allow for varying the amount of binder in the
electrode of the separator-absorber to enhance
strength with minimum effect on cell performance. The
electrochemical cells formed in this way therefore
will have improved mechanical properties and can be
made to be self-supporting i.e., secondary reinforcing
structures do not have to be employed such as a metal
or other conventional battery casing material.
This also leads to ease of fabrication where the
electrochemical cell is enveloped or enclosed in a
vinylidene fluoride homopolymer which will adhere to
the porous electrodes and/or the absorber-separator
structures. Adhesion can be obtained by simple heat
bonding or radio frequency (rf) welding or other
similar processes well known in the art. Adhesives
are not required, but importantly, the exterior part
of the electrochemical cell (i.e., the envelope) is of
the same type or a substantially similar type of
material as the electrodes and absorber-separa~or and
is more compatible therewith and adherent thereto,
thereby simplifying and reducing the cost of
manufacture in that only one type of material is used
for the structural components of the cell as compared
to either conventional dry cell or secondary cell
construction.
Polyvinylidene fluoride in general absorbs rf
frequency and may also ~e heated by dielectric
techniques. Heat guns may also be used for sealing
polyvinylidene fluoride surfaces under pressure.
Welding rods may also be employed to heat seal two
- 23 -
CA 022~1648 1998-10-1
WO 98/38687
PCT/US98/04071
- pieces easily as is done in the fabrication of larger
polyvinylidene fluoride structures. The joints
obtained are usually as strong as the basic resins
employed. Because polyvinylidene fluoride polymers
are abrasion resistant and tough as well as chemical
resistant, they are useful in the internal and
external element of the battery and, as noted
previously, can be assembled by non-adhesive means by
heat bonding.
By selecting vinylidene fluoride polymers of the
present invention for electrodes and such polymers or
conventional VDF polymer for cladding that are either
extremely flexible or somewhat rigid, structures can
be fabricated that are in turn either flexible or
somewhat rigid. Further in this regard, enhanced
rigidity can be obtained by cross-linking the
vinylidene fluoride homo- or copolymers in general
either chemically, but preferably by employing high
energy radiation such as high energy (about 10 to
about 20 Mrad) electron beam radiation, with some
attendant dehydrofluorination. One potential benefit
is the further stabilization of the amorphous regions
in the vinylidene fluoride polymers, i.e., inhibitions
of crystallization over time which is important since
ionic conductivity of the electrolyte is believed to
occur primarily in the amorphous or open regions.
As noted previously, vinylidene fluoride polymers
in general affect ionic conductivity in a manner that
makes them suitable for the fabrication of
electrochemical cells.
Since mobility of charged species is required in
- 24 -
CA 022S1648 1998-10-15
W098/38687
PCT~US98/04071
- electrochemical cells, it is believed that the
migration of charged species in polyvinylidene
fluoride polymers will be through the amorphous phase.
The vinylidene fluoride polymers of the present
invention have been recognized by the invention as
having enhanced amorphous phases which are more stable
and particularly for the HFP and CTFE copolymers
provide this benefit to conductivity and the like at
lower comonomer levels, thus, providing solubility and
temperature advantages approaching that of
homopolymers.
In the triboelectric series, most polymers
stabilize electrons. Vinylidene fluoride polymers,
however, are unique in stabilizing positive holes and
are one of the most effective media in this regard,
presumably due to the highly negative gem-
difluorocar~on group.
In the special case of lithium ion batteries such
as the rocking-chair batteries described herein, the
high specific charge and small ionic size of the
lithium ion may lead to specific interactions in the
host vinylidene fluoride poly~er environment,
considering the extent of the non-polarizable,
negative gem-difluorocarbon groups available.
Since conductivity is inversely related to
crystallinity of the polyvinylidene fluoride polymer,
it has been determined that conventional copolymers of
vinylidene fluoride with about 7 to about 25
hexafluoropropylene sufficiently reduce the
crystalline structure of the polymer without
- 25 -
CA 022S1648 1998-10-1
W098/38687
PCT~S98/04071
sacrificing mechanical properties so that acceptable
ionic conductivity effects of the polymer can be
obtained. The inventors have discovered that the
vinylidene fluoride polymers of the present invention
provide benefits equal to or better than the above
conventional VDF/HFP copolymers at comonomer levels
below 8% by weight, preferably below 6~ by weight.
When employing vinylidene fluoride polymers of
the present invention in the manu~acture of electrodes
or absorber-separators, plasticizers such as organic
carbonates (e.g., ethylene carbonate, propylene
carbonate, dimethylcarbonate and the like) are
utilized in order to minimize the effect of the
crystalline structure and promote ionic conductivity.
Other solvents or plasticizers may also be employed
including diethoxyethane, diethylcarbonate,
dimethoxyethane, dipropyl carbonate and mixtures
~ thereof especially the two or three component
mixtures.
Similarly, and in accord with the present
invention, the various porous or non-porous
structures, depending on the their tensile strength,
can be mechanically oriented by stretching or the
application of tensile forces in order to enhance the
amount of beta conformation within the polymer
structure and thereby possibly promote ionic
conductivity depending upon the electrolyte and
polyvinylidene fluoride composition.
Using solvent and non-solvent combinations,
polyvinylidene fluoride polymers of the present
CA 022S1648 1998-10-lS
W098/38687
PCT~S~8/01~71
invention are cast in thin membranes. This method ls
described by Benzinger et al. in U.S. Patent No.
4,384,047 which is incorporated herein by re~erence.
