Note: Descriptions are shown in the official language in which they were submitted.
CA 02251931 1998-10-16
WO 97/39780 ~ PCT/US97/06603
ABSORBENT MATERIALS HAVING IMPROVED STRUCTURAL STABILITY
IN DRY AND WET STATES AND MAKING METHODS THEREFOR
FIELD OF THE INVENT10N
The present invention relates to absorbent materials that, upon contacting
liquids such as water or body fluids, swell and imbibe such liquids. More
specifically, the present invention relates to improved structural stability
in the
dry and wet states of absorbent materials. The absorbent material of the
present invention has particular applicability to absorbent articles such as
diapers, adult incontinence pads, sanitary napkins, and the like.
BACKGROUND
Water-insoluble, water-swellable, hydrogel-forming absorbent polymers
are capable of absorbing large quantities of liquids such as water, body
fluids
(e.g., urine, blood, menstrual fluid), industrial fluids and household fluids
and
are further capable of retaining such absorbed liquids under moderate
pressures. These absorption characteristics of such polymer materials make
them especially useful for incorporation into absorbent articles such as
disposable diapers, adult incontinence pads and briefs, and catamenial
products such as sanitary napkins, and the like.
The development of highly absorbent members used in such absorbent
articles are the subject of substantial cornmerciai interest. A highly desired
characteristic for such absorbent articles is thinness. For example, thinner
diapers are less bulky to wear, fit better under clothing, and are less
noticeable. They are also more compact in the package, making the diapers
easier for the consumer to carry and store. Compactness in packaging also
results- in reduced distribution costs for the manufacturer and distributor,
including less shelf space required in the store per diaper unit.
The ability to provide thinner absorbent articles such as a diaper has been
contingent on the ability to develop relatively thin absorbent cores or
structures that can acquire and store large quantities of discharged body
fluids, particularly urine. In this regard, the use of certain absorbent
polymers
often referred to as "hydrogels" "superabsorbents" or "hydrocolloid" material,
has been particularly important. See, for example, U.S. Patent 3,699,103
(Harper et. al), issued June 13, 1972, and U.S. Patent 3,670,731 (Harmony,
CA 02251931 1998-10-16
WO 97/39780 2 PCT/US97/06603
issued June 20, 1972, that disclose the use of such absorbent polymers
(hereafter "water-insoluble absorbent hydrogel-forming polymers") in
absorbent articles.
Moreover, prior absorbent articles have generally comprised relatively low
amounts (e.g., less than about 50% by weight) of absorbent gelling particles
of the WAHPs. See, for example, U.S. Patent 4,834,735 (Alemany et. al),
issued May 30, 1989. It discloses that an absorbent structure or core
contains preferably from about 9 to about 50% of WAHP in the fibrous matrix.
Unfortunately several problems are encountered when one attempts to
provide a thin absorbent core having more than 50% concentration of
absorbent gelling particles by weight.
Conventional absorbent articles have the limitation that the absorbent
gelling particles are not immobilized and are free to migrate(shift) during
the
manufacturing process and/or use(wearing). Migrations(shifting) of the
absorbent gelling particles during manufacture can lead to absorbent material
handling losses during manufacturing operations as well as nonhomogerous
incorporation of the particles being used. A more significant problem, though,
occurs when these absorbent gelling particles of WAHPs migrate during or
after swelling. This inability to fix the particles at optimum locations leads
to
an insufficient urine storage capacity in one area and over-capacity in other
areas due to the lack of stability.
One important factor is to minimize and/or eliminate the shifting of
particles of WAHPs from the first applying location to another position and
handling losses during manufacture.
One problem encountered is the shifting and/or leakage of swollen (e.g.,
with urine) particles of WAHP due to wear-related movement and pressure on
the absorbent article. The inability to fix the particles at optimum location
is
another issue that results in insufficient urine storage capacity in one area
and over-capacity in other areas. Subsequently the absorbent article will leak
during use. The shifting of wet particles of WAHPs can cause core shifting
and more incidence of gel leakage when in use, especially from an absorbent
material containing high concentration of WAHPs.
Yet another important factor that has to be considered is the liquid
permeability of WAHPs. It has been discovered that the permeability or flow
conductivity of the gel layer formed by swelling in the presence of body
fluids
is extremely important when these absorbent polymers are used in absorbent
cores or members at a high concentration in localized'or throughout regions
CA 02251931 2003-O1-20
thereof. It should be noted that lack of liquid permeability or flow
conductivity
of absorbent polymers may directly impact on the ability of resultant gel
layers
to acquire and distribute body fluids.
Still another concern of WAHPs used in thinner absorbent article is the
jelly and mushy feel when touching and handling the absorbent article after
usage. When WAHP is dispersed in region or regions at a high concentration,
the swollen gel formed by absorbing body fluids is a gel layer, in which the
particulate is mobile and the gel layer collapses when subjected to forces
such as pushing, squeezing, etc. when handling the absorbent article after
usage. This is why absorbent articles having high concentration of WAHP give
users or consumers "wet/mushy" feel when touching or handling them from
outside.
Therefore, aspects of the present invention seek to resolve the above
problems by providing an absorbent material having improved structural
stability in dry and wet status.
SUMMARY
Briefly stated, the present invention relates to absorbent materials
having improved structural stability in dry and wet states. In one aspect,
'?0 these absorbent materials comprise (a) absorbent gelling particles
comprising
a water-insoluble absorbent hydrogel-forming polymer; (b) a polycationic
polymer; (c) glue microfibers; and (d) a carrier layer; wherein the
polycationic
polymer is bonded to the absorbent gelling particles; and 'the glue
microfibers
act as an adhesive between the absorbent gelling particles and the carrier
'?5 layer. Because the glue microfibers are tacky, the absorbent gelling
particles
comprising a WAHP fix to the desired location on the carrier layer and do not
shift to the another area in dry state. In wet state, when the absorbent
material
contacts liquids such as body fluids, the absorbent gelling particles
contained
in the absorbent material fix to the location first applied due to bonding of
the
:30 polycationic polymer to the absorbent gelling particles comprising a WAHP,
and the absorbent material does not shift.
The bonds between the absorbent gelling particles to the glue
CA 02251931 2003-12-12
4
microfibers, which in turn, are bonded to the carrier layer, prevent the
absorbent gelling particles from shifting during the manufacturing process.
The polycationic polymer bonded to the absorbent gelling particles prevents
the particles from shifting after they swell with liquid. Consequently, the
absorbent material of the invention has improved liquid acquisition speed and
low rewetness when in use. It has been found that when the absorbent
material is contacted with liquids, the absorbent material swells, imbibes
such
liquids into the absorbent gelling particles, and absorbs even under moderate
confining pressures.
In a preferred embodiment of the present invention, the carrier layer is
selected from the group consisting of a woven material and a nonwoven
material.
These absorbent materials may further comprise the cellulose fibers
dispersed in the absorbent gelling particles, wherein the cellulose fibers are
adhered to the absorbent gelling particles by the glue microfibers.
