Note: Descriptions are shown in the official language in which they were submitted.
CA 02252474 2005-07-05
WO 9'7141424
BIOSENSOR DEVICE AND METHOD
Field of ~,gl~nrion
The present invention relates to biosensors, and is particular, to a biosensor
for measuring
a binding event between a ligand and a ligand-binding receptor, and to methods
for producing
such biosensors.
Bac ound o~ae In tip ion
IO Diagnostic tools used for detecting or quantitating biological analytes
typically rely on
ligacul-specific binding between a ligand and a receptor. Ligandlreceptor
b'utding pairs used
commonly in diagnostics include antigen-antibody, hormone-receptor, drug-
receptor, cell
surface antigen-lectin, biotin-avidin, substratelenzyme, and complementary
nucleic acid strands.
The analyze to be detected may be either member of the binding pair;
altetn~ivdY, the aoalyte
may be a liga~ analog that competes with dte ligand for binding to the
oomphna~t receptor.
A variety of devices for detecting ligandlreceptot interactions are known. The
most basic
of these are purely chemicalleazymatic assays in which the presence or amount
of analyte is
detected by measuring or quantitating a detectable reaction product, such as
gold
immunoparticles. Ligandlreceptor interactions can also be detected and
quantitatod by
radiolabel assays.
Quantitative binding assays of this type involve two separate components: a
reaction
substrate, e.g., a solid phase test strip and a separate realer or detector
dice, such as a
sci~illation cower or spectrophotometer. The aubstraae is generally unsu'tted
to multiple
assays, or to miniaturization, for handling multiple analyte assays from a
small amourn of
body fluid sample.
In biosensor diagnostic devices, by contrast, the assay substrate and detector
surface are
integrated into a single device. One general type of biosensor employs an
electrode surface in
combination with current or impedance measuring elements for detecting a
change in current
or impedance in response to the presence of a ligand-receptor binding event.
This type of
biosensor is disclosed, for example, in U.S. Patent No. 5,567,301.
. Gravimetric biosenaors employ a piezoelectric crystal to geneaate a surface
acoustic wave
whose frequency, wavelength and/or resonance state are sensitive to surface
mass an the crystal
surface. The shift in acoustic wave propeeties is therefore indicative of a
change in surface
mass, e.g., due to a ligand-receptor binding event. U.S. Patents Nos.
5,4?8,756 and
4,789,804 describe gravimehic biosensors of this type.
Biosensors based on surface plasmon resonance (SPR) effects have also been
proposed,
for example, in U.S. Patents Nos. 5,485,277 and 5,492,840. These devices
exploit the shift
CA 02252474 2005-07-05
Wl) 971414Z4 YCT/CA9'f100275
Z
in SPR surface reflection angle that occurs with peraubations, e.g., biading
events, at the SPR
interface. Finally, a variety of biosensors that utilize changes in optical
properties at a
biosensor surface are known, e.g., U.S. Pateat No. 5,268,305.
Biosensors have a number of potential advantages over binding assay systems
having
separate reaction substrates and reader devices. One important advantage is
the ability to
manufacture small-scale, but highly reproducible, biosenaor unites using
microchip
manufachrring methods, as described; for example, in U.S. Patents Nos.
5,200,051 and
5,212,050.
Another advantage is ~e potentially large number of different analyte
detection regions
that can be integrated into a siagle biosensor unit, allowing sensitive din of
several
analytes with a very small amount of body-fluid sample. Both of these
adva.~tages can lead to
substantial cost-per-test savings.
A key element in the manufacture of biosensors, particularly mufti-assay
biosensors, is the
placement of analyre-specific binding molecules or enzymes at desired
locatnons oa a biosensor
surface. Ideally, it would be desirable to construcx a universal biosensor
surface under rigorous
microchip maironfacturing conditions, but allow a variety of different sarface-
region formats ho
be achieved under less restrictive manufacturing conditions, which at one
extreme would allow
an end user to tailor the universal chip to a unique mufti-aaalyte format.
Summanr of the Invent
In one aspect, the imrention includes a bioseuaor apparatus for detecting a
binding event
between a ligand and ligand~inding agent. The apparatus has a biosensor
surface, and two-
subunit heterodimer complexes carried on the surface. 'the complexes are
composed of first
and second, preferably oppositely charged p~tides that together form an a-
helical coiled-coil
hMerodimer. The first peptide is attached to the bioseasor surface, and a
ligand is covaleatly
attached to the second p~tide, accessible for binding by a ligand~inding
agent. Binding of
an anti~igand agent to the ligand is detected by a suitable decor in the
apparatus.
