Note: Descriptions are shown in the official language in which they were submitted.
CA 022.7 1783 1998 - 10 - 1.,
Le A 31 749-Foreign Countries / WA/li/S-P
- I -FIL~
oalyl pyn~zoles I '~ ~ , L ~ r, ;J :~
The invention relates to novel 3-cyanoaryl-pyrazoles, to processes for their p,epaldlion
and to their use as herbicides.
It is known that certain substituted 3-aryl-pyrazoles have herbicidal properties (cf.
EP 361114, EP 447055, WO 92/02509, WO 92/06962, WO 94/26109, WO 95/33728,
WO 96/01255). However, the herbicidal activity of these compounds and their
compatibility with crop plants are not always entirely satisfactory. Starting from the
closest prior art, it was an object of the present invention to synthesize herbicides based
on 3-cyanoaryl-pyrazoles having optimized herbicidal activity and at the same time
compatability with crop plants.
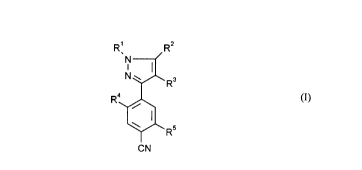
This invention, accordingly, provides the novel 3-cyanoaryl-pyrazoles of the general
formula (I)
R1 R2
Nl ~(R3
R4~ (1)
~Rs
CN
in which
15 R' represents hydrogen, represents optionally cyano-, halogen- or C,-C4-alkoxy-
substituted alkyl having I to 6 carbon atoms, represents respectively optionallyhalogen-substituted alkenyl or alkinyl having in each case 2 to 6 carbon atoms,
or represents respectively optionally cyano-, halogen- or Cl-C4-alkyl-substituted
cycloalkyl or cycloalkylalkyl having in each case 3 to 6 carbon atoms in the
cycloalkyl group and optionally I to 4 carbon atoms in the alkyl moiety,
CA 022~1783 1998-10-1~
- Le A 31 749-Foreign Countries
- 2 -
R2 represents optionally cyano-, halogen-, C,-C4-alkoxy- or C,-C4-alkylthio-
substituted alkyl having I to 6 carbon atoms, represents respectively optionallyhalogen-substituted alkenyl or alkinyl having in each case 2 to 6 carbon atoms,
or represents respectively optionally cyano-, halogen- or C,-C4-alkyl-substituted
cycloalkyl or cycloalkylalkyl having in each case 3 to 6 carbon atoms in the
cycloalkyl group and optionally I to 4 carbon atoms in the alkyl moiety,
R3 represents hydrogen, halogen or optionally cyano-, halogen-, Cl-C4-alkoxy- or
C,-C4-alkylthio-substituted alkyl having I to 6 carbon atoms,
R4 represents hydrogen or halogen and
10 R5 represents hydrogen, hydroxyl, mercapto, amino, hydroxyamino, halogen, or
represents one of the radicals -Q-R6, -NH-R6, -NH-O-R6, -NH-SO2-R6, -CQI-R6,
-cQl-Q2-R6~-cQl-NH-R6~-Q2-cQl-R6~-NH-cQl-R6 Q2 cQI Q2 R6 NH CQI
Q2-R6, -N(SO2-R6)2, -N(SO2-R6)(CQI-R6) or -Q2-CQI-NH-R6, where Q represents
O, S, SO or SO2, Ql and Q2 each represent oxygen or sulphur and R6 represents
optionally cyano-, halogen-, Cl-C4-alkoxy-, Cl-C4-alkylthio-, Cl-C4-alkyl-
carbonyl-, Cl-C4-alkoxy-carbonyl- or Cl-C4-alkylamino-carbonyl-substituted
alkyl having I to 6 carbon atoms, represents respectively optionally cyano-,
carboxyl-, halogen-, Cl-C4-alkylcarbonyl-, C,-C4-alkoxycarbonyl- or C,-C4-
alkylamino-carbonyl-substituted alkenyl or alkinyl having in each case 2 to 6
carbon atoms, represents respectively optionally cyano-, carboxyl-, halogen-,
C,-C4-alkyl-carbonyl- or Cl-C4-alkoxycarbonyl-substituted cycloalkyl or
cycloalkylalkyl having in each case 3 to 6 carbon atoms in the cycloalkyl group
and optionally I to 4 carbon atoms in the alkyl moiety, represents respectively
optionally hydroxyl-, mercapto-, amino-, cyano-, carboxyl-, carbamoyl-,
thiocarbamoyl-, Cl-C4-alkyl-, Cl-C4-halogenoalkyl-, Cl-C4-alkoxy-, Cl-C4-
halogenoalkoxy-, Cl-C4-alkylthio-, Cl-C4-halogenoalkylthio-, Cl-C4-
alkylsulphinyl-, Cl-C4-alkylsulphonyl-, C,-C4-alkylamino- or dimethylamino-
substituted aryl or arylalkyl having in each case 6 or 10 carbon atoms in the
aryl group and optionally I to 4 carbon atoms in the alkyl rnoiety, or
CA 022~1783 1998-10-1~
Le A 31 749-Forei~n Countries
-- 3 --
represents respectively optionally hydroxyl-, mercapto-, amino-, cyano-,
carboxyl-, carbamoyl-, thiocarbamoyl-, C,-C4-alkyl-, Cl-C4-halogenoalkyl-,
C,-C4-alkoxy-, C~-C4-halogenoalkoxy-, C,-C4-alkylthio-, C,-C4-
halogenoalkylthio-, C,-C4-alkylsulphinyl-, Cl-C4-alkylsulphonyl-, C,-C4-
alkylamino- or dimethylamino-substituted heterocyclyl or heterocyclylalkyl
having 2 to 6 carbon atoms and I to 3 nitrogen atoms and/or I or 2 oxygen
atoms and/or one sulphur atom in the heterocyclyl group and optionally I to 4
carbon atoms in the alkyl moiety.
The novel 3-cyanoaryl-pyrazoles of the general formula (I) are obtained when
10 hydrazine or derivatives thereof of the general formula (II)
H2N-NH-R' (II)
in which
R' is as defined above
are reacted with l-cyanoaryl-1,3-dicarbonyl compounds of the general formula (III)
0~ R2
~~ R3
R4~ (Ill)
~~ Rs
CN
in which
R2, R3, R4 and R5 are each as defined above,
if appropriate in the presence of a reaction auxiliary and if appropriate in the presence
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 4 -
of a diluent,
and, if appropriate, further conversions within the limits of the above definition of
substituents are carried out by customary methods on the resulting compounds of the
formula (I).
The compounds of the general formula (I) can be converted by customary methods into
other compounds of the general formula (I) according to the above definition of
substituents, for example by customary alkylation, acylation or sulphonylation reactions
(for example R': H ~ CH3, CHF2, C2H5, CH2CH=CH2; R5: OH ~ OCH3, OC2H5,
OCHF2, OCH2CH=CH2, OCOCH3; SH ~ SCH3, SC2H5; NH2 ~ NHC3H" NHCOCH3,
10 NHSO2CH3), or by electrophilic or nucleophilic substitution reactions (for example R3:
H ~ Cl, Br; R5: F ~ OH, SH, NH2) - cf. also the Preparation Examples.
The novel 3-cyanoaryl-pyrazoles of the general formula (1) have strong herbicidal
activity.
In the definitions, saturated or unsaturated hydrocarbon radicals, such as alkyl, alkenyl
15 or alkinyl, are in each case straight-chain or branched.
Halogen generally represents fluorine, chlorine, bromine or iodine, preferably fluorine,
chlorine or bromine, in particular fluorine or chlorine.