Preferably, the absorbent material of present invention comprises from
about 50% to about 90% of the absorbent gelling particle, from about 0.1 % to
about 10% of the polycationic polymer, from about 1 % to about 10% of the
thermoplastic polymeric microfiber and from about 5% to about 50% of the
carrier layer by weight.
The invention further relates to a method of make the absorbent
materials, and the absorbent articles comprising the absorbent materials.
In accordance with another aspect of the present invention, there is
provided an absorbent material comprising:
(a) absorbent gelling particles comprising more than 50% by weight of
a water-insoluble absorbent hydrogel-forming polymer;
(b) polycationic polymer fibers comprising a polycationic polymer; and
(c) a carrier layer;
wherein the polycationic polymer fibers having a concentration of from
about 80% to 99% by weight are bonded to the absorbent gelling particles;
and the polycationic polymer fibers act as an adhesive between the absorbent
gelling particles and the carrier layer.
CA 02251931 2003-12-12
4a
In accordance with another aspect of the present invention, there is
provided An absorbent material comprising:
(a) absorbent gelling particles comprising more than 50% by weight of
a water-insoluble absorbent hydrogel-forming polymer;
(b) polycationic polymer fibers comprising a polycationic polymer; and
(c) a carrier layer;
wherein the polycationic polymer fibers having a concentration of from about
80% to 99% by weight are bonded to the absorbent gelling particles; and the
polycationic polymer fibers act as an adhesive between the absorbent gelling
particles and the carrier layer.
In accordance with another aspect of the present invention, there is
provided a method of making an absorbent material comprising:
(a) applying absorbent gelling particles comprising more than 50% by
weight of a water-insoluble absorbent hydrogel-forming polymer onto a
carrier layer;
(b) applying glue microfibers onto the carrier layer; and
(c) applying a polycationic polymer onto the absorbent gelling particles
to form a bond between the absorbent gelling particles and the
polycationic polymer;
wherein the absorbent gelling particles adhere to the glue microfibers
prior to the glue microfibers adhering to the carrier layer.
In accordance with another aspect of the present invention, there is
provided a method of making an absorbent material, the method comprising
the steps of:
(a) forming a first air stream comprising absorbent gelling particles
comprising more than 50% by weight of a water-insoluble absorbent
hydrogel-forming polymer;
(b) forming a second air stream comprising glue microfibers;
(c) merging the second air stream with the first air stream to form an
integrated air stream comprising a thorough mixture of the glue
microfibers and the absorbent gelling particles;
(d) directing the integrated air stream onto a carrier layer;
CA 02251931 2003-12-12
4b
(e) forming a third air stream comprising a polycationic polymer; and
(f) directing the third air stream onto the carrier layer so the polycationic
polymer bonds to the absorbent gelling particles.
In accordance with another aspect of the present invention, there is
provided a method of making an absorbent material, the method comprising
the steps of:
(a) forming a first air stream comprising absorbent gelling particles
comprising more than 50% by weight of a water-insoluble absorbent
hydrogel-forming polymer;
(b) forming a second air stream comprising a polycationic polymer;
(c) merging the second air stream with the first air stream to form an
integrated air stream, wherein the polycationic polymer bonds to the
absorbent gelling particles;
(d) forming a third air stream comprising glue microfibers;
(e) merging the integrated air stream with the third air stream to form a
mixture air stream; and
(f) directing the mixture air stream onto a carrier layer so the absorbent
gelling particles bonded to the polycationic polymer adhere to glue
microfibers, and the glue microfibers adhere to the carrier layer.
In accordance with another aspect of the present invention, there is
provided a method of making an absorbent material, the method comprising
the steps of:
(a) forming a first air stream containing polycationic polymer fibers;
(b) forming a second air stream containing absorbent gelling particles
comprising more than 50% by weight of a water-insoluble absorbent
hydrogel-forming polymer;
(c) merging the second air stream with the first air stream to form an
integrated air stream, wherein the polycationic polymer fibers having a
concentration of from about 80% to 99% by weight, bond to the
absorbent gelling particles; and
(d) directing the integrated air stream onto a carrier layer so that the
absorbent gelling particles bonded to the polycationic polymer fibers adhere
to
CA 02251931 2003-12-12
4c
the carrier layer.
In accordance with a further embodiment of the present invention,
there is provided an absorbent article comprising the absorbent material as
described above.
These and other features, aspects, and advantages of the present
invention will become better understood with regard to the following
description, attended claims and accompanying drawing.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic view of an apparatus for making the absorbent
materials of the present invention.
DETAILED DESCRIPTION
The following is a list of definitions for certain terms used herein:
"Comprising" means other steps and other ingredients which do not
affect the result can be added. The term encompasses the terms "consisting
of" and "consisting essentially of".
"Dry state" means the state of the absorbent material during
manufacture.
"Wet state," means the swollen absorbent materials due to absorption
of large quantities of liquids such as water, body fluids, industrial fluids
and
household fluids, when the absorbent materials of the present invention are
used in, e.g., a diaper and worn.
CA 02251931 1998-10-16
WO 97/39780 5 PCT/US97106603
"Structural stability" means immobilizing(fixing) the absorbent materials
onto the right position in a diaper where first located.
"WAHP" means a water-insoluble absorbent hydrogel-forming polymer.
A. Absorbent Material
The absorbent material of the present invention is capable of absorbing
large quantities of liquids such as water, body fluids, industrial fluids and
household fluids at a rapid rate and is capable of retaining such fluids under
moderate pressures. In particular, the absorbent material of the present
invention has an improved structural stability in the dry and wet states,
while
the absorbent material has a high concentration of WAHPs. Preferably, the
absorbent material comprises greater than about 50% by weight of a WAHP.
The absorbent materials are no shifting in the dry and wet states.
If one does not adhere the absorbent gelling particles at the desired
locations in the dry state, the absorbent gelling particles tend to shift
during
manufacturing process, resulting in, e.g., clumping of absorbent gelling
particles and a lack of uniform distribution of the particles.
If one does not adhere the absorbent gelling particles at the desired
locations in the wet state, the particles may shift, resulting in insufficient
urine
storage capacity in one area and over-capacity in other areas. Subsequently
the absorbent article will leak during use(wearing). The shifting of wet
absorbent gelling particles of WAHP can cause core shifting and more
incidence of gel leakage when in use or wearing, especially from an
absorbent article using absorbent materials comprising a high concentration
of WAHP.
It is the structural stability in the dry and wet status having a high
concentration of WAHP at more than 50% by weight which form the basis for
the instant invention over past absorbent materials which have not provided
such structural stability in the dry and wet states to the extent now
achieved.
The absorbent material ,~f the present invention comprises: (a) absorbent
gelling particles comprising a WAHP; (b) a polycationic polymer; (c) glue
microfibers; and (d) a carrier layer; wherein the polycationic polymer is
bonded to the absorbent gelling particles; and the glue microfibers act as an
adhesive between the absorbent gelling particles and the carrier layer.