The first p~tide subunit may be attached to the biosensor surface covalently,
e.g., through
an oligopeptide spacer or a hydrocarbon-chain spacer, oc may be bound to the
biosensor surface
through a stable non-covalent linkage; e.g., a biotinlavidin binding pair. The
biosensor surface
may include multiple regions, each having a diff~nt selected ligand attached
to dte second-
subunit peptide.
In one general embadimettt, the bioseasor surface includes a' monolayer
composed of
hydrocarbon chains anchored at their proximal ends to the biosensor surface,
and having free
CA 02252474 1998-10-22
WO 97/41424 PCT/CA97100275
3
distal ends defining an exposed monolayer surface. The heterodimer complexes
in this
embodiment are preferably embedded in the monolayer, and the ligands are
disposed on or near
the monolayer surface. The monolayer may be formed on a metal, e.g., gold
film, and may
be composed of 8-22 carbon atom chains attached at their proximal ends to the
biosensor
S surface by a thiol linkage. The chains have a preferred molecular density of
about 3 to 5
chains/nm2, and the dielectric constant of the monolayer, in the presence of
such solution but
in the absence of such binding receptor, is preferably less than about 2.
In a biosensor apparatus designed for amperometric detection of binding of a
ligand-
binding agent to the monolayer ligand, the biosensor surface is an electrode,
and the
monolayer, including the heterodimer complexes embedded in the monolayer, is
sufficiently
close-packed and ordered to form an effective barrier to current across the
monolayer mediated
by a redox ion species in an aqueous solution in contact with the monolayer.
Binding of a
ligand-binding agent to the ligand on the monolayer surface is effective to
increase current
across the monolayer, mediated by such redox species. A chamber in the
apparatus is adapted
to contain an aqueous solution of redox species in contact with the monolayer,
and the detector
includes a circuit for measuring ion-mediated current across the monolayer, in
response to
binding events occurring between the receptor and ligand.
In a biosensor apparatus designed for gravimetric detection of binding of a
ligand-binding
agent to the surface-bound ligand, the biosensor surface is a piezoelectric
crystal. The detector
functions to (i) generate a surface acoustic wave in the crystal and (ii)
detect the shift in wave
frequency, velocity, or resonance frequency of the surface acoustic wave
produced by binding
of ligand-binding agent to the ligand.
In a biosensor designed for optical surface plasmon resonance (SPR) detection
of binding
of a ligand-binding agent to the surface-bound ligand, the biosensor surface
is a transparent
dielectric substrate coated with a thin metal layer on which the monolayer is
formed, where the
substrate and metal layer form a plasmon resonance interface. The detector
functions to excite
surface plasmons at a plasmon resonance angle that is dependent on the optical
properties of
the metal film and attached monolayer, and to detect the shift in plasmon
resonance angle
produced by binding of ligand-binding agent to the ligand.
In a biosensor designed for optical detection of binding of a ligand binding
agent to the
surface bound ligand, the detector functions to irradiate the biosensor
surface with a light beam,
and detect a change in the optical properties of the surface layer, e.g.,
monolayer with
embedded heterodimer, produced by binding of ligand-binding agent to the
ligand.
CA 02252474 2005-07-05
WO 97141414 PCTICAf11n0275
4
In another aspect, the invention includes a method for producing a ligand-
specific
biosensor for use in a biosensor apparatus capable of dere~ng a binding event
b~ween a ligand
and ligand binding rector. The method imrolvea contacting together: (a) a
biosensor
elecxrode having a biosensor surface and a first heteTOdimer-subunit peptide
attached to the
biosensor surface, and (b) a second, preferably oppositely charged peptide
capable of binding
to the first peptide to,form a two-subunit or-helical coiled-ooIl
heterodiiner. The second peptide
has an attached ligand capable of binding specifically to a !igand-specific
agent. The contacting
is effective attach liganda to the biosensor surface. The biose~or surface may
include first and
second discrete regions, where the second hetemdimer subunit peptide in each
region has a
different attached ligatui.
In one geaesat etnbodimont of the method, the biosenaor surface has a
tnono!ayer
composed of hydmearbon chains (l) anchored at their proximal ends to the
biosensor surface,
and (ii) having free distal ends defining an exposed motalayer surface. The
first peptide is
embedded in the monolayer, sad binding of the second p~tide to surface-bound
first peptide
is effective to dispose the ligand preferably on or near the monolayer
surface.
More getterslly, the imr~ion proves a n~Od of conet<ucdng an array of
diffetmt,
sele<xed biologics! reagents attached to different, sdecxed regions on an
assay support surface.