The invention preferably provides compounds of the formula (I) in which
R' represents hydrogen, represents respectively optionally cyano-, fluorine-,
chlorine-, methoxy- or ethoxy-substituted methyl, ethyl, n- or i-propyl, n-, i-, s-
or t-butyl, represents respectively optionally fluorine-, chlorine- or bromine-
substituted propenyl, butenyl, propinyl or butinyl, or represents respectively
optionally cyano-, fluorine-, chlorine-, bromine-, methyl-, ethyl-, n- or i-propyl-
substituted cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl,cyclobutylmethyl, cyclopentylmethyl or cyclohexylmethyl,
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
R2 represents optionally cyano-, fluorine-, chlorine-, methoxy-, ethoxy-, methylthio-
or ethylthio-substituted methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl,
represents respectively optionally fluorine-, chlorine- or bromine-substituted
propenyl, butenyl, propinyl or butinyl, or represents respectively optionally
cyano-, fluorine-, chlorine-, bromine-, methyl-, ethyl-, n- or i-propyl-substituted
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl,
cyclobutylmethyl, cyclopentylmethyl or cyclohexylmethyl,
R3 represents hydrogen, fluorine, chlorine, bromine or optionally cyano-, fluorine-,
chlorine-, methoxy-, ethoxy-, methylthio- or ethylthio-substituted methyl, ethyl,
n- or i-propyl, n-, i-, s- or t-butyl,
R4 represents hydrogen, fluorine, chlorine or bromine and
R5 represents hydrogen, hydroxyl, mercapto, amino, hydroxyamino, fluorine,
chlorine, bromine, or represents one of the radicals -Q-R6, -NH-R6, -NH-O-R6,
-NH sO2-R, cQ, R6~-cQl-Q2-R6~-cQl-NH-R6 Q2 CQ' R6 NH CQ' R6 Q2
lS CQ'-Q2-R6, -NH-CQ'-Q2-R6, -N(SO2-R6)2, -N(SO2-R6)(CQ'-R6) or -Q2-CQ'-NH-
R6, where Q represents O, S, SO or SO2, Q' and Q2 each represent oxygen or
sulphur and R6 represents respectively optionally cyano-, fluorine-, chlorine-,
methoxy-, ethoxy-, methylthio-, ethylthio-, acetyl-, propionyl-,
methoxycarbonyl-, ethoxycarbonyl-, methylaminocarbonyl- or ethylamino-
carbonyl-substituted methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl, represents
respectively optionally cyano-, carboxyl-, fluorine-, chlorine-, bromine-, acetyl-,
propionyl-, methoxycarbonyl-, ethoxycarbonyl-, methylaminocarbonyl- or
ethylaminocarbonyl-substituted propenyl, butenyl, propinyl or butinyl, represents
respectively optionally cyano-, carboxyl-, fluorine-, chlorine-, bromine-, acetyl-,
propionyl-, methoxycarbonyl- or ethoxycarbonyl-substituted cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, cyclopropylmethyl, cyclobutylmethyl,
cyclopentylmethyl or cyclohexylmethyl, represents respectively optionally
hydroxyl-, mercapto-, amino-, cyano-, carboxyl-, carbamoyl-, thiocarbamoyl-,
methyl-, ethyl-, n- or i-propyl-, n-, i-, s- or t-butyl-, difluoromethyl-,
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
-- 6 --
trifluoromethyl-, methoxy-, ethoxy-, n- or i-propoxy-, difluoromethoxy-,
trifluoromethoxy-, methylthio-, ethylthio-, difluoromethylthio-,
trifluoromethylthio-, methylsulphinyl-, ethylsulphinyl-, methylsulphonyl-,
ethylsulphonyl-, methylamino-, ethylamino- or dimethylamino-substituted
phenyl, benzyl or phenylethyl, or represents respectively optionally hydroxyl-,
mercapto-, amino-, cyano-, carboxyl-, carbamoyl-, thiocarbamoyl-, methyl-,
ethyl-, n- or i-propyl-, n-, i-, s- or t-butyl-, difluoromethyl-, trichloromethyl-,
difluoromethyl-, trifluoromethyl-, chlorodifluoromethyl-, fluorodichloromethyl-,methoxy-, ethoxy-, n- or i-propoxy-, difluoromethoxy-, trifluoromethoxy-,
methylthio-, ethylthio-, n- or i-propylthio-, difluoromethylthio-,
trifluoromethylthio-, chlorodifluoromethylthio-, fluorodichloromethylthio-,
methylsulphinyl-, ethylsulphinyl-, methylsulphonyl-, ethylsulphonyl-,
methylamino-, ethylamino-, n- or i-propylamino- or dimethylamino-substituted
heterocyclyl or heterocyclylalkyl from the series oxiranyl, oxetanyl, furyl,
tetrahydrofuryl, dioxolanyl, thienyl, tetrahydrothienyl, pyrrolyl, pyrazolyl,
imidazolyl, triazolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, oxadiazolyl,
thi~ 701yl, pyridinyl, pyrimidinyl, triazinyl, pyrazolylmethyl, furylmethyl,
thienylmethyl, oxazolylmethyl, isoxazolylmethyl, thiazolylmethyl,
pyridinylmethyl, pyrimidinylmethyl.
20 The invention in particular relates to compounds of the formula (I) in which
R' represents respectively optionally cyano-, fluorine-, chlorine-, methoxy- or
ethoxy-substituted methyl or ethyl, represents respectively optionally fluorine-or chlorine-substituted propenyl, butenyl, propinyl or butinyl, or represents
optionally cyano-, fluorine-, chlorine-, methyl- or ethyl-substituted cyclopropyl,
25 R2 represents respectively optionally cyano-, fluorine-, chlorine-, methoxy-,ethoxy-, methylthio- or ethylthio-substituted methyl or ethyl, represents
respectively optionally fluorine- or chlorine-substituted propenyl, butenyl,
propinyl or butinyl,
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
R3 represents hydrogen, fluorine, chlorine, bromine or optionally cyano-, fluorine-,
chlorine, methoxy-, ethoxy-, methylthio- or ethylthio-substituted methyl or
ethyl,
R4 represents hydrogen, fluorine or chlorine and
S R5 represents hydroxyl, mercapto, amino, hydroxyamino, fluorine, chlorine,
bromine, or represents one of the radicals -Q-R6, -NH-R6, -NH-O-R6, -NH-
R6 CQ' R6 CQ' Q2 R6 -cQl-NH-R6~-Q2-cQl-R6~-NH-cQ-R~-Q-cQ-
Q2-R6, -NH-CQ'-Q2-R6, -N(SO2-R6)2, -N(SO2-R6)(CQ'-R6) or-Q2-CQ'-NH-R6,
where Q represents O, S, SO or SO2, Q' and Q2 each represent oxygen or
sulphur and R6 represents respectively optionally cyano-, fluorine-, chlorine-,
methoxy-, ethoxy-, methylthio-, ethylthio-, acetyl-, propionyl-,
methoxycarbonyl-, ethoxycarbonyl-, methylaminocarbonyl- or
ethylaminocarbonyl-substituted methyl, ethyl, n- or i-propyl, n-, i-, s- or t-butyl,
represents respectively optionally cyano-, carboxyl-, fluorine-, chlorine-,
bromine-, acetyl-, propionyl-, methoxycarbonyl-, ethoxycarbonyl-,
methylaminocarbonyl or ethylaminocarbonyl-substituted propenyl, butenyl,
propinyl or butinyl, represents respectively optionally cyano-, carboxyl-,
fluorine-, chlorine-, bromine-, acetyl-, propionyl-, methoxycarbonyl- or
ethoxycarbonyl-substituted cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cyclopropylmethyl, cyclobutylmethyl, cyclopentylmethyl or cyclohexylmethyl,
represents respectively optionally hydroxyl-, mercapto-, amino-, cyano-,
carboxyl-, carbamoyl-, thiocarbamoyl-, methyl-, ethyl-, n- or i-propyl-, n-, i-, s-
or t-butyl-, difluoromethyl-, trifluoromethyl-, methoxy-, ethoxy-, n- or i-
propoxy-, difluoromethoxy-, trifluoromethoxy-, methylthio-, ethylthio-,
difluoromethylthio-, trifluoromethylthio-, methylsulphinyl-, ethylsulphinyl-,
methylsulphonyl-, ethylsulphonyl-, methylamino-, ethylamino- or
dimethylamino-substituted phenyl, benzyl or phenylethyl, or represents
respectively optionally hydroxyl-, mercapto-, amino-, cyano-, carboxyl-,
carbamoyl-, thiocarbamoyl-, methyl-, ethyl-, n- or i-propyl-, n-, i-, s- or t-butyl-,
dichloromethyl-, trichloromethyl-, difluoromethyl-, trifluoromethyl-,
_ . .