The present invention in its aspects contemplates the absorbent materials
comprising the absorbent gelling particles, the polycationic polymer bonded
to the absorbent gelling particles, the glue microfibers dispersed in the
CA 02251931 1998-10-16
WO 97/39780 6 PCT/LTS97/06603
absorbent gelling particles and the carrier layer. In particular, its object
is to
fix the absorbent gelling particles to the desired location of the carrier
layer by
an adhesive glue microfiber in the dry state and to fix the absorbent gelling
particles bonding to the polycationic polymer on the surface, when the
absorbent materials contact liquids such as body fluids in the wet state.
The glue microfiber used herein can be meltblown to form fibers that are
tacky in at least one step of the manufacture of the absorbent materials. It
is
possible that the glue microfiber are initially fixed the absorbent gelling
particles to the desired location of the absorbent materials during the
manufacturing process.
Generally, one can use any polymer as glue microfibers that are
sufficiently tacky, to hold onto the particles that contact it, and thereby
qualify
as an adhesive polymer. Preferably, the melt blown adhesive polymers
which can be utilized for forming the absorbent materials include the
elastomeric and non-elastomeric polymers. These polymers must be tacky
enough to be blown into fiber forms. The tackiness can be modified with the
usage of tackifying resins, which include rosin esters, mixed polyalkenes,
polyterpenes, waxes, or incorporating carboxylic acid contained polymers or
oligomers within the adhesive resin. Also contemplated by the invention are
the use of blends of adhesive polymers, or blends of adhesive polymers and
other polymers.
Useful elastomeric polymers include polyolefins and blends {e.g.,
polypropylene, polybutylene, or ethyleneacrylic acid copolymers),
ethylenevinyl acetate copolymers, poiyamides, polyesters, and reactive
polyamide and polyesters.
Pressure sensitive adhesives are also useful for forming the absorbent
structure of this invention. They are permanently tacky and do not change
their physical state from an initial liquid to a solid after final bond
formation.
Exemplified eiastomeric polymers are ethylenevinyl acetate copolymer,
styrene/diene triblock copolymer, poly(vinylether)s, polyacrylates, and
silicones. Thermoplastic elastomeric triblock copolymers of the ABA type
have great adhesive capability and processing convenience in this invention.
The end block (A) in these polymers are plastic in nature with a high glass
transition (or melt) temperature, which the block (B) is rubbery. In
particular,
the styrene-butadiene-styrene, styrene-isoprene-styrene, styrene-ethylene-
co-propylene-styrene copolymers are very useful in this invention.
CA 02251931 1998-10-16
WO 97/39780 ~ PCT/US97/06603
The non-elastomeric polymer may be a non-elastomeric fiber forming
resin or blend containing the same. For example, such polymers include
polyolefins, non-elastomeric polyamides, cellulosic derived polymers, vinyl
chlorides, and polyvinyl alcohols.
In a preferred embodiment of the present invention, the types of
elastomeric Styrene-Isoprene-Styrene block copolymers are HL-1358 or
Finely H-6752A supplied by Fuller Co. The types of non-elastomeric glue
microfiber include polyethyloxazoline, for example XR-2676 (Fuller Co.,),
polyvinylpyrolidone, for example H-1716 (Fuller Co.,) and
ethylenevinyacetate copolymer, for example HT-480 (Fuller Co.,). The glue
microfrbers comprising polyethyloxazoline would provide the absorbent article
comprising the absorbent material of the present invention sufficient
structural
integrity in the dry state, while in the wet state the polycationic polymers
comprising polyethyleneimine are activated to maintain the structural
integrity
1 S of the absorbent article.
The polycationic polymer used herein is a polymer which has multiple
functional groups that are capable of bonding to the surface of the absorbent
gelling particles. In a preferred embodiment, an amino-group or iminegroup
containing polymer is used as the polycationic polymer. Such polycationic
polymers include polyamines, polyimines and mixtures thereof. More
preferably, the polyamine is selected from the group consisting of polymers
having first amine groups (e.g., polyvinylamine, polyailylamine), polymers
having second amine groups (e.g., poiyethyleneamines) and polymers having
third amine groups (e.g., poly-N, N- dimethylalkyl amine, poly-N-alkylamine).
2S The polyimines preferably used include polyethyleneimines, modified
polyethyleneimines crosslinked with epihalohydrine, poiyamidoamines grafted
with ethyleneimine and mixtures thereof. Other suitable polycationic
polymers include modified polyamidoamine grafted with ethyleneimine,
polyet~eramine, polyvinylamine, polyallylamine, polyamidopolyamine and
mixtures thereof.
In a preferred embodiment, the polycationic polymer is a cationic polymer
having an average molecular Weight of at least about 200, more preferably of
at least more than 5,000, and most preferably of more than about 10,000.
The polycationic polymers useful in the invention include those polymers
having a single maximum value (a peak) in molecular weight distribution, as
well as those polycationic polymers having one or more maximum values.
CA 02251931 1998-10-16
WO 97/39780 8 PCT/L1S97/06603
The molecular weight distribution can be analyzed by, for example, gel
permeation chromatography.
Preferably, the amount of polycationic polymer used in the absorbent
material is from about 0.1 % to 10% by weight of the absorbent materials.
For providing a high concentration of a WAHP, such as more than 50% by
weight of the absorbent material, the polycationic polymer used for the
present invention has a concentration of from about 80% to 99% by weight so
that it can be tacky by itseif. The polycationic polymers having the
characteristic of tackiness can be meltblown without glue microfibers,
consequently acting as an adhesive between the absorbent gelling particles
and the carrier layer. Preferably, the polycationic polymer fiber has a
molecular weight of at least about 70,000.
B. Water-insoluble Absorbent Hydrogel-forming Polymer
1. Chemical Composition
The WAHPs useful in the present invention are commonly referred to as
"hydrogel-forming", "hydrocolloid", or "superabsorbent" polymers and can
include polysaccharides such as carboxymethyl starch, carboxymethyl
cellulose, and hydroxypropyl cellulose; nonionic types such as polyvinyl
alcohol, and polyvinyl ethers; cationic types such as polyvinyl pyridine,
polyvinyl morpholinione, and N,N-dimethylaminoethyi or N,N-
diethylaminopropyl acrylates and methacrylates, and the respective
quaternary salts thereof. Typically, WAHPs useful in the present invention
have a plurality of anionic, functional groups, such as sulfonic acid, and
more
typically carboxy, groups. Examples of polymers suitable for use herein
include those which are prepared from polymerizable, unsaturated, acid-
containing monomers. Thus, such monomers include the olefinically
unsaturated acids and anhydrides that contain at least one carbon to carbon
olefinie double bond. More specifically, these monomers can be selected
from olefinically unsaturated carboxylic acids and acid anhydrides,
olefinically
unsaturated sulfonic acids, and mixtures thereof.
Some non-acid monomers can also be included, preferably in minor
amounts, in preparing the WAHPs herein. Such non-acid monomers can
include, for example, the water-soluble or water-dispersible esters of the
acid-
containing monomers, as well as monomers that contain no carboxylic or
sulfonic acid groups at all. Optional non-acid monomers can thus include
monomers containing the following types of functional groups: carboxylic
CA 02251931 1998-10-16
WO 97/39780 PCT/US97/06603
9
acid or sulfonic acid esters, hydroxyl groups, amide-groups, amino groups,
nitrite groups, quaternary ammonium salt groups, aryl groups (e.g., phenyl
groups, such as those derived from styrene monomer). These non-acid
monomers are well-known materials and are described in greater detail, for
example, in U.S. Patent 4,076,663 (Masuda et. al), issued February 28,
1978, and in U.S. Patent 4,062,817 (Westerman), issued December 13,
1977.