The electrode materials or the electrolyte materials
as described herein can be incorporated into
polyvinylidene fluoride solution prior to casting it
into a film or sheet, after which the solution may, if
desired, be converted to a porous polyvinylidene
fluoride membrane combined with the electrode of
electrolyte materials. These films or sheets, either
with or without the electrode or electrolyte materials
can be any where from about 0.25 to about 100,
particularly from about 0.5 to about 10, and
especially from about 1 to about 8 mils thick, and are
especially suitable for further treatment by
stretching or the application of tensile forces in
order to promote the beta conformation necessary to
achieve ferroelectric properties in polyvinylidene
fluoride.
There are three classes of organic liquids, that
may be used to make solutions or dispersions of
vinylidene fluoride polymers. Active solvents are
those organic li~uids that dissolve or swell
vinylidene fluoride polymers at room temperature and
typically consist of lower alkyl ketones, esters and
amides. Latent solvents are those organic liquids
that do not dissolve vinylidene fluoride homo- or
copolymers at room temperature; however, will dissolve
polyvinylidene fluoride at elevated temperatures and
typically are medium chain length alkyl ketones,
CA 022~1648 1998-10- lS
WO 98/38687
PCT~S98/04071
- esters, glycol ethers and organic carbonates. Non-
solvents are organic liquids that do not dissolve or
swell vinylidene fluoride polymers up to the boiling
point of the liquid or the crystalline melting point
of the vinylidene fluoride polymer, whichever
condition is met first. These liquids typically are
aromatic hydrocarbons, aliphatic hydro-carbons and
chlorinated hydrocarbons or other chlorinated organic
liquids. The solvents and latent solvents are used in
the manufacture of the polyvinylidene fluoride films
or sheets of the present invention.
Examples of these organic liquids are given in
Table A which follows.
- 28 -
CA 022S1648 1998-10-lS
W098/38687
PCT~S98/04071
- TABLE A
LIQUIDS TO PREPARE SOLUTIONS OR DISPERSIONS OF PVDF
ACTIVE SOLVENTS LATENT SOLVENTS NON SOLVENTS
(APPROX.) DISSOLUTION
TEMPERATURE IN ~C
Acetone Butyrolactone (65) Hexane
TetrahydrofuranIsophorone (75) Pentane
5Methyl Ethyl KetoneMethyl Isoamyl Ketone Benzene
(102)
Dimethyl FormamideCycloh~n~ (70) Toluene
Dimethyl Acetamide Dimethyl Phthalate (110) Methanol
Tetramethyl Urea Propylene Glycol Methyl Ethanol
Ether (115)
Dimethyl Sulfcxide Propylene Carbonate (80) Car~on
Tetrachloride
Trimethyl Phosphate Diacetone Alcohol (100) o-Dichlorobenzene
N-Methyl Glycerol Tricetate (100) Trichloroethylene
Pyrrolidone
The suitability of any given liquid depends upon the
exact PVDF resin type and grade.
Other methods have been developed for the
manufacture, when desired, of open cell foam porous
polyvlnylidene fluoride polymers which are formulated
to contain chemical or physical blowing agents such as
absorbed carbon dioxide. It is preferred to use
physical blowing agents in the manufacture of
electrochemical cells since trace amounts of the
chemical ~lowing agents in the foam structure could
adversely affect the functioning of the cell. Where
carbon dioxide or comparable physical blowing agents
are employed, they are incorporated into the
polyvinylidene fluoride at super critical pressures
- 29 -
CA 022~l648 l998- lO- l~
WO 98/38687
PCT/US3~i~1C 1'71
followed by heat treatment to expand the article thus
produced. Open cell film5 of varying thickness have
been made in this manner with excellent mechanical
integrity and which have specific gravities about one
as compared to solid polyvinylidene fluoride which has
a specific gravity of from about 1.76 about 1.78.
Similarly, polyvinylidene fluoride powders can be
sintered to form a porous structure by heating the
powders in a non-solvent slurry, or under pressure
between opposed platens, until the individual
particles sufficiently melt flow into one another to
form the desired open cell structure. Other art known
methods for sintering powder polymers such as PT~E for
forming open cell porous structures as described by
Menassen et al . "A Polymer Chemist ' s View on Fuel Cell
Electrodes,~' Procee~ing of the 34th Internation~l
Power Source SymDosium, June 25-28, 1990, pp. 408-10
can also be used.
A porous film made by casting polyvinylidene
provide polymers of the present invention from a
mixture of solvents and non-solvents as described by
Benzinger et al. in U.S. Patent 4,383,047, which is
about 10 mils thick after formation from the casting
solution, may be utilized for the manufacture of an
electrochemical cell. The polymer comprises a
copolymer of Example 1 herein below. This film is
used in fabricating an absorber-separator of solid
electrolyte by making a solution of LiPF6 in 1:1
mixture by weight of ethylene carbonate:propylene
carbonate which is heated to about 125~C and the
- 30 -
CA 022~l648 lsss-lo-
W098/38687
PCT~S98/04071
- porous copolymer film immersed in the solution until
it is com~ined with the film.
Similarly, a positive electrode is made from the
same porous copolymer. A dispersion of LiMn2O4, SS
carbon black and LiPF6 in a 1:1 mixture of ethylene
carbonate and propylene carbonate along with
tetrahydrofuran (THF) was combined with the porous
film by soaking the film in the suspension which is
agitated in a vibrating beaker in order to keep solid
material in suspension until adequately combined with
the film. The film is then placed on an aluminum
foil.
A negative electrode is prepared by making a
dispersion or suspension of petroleum coke, SS carbon
black and LiPF6 in a 1:1 ethylene carbonate propylene
carbonate solution in the same manner as was done for
the preparation of the positive electrode and after
combining the suspension with the porous film, a
copper foil was placed on the film.