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 8 -
chlorodifluoromethyl-, fluorodichloromethyl-, methoxy-, ethoxy-, n- or i-
propoxy-, difluoromethoxy-, trifluoromethoxy-, methylthio-, ethylthio-, n- or i-propylthio-, difluoromethylthio-, trifluoromethylthio-, chlorodifluoromethylthio-,
fluorodichloromethylthio-, methylsulphinyl-, ethylsulphinyl-, methylsulphonyl-,
ethylsulphonyl-, methylamino-, ethylamino-, - n- or i-propylamino- or
dimethylamino-substituted heterocyclyl or heterocyclylalkyl from the series
oxiranyl, oxetanyl, furyl, tetrahydrofuryl, dioxolanyl, thienyl, tetrahydrothienyl,
pyrrolyl, pyrazolyl, imidazolyl, triazolyl, oxazolyl, isoxazolyl, thiazolyl,
isothiazolyl, oxadiazolyl, tl~ 701yl, pyridinyl, pyrimidinyl, pyrazolylmethyl,
furylmethyl, thienylmethyl, oxazolylmethyl, isoxazolylmethyl, thiazolylmethyl.
Very particular preference is given to 3-cyanoaryl-pyrazoles of the formulae
R \ CH3 R \ CF3 R \ CH3
N~CI N~(CI NN~H
F ~ Rs F ~ Rs F ~' Rs
CN CN CN
R \ CF3 R \ CH3 R \ CF3
N ~( H NN ~( Br N ~ ( Br
R's ~lRs F~ Rs
CN CN CN
where R' and R5 are each as defined above.
The radical definitions listed above, whether general or listed in ranges of preference,
15 apply both to the end products of the formula (I) and, correspondingly, to the starting
materials or intermediates required in each case for the preparation. These radical
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
g
definitions can be combined with one another as desired, thus including combinations
between the preferred ranges indicated.
Using, for example, methylhydrazine and 2-chloro- 1 -(4-cyano-2-fluoro-5-methoxy-
phenyl)-4,4-difluoro-butane-1,3-dione as starting materials, the course of the reaction
S in the process according to the invention can be represented by the following equation:
O~CHF2 H3C_N ~CHF2
INH~CH3 ~J~CI N~CI
NH2 F~OCH3 ~OCH3
CN CN
The formula (II) provides a general definition of the hydrazine derivatives to be
employed as starting materials in the process according to the invention for preparing
compounds of the formula (I). In the formula (II), R' preferably or in particular has
10 those meanings which have already been indicated above, in connection with the
description of the compounds of the formula (I) according to the invention as being
preferred or as being particularly preferred for R'.
The starting materials of the formula (II) are known chemicals for synthesis.
The formula (III) provides a general definition of the l-cyanoaryl-1,3-dicarbonyl
15 compounds further to be employed as starting materials for the process according to the
invention. In the formula (III), R2, R3, R4 and R5 each preferably or in particular have
those meanings which have already been indicated above, in connection with the
description of the compounds of the formula (I) according to the invention, as being
preferred or as being particularly preferred for R2, R3, R4 and R5.
20 The starting materials of the formula (III) have hitherto not been disclosed in the
literature; as novel compounds, they form part of the subject matter of the present
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
~ ~ ' - 10 -
application.
The novel l-cyanoaryl-1,3-dicarbonyl compounds of the formula (111) are obtainedwhen
(a) carboxylic esters of the general formula (IV)
Oq~R2
(IV)
O'R
in which
R2 is as defined above and
R represents alkyl (preferably Cl-C4-alkyl, in particular methyl or ethyl)
are reacted with cyanophenyl ketones of the general formula (V)
~R
R4~ (V)
~Rs
CN
in which
R3, R4 and R5 are each as defined above,
if appropriate in the presence of a reaction auxiliary, such as, for example, sodium
methoxide, and if appropriate in the presence of one or more diluents, such as, for
15 example, diethyl ether and methanol, at temperatures between -20~C and +50~C (cf. the
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
Preparation Examples),
or when
(b) keto esters of the general formula (VI)
o~,R2
qJ~ (Vl)
O'R
5 in which
R2 and R3 are each as defined above and
R represents alkyl (preferably C,-C4-alkyl)
are reacted with cyanobenzyl halides of the general formula (VII)
R4 O ~,X
(VII)
R5
CN
10 in which
R4 and R5 are each as defined above and
X represents halogen (preferably fluorine, chlorine or bromine, in particular
chlorine),
.
CA 022~1783 1998-10-1~
Le A 31 749-Forei~n Countries
- 12 -
if appropriate in the presence of reaction auxiliaries, such as, for example, triethylamine
and magnesium chloride, and if appropriate in the presence of a diluent, such as, for
example, acetonitrile, at temperatures between -20~C and +50~C and the resultingcyanobenzoyl keto esters of the general formula (VIII)
0~~
S R4~ R R (VIII)
~R5
CN
in which
R, R2, R3, R4 and R5 are each as defined above
are reacted with a strong acid, such as, for example, trifluoroacetic acid, if appropriate
in the presence of a diluent, such as, for example, methylene chloride, at temperatures
10 between -20~C and +50~C (cf. the Preparation Examples).
The carboxylic esters of the general formula (IV) to be used as intermediates are
known organic chemicals for synthesis.
The cyanophenyl ketones of the general formula (V) further to be used as intermediates
are known and/or can be prepared by known processes (cf. Chem. Pharm. Bull. 33
(1985), 1360-1366; EP 166609; EP 628550; WO 94/05153).
Not yet known from the literature and as novel compounds part of the subject matter
of the present application are the compounds of the general formula (Va)
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 13 -
q'~ R
X~ (Va)
R5
CN
in which
R3 and R5 are each as defined above and
X' represents halogen (preferably fluorine or chlorine).
S The novel cyanophenyl ketones of the formula (Va) are obtained when
nitroalkylbenzonitriles of the general formula (IX)
02N~' R3
X~ (IX)
~Rs
CN
in which
R3, R5 and X' are each as defined above,
10 are reacted with an acid, such as, for example, acetic acid, at temperatures between 0~C
and 120~C (cf. the Preparation Examples).
The nitroalkylbenzonitriles of the formula (IX) required here as intermediates are not
yet known from the literature; as novel compounds, they form part of the subjectmatter of the present application.
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 14 -
The novel nitroalkylbenzonitriles of the formula (IX) are obtained when
halogenobenzonitriles of the general formula (X)
X~
l l (X)
R5
CN
in which
5 R5 and X' are each as defined above and
X2 represents halogen (preferably fluorine or chlorine)
are reacted with nitroalkanes of the general forrnula (XI)
02N R3 (XI)
in which
10 R3 is as defined above,
in the presence of an acid acceptor, such as, for example, 1,8-diazabicyclo-[5,4,0]-
undec-7-ene (DBU) and if appropriate in the presence of a diluent, such as, for
- example, ethyl acetate, at temperatures between -20~C and +40~C (cf. the Preparation
Examples).
15 The intermediates of the forrnula (X) are known and/or can be prepared by processes
known per se (cf. EP 191185, EP 433124, EP 431373, EP 497239, EP 557949, EP
635486).
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 15 -
The intermediates of the formula (XI) are known organic chemicals for synthesis.
The keto esters of the formula (VI) which are, if appropriate, employed as
intermediates are also known organic chemicals for synthesis.
The cyanobenzoyl halides of the formula (VII) further, if appropriate, to be employed
5 as intermediates are known and/or can be prepared by known processes (cf. Arch.
Pharm. 323 (1990), 507-512; EP 166609; WO 96/01255).