The medwd includes attaching mold:ules of a first heterodin~er-aubunit peptide
to the support
surface, effective to cover the different regions on the surface with the
first peptide molecules.
The subunit peptide has protecting gmups which when photo-released, allow the
p~tlde to
interact with a aernad, preferably opposltely charged heterodiaser-subunut
peptide, to form a
twa-~subunit a-helical coiled-coil hmerodimer.
The surface is irradiated in a selected region of the surface under conditions
effecxive to
deprotect the first p~tide in the irradiated region ody, then cantactod with a
second subunit
peptide carrying the assay reagent. This contacting is effective to attach the
selected reag~t
to the exposed region of the surface only. The above steps are repeated for
different aeloaed
regions and assay reagems, -until the desired array of different, selected
biological reagents
disposed at different selected regions on as assay support surface is
produced.
In one embodiment, the first subunit peptide contains amino acid residues with
one or
more protected carboxyl groups, e.g., glutamate groups with nitrophenolate
protecting groups,
Those and other objects and f~ures of the invention w01 become more fully
apparent
whey the following detailed . description of the invention is read in
conjunction with the
accompanying drawings.
CA 02252474 1998-10-22
WO 97/41424 PCT/CA97/00275
Brief Description of the Drawings
Figs. 1A and 1B show elements of a biosensor apparatus in accordance with of
the
invention, illustrating the apparatus before (1A) and after (1B) binding of a
ligand-binding agent
to the biosensor surface in the apparatus;
5 Figs 2A-2C show helical wheel representations of (2A) terminal heptads of
two exemplary
heterodimer-subunit peptides in a parallel a-helical heterodimer
configuration; (2B) terminal
heptads of two exemplary heterodimer-subunit peptides in an antiparallel a-
helical heterodimer
configuration; and (2C) helical wheel representations of specific peptides in
an a-helical
heterodimer configuration;
Figs. 3A-3E show schematic representations of adjacent heptads of two
heterodimer-
subunit peptides in a parallel configuration comparing the
stabilizing/destabilizing effects of
charged residues at the a and g positions in homodimers vs. heterodimers;
Figs. 4A and 4B illustrate alternative methods for coupling an HSP1 subunit
peptide to a
biosensor surface in a biosensor;
Figs. 5A and SB illustrate hydrocarbon-chain monolayers formed on a biosensor
surface
in a biosensor with an K-coil peptide alone embedded in the monolayer (5A) and
a K-coil/E-
coil heterodimer embedded in the monolayer (5B);
Fig. 6 shows elements of an amperometric biosensor constructed in accordance
with one
embodiment of the invention;
Fig. 7 shows the change in oxidation (solid circles) and reduction (open
squares) current
as a function of time after addition of E-coil peptide subunit to an electrode
of the type
illustrated in Fig. 5A containing an embedded K-coil peptide subunit;
Fig. 8 shows changes in oxidation of Fe(CN)6''/" (open circles) and reduction
(open
squares) as a function of time after addition of PAK peptide to an electrode
containing di-
saccharide ligands on a K-coil/E-coil lipid monolayer;
Fig. 9 shows changes in oxidation of Fe(CN)6~/' (open circles) and reduction
(open
squares) as a function of time after addition of Verotoxin peptide to an
electrode containing
trisaccharide ligands on a K- coil/E coil lipid monolayer;
-Fig. 10 shows elements of a gravimetric biosensor constructed in accordance
with an
embodiment of the invention;
Fig. 11 shows elements of a surface plasmon resonance biosensor constructed in
accordance with an embodiment of the invention;
Fig. 12 shows elements of an optical biosensor constructed in accordance with
an
embodiment of the invention;
CA 02252474 2005-O1-13
6
Figs. 13A-13C illustrate steps in the attachment of an assay reagent to an
irradiated region
of a biosensor surface, in accordance with a method of the invention; and
Fig. 14 is a cross-sectional view of a portion of a mufti-test amperometric
biosensor
constructed in accordance with an embodiment of the invention.
S
Detailed Descrig~ion of Invention
I. ~iosensor A app rates
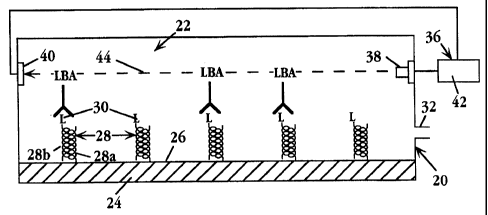
Figs. lA and 1B show a simplified schematic view of a biosensor apparatus 20
for
detecting a binding event between a ligand and a ligand binding receptor, in
accordance with
the invention. The apparatus includes a reaction chamber 22 defined in part by
a substrate 24
which has a biosensor surface 26 within the chamber.