Olefinically unsaturated carboxylic acid and carboxylic acid anhydride
monomers include the acrylic acids typified by acrylic acid itself,
methacrylic
acid, ethacrylic acid, -chloroacrylic acid, -cyanoacrylic acid, -methylacrylic
acid (crotonic acid), -phenylacryiic acid, -acryloxypropionic acid, sorbic
acid, -chlorosorbic acid, angelic acid, oinnamic acid, p-chlorocinnamic
acid, -sterylacrylic acid, itaconic acid, citroconic acid, mesaconic acid,
glutaconic acid, aconitic acid, malefic acid, fumaric acid, tricarboxyethylene
and malefic acid anhydride.
Olefinically unsaturated sulfonic acid monomers include aliphatic or
aromatic vinyl sulfonic acids such as vinylsulfonic acid, allyl sulfonic acid,
vinyl toluene sulfonic acid and-styrene sulfonic acid; acrylic and methacrylic
sulfonic acid such as sulfoethyl acrylate, sulfoethyl methacrylate,
sulfopropyl
acrylate, sulfopropyl methacrylate, 2-hydroxy-3-methacryloxypropyl suifonic
acid and 2-acrylamide-2-methylpropane sulfonic acid.
Preferred WAHPs for use in the present invention contain carboxy groups.
These polymers include hydrolyzed starch-acrylonitrile graft copolymers,
partially neutralized hydrolyzed starch-acrylonitrile graft copolymers, starch-
acrylic acid graft copolymers, partially neutralized starch-acrylic acid graft
copolymers, saponified vinyl acetate-acrylic ester copolymers, hydrolyzed
acrylonitrile or acrylamide copolymers, slightly network crosslinked polymers
of any of the foregoing copolymers, partially neutralized polyacrylic acid,
and
slightly network crosslinked polymers of partially neutralized polyacrylic
acid.
These polymers can be used either solely or in the form of a mixture of two or
more different polymers. Examples of these polymer materials are disclosed
in U.S. Patent 4,076,663 (Masuda et. al), issued February 28, 1978, U.S.
Patent 4,093,776 (Aoki et. al), issued June 6, 1978, U.S. Patent 4,666,983
(Tsubakimoto et. al), issued May 19, 1987, and U.S. Patent 4,734,478
(Tsubakimoto et. al), issued March 29, 1988.
More preferably polymer materials used in making the WAHPs are slightly
network crosslinked polymers of partially neutralized polyacrylic acids and
CA 02251931 1998-10-16
WO 97/39780 ~ ~ PCT/US97/066b3
starch derivatives thereof. More preferably still, the WAHPs comprise from
about 50 to about 95%, more preferably about 75%, neutralized, slightly
network crosslinked, polyacrylic acid (i.e., poly (sodium acrylate/acrylic
acid) ). Network crosslinking renders the polymer substantially water-
s insoluble and, in part, determines the absorptive capacity and extractable
polymer content characteristics of the WAHPs. Processes for network
crosslinking these polymers and typical network crosslinking agents are
described in greater detail in U.S. Patent 4,076,663 (Masuda et. al), issued
February 28.
Surface crosslinked WAHPs are used in a preferred embodiment of the
present invention. They have a higher level of crosslinking in the vicinity of
the surface than in the interior. As used herein, "surface" describes the
outer-facing boundaries of, e.g., the particle, fiber. For porous WAHPs (e.g.,
porous particles), exposed internal boundaries can also be included. By a
1 S higher level of crosslinking at the surface, it is meant that the level of
functional crosslinks for the WAHP in the vicinity of the surface is generally
higher than the level of functional crosslinks for the WAHP in the interior.
The gradation in crosslinking from surface to interior can vary, both in
depth and profile. Thus, for example, the depth of surface crosslinking can
be shallow, with a relatively sharp transition to a lower level of
crosslinking.
Alternatively, for example, the depth of surface crosslinking can be a
significant fraction of the dimensions of the WAHP, with a broader transition.
Depending on size, shape, porosity as well as functional considerations,
the degree and gradient of surface crosslinking can vary within a given
WAHP. For particulate WAHPs, surface crosslinking can vary with particle
size, porosity, etc. Depending on variations in surface/volume ratio within
the
WAHP (e.g., between small and large particles), it is not unusual for the
overall level of crosslinking to vary within the material (e.g., be greater
for
smaller particles).
Surface crosslinking is generally accomplished after the final boundaries
of the WAHP is essentially established (e.g., by grinding, extruding, foaming,
etc.) However, it is also possible to effect surface crosslinking concurrent
with the creation of final boundaries. Furthermore, some additional changes
in boundaries can occur even after surface crosslinks are introduced.
The surface crosslinking can be accomplished before or, simultaneously,
with the covalent bonding of the polycationic polymer to the surface of the
absorbent gelling particles.
CA 02251931 1998-10-16
WO 97/39780 11 PCT/US97/06603
While the WAHP is preferably of one type (i.e., homogeneous), mixtures
of polymers can also be used in the present invention. For example, mixtures
of starch-acrylic acid graft copolymers and slightly network crosslinked
polymers of partially neutralized polyacrylic acid can be used in the present
invention.
2. Physical Forms
The absorbent gelling particles used in the present invention can have a
size, shape and/or morphology varying over a wide range. The absorbent
gelling particles may have a large ratio of greatest dimension to smallest
dimension (e.g., granules, flakes, pulverulents, interparticle aggregates,
interparticle crosslinked aggregates, and the like) and can be in the form of
fibers, foams, and the like.
For particles of WAHPs useful in the present invention, the particle size is
in the range of from about 10 to about 1000 microns. The WAHPs can also
comprise mixtures with low levels of one or more additives, such as, for
example, powdered silica, surfactants, celloluse microfiber and the like. The
components in this mixture can be physically and/or chemically associated in
a form such that the WAHP component and the non-hydrogel-forming
polymer additive are not readily physically separable. The WAHPs can be
essentially non-porous or have substantial internal porosity.
For particles as described above, particle size is defined as the dimension
determined by sieve size analysis. Thus, for example, a particle that is
retained on a U.S.A. Standard Testing Sieve with 710 micron openings (e.g.,
No. 25 U.S. Series Alternate Sieve Designation) is considered to have a size
greater than 710 microns; a particle that passes through a sieve with 710
micron openings and is retained on a sieve with 500 micron openings (e.g.,
No. 35 U.S, Series Alternate Sieve Designation) is considered to have a
particle size between 500 and 710 microns; and a particle that passes
through a sieve with 500 micron openings is considered to have a size less
than 500 microns.