The proportions of the various components of the
electrode and the absorber-separator or solid
electrolyte are substantially the same as those set
forth in Examples 1 and 8 of Gozdz et al., U.S. Patent
No. 5,296,318.
The electrodes and electrolyte can also be made
from sintered polyvinylidene fluoride by forming a dry
blend of the electrode or electrolyte materials with
powdered polyvinylidene fluoride. Dry mixing
techniques, known in the art may be employed, such as
tumbler type mixing. For example, the mixture of
_ . .
CA 022~1648 1998-10-1
WO 98/38687
PCT~S~0l~71
polyvinylidene fluoride powder and the electrode or
electrolyte materials can be subjected to tumbling or
ball milling for a time to sufficiently ensure that a
good mixture is obtained. A steel or other metal
vessel, or ceramic vessel is employed, especially
where either is lined with a polyvinylidene fluoride
or PTFE layer. In the case of ball milling, steel or
other metal, or ceramic grinding balls, also coated
with a polyvinylidene fluoride or PTFE layer are used.
The polyvinylidene fluoride or PTFE coating is
employed to substantially minimize or substantially
eliminate the introduction of impurities into the
system. The milled mixtures are formed into
electrodes and electrolytes by the application of heat
and pressure as noted herein.
One of skill in the art will recognize that non-
porous VDF/HFP copolymer of the 1st composition aspect
of the invention or other vinylidene fluoride polymers
of the invention, porous or non-porous may be
substituted for the described VDF/HFP of Example 1 to
make analogous batteries.
Solvents such as ethylene carbonate and propylene
carbonate, and their equivalents, especially as noted
herein, including mixtures thereof, which are employed
in the electrode or electrolyte can be added
afterwards by soaking the electrodes and electrolyte
structures in such solvents. The soaking can be
carried out at room temperature or above to maximize
the solvating effect of these materials and to produce
optimum ionic conductivity n the electrodes or
electrolyte.
- 32 -
CA 02251648 1998-10-lS
W O 98/38687
PCT~S98/0~~71
~ The positive electrode and the negative electrode
thus prepared, are then placed on opposite sides of
the absorber-separator prepared as described above
with the copper and aluminum surfaces facing outwardly
to form a cell as illustrated in Fig. 4 in which
copper film 14 is shown as extending along one surface
of negative electrode 16 which is operatively
associated with absorber-separator 18 combined with
the electrolyte. aluminum film 22 is in contact with
positive electrode 20 which is in turn in contact with
the other face of absorber-separator 18, all of the
elements being operatively associated with one
another. An envelope 12 of polyvinylidene fluoride
homopolymer extends completely around the cell.
Envelope 12 may be a single film or a plurality of
films e.g., two or three films and extends around all
sides and completely envelops cell 10. Copper and
aluminum leads (not shown) are passed through envelope
10 to make electrical contact with films 14 and 22,
respectively and are connected to a load (not shown)
to for~ an electric circuit.
The other electrolytes described herein for the
rocking-chair cells may also be employed in lieu of
the LiPF6 salt and LiNiO2 or LiCoO2 materials
substituted for the LiMn2O4 materials in the forgoing
example. Additionally, graphite rather than petroleum
coke may be employed in the manuf acture of the
negative electrode although, petroleum coke is
especially preferred.
The vinylidene fluoride polymers of the present
invention may also be employed in cells having a
- 33 -
CA 022~1648 1998-10-1
WO 98/38687
PCT/US~)81'~ 71
- lithium organic electrolyte where the polymer is used
either as a binder for particular electrode active
materials, as a solid electrolyte for polymeric cells,
a porous mesh supporting a quasi-solid state gel
electrolyte and as the cell base material.
The vinylidene fluoride polymers of the present
invention as described herein can also be used in
lithium/oxyhalide cells as a bottom insulator. They
may also be used in zinc bromide cells as a binder for
bipolar electrodes or in nickel-metal hydride cells as
a binder for the hydride electrode or for the nickel
electrode.
The vinylidene fluoride polymers of the present
invention are also suitable for use in a silver-zinc
cell where the vinylidene fluoride polymers are used
as a binder for the zinc electrode or in a lead-acid
cell as a spacer between the electrodes and as a
separator. The vinylidene fluoride polymers may also
be used in thermal batteries for cathode active
materials. In addition to nickel-metal hydride cells,
the vinylidene fluoride polymers may also be used in
other alkaline cells such as nickel-cadmium cells, and
zinc-air cells, especially where a buffered
electrolyte is employed to counteract the
dehydrohalogenation effect of the alkali medium of
these cells.
The following Examples further illustrate the
best mode contemplated by the inventors for carrying
out their invention and are to be construed as
illustrative and not as in limitation thereof.
Melt viscosity measurements are by ASTM D3835 at
- 34 -
CA 022S1648 1998-10-lS
W O 98/38687
PCT~US98/04071
~ 232~C and 100 s-1.
Thermal properties are measured with a
Differential Scanning Calorimeter (DSC) according to
ASTM ~3418.
HFP content was determined by 19F NMR according
to the signal assignments and method described in
Pianca et al., Polymer, vol. 28, 224-230 (Feb. 1987).
A Unity 400 spectrometer at 376.3 MHz was used.
Spectra were obtained either in deuterated dimethyl
formamide at 50~ C with an excitation pulse width of
8.0 microseconds and a recycle delay of 10 seconds, in
deuterated dimethyl sulfoxide at 80~ C with an
excitation pulse width of 6.0 microseconds and recycle
delay of 5 seconds, or in deuterated acetone at 50~ C
with an excitation pulse width of 8.0 microseconds and
a recycle delay of 20 seconds.