The cyanobenzoyl halides of the formula (VII) are obtained when cyanobenzoic acids
of the general formula (XII)
O~,,OH
R4~ (XII)
~R5
CN
in which
R4 and R5 are each as defined above
are reacted with halogenating agents, such as, for example, phosgene (or its dimer or
trimer) or thionyl chloride, if appropriate in the presence of a reaction auxiliary, such
as, for example, N,N-dimethylformamide, and if appropriate in the presence of a
diluent, such as, for example, tetrachloromethane, at temperatures between 0~C and
120~C (cf. the Preparation Examples).
The cyanobenzoic acids of the formula (XII) required as intermediates are known
and/or can be prepared by known processes (cf. Arch. Pharm. 323 (1990), 507-512;Collect. Czech. Chem. Commun. 40 (1975), 3009-3019; Chem. Pharm. Bull. 27 (1979),
3039-3048; J. Chem. Soc., Perkin Trans. I 1994, 1679-1684; Tetrahedron Lett. 31
(1990), 7223-7226; EP 166609; EP 351856; WO 93/15078; Preparation Examples).
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
~ ~ - 16 -
Particularly suitable diluents for carrying out the process according to the invention for
preparing the novel compounds of the formula (I) are organic solvents. These include
in particular aliphatic, alicyclic or aromatic, optionally halogenated hydrocarbons, such
as, for example, benzine, benzene, toluene, xylene, chlorobenzene, dichlorobenzene,
5 petroleum ether, hexane, cyclohexane, dichloromethane, chloroform, carbon
tetrachloride; ethers, such as diethyl ether, diisopropyl ether, dioxane, tetrahydrofuran
or ethylene glycol dimethyl ether or ethylene glycol diethyl ether; ketones, such as
acetone, butanone or methyl isobutyl ketone; carboxylic acids, such as, for example,
acetic acid or propionic acid, nitriles, such as acetonitrile, propionitrile or butyronitrile;
10 amides, such as N,N-dimethylformamide, N,N-dimethylacetamide, N-methyl-
formanilide, N-methyl-pyrrolidone or hexamethylphosphoric triamide; esters such as
methyl acetate or ethyl acetate, sulphoxides, such as dimethyl sulphoxide, alcohols,
such as methanol, ethanol, n- or i-propanol, ethylene glycol monomethyl ether, ethylene
glycol monoethyl ether, diethylene glycol monomethyl ether or diethylene glycol
15 monoethyl ether.
Suitable reaction auxiliaries for the process according to the invention are generally the
customary inorganic or organic bases or acid acceptors. These include preferably alkali
metal or alkaline earth metal acetates, amides, carbonates, bicarbonates, hydrides,
hydroxides or alkoxides, such as, for example, sodium acetate, potassium acetate or
20 calcium acetate, lithium amide, sodium amide, potassium amide or calcium amide,
sodium carbonate, potassium carbonate or calcium carbonate, sodium bicarbonate,
potassium bicarbonate or calcium bicarbonate, lithium hydride, sodium hydride,
potassium hydride or calcium hydride, lithium hydroxide, sodium hydroxide, potassium
hydroxide or calcium hydroxide, sodium methoxide or potassium methoxide, sodium
25 ethoxide or potassium ethoxide, sodium n- or i-propoxide or potassium n- or
i-propoxide, sodium n-, i-, s- or t-butoxide or potassium n-, i-, s- or t-butoxide;
furthermore also basic organic nitrogen compounds, such as, for example
trimethylamine, triethylamine, tripropylamine, tributylamine, ethyldiisopropylamine,
N,N-dimethyl-cyclohexylamine, dicyclohexylamine, ethyl-dicyclohexylamine,
30 N,N-dimethyl-aniline, N,N-dimethyl-benzylamine, pyridine, 2-methyl-, 3-methyl-,
4-methyl-, 2,4-dimethyl-, 2,6-dimethyl-, 3,4-dimethyl- and 3,5-dimethyl-pyridine,
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
5-ethyl-2-methyl-pyridine, 4-dimethylamino-pyridine, N-methyl-piperidine,
1,4-diazabicyclo[2,2,2]-octane (DABCO), 1,5-diazabicyclo[4,3,0]-non-5-ene (DBN), or
1,8 diazabicyclo[5,4,0]-undec-7-ene (DBU).
In the practice of the process according to the invention, the reaction temperatures can
5 be varied over a relatively wide range. Generally, the reaction is carried out at
temperatures between 0~C and 150~C, preferably between 20~C and 120~C.
The process according to the invention is generally carried out at atmospheric pressure.
However, it is also possible to carry out the process according to the invention under
elevated or reduced pressure - generally between 0.1 bar and 10 bar.
10 In the practice of the process according to the invention, the starting materials are
generally employed in approximately equimolar amounts. However, it is also possible
to use a relatively large excess of one of the components. The reaction is generally
carried out in a suitable diluent, and the reaction mixture is generally stirred for several
hours at the temperature required. Work-up is carried out by conventional methods (cf.
15 the Preparation Examples).
The active compounds according to the invention can be used as defoliants, desiccants,
haulm killers and~ especially, as weed-killers. By weeds in the broadest sense, there are
to be understood all plants which grow in locations where they are undesirable.
Whether the substances according to the invention act as total or selective herbicides
20 depends essentially on the amount used.
The active compounds according to the invention can be used, for example, in
connection with the following plants:
Dicotyledonous weeds of the ~enera: Sinapis, Lepidium, Galium, Stellaria, Matricaria,
Anthemis, Galinsoga, Chenopodium, Urtica, Senecio, Amaranthus, Portulaca,
25 Xanthium, Convolvulus, Ipomoea, Polygonum, Sesbania, Ambrosia, Cirsium, Carduus,
Sonchus, Solanum, Rorippa, Rotala, Lindernia, Lamium, Veronica, Abutilon, Emex,
Datura, Viola, Galeopsis, Papaver, Centaurea, Trifolium, Ranunculus and Taraxacum.
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
Dicotyledonous crops of the genera: Gossypium, Glycine, Beta, Daucus, Phaseolus,Pisum, Solanum, Linum, Ipomoea, Vicia, Nicotiana, Lycopersicon, Arachis, Brassica,
Lactuca, Cucumis and Cucurbita.
Monocotyledonous weeds of the genera: Echinochloa, Setaria, Panicum, Digitaria,
5 Phleum, Poa, Festuca, Eleusine, Brachiaria, Lolium, Bromus, Avena, Cyperus,
Sorghum, Agropyron, Cynodon, Monochoria, Fimbristylis, Sagittaria, Eleocharis,
Scirpus, Paspalum, Ischaemum, Sphenoclea, Dactyloctenium, Agrostis, Alopecurus and
Apera.
Monocotyledonous crops of the genera: Oryza, Zea, Triticum, Hordeum, Avena,
10 Secale, Sorghum, Panicum, Saccharum, Ananas, Asparagus and Allium.
However, the use of the active compounds according to the invention is in no wayrestricted to these genera, but also extends in the same manner to other plants.
The compounds are suitable, depending on the concentration, for the total control of
weeds, for example on industrial terrain and railway tracks, and on paths and squares
15 with or without tree plantings. Equally, the compounds can be employed for controlling
weeds in perennial cultures, for example forests, decorative tree pl~nting~, orchards,
vineyards, citrus groves, nut orchards, banana plantations, coffee plantations, tea
plantations, rubber plantations, oil palm plantations, cocoa plantations, soft fruit
pl~nting~ and hopfields, on lawns, turf and pasture-land, and for the selective control
20 of weeds in annual cultures.
The compounds of the formula (I) according to the invention are suitable in particular
for selectively controlling monocotyledonous and dicotyledonous weeds in
monocotyledonous crops, both pre-emergence and post-emergence.
The active compounds can be converted into the customary formulations, such as
25 solutions, emulsions, wettable powders, suspensions, powders, dusting agents, pastes,
soluble powders, granules, suspo-emulsion concentrates, natural and synthetic materials
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- . - 19 -
impregnated with active compound, and very fine capsules in polymeric substances.
These formulations are produced in a known manner, for example by mixing the active
compounds with extenders, that is liquid solvents and/or solid carriers, optionally with
the use of surfactants, that is emulsifiers and/or dispersing agents and/or foam-forming
5 agents.