C. Absorbent Article comprising the Absorbent Materials
The absorbent materials according to the present invention can be used
for many purposes in many fields of use. For example, the absorbent
material can be used for packing containers; drug delivery devices; wound
cleaning devices; burn treatment devices; ion exchange column materials;
CA 02251931 1998-10-16
WO 97/39780 ~ 2 PCT/US97/06603
construction materials; agricultural or horticultural materials such as seed
sheets or water-retentive materials; and industrial uses such as sludge or oil
dewatering agents, materials for the prevention of dew formation, desiccants,
and humidity control materials. In these environments, the absorbent
material of the invention can have a number of shapes and sizes. For
example, the absorbent material can be in the form of sheets, films,
cylinders,
blocks or other shaped elements. The absorbent material can comprise a
cellulosic material for enhancing absorbency and/or be in a form amenable to
these and other applications as described hereinafter.
Because of the unique absorbent properties of the absorbent material of
the present invention, it is especially suitable for use as an absorbent core
in
absorbent articles, especially disposable absorbent articles. As used herein,
the term "absorbent article" refers to articles which absorb and contain body
fluids and more specifically refers to articles which are placed against or in
I S proximity to the body of the wearer to absorb and contain the various
fluids
discharged from the body. Additionally, "disposable" absorbent articles are
those which are intended to be discarded after a single use (i.e., the
original
absorbent article in its whole -is not intended to be laundered or otherwise
restored or reused as an absorbent article, although certain materials or all
of
the absorbent article may be recycled, reused, or composted).
In general, an absorbent article comprise (a) a liquid pervious topsheet;
(b) a liquid impervious backsheet; and (c) an absorbent core positioned
between the topsheet and the backsheet wherein the absorbent core
comprises at least one absorbent material. As used herein, the term
"absorbent core" refers to the component of the absorbent article that is
primarily responsible for fluid handling properties of the article, including
acquiring, transporting, distributing and storing body fluids. As such, the
absorbent core preferably does not include the topsheet or backsheet of the
absorbent article.
In a more preferred embodiment, the absorbent core or absorbent
member can further comprise fibers or fluff pulp (fibrous or fiber material);
more specifically, non-absorbent-gelling fibers. Such fiber material can be
used as a reinforcing or absorbent member in the absorbent core, improving
fluid handling of the core, as well as serving as a co-absorbent with the
absorbent polymers. As used herein, the term "absorbent member" refers to
the components of the absorbent core that typically provide one or more fluid
handling properties, e.g., fluid acquisition, fluid distribution, fluid
CA 02251931 2003-O1-20
13
transportation, fluid storage, etc. The absorbent member can comprise the
entire absorbent core or only a portion of the absorbent core, i.e., the
absorbent core can comprise one or more absorbent members.
Any type of fiber material which is suitable for use in conventional
absorbent products can be used in the absorbent core or absorbent member
herein. Speck examples of such fiber material include cellulose fibers,
improved cellulose fibers, rayon, polypropylene, and polyester fibers such as
polyethylene terephthalate (DACRONj M hydrophilic nylon (HYDROFII_)TMand
the like. Examples of other fiber materials for use in the present invention
in
addition to some already discussed are hydrophilized hydrophobic frbers,
such as surfactant-treated or silica-treated thermoplastic fibers derived, for
example, from polyolefins such as polyethylene or polypropylene,
polyacrylics, poiyamides, polystyrenes, polyurethanes and the like. In fact,
hydrophilized hydrophobic fibers which are in and of themselves not very
absorbent and which, therefore, do not provide webs of sufficient absorbent
capacity to be useful in conventional absorbent structures, are suitable for
use in the absorbent core by virtue of their good wicking properties. This is
because, in the absorbent core herein, the wicking propensity of the fibers is
as important, if not more important, than the absorbent capacity of the fiber
material itself due to the high rate of fluid uptake and lack of gel blocking
properties of the absorbent core. Synthetic fibers are generally preferred for
use herein as the fiber component of the absorbent core. More preferred are
polyolefin fibers, preferably polyethylene fbers.
Other cellulosic fiber materials which can be useful in certain absorbent
cores or absorbent members herein are chemically stiffened cellulosic fibers.
Preferred chemically stiffened cellulosic fibers are the stiffened, twisted,
curled celluiosic fibers which can be produced by internally crosslinking
cellulose fibers with a crosslinking agent. Suitable stiffened, twisted,
curled
cellulose fibers useful as the hydrophilic fiber materials herein are
described
in greater detail in U.S. Patent 4,888.093 (Dean et. al), issued December 19,
1989; U.S. Patent 4,889,596 (Herron et. al), issued December 26, 1989; U.S.
Patent 4,889,596 (Schoggen et. al), issued December 26, 1989; U.S. Patent
4,889,597 (Bourbon et, al), issued December 2Ei, 1989; and U.S. Patent
4,898,647 (Moors et. al), issued February 6, 1990.
A preferred embodiment of the disposable absorbent article is a diaper.
As used herein, the term "diaper" refers to a garment, generally worn by
infants and incontinent persons, that is worn about the lower torso of the
...,M ~...._..~ ..~~.n,~.~. ~,..~~.,.~.~m..~~. .... .". . _ .... ....
w.............
CA 02251931 2003-O1-20
14
wearer. A preferred diaper configuration for a diaper comprising an
absorbent core is described generally in U.S. Patent 3,860,003 (Buell),
issued January 14, 197 5. Altemativeiy preferred configurations for
disposable diapers herein are also disclosed in U.S. Patent 4,808,178 (Aziz
et. al), issued February 28, 1989; U.S. Patent 4,fi95,278 (Lawson), issued
September 22, 1987; U.S. Patent 4,816.025 (Foreman), issued March 28,
1989; and U.S. Patent 5,151,092 (Buell et. al.), issued September 29, 1992.
Another preferred embodiment of the disposable absorbent article is a
catamenial product. Preferred catamenial products comprise a formed-film,
apertured topsheet as disclosed in U.S. Patent 4,285,343 (McNair), issued
August 25, 1981; U.S. Patent 4,608,047 (Mattingly), issued August 26, 1986;
and U.S. Patent 4,687,478 (Van Tilburg), issued August 18, 1987.
Preferred catamenial products c:an comprise wings, side flaps, and other
structures and elements, as described in Canadian Patent Application No.
2,123,603 to Yasuko Morita, entitled "Absorbent Article Having Elasticized
Side Flaps", filed November 30, 1992.
It should be understood, however, that the present invention is also
applicable to other absorbent articles known commercially by other names,
such as incontinent briefs, adult incontinent products, training pants, diaper
inserts, facial tissues, paper towels, and the like.
D. Process for Making Absorbent Material
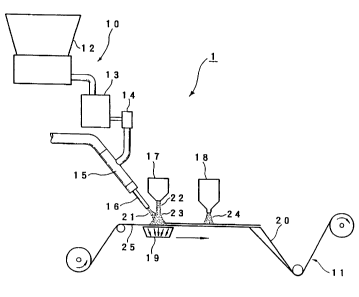
FIG. 1 illustrates a preferred apparatus useful in the process of the
present invention. The forming apparatus generally indicated as 1, is
composed of a particle applying unit 10, and a carrier layer apparatus 11.