Molecular weights were measured by size exclusion
chromatography ~SEC). A Waters 150 C chromatographic
device with a set of PL gel 2 mixed B columns with
bead size of 10 microns was used at an operating
temperature of 105 degrees C. HPLC grade dimethyl
sulfoxide (DMS0) was used as the eluant at flow rate
of 1.0 mL/min. The samp}es were prepared by
dissolution in DMS0 for 5 hours at 100 degrees C,
followed by filtration.
.
CA 022~l648 l998- lO- l~
WO 98/38687
PCT/US~lU,'~ 1' 71
MPT.F! 1
Into a 7.5 liter, stainless steel reactor were
charged 4.799 kg of deionized water, 0.230 kg of a 1
wt~ solution of a mixture of perfluoroalkanoate salts,
and 0.004 kg of paraffin wax. The mixture was purged
with nitrogen and agitated for 30 minutes. The
reactor was sealed and heated to 80 degrees Celsius.
The reactor was charged with 0.355 kg of vinylidene
fluoride, 0.049 kg of hexafluoropropylene (a ratio of
88 vinylidene fluoride/12 hexafluoropropylene), and
0.120 kg of a 5 wt~ solution of ethyl acetate in
deionized water. The reaction conditions were
stabilized at 80 degrees Celsius and 4480 kPa, and
then the polymerization was begun by introducing 0.026
kg of an initiator emulsion consisting of 2 wt~ di-n-
propyl peroxydicarbonate and 0.15 wt~ mixed
perfluoroalkanoate salts dispersed in deionized water.
The pressure rose to 4550 kPa with the addition of the
initiator emulsion. The polymerization was maintained
by the addition of the initiator emulsion at the rate
of 0.112 kg per hour, and by the addition of a mixture
of vinylidene fluoride/hexafluoropropylene in the
ratio 95 vinylidene fluoride/5 hexafluoropropylene so
as to maintain pressure. After 4.2 hours, totals of
1.890 kg of vinylidene fluoride and 0.140 kg of
hexafluoropropylene had been charged to the reactor.
All feeds were stopped, and the reactor was cooled.
After 5 minutes of cooling, agitation speed was
reduced by 78~ and surplus gases were vented.
Agitation was stopped, the reactor was further cooled,
- 36 -
CA 022~1648 1998-10- lS
WO 98/38687
PCT~S98/04071
- and then it was emptied of latex. Polymer resin was
isolated by coagulating the latex, washing the
resulting solids with boiling water, and drying the
solids at 110 degrees Celsius to yield fine powder.
The resin so made had a melt viscosity of 2770 Pa.s,
had a DSC melting point of 152 degrees Celsius, and
had a hexafluoropropylene content as measured by NM~
of 5.4 wt~.
MP~F! 2
Into a 7.5 liter, stainless steel reactor were
charged 4.913 kg of deionized water, 0.230 kg of a 1
wt~ solution of a mixture of perfluoroalkanoate salts,
and 0.004 k~ of paraffin wax. The mixture was purged
with nitrogen and agitated for 30 minutes. The
reactor was sealed and heated to 80 degrees Celsius.
The reactor was charged with 0.415 kg of vinylidene
fluoride, 0.215 kg of hexafluoropropylene (a ratio of
66 vinylidene fluoride/34 hexafluoropropylene), and
o.olO kg of ethyl acetate. The pressure was at 4895
kPa. The reaction conditions were stabilized at 80
degrees Celsius, and then the polymerization was begun
by introducing 0.040 kg of an initiator emulsion
consisting of 2 wt~ di-n-propyl peroxydicarbonate and
0.15 wt% mixed perfluoroalkanoate salts dispersed in
deionized water. The pressure dropped upon initiation
and it was then maintained at 4825 kPa. The
polymerization was maintained by the addition of the
initiator emulsion at the rate of 0.176 kg per hour,
and by the addition of a mixture of ~inylidene
fluoride/hexafluoropropylene in the ratio 84
vinylidene fluoride/16 hexafluoropropylene so as to
- 37 -
CA 022~1648 1998-10-1
W098/38687
PCT~S98/04071
- maintain pressure. After 2.2 hours, totals of 1.585
kg of vinylidene fluoride and 0.445 kg of
hexafluoropropylene had been charged to the reactor.
Monomer feeds were stopped, and residual monomer was
consumed by maintaining the initiator emulsion feed
and 80 degrees Celsius for 20 minutes. The initiator
feed and agitation were stopped and the reactor was
allowed to settle 10 minutes. The reactor was cooled
to 45 degrees Celsius, vented, and then it was emptied
of latex. Polymer resin was isolated by coagulating
the latex, washing the resulting solids with boiling
water, and drying the solids at 80 degrees Celsius to
yield fine powder. The resin so made had a melt
viscosity of 1220 Pa.s, had a DSC melting point of 114
degrees Celsius, and had a hexafluoropropylene content
as measured by NMR of 17.2 wt.~.
~X~MPT.~ 3 (Comparative Example to Example 1)
Into a 7.5 liter, stainless steel reactor were
charged 4.799 kg of deionized water, 0.230 kg of a 1
wt~ solution of a mixture of perfluoroalkanoate salts,
and 0.004 kg of paraffin wax. The mixture was purged
with nitrogen and agitated for 30 minutes. The
reactor was sealed and heated to 80 degrees Celsius.
The reactor was charged with 0.400 kg of vinylidene
fluoride, 0.030 kg of hexafluoropropylene (a ratio of
93 vinylidene fluoride/7 hexafluoropropylene), and
0.120 kg of a 5 wt.~ solution of ethyl acetate in
deionized water. The reaction conditions were
stabilized at 80 degrees Celsius and 4480 kPa, and
then the polymerization was begun by introducing 0.026
kg of an initiator emulsion consisting of 2 wt~ di-n-
- 38 -
CA 022~1648 1998-10-1~
W098/38687 PCT~S98/04071
- propyl peroxydicarbonate and 0.15 wt~ mixed
perfluoroalkanoate salts dispersed in deionized water.