The biosensor surface has attached thereto, two-subunit heterodimer complexes,
such as
complexes 28, each complex carrying a ligand, such as ligands 30, which forms
one of the two
binding pairs of a ligand/anti-ligand agent whose binding serves as the
"trigger" of a
measurable biosensor event, as will be described below. Fig. IB shows the
condition of the
biosensor surface after binding of ligand binding agent to a portion
of the ligands on the biosensor surface.
According to an important feature of the invention, each heterodimer complex,
such as
complex 28, includes a first peptide subunit, such as subunit 28a, which is
attached to the
biosensor surface, e.g., by covalent attachment, and a second, preferably
oppositely charged
subunit, such as subunit 28b, to which the ligand is attached. The two
peptides are
constructed, as will be detailed below, for self assembly into stable, two-
subunit alpha-helix
coiled-coil heterodimer complexes, and when so assembled, serve to anchor the
ligand on the
biosensor. surface as shown.
The chamber includes at least one port or opening 32 for introducing a
solution or
suspension into the chamber. Where the biosensor has a closed chamber, as
here, the chamber
may additionally include a vent or outlet port. The analyte introduced into
the chamber, i.e.,
the compound or material to be assayed, will be either an anti-ligand binding
agent, or a ligand
or ligand analog which is capable of competing with surface-bound ligand for
binding to a
ligand binding agent. The analyte-i.e., the ligand, ligand analog or anti-
ligand agent-may be
in free molecule form or may be part of a complex, e.g., a cell or
macromolecular complex.
Where the analyte is a ligand or ligand analog, the apparatus further includes
a ligand-binding
agent which may be introduced with the analyte or may be present in the
chamber, e.g.,
immobilized on the chamber walls or present in dried, unbound form within the
chamber.
CA 02252474 1998-10-22
WO 97/41424 PCT/CA97/00275
7
The biosensor apparatus also includes a detector or detector means 36 for
detecting the
presence and/or level or binding of ligand binding agent to the surface
ligands. A variety of
detectors are described below. For simplicity, the detector in Fig. 1 is
illustrated
schematically, and includes a beam source 38 for producing a beam 4.4, a beam
detector 40,
and a control unit 42 operatively connected to the beam source and detector
for measuring
changes in the beam, e.g., beam intensity, in response to binding of ligand-
binding agent to
surface-bound ligand, as illustrated in Fig. 1B.
A. Heterodimer Subunit Peptides
The heterodimer-subunit peptides employed in the biosensor invention are two
non-
identical, preferably oppositely charged polypeptide chains, typically each
about 21 to about
70 residues in length, having an amino acid sequence compatible with their
formation into two-
stranded a-helical heterodimeric coiled-coils. They are designated herein as
HSP1
(heterodimer-subunit peptide 1), and HSP2 (heterodimer-subunit peptide 2). In
the discussion
below, HSP1 will refer to the peptide attached to the biosensor surface in the
biosensor, and
HSP2, to the peptide having an attached ligand. It will be understood that
these designations
refer to the functional role played by the subunit peptide, not the actual
peptide sequence.
In aqueous medium, the isolated heterodimer-subunit peptides are typically
random coils.
When HSP1 and HSP2 are mixed together under conditions favoring the formation
of a-helical
coiled-coil heterodimers, they interact to form a two-subunit a-helical coiled-
coil heterodimeric
complex.
Peptides in an a-helical coiled-coil conformation interact with one another in
a
characteristic manner that is determined by the primary sequence of each
peptide: The tertiary
structure of an a-helix is such that 7 amino acid residues in the primary
sequence correspond
to approximately 2 turns of the a-helix. Accordingly, a primary amino acid
sequence giving
rise to an a-helical conformation may be broken down into units of 7 residues
each, termed
heptads. The heterodimer-subunit peptides are composed of a series of heptads
in tandem.
When the sequence of a heptad is repeated in a particular heterodimer-subunit
peptide, the
heptad may be referred to as a "heptad repeat", or simply "repeat".
Specific types of amino acid residues at defined positions in each heptad act
to stabilize
the two-stranded a-helical coiled-coil heterodimeric structure or complex.