If the extender used is water, it is also possible to employ for example organic solvents
as auxiliary solvents. Essentially, suitable liquid solvents are: aromatics, such as xylene,
toluene or alkylnaphthalenes, chlorinated aromatics and chlorinated aliphatic
hydrocarbons, such as chlorobenzenes, chloroethylenes or methylene chloride, aliphatic
10 hydrocarbons, such as cyclohexane or paraffins, for example petroleum fractions,
mineral and vegetable oils, alcohols, such as butanol or glycol and also their ethers and
esters, ketones, such as acetone, methyl ethyl ketone, methyl isobutyl ketone orcyclohexanone, strongly polar solvents, such as dimethylformamide and dimethyl
sulphoxide, and also water.
15 Suitable solid carriers are: for example ammonium salts and ground natural minerals,
such as kaolins, clays, talc, chalk, quartz, attapulgite, montmorillonite or diatomaceous
earth, and ground synthetic minerals, such as finely divided silica, alumina andsilicates; suitable solid carriers for granules are: for example crushed and fractionated
natural rocks such as calcite, marble, pumice, sepiolite and dolomite, and also synthetic
20 granules of inorganic and organic meals, and granules of organic material such as
sawdust, coconut shells, maize cobs and tobacco stalks; suitable emulsifiers and/or
foam-forming agents are: for example nonionic and anionic emulsifiers, such as
polyoxyethylene fatty acid esters, polyoxyethylene fatty alcohol ethers, for example
alkylaryl polyglycol ethers, alkylsulphonates, alkyl sulphates, arylsulphonates and also
25 protein hydrolysates; suitable dispersing agents are: for example lignin-sulphite waste
liquors and methylcellulose.
Tackifiers such as carboxymethylcellulose and natural and synthetic polymers in the
form of powders, granules or latexes, such as gum arabic, polyvinyl alcohol and
CA 022~1783 1998-10-1~
Le A 31 749-Forei~n Countries
- ~ - 20 -
polyvinyl acetate, as well as natural phospholipids, such as cephalins and lecithins, and
synthetic phospholipids, can be used in the formulations. Other possible additives are
mineral and vegetable oils.
It is possible to use colorants such as inorganic pigments, for example iron oxide,
5 titanium oxide and Prussian Blue, and organic dyes, such as alizarin dyes, azo dyes and
metal phthalocyanine dyes, and trace nutrients such as salts of iron, manganese, boron,
copper, cobalt, molybdenum and zinc.
The formulations in general contain between 0.1 and 95 per cent by weight of active
compound, preferably between 0.5 and 90%.
10 For controlling weeds, the active compounds according to the invention, as such or in
the form of their formulations, can also be used as mixtures with known herbicides,
finished formulations or tank mixes being possible.
Possible components for the mixtures are known herbicides, for example
acetochlor, acifluorfen(-sodium), aclonifen, alachlor, alloxydim(-sodium), ametryne,
15 amidochlor, amidosulfuron, asulam, atrazine, azimsulfuron, benazolin, benfuresate,
bensulfuron(-methyl), bentazon, benzofenap, benzoylprop(-ethyl), bialaphos, bifenox,
bromobutide, bromofenoxim, bromoxynil, butachlor, butylate, cafenstrole, carbetamide,
chlomethoxyfen, chloramben, chloridazon, chlorimuron(-ethyl), chlornitrofen,
chlorsulfuron, chlortoluron, cinmethylin, cinosulfuron, clethodim, clodinafop(-
20 propargyl), clomazone, clopyralid, clopyrasulfuron, cloransulam(-methyl), cumyluron,
cyanazine, cycloate, cyclosulfamuron, cycloxydim, cyhalofop(-butyl), 2,4-D, 2,4-DB,
2,4-DP, desmedipham, diallate, dicamba, diclofop(-methyl), difenzoquat, diflufenican,
dimefuron, dimepiperate, dimethachlor, dimethametryn, dimethenamid, dinitramine,diphenamid, diquat, dithiopyr, diuron, dymron, EPTC, esprocarb, ethalfluralin,
25 ethametsulfuron(-methyl), ethofumesate, ethoxyfen, etobenzanid, fenoxaprop-ethyl,
flamprop(-isopropyl), flamprop(-isopropyl-L), flamprop(-methyl), flazasulfuron,
fluazifop(-butyl), flumetsulam, flumiclorac(-pentyl), flumioxazin, flumipropyn,
fluometuron, fluorochloridone, fluoroglycofen(-ethyl), flupoxam, flupropacil, flurenol,
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
fluridone, fluroxypyr, flurprimidol, flurtamone, fomesafen, glufosinate(-ammonium),
glyphosate(-isopropylammonium), halosafen, haloxyfop(-ethoxyethyl), hexazinone,
imazamethabenz(-methyl), im~7~methapyr, im~7~mox, imazapyr, imazaquin,
imazethapyr, imazosulfuron, ioxynil, isopropalin, isoproturon, isoxaben, isoxaflutole,
5 isoxapyrifop, lactofen, lenacil, linuron, MCPA, MCPP, mefenacet, metamitron,
metazachlor, methaben7thi~7llron, metobenzuron, metobromuron, metolachlor,
metosulam, metoxuron, metsulfuron(-methyl), metribuzin, molinate, monolinuron,
naproanilide, napropamide, neburon, nicosulfuron, norflurazon orbencarb, oryzalin,
oxadiazon, oxyfluorfen, paraquat, pendimethalin, phenmedipham, piperophos,
10 pretilachlor, primisulfuron(-methyl), prometryn, propachlor, propanil, propaquizafop.
propyzamide, prosulfocarb, prosulfuron, pyrazolate, pyrazosulfuron(-ethyl),
pyrazoxyfen, pyributicarb, pyridate, pyrithiobac(-sodium) quinchlorac, quinmerac,
quizalofop(-ethyl), quizalofop(-p-tefuryl), rimsulfuron, sethoxydim, simazine, simetryn,
sulcotrione, sulfentrazone, sulfometuron(-methyl), sulfosate, tebutam, tebuthiuron,
15 terbuthylazine, terbutryn, thenylchlor, thiafluamide, thiazopyr, thidi~7imin,thifensulfuron(-methyl), thiobencarb, tiocarbazil, tralkoxydim, triallate, triasulfuron,
tribenuron(-methyl), triclopyr, tridiphane, trifluralin and triflusulfuron.
Mixtures with other known active compounds, such as fungicides, insecticides,
acaricides, nematicides, bird repellents, plant nutrients and agents which improve soil
20 structure, are also possible.
The active compounds can be used as such, in the form of their formulations or in the
use forms prepared therefrom by further dilution, such as ready-to-use solutions,
suspensions, emulsions, powders, pastes and granules. They are used in the customary
manner, for example by watering, spraying, atomizing or scattering.
25 The active compounds according to the invention can be applied either before or after
emergence of the plants. They can also be incorporated into the soil before sowing.
The amount of active compound used can vary within a substantial range. It depends
essentially on the nature of the desired effect. In general, the amounts used are between
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
1 g and 10 kg of active compound per hectare of soil surface, preferably between 5 g
and 5 kg per ha.
The preparation and use of the active compounds according to the invention can be
seen from the Examples below.
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 23 -
Preparation Examples
Example 1
H CF3
N ~/
F ~" F
CN
With stirring, a solution of 8.3 g (30 mmol) of 4-(4-cyano-2,5-difluorophenyl)-1,1,1-
5 trifluorobutane-2,4-dione in 20 ml of anhydrous acetic acid is added to a mixture of
2.1 g (30 mmol) of hydrazine hydrochloride, 2.5 g (30 mmol) of sodium acetate
(anhydrous) and 60 ml of acetic acid (anhydrous). The reaction mixture is then heated
at 95~C for approximately 30 minutes and subsequently poured onto ice-water. Theprecipitated solid is isolated by filtration with suction, washed with dilute sodium
10 bicarbonate solution and with water and dried under reduced pressure.
This gives 6.3 g (77% of theory) of 3-(4-cyano-2,5-difluoro-phenyl)-5-trifluoromethyl-
lH-pyrazole of melting point 138~C.