The polymerization was maintained by the addition of
the initiator emulsion at the rate of 0.112 kg per
hour, and by the addition of a mixture of vinylidene
fluoride/hexafluoropropy~ene in the ratio 93
vinylidene fluoride/7 hexafluoropropylene so as to
maintain pressure. After 3.1 hours, totals of 1.890
kg of vinylidene fluoride and 0.140 kg of
hexafluoropropylene had been charged to the reactor.
Monomer feeds were stopped, and residual monomer was
consumed by maintaining the initiator emulsion feed
and 80 degrees Celsius for 20 minutes. The initiator
feed and agitation were stopped, and the reactor was
allowed to settle for 10 minutes. The reactor was
cooled to 45 degrees Celsius, vented, and then it was
emptied of latex. Polymer resin was isolated by
coagulating the latex, washing the resulting solids
with boiling water, and drying the solids at 110
degrees Celsius to yield fine powder. The resin so
made had a melt viscosity of 2550 Pa.s, had a DSC
melting point of 154 degrees Celsius, and had a
hexafluoropropylene content as measured by NMR of 6.0
wt.~.
~!lrl~MPT.~;! 4
Into a 293 liter stainless steel reactor were
charged 200.0 kg of deionized water, 1.00 kg of a 10
wt~ solution of a mixture of perfluoroalkanoate salts,
and 0.015 kg of paraffin oil. The reactor was
evacuated and heated to a temperature of 91 degrees
Celsius during the charging, and agitation was used.
- 39 -
CA 022~1648 1998-10-1~
W098/38687 PCT~S98/04071
To the reactor were added 12.6 kg of vinylidene
fluoride, 0.8 kg of hexafluoropropylene (a ratio of 94
vinylidene fluoride/6 hexafluoropropylene), and 0.5 kg
of ethyl acetate, which brought the reactor pressure
to 4480 kPa. During the pressurization, when the
pressure reached 3445 kPa, a feed of initiator
emulsion consisting of 2 wt~ di-n-propyl
peroxydicarbonate and 0.15 wt~ mixed
perfluoroalkanoate salts dispersed in deionized water
was begun and was maintained at 9.0 kg/h until 4.6 kg
of initiator emulsion had been added. The rate of
further initiator emulsion addition was adjusted so as
to maintain a total monomer feed rate of 27.0 kg/h. A
monomer mixture in the ratio 94 vinylidene fluoride/6
hexafluoropropylene was fed to the reactor so as to
maintain pressure at 4480 kPa until the totals of 85.3
kg of vinylidene fluoride and 5.4 kg of
hexafluoropropylene had been charged to the reactor.
All feeds were stopped, and residual monomer was
consumed by maintaining 91~ Celsius and agitation for
20 minutes and then by maintaining 91~ C for 35
minutes. The reactor was cooled, vented, and emptied
of latex. Polymer resin was isolated by coagulating
the latex, washing the resulting solids with water,
and drying the solids to yield fine powder. The resin
so made had a melt viscosity of 1740 Pa.s, had a DSC
melting point of 155 degrees Celsius, and had a
hexafluoropropylene content as measured by NMR of 4.7
wt.~.
~MPT.~.~ 5 to 12
Copolymers of examples 5 to 8 are made similarly to
- 40 -
CA 02251648 1998-10-15
W098/38687 PCT~,8~'71
copolymers of Examples 1 or 2, and copolymers of
examples 9 to 12 are made similarly to copolymers of
Examples 3 or 4 and are shown in Table I.
- 41 -
CA 02251648 1998-10-15
WO 98/38687
PCT/US98/04071
o ~ u~ ~ ~ .~r ~ u~ ~
0 ~ ~ r-~ N I O 111 ~ U'l O ~o 3
_1 0 ~ ~P O O O O ~ O 0 0 r1 ~-~ N
O o ~ r r-~ ~~ ~ r
~i ~ O r~r "~ 0 o
a ~
0 u~ O O r~
,t) ~D VJ ~~ ,~, o r~ N .r '~ 'I
r~ 0 ~r ~ O O o o ,~ o o ~ ~ _~
~ o r o~D m o
~ ~ ~ o o,o ~ o ~ ~r ~ c~ "
0 ~r .r O O O O ~ O O ~ r~ ~r
o ~ ~ o
o ~ o ~ o ~ ~ 0 ~ ul ~
O~ O O O ~D O O U- C
~ r .~~ O ~, 0 0 'r ~ 0
~-~ ~ ' 0"~ "~ o o o o ~1 0 0 N ~ 0 X
3 o ~ o o o ~ ~ ~ ~ o
H X 0 ~ O ~, . ~ ~.In ~r ~ m ~ ~
~ ~ ~ ~ D o ,~
O ~ r ~ I o
~4 0 ~r~r o o o o ,~ o o
0 ~ o r-U~ Ul N
0 r ,~, O ~ m ~ o u~
~3 m ~ ~r o o o o ,~ o o ,~
C r m o o ~o ul
O 0 ~D ~o OmI rO~ ~ O ~ N
0 ~ ~r O O O O _I O O ~ ~1 ~.~
0 Ul Ul O o 0 Ul ~D o N
m ~ o oo o
o ~n 0 ~r N N 0~ r O O . ~,
~n r ~ o ~ I o 0 ~ ~n r ~ .r
0 .r~r O o o o _~ o o N ~ ~I r
L
,; ~n ~ ~ 1~ s
_Y ~ o L~ L~
L~ 0 ' ' >~ ) 3
L v
S _ " i" y ~ o~ y, ~ ~, , ~ :~ ,
- S ' ~ ~ U U I ~ C Y I j ~ ~ E
~rl ~ L L~ 0 0 O~ 0 1~ L~ .