The particle applying unit 10 includes an absorbent gelling particles applying
unit 12, a glue microfibers applying unit 16 and a polycationic polymer spray
unit 17. The absorbent gelling particles are first loaded in, for example, a K-
tron screw feeder 12 for continuously feeding absorbent gelling particles to
Vibratory feeder 13 and then hopper 14. After absorbent gelling particles are
carried away from the outlet of hopper i4 into an eductor 15, absorbent
gelling particles leave the nozzle 16 as the first air stream 2'1 by about 50-
psi
air stream. The eductor 15 and the nozzle 16 concentrates the absorbent
gelling particles into a constant flow in order to inject the absorbent
gelling
particles through the glue microfibers. Preferably the average diameter of the
absorbent gelling particle is usually from about 10 microns to about 1,000
microns. While the absorbent gelling particles are predominately
CA 02251931 2003-O1-20
l5
discontinuous, they generally have a length excec ding that normally
associated with particles.
The glue microtibers are extruded ~ is the polycationic pol.,~mer spray unit I
? (also
referred to as a glue gun (J&)VI C'o.)) with rate between about 0.2 to about
2.0 Kgcm-'hr=' as
the second air stream 2?. The glue rnicrotibers oxtrusi<» ~ thins out when
guided through a
second air stream. The temperature range i~, sc:t trp enough to solute and
spray the glue
microfibers. The air gap is preferable kept about 0. I ~ mm. The second air
stream of glue
microfibers is controlled to preferably deli~~er ahaut 1 (i ,.rim- basis
weight of the resulting
absorbent material and the operation range is preferably ti'om about 3.0 gm/m'
to about 50.0
gm%m-.
The first air stream 21 is merged with the second air stream 22 to form an
integrated
air stream 23. The integrated air stream 23 is rnjected unto a carrier layer
in mechanical
direction, preferably about 70 meterimm. 'hl~~e injecti<an rate of integrated
air stream 23
preferably is about 1.0 miser which is adjusted to match the carrier unit's
speed.
A vacuum conveyor ', 9 is placed beneath the nozzle l6 and the glue gun 1'~.
As the
carrier layer 25 is run through the vacuum conseyor I9, the incoming
integrated air stream 23
is attracted and firmly attached to tire carrier layer 2a l'he absorbent
gelling particles cover
the center line of the carrier layer, preferably at least one half of the
width.
A polycationic polymer unit 18 applies a third air stream 24 containing pre-
agitating
polycationic polymers. fhe third air stream 24 i , located after the absorbent
gelling particles
are laid on the carrier layer. The third air stream is slrrayed onto the
absorbent gelling
particles attached to the carrier layer and the potycationi~ polymer bonds to
the absorbent
gelling particles on the surface. The line speed ~s contr<alled preferably at
about 8 gm/m'.
A folding board 20 is placed adjacent to the pc>lycationic polymer unit 18.
The
absorbent material comprising the absorbent gelling particles, the glue
microfibers, the
polycationic polymer and the carrier layer are folded to form an edge closed
laminate
structure of final width. fhe laminated product <7i~ absorbent matena~l is
wound at tire end of
the line.
The present invention also provides a method for making the absorbent
material. The
method comprises (a) applying absorbent gelling particles comprising; a WAHP
onto a carrier
layer; (b) applying glue microfibers onto the carrier laser: and (c) applying
a polycationic
polymer onto the absorbent c;elling particles to form a bond between the
absorbent gelling
particles and the potycatzonic polyme~~; where~rr the absorbent gelling
particles adhere to the
glue microfibers prior to the glue micrcrtibers <~dhering to tl~e carrier
layer.
CA 02251931 1998-10-16
WO 97/39780 ~ 6 PCT/US97/06603
In one embodiment, the method further comprises the step of dispersing
cellulose fibers into the absorbent gelling particles, wherein the glue
microfibers act as an adhesive between the cellulose fibers and the
absorbent gelling particles.
In a preferred embodiment, the absorbent gelling particles are applied via
a first air stream on a carrier layer.
In more preferred embodiment, the glue microfibers are applied via the
second air stream. The second air stream comprising glue microfibers
preferably has a temperature of from about 100°C to about 400°C.
In a preferred embodiment, the polycationic polymer is applied via the
third air stream. The third air stream comprising the polycationic polymer is
used as a solution having a concentration preferably from about 0.1 % to
about 10% by weight.
The solution containing the polycationic polymer is then applied to a
plurality of the absorbent gelling particles. In particular, at least two,
preferably all, of the absorbent gelling particles have at least some portion
covered with the solution. In a preferred embodiment, at least 70% of the
surface area of the gelling particles are covered with the solution applied
thereon. The solution can be applied using any of the various techniques
and apparatus well known in the art which are suitable for applying a solution
to a material including coating, dumping, pouring, dropping, spraying,
atomizing, condensing, or immersing the solution onto the absorbent gelling
particles. After the polycationic polymer is applied, preferably greater than
about 90% of the surface area of the gelling particles is covered with the
solution.
In a preferred embodiment, the method further comprises the step of
heating the resulting material of step (c) at a temperature of from about
50°C
to about 300°C so as to covaiently bond the polycationic polymer to the
WAHR of the absorbent gelling particles.
In a preferred embodiment, the polycationic polymer are reacted with the
absorbent gelling particles such that the polycationic polymer becomes
covalently bonded to the absorbent gelling particles at the surface area of
the
absorbent gelling particles. More preferably, the covalent bonds are made
between the surface-located carboxy groups of the absorbent gelling particles
and the amino groups of the polycationic polymer. Preferably, at least about
80% more preferably more than about 90% by weight of the polycationic
polymer is covalently bonded to the absorbent gelling particles. When
CA 02251931 1998-10-16
WO 97/39780 1 ~ PCT/ITS97/06603
supplied with higher thermal energy, the absorbent articles comprising the
absorbent material have more fluid permeable. With the improved fluid
permeability the spreading of, e.g., urine throughout the absorbent articles
comprising the absorbent material is increased, and therefore the absorbent
gelling particles fluid absorption efficiency can be raised.
The present invention also relates to a method of making an absorbent
material. The method comprises (a) forming a first air stream comprising
absorbent gelling particles comprising a WAHP; (b) forming a second air
stream comprising glue microfibers; (c) merging the second air stream with
the first air stream to form an integrated air stream comprising a through
mixture of the glue microfibers and the absorbent gelling particles; (d)
directing the integrated air stream onto a carrier layer; (e) forming a third
air
stream comprising a polycationic polymer; and (f) directing the third air
stream onto the carrier layer so the polycationic polymer bonds to the
absorbent gelling particles.
Preferably, the method of forming an absorbent material comprises (a)
forming a first air stream comprising absorbent gelling particles comprising a
WAHP; (b) forming a second air stream comprising a polycationic polymer;
(c) merging the second air stream with the first air stream to form an
integrated air stream, wherein the polycationic polymer bonds to the
absorbent gelling particles; (d) forming a third air stream comprising glue
microfibers; (e) merging the integrated air stream with the third air stream
to
form a mixture air stream; and (f) directing the mixture air stream onto a
carrier layer so the absorbent gelling particles bonded to the polycationic
polymer adhere to the glue microfibers, and the glue microfibers adhere to
the carrier layer.