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 24 -
Example 2
H ~ CH3
N ~/
N~
CN
With stirring, a solution of 6.0 g (27 mmol) of 4-(4-cyano-2,5-difluorophenyl)-butane-
2,4-dione in 20 ml of anhydrous acetic acid is added to a mixture of 1.9 g (27 mmol)
of hydrazine hydrochloride, 2.3 g (27 mmol) of sodium acetate (anhydrous) and 60 ml
of acetic acid (anhydrous). The reaction mixture is then heated at 95~C for
approximately 30 minutes and subsequently poured onto ice-water. The precipitated
solid is isolated by filtration with suction, washed with dilute sodium bicarbonate
solution and with water and dried under reduced pressure.
This gives 5.0 g (85% of theory) of 3-(4-cyano-2,5-difluoro-phenyl)-5-methyl-lH-pyrazole of melting point 167~C.
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 25 -
Example 3
H3C_ CF3
I
N~
CN
A solution of 6.0 g (22 mmol) of 3-(4-cyano-2,5-difluoro-phenyl)-5-trifluoromethyl- I H-
pyrazole in 70 ml of toluene is heated using a water separator for one hour. The hot
5 solution is admixed with 4.0 g (31 mmol) of dimethyl sulphate and heated under reflux
for a further 6 hours. The reaction mixture is cooled to room temperature
(approximately 20~C) and washed successively with 10% strength aqueous sodium
hydroxide solution and with water, dried over magnesium sulphate and freed of solvent
using waterpump vacuum.
This gives 5 .0 g (80% of theory) of 3-(4-cyano-2,5-difluoro-phenyl)- 1 -methyl-5-
trifluoromethyl-lH-pyrazole of melting point 83~C.
CA 022~1783 1998-10-1~
Le A 31 749-Forei~n Countries
- 26 -
Example 4
H3C ~CF3
N~CI
'C '
CN
At 75~C to 85~C, approximately 40 g (0.56 mol) of chlorine gas are introduced with
stirring over a period of about 8 hours into a solution of 10.0 g (35 mmol) of 3-(4-
cyano-2,5-difluoro-phenyl)-1-methyl-5-trifluoromethyl-lH-pyrazole in 60 ml of
anhydrous acetic acid. The reaction mixture is cooled, poured onto approximately500 ml of ice-water and extracted with diethyl ether. The organic phase is separated off
and washed successively with saturated sodium bicarbonate solution and sodium
chloride solution, dried over magnesium sulphate and freed from solvent using
10 waterpump vacuum.
This gives 10. 5 g (93.5% of theory) of 4-chloro-3-(4-cyano-2,5-difluoro-phenyl)-1-
methyl-5-trifluoromethyl-lH-pyrazole as a crystalline solid of melting point 37~C.
CA 022~1783 1998-10-1~
Le A 31 749-Forei~n Countries
Example 5
CN
~O~CH
F/~j/
N~--
C~H3 CF3
1.9 g (6 mmol) of 4-chloro-3-(4-cyano-2,5-difluoro-phenyl)- 1 -methyl-5-trifluoromethyl-
lH-pyrazole are added to a solution of 1.0 g (18 mmol) of sodium methoxide in 60 ml
5 of methanol. The mixture is stirred at 40~C for 16 hours and then poured into water
and acidified with hydrochloric acid and the precipitated solid is filtered off, washed
with water and dried under reduced pressure.
This gives 1.5 g (75% of theory) of 4-chloro-3-(4-cyano-2-fluoro-5-methoxy-phenyl)-1-
methyl-5-trifluoromethyl- I H-pyrazole of melting point 1 39~C.
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 28 -
Example 6
CN
FJ~OH
N
C/H3 CF3
At room temperature (approximately 20~C), 180 ml (0.18 mol) of a IM solution of
boron tribromide in dichloromethane is added to a solution of 20.0 g (0.06 mol) of 4-
5 chloro-3-(4-cyano-2-fluoro-5-methoxy-phenyl)- 1 -methyl-S-trifluoromethyl- I H-pyrazole
in 100 ml of dichloromethane. The reaction mixture is stirred at room temperature for
16 hours and then admixed with water and dichloromethane. The organic phase is
separated off and washed successively with water, saturated aqueous sodium
bicarbonate solution and with sodium chloride solution, dried over magnesium sulphate
10 and freed from the solvent using waterpump vacuum. The resulting crude product is
purified by column chromatography using dichloromethane as eluent.
This gives 6.1 g (32% of theory) of 4-chloro-3-(4-cyano-2-fluoro-5-hydroxy-phenyl)- 1-
methyl-S-trifluoromethyl-lH-pyrazole of melting point 151~C.
CA 022~1783 1998-10-1~
Le A 31 749-Forei~n Countries
- 29 -
Example 7
H3C_ CF3
N~
N~CI
F~ CN
11
--0 CH3
CN
4.2 g (30 mmol) of potassium carbonate and 2.0 g (15 mmol) of 2-bromo-propionitrile
are added successively to a solution of 3.5 g (l l mmol) of 4-chloro-3-(4-cyano-2-
S fluoro-5-hydroxy-phenyl)-1-methyl-5-trifluoromethyl-lH-pyrazole in 60 ml of
acetonitrile. The mixture is stirred at 50~C for 6 hours and most of the solvent is then
removed using waterpump vacuum, the residue is admixed with 50 ml of water,
acidified with 2N hydrochloric acid and extracted with dichloromethane. The organic
phase is washed successively with saturated sodium bicarbonate solution and with10 sodium chloride solution, dried over magnesium sulphate, freed from the solvent using
waterpump vacuum and purifled by column chromatography using
n-hexane/dichloromethane (vol.: 1:1) as eluent.
This gives 1.3 g (32% of theory) of 2-[5-(4-chloro-1-methyl-5-trifluoromethyl-lH-
pyrazol-3-yl)-2-cyano-4-fluoro-phenoxy]-propionitrile of melting point 66~C.
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 30 -
Example 8
N ~/
~F
CN
A solution of 3.1 g (14 mmol) of 3-(4-cyano-2,5-difluoro-phenyl)-5-methyl- I H-pyrazole
in 50 ml of dimethylformamide is ~mixed with 8.8 g of caesium carbonate and heated
5 at 50~C for one hour. The temperature is then increased to 60~C and approximately
10 g (0.12 mol) of chlorodifluoromethane are introduced over a period of 6 hours.
After the reaction has ended, the predominant part of the solvent is removed using
waterpump vacuum and the residue is taken up in 50 ml of water. The aqueous
suspension is acidified with 2N hydrochloric acid and extracted with dichloromethane.
10 The organic phase is washed with water, dried over magnesium sulphate and freed
from the solvent using waterpump vacuum. The resulting crude product is purified by
column chromatography using n-hexane/dichloromethane (vol.: 7:1) as eluent.
As a first fraction, 0.9 g (24% of theory) of 3-(4-cyano-2,5-difluoro-phenyl)- 1 -
difluoromethyl-5-methyl-lH-pyrazole is obtained as colourless crystals of melting point
133~C.
As a second fraction, 0.4 g (11% of theory) of 5-(4-cyano-2,5-difluoro-phenyl)-1-
difluoromethyl-3-methyl- I H-pyrazole is obtained as colourless crystals of melting point
82~C.
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
Example 9
F2CH CH3
IN ~/
N~CI
'~ "
CN
At room temperature (approximately 20~C), 2.5 g (18.5 mmol) of sulphuryl chloride
are added with stirring to a solution of 0.7 g (2.6 mmol) of 3-(4-cyano-2,5-difluoro-
5 phenyl)- I -difluoromethyl-5-methyl- I H-pyrazole in 20 ml of dichloromethane. The
reaction mixture is stirred at 35~C for six hours, diluted with 20 ml of dichloromethane
and washed successively with saturated sodium bicarbonate solution and with sodium
chloride solution, dried over magnesium sulphate and freed from solvent using
waterpump vacuum.