._ g L L 2 0 g ~ 2 C) 111 S
U') O U'l O
-42 -
CA 022~1648 1998-10-1~
W098/38687 PCT~S98/04071
The term "solution(s) having improved clarity
and fluidity" as used in the specification and
claims of this application means that the
solution(s) of any particular copolymer of this
invention having a particular nominal HFP content
will provide solution(s) having descriptive
properties analogous to those shown by Example 2 in
Table II when dissolved in any of the solvents
listed at the same concentration levels at which a
copolymer having about the same particular nominal
HFP content made by a typical process described in
detail in the prior art provides solution
descriptive properties analogous to those shown in
Table II for Example 12.
T~'VAT-UATION OF T~ SOT~UTION PROP~TT~ OF T}
1 5 F~MPT,P~.~
The solution properties of examples 2 and 12
are shown in Table II. Mixtures of the indicated
weight percent were prepared, using heat when
necessary to dissolve the polymer completely and
form a clear solution. Solutions were then allowed
to cool and observed daily over a period of two
weeks. The copolymer 2 showed a reduced tendency to
gel and to be clearer than the copolymer 12. The
retention of fluidity and clarity by the copolymer 2
is advantageous in applications which rely on
polymer solutions, such as in the production of cast
films and membranes.
The reduction in tendency toward gelation by
the copolymers of the present invention is further
- 43 -
CA 022~l648 lggs-lo-l~
W098/38687 PCT~S98/04071
shown in Table II A. The gelation times of
propylene carbonate solutions of some of the
examples are shown in the table. A Rheometrics
dynamic stress rheometer DSR-200 was used to measure
the gelation times of 20 wt~ solutions of the
polymers in propylene carbonate (the propylene
carbonate was of nominal 99.7~ purity). The
rheometer was fitted with a Peltier fixture and
solvent trap. A 40 mm parallel plate geometry was
used with a gap of 1 mm. Solid copolymer was mixed
with propylene carbonate at room temperature on the
day of measurement, the container was sealed, and
the solution was formed by heating and stirring the
mixture in the sealed container for l.0 hour in a
Pierce Reacti-Therm Heating/Stirring Module set at
120~C. The solutions were quickly loaded at the end
of the dissolution period into the test fixture,
which was preset at 100~C. A temperature cooling
ramp in dynamic oscillatory mode at 1 Hz was begun
as soon as the fixture temperature re-equilibrated
at 100~C; re-equilibration typically required a
minute or less. The cooling ramp was from 100~C to
15~C at a rate of 30~C/m. When 15~C was reached, a l
minute equilibration time was used, and then a time
sweep measurement was begun. The sample was held at
15~C during the time sweep measurement performed at
l radian/s. The time sweep was continued until the
gel point was reached. The gel point was taken as
the point at which the solution storage modulus, G',
and the loss modulus, Gn, became equal. The gel
- 44 -
CA 022~1648 1998-10-1~
W098/38687 PCTtUS98tO4071
time was taken as the time duration in the time
sweep to reach the gel point.
The relation between HFP content and the
logarithm of the gel time of the 20 wt~ propylene
carbonate solutions is shown in Figure 3. It can be
seen that the copolymers prepared according to the
present invention have longer gelation times than
the copolymers prepared according to the prior art
synthesis over the whole range of HFP content. The
reduced tendency toward gelation by the copolymers
of the present invention is advantageous in
processing such solutions for film casting and other
solution applications.
- 45 -
CA 022~l648 l998-l0-l~
WO 98/38687 PCT/US98/04071
TABLE II
SOLUTION PRO~l~:K~ S
Polymer ~pp~rPnre
concentration
and solvent [a] Example 2 Example 12
10% in MEK fluid, clear fluid clear
20~ in MER fluid, clear by day 2, loose gel, clear
30% in MEKby day 14,some gel,clear by day l, loose qel,
10 cloudy; by day 4, gel,
cloudy
10% in MPK fluid, clear fluid, clear
20% in MPR fluid, clear by hour 2, some gel,
clear; by day l, gel,
slightly cloudy
10% in MiBK fluid, clear by day 4, gel, clear
10~ in CPO fluid, clear fluid, clear
10% in CHO fluid, clear fluid, clear
20% in CHOby day 2,some gel,clear by day 1, some gel, clear;
20 by day 2, some gel, cloudy
10~ in EtoAC fluid, clear by day 7, some gel, clear
20% in EtoAC fluid, clear by day l, fluid, cloudy;
by day 3, some gel,
cloudy
10% in n-PrOAc fluid, clear fluid, clear
10% in i-PrOAc fluid, clear by day 6, some gel, clear
10% in EGMEA fluid, clear by day 6, gel, clear
10~ in DMC fluid, clear by day 7, some gel, clear
20~ in DMC fluid, clear by day l, some gel,
cloudy; by day 2, mostly
gel, cloudy
20% in Blend 2 fluid, clear by day 14, fluid, cloudy
[a] Polymer concentrations are Wt~ unless stated otherwise. MEK is methyl
ethyl ketone, MPR is methyl propyl ketone, MiBK is methyl isobutyl ketone,
CPO is cyclopentanone, CHO is cycloh~Y~none, EtOAc is ethyl acetate, N-
PrOAc is n-propyl acetate, i-PrOAc is isopropyl acetate, EGMEA is ethylene
glycol monomethyl ether acetate, DMC is dimethyl carbonate, Blend 2 is
composed of 35.4 parts MiBK, 29.8 parts CHO, and 30 parts DMC by weight.