In a more preferred embodiment, the second air stream is formed a
temperature of at least about 400°C and about 50 psi air pressure at
sonic
velocity.
In another embodiment, the method comprises (a) applying polycationic
polymer fibers comprising a polycationic polymer having a concentration of
from about 80% to about 99% by weight onto absorbent gelling particles
comprising a WAHP; and (b) applying the absorbent gelling particles onto a
carrier layer; wherein the polycationic polymer fibers act as an adhesive
between the absorbent gelling particles and the carrier layer. Preferably, the
polycationic polymer fiber has the molecular weight of at least about 70,000.
CA 02251931 2003-O1-20
18
In a preferred embodiment, the potycationic polymer fibers form a first air
stream containing the polycationic polymer fibers and the absorbent gelling
particles from the second air stream to form an integrated air stream
containing a thorough mixture of the polycationic polymer fibers and the
absorbent gelling particles.
In a more preferred embodiment, the method comprises (a) forming a first
air stream containing polycationic polymer fibers; (b) forming a second air
stream containing absorbent gelling particles comprising a WAHP; (c)
merging the second air stream with the first air stream to form an integrated
air stream, wherein the potycationic polymer frbers bond to the absorbent
gelling particles; and (d) directing the integrated air stream onto a carrier
layer so that the absorbent gelling particles bond to the polycationic polymer
fibers on the carrier layer.
E. Test Methods
1. Synthetic Urine
The specific synthetic urine used in the test methods set forth herein is
referred to as "Synthetic Urine". The Synthetic Urine is commonly known as
Jayco SynUrine Mor Jayco Synthetic Urine and is available from Jayco
Pharmaceuticals Company of Camp Hill, Pennsylvania. The formula for the
Synthetic Urine is: 2.0 gtt of KCI; 2.0 g/i of Na2SO4; 0.85 g!I of (NH4)H2P04;
0.15 g/l (NH4)H2P04; 0.19 g/l of CaCl2 and 0.23 g/t of MgCl2. All of the
chemicals are of reagent grade. The PH of the Synthetic Urine is in the
range of 6.0 to 6.4.
2S
2. Wet Burst Strength Measurement
The standard burst test program measures toad, deflection and energy at
peak load, and fail toad at test end. The purpose of this test is to evaluate
the gram force of a absorbent-gelling-particle-contained laminate structure
after subjecting to a constant loading of synthetic urine. Laminate samples
with a dimension of 10 cm x 10 cm and of 310 gm/m2 absorbent gelling
particle basis weights, typically weigh at 3.6 t 0.3 gm and are allowed to
soak
in 70m1 of synthetic urine. Synthetic urine is fully absorbed by the
absorbent-gelling-particle-contained laminate, with 20 times synthetic urine
loading of its original weight. A burst tester, Thwing-Albert Instrument Co.
No. 177-1-B, is used to measure the gram force needed to puncture the 20
time's synthetic urine-loaded laminate samples. The sample holder is a plexi
CA 02251931 1998-10-16
WO 97/39780 ~ 9 PCT/US97/06603
glass disk of 4.5-inch diameter and 0.125 inch of thickness, and with a hole
of
0.75 inch diameter in the center of plexiglass. A stainless ball head of 0.25-
inch diameter is used to puncture the samples.
3. Tea Bag Gel Volume
Gel volume of a WAHP is defined as its retention absorbent capacity after
swollen in an excess of Jaycee Synthetic Urine. It provides a measure of the
maximum absorbent capacity of the polymer under conditions of use where
the pressures on the polymer are relatively low. Gel volume is determined by
centrifuge capacity method described below by using the Jaycee Synthetic
Urine. The gel volume is calculated on a dry-weight basis. The dry weight
used in the gel volume calculation is determined by oven drying the WAHP at
105°C for three hours. All of the chemicals are of reagent grade. The
pH of
the Jaycee Synthetic Urine is in the range of 6.0 to 6.4.
Heat-sealable tea-bag paper is cut into 6cm x 12cm, folded in half
lengthwise and sealed close to the edge along two sides with a T-bar sealer
to produce 6cm x 6cm tea bag squares. 0.200(~ 0.005) gm of a WAHP is
transferred into a tea-bag, and the top of the bag is sealed at its edge. The
top of an empty tea-bag is sealed and is used as a blank. Approximately
300m1 of Jayco Synthetic Urine is poured into a 1,OOOmI beaker, and the tea-
bag containing WAHP and the blank are submerged into the beaker. After
being soaked for 30 minutes, the blank and the WAHP-filled tea bag are
removed from the solution by using tongs. A centrifuge (H-122 type,
Kokusan Enshinki Co. Ltd., Tokyo, Japan) with a direct read tachometer,
electric timer is used for this measurement. The sample tea bags and the
blank tea bags are positioned in the centrifuge basket and centrifuged at
1100rpm for three minutes. Gel volume is calculated as follows:
Gel volume (g/g) _ (Ws - Wb-Wo) / Wo
wherein Ws is the sample tea bag weight after centrifuge, Wb is the blank tea
bag weight after centrifuge, Wo is the WAHP weight (0.200g).
The average of at least two determinations should be reported.
4. Acquisition Speed and Re-wet Test
The Acquisition Speed and Rewetness, which are the laminate production
properties comprising this absorbent material made according to present
CA 02251931 1998-10-16
WO 97139780 2~ PCT/US97/06603
invention, are evaluated in diapers. The typical diaper design includes
airfilt
as the acquisition layer and the laminate production as fluid storage core of
at
least 310 gm/m2 absorbent gelling particle basis weight. The acquisition
speed and rewet measurements are performed with 0.30 psi external
pressure in a flat configuration. After continuous loading of 200 mL synthetic
urine, several pieces of filter papers are placed on the wet pad and allowed
to
soak for 30 min under 0.40 psi. The rewet values, as measured from the
weight increase of filter paper, are summed up from front, middle, to back
part of diaper.
F. Examples
The following examples are presented for purposes of illustrating various
aspects of the absorbent material of the invention and are not intended as
limiting the scope of the appended claims in any way.
A composite in accordance with the present invention is prepared on a
process line for laminate production illustrated in FIG 1.
All raw materials used in this example are obtained from commercial
sources. Styrene-Isoprene-Styrene block copolymer (HL-1358-XZP)
produced by H.B. Fuller Co. is used as a glue microfiber, and is heated and
kept at least 350°C during laminate production process. L761f produced
by
Nippon Shokubai Co. Ltd. is used as absorbent gelling particles, and has
particle size distribution ranging from 300pm to 600~m. The
Polyethyieneimine produced by Wako Chem Co., is used as a polycationic
polymer, and is a 30% solid and has molecular weight of 70,000 Daltons. A
tissue produced by Havix Company LTD is used as a wet laid tissue of 18
gms and has the tensile strength of 1.1 Kg/in in mechanical direction.