1fie heterodimer
peptides may also contain residues that can be reacted (either intro- or inter-
helically) to
- stabilize the a-helical or coiled-coil nature of the polypeptides. One
example of a stabilizing
modification is the incorporation of lactam bridges in the first and last
(terminal) repeats of
CA 02252474 2005-07-05
WO 99141421 PCTICA9'f1~75
8
heterodimer-subunit peptides, as detailed in PCT application WO CA95100293 f~
"Heterodimer Polypeptide Imnnuaogen Carrier Composition and Method";
publication date 23
November 1995,
The dimerization of HSPI and IiSP2 is due to the pt~esence of a repeated
heptad motif of
conserved amino acid residaas in each p~tide's prinnary ami~w acid sequence.
The individual
positions in each heptad are designated by the letters a through g for HSPl,
and a' through g'
for HSP2, as shown in Figures 2A and 2B. Repeating h~tad motifs having
appropriate amigo
acid sequences direct the HSPI and HSP2 polypeptides to assemble into a
heterodim~ic a-
helical coiled-coil sr<uaure m~der permissible conditions. The iadividusi a-
helical p~tides
coarser one another along their respective hydrophobic faces, defined as the s
and d positions
of each heptad.
HSP1 and HSP2 may assemble into a heterodimer coiled-coil helix (coiled-coil
heterodimer) in either parallel or antiparalld configorations. 1n a parallel
configuration, the
two heterodimer-subunit peptide helixes are aligned such that they have the
scone orientation
(amino-terminal to carboxyl-terminal). 1n an antiparallel configaration, the
helixes are an~mged
such that the araino-termlnal end of one helix is aligned with the carboxyl-
terminal end of the
other helix, and vice ~itrsa.
Diagraaas of the relative orientations of the a-g poshions of two interacting
a-helices are
shown in Figures 2A and 2H. Figure 2A shows m end~n schematic of fho first two
tunes (one
heptad) of two exemplary heterodimer subunit peptides, EE and ICK, arranged in
a parallel
contignration. Figure 2H shows an end-on schematic of the same heterodimer-
subunit peptides
arranged in an asitiparallel configuration. '
Hetemdimer-subunit peptides designed in accord with the guidance presented
herein
typically show a preference for assembling in a parallel orient~ion vs. an
antiparallel
orientation. For exa~le, the exemplary peptides identified by SED ID NO:1 and
SEQ ID
N0:2 in the above CA95100293 PCT patent application, form .parallel-
configuration
heterodimers as do other peptide sequences discussed in the PCT application.
Wham ding
a ligand to HSP2, it is ge~aerally desirable to attach the ligand at or near
the end of the p~tide
that wilt form the distal end of the heterodimer. In particular, where the
heterodimer forms
a parallel ~guration, the ~Pl peptide is preferably anchored to the biosensor
surface at
its C terminus, and the liga~ attached to the HSP2 peptide at its N terminus.
1n Figures 2A, 2B and 2C, amino acids are circled and indicated by the
onedetter code, .
and consecutive amino acid positions are numbered and joined by lines with
arrow heads
indicating the N-terminal to C-terminal direcxlon. Interactions between the
two helixes are
CA 02252474 1998-10-22
WO 97/41424 PCT/CA97/00275
9
indicated by arrows. Wide arrows crossing between the helixes depict
hydrophobic interactions
between the a and d positions of adjacent helixes.
Ionic interactions between the a and g positions of adjacent helixes are
indicated as curving
arrows above and below the nexus of the helixes. In Figs. 2A and 2B, position
a of peptide
EE is a Gln in the fast and last heptad, and a Glu in the internal heptads.
The (bottom)
curving arrow depicting ionic interactions with this position is drawn with a
dashed line to
indicate that ionic interactions are present between internal heptads of the
helixes, but not
between the first and last, or terminal, heptads. Lactam bridges in Figs. 2A
and 2B are
indicated as a right-angle line between the f and b positions within each
helix.
The hydrophobic interactions between the helixes are due to hydrophobic
residues at the
a and d positions of the heterodimer-subunit peptides. Residues at these
positions, effective
to maintain the helixes in contact, include leucine, isoleucine, valine,
phenylalanine,
methionine, tryptophan, tyrosine, alanine and derivatives of any of the above.
Other residues,
including alanine, cysteine, serine, threonine, asparagine and glutamine may
also occupy a or
d positions in some heptads, so long as others are occupied by hydrophobic
residues.
Appropriate selection of the specific residues to occupy the a and d positions
is important.