This gives 0.45 g (70% of theory) of 4-chloro-3-(4-cyano-2,5-difluoro-phenyl)-1-difluoromethyl-5-methyl-lH-pyrazole as a crystalline solid of mp. 85~C.
Similar to the methods of Preparation Examples I to 9, and in accordance with the
general description of the preparation process according to the invention, it is also
possible to prepare, for example, the compounds of the formula (I) listed in Table I
1 5 below.
R R
N ~ 3
R4~
l ll
s
CN
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- - 32 -
Table 1: Examples of compounds of the formula (I)
Ex. R' R2 R3 R4 R5 Melting
No. point
(~C)
CH3 CF3 Cl F OC3H,-n 48
I l CH3 CF3 Cl F 108
~0~
1 2 CH3 CF3 Cl F OC2Hs 11 4
13 CH3 CF3 Cl F OC3H7-i 88
14 CH3 CF3 Cl F OCH2CH2OCH3 58
CH3 CF3 Cl F O(CHzCH2O)2CH3 52
16 CH3 CF3 Cl F ~O (amor-
~ phous)
17 CH3 CF3 Cl F O (amor-
\ o phous)
'- . '
18 CH3 CF3 Cl F OcH2cH=cH2 51
19 CH3 CF3 Cl F ~ 126
0~~
CH3 CF3 Cl F OCH2CF3 142
CA 02251783 1998-10-15
Le A 31 749-Foreign Countries
Table 1 - continued - 33 -
Ex. R' R2 R3 R4 R5 Melting
No. point
(~C)
21 CH3 CF3 Cl F CH3 (amor-
'~ phous)
CH3
22 CH3 CF3 Cl F OCH(CH2F)2 87
23 CH3 CF3 Cl F ~O 87
24 CH3 CF3 Cl F ~O 56
b
CH3 CF3 Cl O 82
26 CH3 CF3 Cl F SC2Hs 69
27 CH3 CF3 Cl F NHSO2CH3 250
CA 022~1783 1998-10-1
Le A 31 749-Foreign Countries
- Table I - continued - 34 -
Ex. R' R2 R3 R4 R5 Melting
No. point
(~C)
28 CH3 CF3 Cl F CH3 55
~0~
29 CH3 CF3 Cl t 132
o~o
CH3 CF3 Cl F 1 1 99
~O~F
31 CH3 CF3 Cl F OCH2COOC2H5 11 5
32 CH3 CF3 Cl F CH3 70
0~ C2Hs
33 CH3 CF3 Cl F CH3 92
~ ~~'
34 CH3 CF3 Cl F OCH2COOCH3 142
CH3 CF3 Cl F OCH2CH2F 100
36 CH3 CF3 Cl F OCH2CHF2 11 2
37 CH3 CF3 Cl F NHSO2C2Hs 154
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- ~ Table 1 - continued - 35 -
Ex. R' R2 R3 R4 R5 Melting
No. point
(~C)
38 CH3 CF3 Cl F ~O 106
~?.
39 CH3 CF3 Cl F OCH2CN 101
CH3 CF3 Cl F OCH2COOC3H,-i 103
41 CHF2 CF3 Br F OCH2CH2OCH3 66
42 CH3 CF3 Cl F N(SO2CH3)2 165
43 CH3 CF3 Cl F N(SO2CH3) 149
(COC6H5)
44 CH3 CF3 Br F COOC3H,-i 97
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
~ - 36 -
Starting materials of the formula (III):
Example (III-I)
Oq~ CF3
0~
"[~ F
CN
Initially at approximately 20~C, 8.0 g (56 mol) of ethyl trifluoroacetate and then, at
approximately -5~C, 10.8 g (60 mmol) of 30% strength methanolic sodium methoxidesolution are added successively to a solution of 7.2 g (40 mmol) of 1-(2,5-difluoro-4-
cyano-phenyl)-ethanone in 100 ml of diethyl ether. The reaction mixture is stirred at
0~C for one hour and then acidified with 2N hydrochloric acid. The organic phase is
separated off and washed successively with saturated sodium bicarbonate solution and
with sodium chloride solution, dried over magnesium sulphate and freed from the
solvent using waterpump vacuum.
This gives 10.3 g (93 .5% of theory) of 4-(4-cyano-2,5-difluoro-phenyl)- 1, I, I ,-
trifluorobutane-2,4-dione as a crystalline solid of melting point 29~C.
CA 022~1783 1998-10-1~
Le A 31 749-Forei~n Countries
- - 37 -
Example (111-2)
0~ CH3
O~J
CN
At approximately 15~C, 9.6 g (9S mmol) of triethylamine and 11.4 g (0.12 mol) ofmagnesium chloride (anhydrous) are added successively to a solution of 15 g
(9S mmol) of tert-butyl acetoacetate in 100 ml of acetonitrile. The suspension is stirred
at room temperature (approximately 20~C) for 3 hours, cooled to -10 to -5~C and, at
this temperature, admixed with 13.7 g (68 mmol) of 4-cyano-2,5-difluoro-benzoyl
chloride. The reaction mixture is then stirred at room temperature for 18 hours. The
predominant part of the solvent is then removed using waterpump vacuum and the
residue is admixed with 100 ml of dichloromethane and 50 ml of 13% strength
hydrochloric acid. The organic phase is separated off, washed with 2N hydrochloric
acid and then with water and dried over magnesium sulphate. Removal of the solvent
using waterpump vacuum gives 21 g (96% of theory) of tert-butyl 2-aceto-3-(4-cyano-
2,5-difluoro-phenyl)-3-oxo-propionate as a bright yellow liquid. The resulting crude
product is employed for further reaction without any further purification.
21 g (65 mmol) of tert-butyl 2-aceto-3-(4-cyano-2,5-difluoro-phenyl)-3-oxo-propionate
are dissolved in 200 ml of dichloromethane and, at 0~C, admixed dropwise with 19.4 g
(0.17 mol) of trifluoroacetic acid. The reaction mixture is stirred at room temperature
for 18 hours and then washed with water and dried over magnesium sulphate. The
solvent is removed using waterpump vacuum and the resulting crystal magma is stirred
with a little diethyl ether, filtered off and dried.
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 38 -
This gives 9.2 g (63.5% of theory) of 4-(4-cyano-2,5-difluoro-phenyl)-butane-2,4-dione
as a crystalline solid of melting point 94~C.
Similar to the methods of Examples (III-I) and/or (III-2), it is also possible to prepare,
for example, the compounds of the formula (III) listed in Table 2 below.
o~R2
q/~ R3
R4~ (III)
11
R5
CN
Table 2: Examples of compounds of the formula (III)
Ex. No.R2 R3 R4 R5 Melting
point (~C)
III-3 CH3 H Cl F
III-4 CH3 Cl F F
III-5. CH3 CH3 F F
III-6 CF3 H Cl F
III-7 CF3 Cl F F
III-8 CF3 CH3 F F
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 39 -
Starting materials of the formula (V):
Example (V- I )
Oq~CH3
'~'
CN
5.16 g (24 mmol) of 2,5-difluoro-4-(1-nitro-ethyl)-benzonitrile are dissolved in 50 ml
of acetic acid and the mixture is heated under reflux for 8 hours and, after cooling,
introduced into approximately 200 ml of water. The mixture is then extracted with
dichloromethane and the organic phase is washed successively with water, saturated
sodium bicarbonate solution and with saturated sodium chloride solution, dried with
magnesium sulphate and filtered. The filtrate is concentrated using waterpump vacuum
and the residue is extracted repeatedly with hot n-hexane. The solvent of the combined
extraction solutions is carefully distilled off using waterpump vacuum.
This gives 2.72 g (62% of theory) of 1-(2,5-difluoro-4-cyano-phenyl)-ethanone as a
colourless crystalline residue of melting point 42~C.
Example (V-2)
Oq~ CH3
F ~' F
CN
At 25~C, 5.4 g (0.11 mol) of sodium cyanide (ground) in 200 ml of N,N-
dimethylformamide are stirred with 17.4 g (0.10 mol) of 2,4,5-trifluoro-acetophenone
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 40 -
for 12 hours. The mixture is filtered off with suction, the filtrate is filtered through
activated carbon and the solvent is carefully distilled off from the filtrate using
waterpump vacuum.