The properties of the absorbent material, Wet Burst Strength (BBS) and
absorbent capacity (G~ are evaluated and presented in Table 1. In a diaper
including the absorbent material of present invention. Acquisition speed and
Rewet performances are evaluated and presented in Table 2.
EXAMPLE 1
L761f is prepared in K-trop screw feeder. L761f is loaded in a K-tron screw
feeder for continuously feeding L761f into a vibrator feeder and then a
hopper.
A compressed air stream is kept at 50 psi air pressure. L761f is carried away
from the outlet of the hopper into an educator and is combined with the
compressed air stream, so as to provide a first air stream. Injection rate of
CA 02251931 1998-10-16
WO 97/39780 21 PCT/US97106603
the first air stream of L761f is kept about 1.0 msec-1 which is adjusted to
match the web line speed.
Styrene-Isoprene-Styrene block copolymer (HL-1358-XZP), is prepared in
the apparatus of glue gun (J&M Co.). HL-1358-XZP is extruded through glue
gun at a rate between about 0.2 to about 2.0 Kgcm-1 hr1. The air gap of the
glue gun is kept at about 0.18 mm, as the glue block-copolymer becomes thin
fibers.
The extruded HL-1358-XZP is combined with an air stream, so as to
provide a second air stream. The second air stream is kept at a temperature
of about 400°C and about 50 psi air pressure at about sonic velocity.
The
second air stream of HL-1358-XZP is controlled to deliver 10 g/m2 basis
weight of the laminate production. The operation range of the second air
stream can be between about 3.0 gm/m2 and about 50.0 gm/m2.
The first air stream of L761f is subsequently injected through the second
air stream of HL-1358-XZP, to form an integrated air stream, onto a vacuum
conveyer.
The vacuum conveyor is placed beneath the glue gun and the educator.
At the same time, a tissue is introduced to the vacuum conveyor at a
typical speed of about 70 meter/min.. As the tissue is run over the vacuum
conveyor, the incoming integrated air stream is attracted and firmly attached
to the tissue. The tissue width is at least about 23 cm and a coverage width
of the integrated air stream is at least about 9.50 cm.
A polyethyleneimine is dissolved in distilled water at a concentration of
from about 10 to about 20% by weight. A third air stream spray is forwarded
an air pressure spray system (B1/8 BAU-SS+SUV 67-SS from Spraying
System Co. of 0.5-1.2 Kg/cm2) containing a pre-agitated solution of
polyethyleneimine and water is located after the integrated air stream
containing HL-1358-XZP and L761f laydown. According to the line speed, the
speed-of spraying and the level of polyethyleneimine solution is controlled at
8 gm/m2, which is equal to about 2.0% by weight of the laminate production.
A folding board is placed next to the third air stream spray of
polyethyleneimine solution. Tissue, L761f, HL-1358-XZP and
polyethyleneimine are folded to form an edge closed laminate structure of
about 10 cm final width. The laminate production is wound at the end of the
line. The thickness of a formed laminate of about 310 gm/m2 absorbent
gelling particle is about 1.3 mm.
CA 02251931 2003-O1-20
22
The laminate production properties comprising this absorbent material
made according to this example are evaluated. The Wet Burst Strength and
The Tea Bag gel Volume is 61 gm and 33 glg. The results surprisingly
illustrate the higher gel strength (BBS) and high absorbent capacity (GV)
achieved by the absorbent material according to the present invention.
EXAMPLE 2
The basic composition of sample is similar to Example 1, except no third
air stream spray of polyethyleneimine additive is used.
The laminate production properties comprising this absorbent material
made according to this example are evaluated. The Wet Burst Strength and
The Tea Bag gel Volume is 25 gm and 35 glg.
EXAMPLE 3
In this example, the basic composition of sample is similar to Example 1,
except as set forth below.
(1) "URIC absorbent gelling particMe" is prepared in K-trop screw feeder for
use in the first air stream. "URIC absorbent gelling particle" is an absorbent
gelling particle having improved absorbent property by the absorbent property
modfication polymer, such as the potycationic polymer, bonded to the
absorbent gelling particles.
(2) There is no third air stream spray.
The laminate production properties comprising this absorbent material
made according to this example are evaluated. The Wet Burst Strength and
The Tea Bag gel Volume is 45 gm and 31 glg. The results surprisingly
illustrate the higher gel strength (BBS) and high absorbent capacity (GV)
achieved by the absorbent material according to the present invention.
EXAMPLE 4.
The basic composition of the sample is similar to Example 1, except no
second air stream of the thermoplastic polymeric microfiber and the third air
stream spray of polyethyieneimine additive are used.
The laminate production properties comprising this, absorbent material
made according to this example are evaluated. The Wet Burst Strength and
The Tea Bag gel Volume is Ugm and 38g1g.
CA 02251931 1998-10-16
WO 97/39780 23 PCT/C1S97/06603
Table-1. Tea bag gel volume and effects on wet strength by adding
polyethyleneimine
Sam les PolvethvleneWet Burst Tea baa
pel
-imine Stren th volume
% m /
Sample Polycationic polymer 2 61 33
#1 is added in situ
Burin laminate makin
process.
Sample No pol cationic polymer 0 25 35
#2 is included.
Sample Polycationic polymer 2 45 31
#3 is added during
absorbent gelling particle
making
process. Subsequently
this absorbent
gelling particle is used
to make
laminate structure.
~ SampleNo glue microfiber. j 0 0 38~
#4 I
The acquisition speeds and rewet values are evaluated in a diaper. The
diaper is made of by laminate production comprising the absorbent material
according to above examples. The properties of the diaper are evaluated
and presented in Table 2.
Table-2. Acquisition and rewet performances of the invention in diaper
application.
Samples Acouisition Speed (sec) Pad Rewet
l Qm)
50 mL, 100mL, 150mL, 200mLat 200 mL loading
of of
s nthetic urine loadin synthetic urine
Sample 20 31 35 43 ~ 0.50
#1
Sample 18 28 39 55 0.50
#2
Sample 16 20 23 28 0.50
#3
Diapers of this invention (the sample #1 and #3) show faster acquisition
speeds at high urine loading, e.g., urine volume levels of at least 150mL than
the Sample #2. The improved acquisition speeds are caused by faster fluid
transportation among the well-bonded absorbent gelling particle particulate in
the wet state. In Sample #3, where absorbent gelling particle is treated with
polyethyleneimine alternatively during absorbent gelling particle production
process, the laminate shows even faster acquisition speeds. The degree of
bonding forces in Sample #3 is higher than that of Sample #1. Also shown in
Table-2, the rewet values of the invention maintain control diaper that is
used
to the absorbent material of no polycationic polymer.
All publications, patent applications, and issued patents mentioned
hereinabove are hereby incorporated in their entirety by reference.
CA 02251931 1998-10-16
WO 97/39780 PCT/US97106603
24
It is understood that the examples and embodiments described herein are
for illustrative purposes only and that various modifications or changes in
light
thereof will be suggested to one skilled in the art and are to be included in
the
spirit and purview of this application and scope of the appended claims.