If the hydrophobic interactions are strong, as is the case, for example,
between helixes contain-
ing De at one of the positions and Leu at the other position, a significant
fraction of the helixes
will form as homodimers at pH 7, even if like-charged residues are present at
the a and g
positions to discourage homodimer formation. If, on the other hand, residues
at the a and d
positions are selected such that the hydrophobic interactions are too weak
(for example, Ala
at both positions), the helixes may not form coiled-coil dimers at all.
Preferably, residue pairs
are selected that promote the formation z 95% heterodimers at pH 7. An
exemplary pair of
residues at the a and d positions, that results in hydrophobic interactions
conducive to z 95 %
heterodimer formation at pH 7, comprises Leu at one of the positions and Val
at the other
position. These residues are present at the a and d positions of exemplary
heterodimer-subunit
peptides.
Dimeric coiled-coil conformations of a-helixes are preferably also stabilized
by ionic
interactions between residues at the a and g positions of adjacent helixes, as
is illustrated in
Figs 3A and 3D. If each helix of a dimer has a positively-charged residue at
one position, for
example, e, and a negatively-charged residue at the other position, for
example, g, homodimer
formation is favored (Fig. 3A; compare with heterodimer in Fig. 3B). However,
if each helix
has like-charged residues at both positions, then two oppositely-charged
helixes will tend to
associate into heterodimers (Fig. 3D), as opposed to forming homodimers (Fig.
3C, 3E). The
CA 02252474 2005-07-05
reader is referred to .WO 95/31480 for exemplary heterodimer sequences and
methods of synthesis.
B. Li~and Attachment to the BiosensQr Surface . ;
5 As noted above, one of the two subunit peptides (HSPI) in the heterodimer is
attached to
the biosensor surface, and the second peptide (HSP2) contains a ligand
intended to participate .
in an analyte-dependent ligand/anti-Iigand binding reaction. In both cases,
the peptide is
synthesized, or derivatized after synthesis, to provide the requisite
attachment function and
ligand, respectively.
10 Considering the modification of HSP1, the peptide may be synthesized, at
either its N or .
C terminus, to carry additional terminal peptides that can function as a
spacer between the
biosensor surface and the helical-forming part of the peptide. Fig. 4A shows
an HSPl peptide
attached.to a metal, e.g, gold, surface. 50 through a polypeptide spacer 51
terminating in a
cysteine or methionine residue which provides for covalent coupling to the
surface through a
thiolate linkage, under standard conditions (e.g., Dakkouri, A.S., et al., an
i (199
12:2849-2852).
For HSP1 coupling to a glass or polymer surface, the C or N terminal residue
can be
derivatized with a suitable activated functional group that allows direct
coupling of the peptide
end to. a selected amine, acid, alcohol, or aldehyde group on the surface.
These groups can
be introduced during solid phase synthesis according to standard methods, with
other reactive
side chains in the peptide being protected with suitable protecting groups.
Alternatively, the
HSP 1 peptide can be attached to the biosensor surface thorough a high-
affinity binding reaction;
such as between a biotin moiety carried on the peptide and an avidin molecule
covalently
attached to the surface.
Where the heterodimer is embedded in a hydrocarbon-chain monolayer, as
described
below, the spacer anchoring the HSPl peptide to the biosensor surface may be a
hydrocarbon
chain; such as spacer chain 51 anchoring HSP1 to metal surface 50 in Fig. 4B.
The chain
is preferably a fractional length of the chains making up the bilayer, such
that the distal ends
of the heterodimer peptides in the assembled monolayer are at or near the
exposed surface of
the monolayer. Thus, for example, if the monolayer is made up of 18-carbon
chains, the -
spacer is preferably 2-10 carbons in length, depending on the length of the
assembled
heterodimer. -
- The hydrocarbon-chain spacer, in the form of a omega-thio fatty acid, may be
coupled to
a terminal hydroxyl or amine coupling during solid~hase synthesis, as outlined
above. The
CA 02252474 1998-10-22
WO 97!41424 PCT/CA97/00275
11
derivatized peptide, in turn, can be attached to a metal surface by standard
thiolate coupling
(Dakkouri, supra).
Considering the ligand-attachment to HSP2, the ligand selected will be
determined by the
analyte to be tested. Ligand-receptor binding pairs, i.e., ligand/ligand-
binding agent pairs used
commonly in diagnostics include antigen-antibody, hormone-receptor, drug-
receptor, cell
surface antigen-lectin, biotin-avidin, substcate/enzyme, and complementary
nucleic acid strands.
The ligand is typically the smaller of the two binding pair members,
particularly where the
ligand is attached to a hydrocarbon-chain monolayer, as described below.
However, attachment
of either binding pair is contemplated herein.