This gives 10.4 g (57% of theory) of (2,5-difluoro-4-cyano-phenyl) methyl ketone as
an amorphous residue.
IR spectrum: 1698 cm~' (CO), 2240 cm~' (CN).
Similar to the method of Example (V-l) or (V-2), it is also possible to prepare, for
example, the compounds of the formula (V) listed in Table 3 below.
4 ~
R~ (V)
~R5
CN
Table 3: Examples of compounds of the formula (V)
Ex. No R3 R4 R5 Melting
point (~C)
V-3 CH3 F F 49
V-4 H Cl F (amor-
phous)
V-5 H F Cl (amor-
phous)
V-6 C2H5 F F
V-7 CH3 Cl F
V-8 CH3 F Cl
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 41 -
Starting materials of the forrnula Vlll):
Example (Vll-l)
O~Cl
CN
6.7 g (56 mmol) of thionyl chloride are added dropwise to a mixture of 3.4 g
(19 mmol) of 4-cyano-2,5-difluoro-benzoic acid, 30 ml of carbon tetrachloride and one
drop of N,N-dimethyl-formamide. The reaction mixture is then heated under reflux for
approximately 6 hours. After the evolution of gas has ceased, the mixture is allowed to
cool and filtered. The volatile components are then carefully distilled off from the
filtrate using waterpump vacuum.
This gives 3.6 g (95.5% of theory) of 4-cyano-2,5-difluoro-benzoyl chloride as ayellow liquid.
'H NMR (CDC13, d, ppm): 7.95 (dd, IH), 7.54 (dd, IH).
Similar to the method of Example (Vll-l), it is also possible to prepare, for example,
the compounds of the formula (Vll) listed in Table 4 below.
R4 O~,X
(Vll)
~R5
CN
CA 02251783 1998-10-15
Le A 31 749-Foreign Countries
- 42 -
Table 4: Examples of compounds of the formula (VII)
Ex. No. R4 R5 X Melting
point (~C)
VII-2 F Cl Cl
VII-3 Cl F Cl
VII-4 F F F
VII-5 F F Br
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 43 -
Starting materials of the formula (IX):
Example (IX-I)
'$ '
CN
At approximately -5~C, 7.9 g (50 mmol) of 2,4,5-trifluoro-benzonitrile are addeddropwise to a mixture of 4.5 g (60 mmol) of nitroethane, 80 ml of ethyl acetate and
18.2 g (120 mmol) of 1,8-diazabicyclo-[5.4.0]-undec-7-ene. The reaction mixture is
then stirred approximately 20~C for 5 hours and subsequently washed with 2N
hydrochloric acid and with water, dried with magnesium sulphate and filtered. The
solvent is carefully distilled off from the filtrate using waterpump vacuum.
This gives 8.5 g (83% of theory) of 2,5-difluoro-4-(1 -nitro-ethyl)-benzonitrile as a dark-
yellow liquid.
'H NMR (CDCI3, d, ppm): 1.96 (d, 3H), 5.90 (q, lH), 7.39 (dd, IH), 7.46 (dd, lH).
Similar to the method of Example (IX-I), it is also possible to prepare, for example,
the compounds of the formula (IX) listed in Table 4 below.
02N~' R3
X~ (IX)
R5
CN
CA 02251783 1998-10-15
Le A 31 749-Foreign Countries
- 44 -
Table 4: Examples of compounds of the formula (IX)
Ex. No. R3 Rs X' Properties
IX-2 H Cl F (amor-
phous)
IX-3 H F Cl (amor-
phous)
IX-4 CH3 F F (amor-
phous)
IX-5 CH3 F Cl
IX-6 CH3 Cl F
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 45 -
Starting materials of the forrnula (Xll):
Example (Xll- I )
O~,OH
'~"
CN
At a temperature not exceeding 40~C, a solution of 5.74 g (29 mmol) of 2,5-difluoro-4-
nitromethyl-benzonitrile in 30 ml of acetone is added dropwise to a suspension of 8.0 g
(50 mmol) of potassium permanganate (finely ground in a mortar) in 100 ml of
acetone. The reaction mixture is then stirred at room temperature (approximately 20~C)
for approximately 16 hours and subsequently acidified with 2N hydrochloric acid. The
precipitated m~ng~nese dioxide is removed by filtration and the filtrate is concentrated
using waterpump vacuum. The residue is taken up in ethyl acetate, washed with water,
dried with magnesium sulphate and filtered. The filtrate is concentrated and the residue
is stirred with a little dichloromethane and filtered off with suction.
This gives 3.4 g (64% of theory) of 4-cyano-2,5-difluoro-benzoic acid as a crystalline
product of melting point 153~C.
'H NMR (DMSO-D6, d, ppm): 7.92 (dd, IH), 8.15 (dd, IH), 14.10 (s - broad, IH).
Similar to the method of Example [lacuna], it is also possible to prepare, for example,
the compounds of the 5-chloro-4-cyano-2-fluoro-benzoic acid (melting point: 147~C)
and 2-chloro-4-cyano-5-fluoro-benzoic acid (melting point: 163~C).
CA 022~1783 1998-10-1~
Le A 31 749-Forei~n Countries
- 46 -
Use Examples:
Example A
Pre-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: I part by weight of alkylaryl polyglycol ether
To produce a suitable plepa-~lion of the active compound, 1 part by weight of active
compound is mixed with the stated amount of solvent, the stated amount of emulsifier
is added and the concentrate is diluted with water to the desired concentration.
Seeds of the test plants are sown in normal soil. After about 24 hours, the soil is
watered with the preparation of the active compound. Advantageously, the amount of
water per unit area is kept constant. The active compound concentration in the
preparation is not important, only the active compound application rate per unit area
matters.
After three weeks, the degree of damage to the plants is rated in % damage in
comparison to the development of the untreated controls.
The figures denote:
0% = no action (like untreated control)
100% = total destruction
In this test, very strong activity against weeds such as Digitarie (60 to 100%),Echinochloa (50 to 100%), Setaria (70 to 100%), Amaranthus (80 to 100%), Matricaria
(80 to 100%), Solanum (95 to 100%), is shown, for example, by the compounds of
Preparation Example 5, 6, 7, 10, Il, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 24, 25,
26, 27, 28, 29, 30, 32, 33, 35, 36, 37, 38 and 39, combined with good tolerance by
CA 02251783 1998-10-15
Le A 31 749-Foreign Countries
- 47 -
crop plants such as, for example, wheat (0 to 20%).
CA 022~1783 1998-10-1~
Le A 31 749-Foreign Countries
- 48 -
Example B
Post-emergence test
Solvent: 5 parts by weight of acetone
Emulsifier: I part by weight of alkylaryl polyglycol ether
To produce a suitable prepal~lion of the active compound, I part by weight of active
compound is mixed with the stated amount of solvent, the stated amount of emulsifier
is added and the concentrate is diluted with water to the desired concentration.
Test plants which have a height of 5 - 15 cm are sprayed with the preparation of the
active compound in such a way as to apply the particular amounts of active compound
desired per unit area. The concentration of the spray liquor is chosen so that the
particular amounts of active compound desired are applied in 1000 1 of water/ha.
After three weeks, the degree of damage to the plants is rated in % damage in
comparison to the development of the untreated controls.
The figures denote:
0% = no action (like untreated control)
100% = total destruction
In this test, very strong activity against weeds such as Echinochloa (50 to 100%),
Setaria (50 to 100%), Amaranthus (100%), Datura (95 to 100%), Solanum (100%),
Abutilon (100%), Ipomoea (100%), Xanthium (100%), Sorghum (80%), Veronica
(100%), is shown, for example, by the compounds of Preparation Example 5, 7, 10, I l,
12, 13, 14, 15, 16, 17, 18, 19,20,21,22,23,24,25,26,28,29,32,33,34,35,36,37,
38 and 39, combined with good tolerance by crop plants such as, for example, wheat
(5 to 30%).