- 46 -
- CA 022~1648 1998-10-1~
W O 98/38687 PCTAUS98/04071
Tli3LE II A
SOLlrrION GEI~TION TI~nE ta]
Fxam~1e N~mher Gel~tion T~me
1 425
1 512
3 342
3 394
6 4,913
6 8,322
6 12,924
934
1,553
3,191
2 77,000
2 52,400
12 14,100
12 47,500
[a] 20 wt~ solutions at 15~C in propylene
carbonate. Gelation time is in seconds.
E~AT.UATTON OF FTTM GLOSS ANn ~T.AT~TTy
Some of the non-gelled solutions from the
solution property tests were used to make films
which were tested for gloss and clarity. The films
were cast on a Leneta Form 2A opacity chart using a
0.127 meter draw down applicator having a 250
micrometer gap. The cast films were dried for three
days at room temperature. Film gloss was determined
using a HunterLab Progloss PG-2 gloss meter, and the
results are shown in Table III. Film haze was
measured by determining the whiteness index (CIEL~3
L* value) of the film on the black portion of the
opacity chart using a HunterLab Labscan 2
colorimeter, and the results are shown in Table IV.
- 47 -
CA 022~1648 1998-10-1~
W098t38687 PCT~S98/04071
Films from copolymer 2 showed higher gloss from a
wider range of solvents than films from copolymer
12. The haze in films from 2 and 12 was generally
similar, but noticeably less haze was observed in
films from 2 in several instances. The results,
taken together, show that VDF/HFP copolymer of the
present invention demonstrates an increased utility
for high-gloss, high-transparency film applications.
- 48 -
CA 022~1648 1998-10-1~
WO 98/38687 PCT/US98/04071
- TABLE III
GLOSS OF CAST FILMS
Polymer Gloss, 20 degree / 60 degree
concentration
and solvent [a]Example 2 Example 12
20~ in MEK33.6 / 69.0 31.3 / 68.7
10% in MPK31.4 / 68.9 1.3 / 18.7
10~ in CPO0.7 / 16.9 2.0 / 27.7
10~ in EtOAc29.4 / 66.6 29.4 / 68.0
10% in n-PrOAc31.9 / 70.1 16.0 / 57.0
10% in i-PrOAc31.6 / 69.4 15.4 / 56.2
10% in DMC35.4 / 70.6 30.1 / 68.6
20% in Blend 234.6 / 71.2 0.1 / 2.4
[a] Polymer concentration and solvent indicates the wt% and
solvent the films were cast from. MEK is methyl ethyl ketone,
MPK is methyl propyl ketone, CPO is cyclopentanone, EtOAc is
ethyl acetate, n-PrOAc is n-propyl acetate, i-PrOAc is
isopropyl acetate, DMC is dimethyl carbonate, Blend 2 is
composed of 35.4 parts methyl isob~tyl ketone, 29.8 parts
cycloh~none, and 30 parts DMC by weight.
- 49 -
CA 02251648 1998-10-15
WO 98/38687 PCT/US33/0 1' 71
'rABLE IV
CLARITY OF CAST FILMS
Polymer Clarity, CIELA~3 L* [b]
concentration
and solvent [a] Example 2 Exampie 12
20'~ in MEK 6.59 6.22
10% in MPK 6.19 14.48
10% in CPO 15.18 15.56
10% in EtOAc 7.38 5.84
1010% in n-PrOAc 5.64 7.34
10'~ in i-PrOAc 5.61 7.79
10% in DMC 6.21 5.73
20~ in Blend 2 5.36 17.85
[a] Polymer concentration and solvent indicates the wt'~ and
solvent the films were cast from. MEK is methyl ethyl ketone,
MPK is methyl propyl ketone, CPO is cyclopentanone, EtOAc is
ethyl acetate, n-PrOAc is n-propyl acetate, i-PrOAc is
isopropyl acetate, DMC is dimethyl carbonate, Blend 2 is
composed of 35.4 parts methyl isobutyl ketone, 29.8 parts
cycloh~n~ne, and 30 parts DMC by weight.
[b] Guide to haze:
L* ~ 7 no haze
257 ~ L* c 9 very slight haze
9 ~ L~ < 11 slight haze
11 ~ L* ~ 15 moderate haze
15 < L* severe haze
- 50 -
CA 022~1648 1998-10-1~
W098/38687 PCT~S98/04071
EVALUATION QF T~T~' T~T~'T~M~T PROPT~'T~TI~ OF THE T~'~MPLES
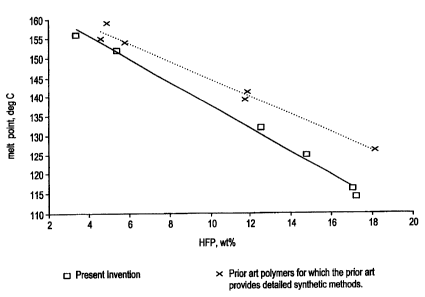
The final melting point is an important
parameter ln the use and processing of
semicrystalline polymers. It is known that the
final melting point of VDF/HFP copolymers is related
to the HFP content in the copolymers. The relation
between HFP content and final melting point of the
VDF/HFP copolymer examples is shown in Figure l.
The copolymers of the present invention and the
copolymers prepared according to the prior art
synthesis which details are available can be seen to
fall on different melting point curves, indicating
that they are different materials, with the prior
art copolymers having a higher melting point at a
given HFP content. The lower melting point property
of the copolymers of the present invention can allow
lower processing temperatures than for the prior art
synthesis copolymers.