Where the ligand is a polypeptide, e.g., peptide antigen, the antigen can be
synthesized
by either solid-state or recombinant methods, to include the peptide antigen
at the end of the
HSP2 peptide that will orient distally in the assembled heterodimer. Where the
ligand is a non-
peptide moiety, e.g., a non-peptide hormone, drug, or nucleic acid, the HSP2
peptide can be
synthesized to include one or more residues that can be specifically
derivatized with the ligand.
The ligand is preferably covalently attached to an amino-acid coupling
residues at positions b,
c and/or f of one or more heptads in HSPZ (Fig. 2A). These positions lie along
the outward
face of a coiled-coil heterodimer. In an exemplary embodiment, a single
coupling residue is
placed at the f position of a terminal heptad of HSP2, or at the terminal
residue. This residue
may be derivatized during solid-state synthesis according to known methods,
allowing selective
deprotection of the residue to be reacted.
Preferred coupling groups are the thiol groups of cysteine residues, which are
easily
modified by standard methods. Other useful coupling groups include the
thioester of
methionine, the imidazolyl group of histidine, the guanidinyl group of
arginine, the phenolic
group of tyrosine and the indolyl group of tryptophan. These coupling groups
can be
derivatized using reaction conditions known to those skilled in the art.
To attach the ligand-derivatized HSP2 peptide to the surface-immobilized HSP1
peptide,
the twp peptides are contacted under conditions that favor heterodimer
formation. A medium
favoring coiled-coil heterodimer formation is a physiologically-compatible
aqueous solution
typically having a pH of between about 6 and about 8 and a salt concentration
of between about
50 mM and about 500 mM. Preferably, the salt concentration is between about
100 mM and
about 200 mM. An exemplary benign medium has the following composition: 50 mM
potassium phosphate, 100 mM KCI, pH 7. Equally effective media may be made by
substituting, for example, sodium phosphate for potassium phosphate and/or
NaCI for KCI.
Heterodimers may form under conditions outside the above pH and salt range,
medium, but
~,~,~..
CA 02252474 2005-07-05
WO 97141424 PCT/CA9'1100Z7S
12
some of the molecular interactions and relative stability of heterodimers vs.
homodimers may
differ from characxeristlcs detailed above. For exa~le, ionic interactions
between the a and
g positions that lead to stabilize >uaaodimas may break down at low or high pH
values due
to the pmtonation of, for example, Glu ale drains ~ acidic pH, or the
deprobonation of, for
S example, Lys side chains at basic pH. Such effects of low and high pH values
on coiled-coil
hetecodimer formation may be overcome, however, by increasing salt
concxatration.
Increasing the salt concentration can neutralize the stabilizing ionic
attractions or suppress
the destabilizing ionic repulsions. Certain salts have greamr eiEcacy at
n~trallzing the ionic
interactions. For example; in the case of the K-coil pee in Fig. 2A, a iM or
greater
concentration of C10; anions is required to induce maximal a-helical structure
(as determined
by CD measurements performed as detailed in Example Z), whereas a 3M or
greater
concentration of Cf ions is required for the same effect. The effects of high
salt on coiled-coil
formation aE low and high pH also show that interhelical ionic attractions are
not essential for
helix formation, but rather, control whether a coiled-coil tends to form as a
h~~odimer vs a
homodimer.
C. Hiosensor,~urface with Hydrocarbon-Chain Mod
In one preferred embodiment, for use is a variety of the biosensors described
below, the
biosensor surface is modified to contain a hydrocarbon-chain mwwlayer, as
illustrated is Pigs.
SA and SB. The figures are eNarged views of a portion of a biosensor surface
48, iacludio~g
a thin electrode film SO on a substrate 52, and a nwnolaye~r 54 formed of
hydrocarbon chains,
such as chains 56, attached to the fitra through thioether linkages. Embedded
is the monolayer
are molecules of the HSP1 peptide, such as molecules 58 (Fig. 5A, before
addition of HSP2
p~tide), anchored w the surface as described above, and heterodimer complexes,
such as
complexes 59 (Fig. 5B, after addition of HSP2 ).
The chains forming the ~nolayer are typically 8-22 carbon, saturated hydmcubon
chains,
although longer chains, chains with some unsaduatioa, chains with non-carbon
chain atoms,
such as lipid ethers, andlor chains with minor branching, such as by non-chain
methyl grips,
may be employed. _ In an amperometric biosensor embodiment, to be descn'bed
below, the
chaiac are suffcieatly close packed and ordered to form an effective a,barrier
to electron
transfer flaw, under biosensor operating conditions, as discussed below. '
This density is
calculated to be between 3-5 chainslnms.