T~VALUATION OF E~TRACT~RT~FS IN Dl~ ~Y I ~ CARBON~TE
Gener~l Procedllre
lg of polymer and 9g of dimethyl carbonate were
placed in a closed 25 ml container. The contents of
the container were continually agitated by
appropriate means while maintaining the desired
temperature by appropriate means for 24 hours. The
entire contents of the container were then
transferred to a centrifuge tube and centrifuged to
separate undissolved polymer. The liquid phase was
transferred to a suitable tared container and the
solvent evaporated. The residue in the container
CA 02251648 1998-10-15
W098/38687 PCT~S98/04071
was weighed and reported as percent by weight
extractables.
The amount of polymer extracted into dimethyl
carbonate at 40~C was measured. The data is shown
in Table V. Copolymers prepared according to
synthetic methods in the prior art for which details
are available are labeled "N". Copolymers prepared
according to the methods described for the present
invention are labeled "U".
CA 022S1648 1998-10-15
W098/38687 PCT~S98~IC71
TABLE V
~ffect of HFP Content, Molecular Number and
Uniformity of Compositional Distribution on Polymer
Dissolution in DMC
Sample }IFP ~w MnExtracta~leComposition
Lot # (mole%) (40~C) DMC
K2801 4.5 460000 145000 12.0% N
K2801 4.5 495000 157000 10.5~ N
9521 2.1 427000 167000 3.11% N
9527 3.6 473000 150000 14.30~ N
9529 2.8 417000 148000 9.29% N
88 3.6 375000 138000 4.04% U
2.3 483000 188000 0.23% U
94 2.4 676000 240000 0.41~ U
96 (Exl) 2.4 409000 159000 0.28~ U
98 2.4 351000 14~000 1.11% U
100 (Ex5) 1.5 523000 194000 0.41% U
104 3.1 433000 157000 1.61~ U
A cursory ex~mi~tion shows that all N samples
have higher levels of polymer extracted into
dimethyl carbonate. Figure 2 shows a plot of the
extractables as a function of HFP content (mole%).
Two distinct curves are outlined for the two classes
of materials. The upper curve (N samples) shows
significantly higher levels of extractables for a
given level of HFP compared to the U curve.
Measured slopes for these curves are 3%
extractables/mole % HFP for the N polymers and l.7%
extractables/mole % HFP for the U polymers.
- 53
CA 022~l648 lsss-lo-l~
:W098/38687 PCT~S98/04071
The observed and calculated % extractables
under both the single and dual functional model are
shown for the N polymers in Table VI and for the U
polymers in Table VII.
Table VI
Comparison of Wt. % Extractables of N polymer as a
function of HFP content or HFP content and Mn
% Extractable % Extractable % Extractable
(mea~) (calc model 1) (calc model 2)
12.0% 12.6% 13.4%
10.5% 12.6% 10.1%
3.11% 5.7% 3.2~
14.30% 10.0% 10.5%
9.29% 7.7% 9.7%
(Model 1) ~ Extractable = 2.9~HFP mole~) -0.4
(Model 2) ~ Extractable = 46.4 + 1.7(HFP mole~) - 0.00020(Mn)
CA 022~l648 lsss-lo-l~
W098/38687 PCT~S98/04071
Table VII
Comparison of Wt.% Extractables of U polymer as a
function of HFP content of HFP content of Mn
% Extractable % Extractable ~ Extractable
(meas) (calc model 1) (calc model 2)
4.04~ 2.9% 3.1%
0.23~ 0.71% 0.
0.41~ 0.88% 0.48
0.28% 0.88% 1.1
1.11~ 0.88~ 1.2
0.41~ -0.65~ -0.50
1.61% 2.1% 2.2
(Model 1) ~ Extractable 5 1.7(HFP mole~) -3.2
(Model 2) ~ Extractable = -1.2 + 1.5(HFP mole~) - 8 x 10~6(Mn)
In the specification and the attached claims,
the expression "having weight percent extractables
within 1.5~ of the percent by weight extractables
calculated by an equation selected from the group
consisting of:
a) wt~ Extractable = 1.7(HFP mole~) - 3.2, and
b) wt~ Extractable = -1.2 + 1.5(HFP mole~ x 10~6(Mn)
means that the measured value of percent
extractables in dimethylcarbonate at 40~C must be
within 1.5 absolute percentage points from the
extractable value calculated for the particular
polymer by either equation.
That is, if the calculated value of ~
extractables by either equation 1 or 2 is 3.0 and
the observed value is between 1.5 and 4.5% it falls
~ . ~
CA 02251648 1998-10-15
W098/38687 PCT~S98/04071
- within the intended coverage value. Similarly if
the observed value is 8.0~ it will be within the
intended coverage if the calculated value from
either equation ranges from 6.5~ to 9.5~.
~Y~ple 13 - Poly~inylidene Fluoride/ Chlorotri-
fluorethylene Copolymer Ha~ing Substantially
Homogeneous Monomer Distribution
Following a procedure analogous to that of
Example l provide an initial charge containing 0.40
kg of vinylidene fluoride and 0.0124 kg of
chlorotrifluorethylene (97 VDF/3 CTFE) and ~aintain
the reaction by a continuous feed of 96 VDF to 4
CTFE together with initiator emulsion for a total
feed convenient for the reactor size of about l.9525
kg of VDF and 0.0775 kg CTFE to obtain the title
copolymer having about 4.0~ CTFE content.
In the abo~e described proceedure for
determining extractables in dimethyl carbonate,
centrifugation for thirty minutes at 1500 rpm at
ambient temperature was employed to separate the
solution from the insoluble matter and drying at
50 deg. C for 70 hours under mechanical pump vacuum
was used to determine the weight of solids in the
separated solution.
-56-