With reference to Fig. 5A, the HSPI peptide is included in the monolayer in a
mole ratio
peptidelhydrocarbon chains of preferably between 1:100 to 1:5. As indicated in
the figure, and
CA 02252474 1998-10-22
WO 97/41424 PCT/CA97/00275
13
discussed below, the Fig. 5A monolayer is leaky to ion carriers, such as
Fe(CN)63, and as a
result, gives a measurable detector current in the absence of analyte. The
leakiness of the
membrane is presumably due to the disruption of the monolayer by charge-charge
repulsion
between the charged peptides in the monolayer, as shown, and a diminution of
the electrostatic
potential barrier in the monolayer.
With reference to Fig. 5B, addition of an oppositely charged HSP2 peptide
neutralizes the
HSP1 peptide charges, with the result that the membrane assumes a low
conductance property,
as evidenced by substantially reduced current in the presence of charge
carriers. This property
of the biosensor surface will be described further below with respect to Figs.
7-9.
In a preferred method for forming the monolayer, a mixture of thiol-containing
chains and
thiol-terminated HSP1 peptide, at a selected mole ratio, is actively driven to
the surface by
applying a positive voltage potential to the substrate surface, e.g., gold
film. In practice, the
hydrocarbon chain mixture (about 1 mM hydrocarbon chains) in an ethanolic
solution of 100
mM Li perchlorate, neutral pH, is placed over the electrode, and a selected
potential is applied
to the electrode. The buildup of the monolayer can be monitored by increase in
layer
thickness. Alternatively, monolayer formation is monitored by measuring
current across the
monolayer, as described below. In this case, formation of the monolayer will
be characterized
by a steady drop in electrode current, until minimum current is reached, at
which point
maximum chain packing has been achieved.
a
The time required to achieve saturation packing density will vary with applied
voltage, and
can be as short as 10 seconds--about 4 orders of magnitude faster than
monolayer formation
by diffusion. Complete or nearly complete monolayer formation (30 A thickness)
occurs within
10 minutes at about 1V potential and above. At lower positive voltages,
additional reaction
time is required. Preferably the voltage applied to the electrode is at least
between about +250
mV relative to a normal hydrogen electrode (+250 vs. NHE) and 1.2V (vs. NHE).
Not only are rapid monolayer formation times achieved, but the percentages of
peptide and
hydrocarbon chains present in the reaction mixture are precisely represented
in the monolayers,
giving highly reproducible electrode characteristics.
To complete formation of the monolayer with attached ligand, the ligand-
derivatized HSP2
peptide is contacted with the monolayer under conditions favoring heterodimer
formation, as
detailed above, where the HSP2 peptide is preferably added in excess. The
formation of
heterodimers can be followed by measuring current across the monolayer.
Because heterodimer
formation tends to "tighten" the monolayer, as discussed above, heterodimer
formation will
CA 02252474 2005-O1-13
14
lead to a steady drop in measurers electrode current, until a stadte low
current is reacneu, at
which point maximum heterodimer formation has occurred.
The subsections below illustrate several types of biosensors for which the
biosensor
surfaces described above are suitable.
S
D. Am~erometric Biosensor
Fig. 6 illustrates, in simplified view, elements of an amperometric biosensor
60
constructed in accordance with one embodiment of the invention. The apparatus
has a closed
chamber 62 housing a biosensor surface 64 formed of a gold film 66, which
forms the surface
electrode in the biosensor. The electrode surface is covered by a monolayer 66
of hydrocarbon
chains which define an exposed monolayer surface 68. . The monolayer includes
ligand-
bearing heterodimers embedded therein, with the ligands disposed at
or near the monolayer surface, for accessibility to reaction with ligand-
binding agents. The
monolayer is formed as above, e.g., with thioether attachment of monolayer
components to the
1S gold film.
The biosensor chamber serves to hold an aqueous electrolyte solution required
for
biosensor operation, as will be described. Liquid may be introduced into or
withdrawn from
the chamber through a valued port 72, and chamber may include a second port or
vent (not
shown) to facilitate liquid flow through the port.
A reference electrode 74 and a -counter electrode 76 in the apparatus are
carried on the
upper chamber wall, as shown, and are both in conductive contact with
electrode 64 when the
chamber is filled with electrolyte solution. The reference electrode, which is
held at ground,
serves as the voltage potential reference of the working electrode, when a
selected potential is
placed an the working electrode by a voltage source 78. This potential is
measured by a
2S voltage measuring device 80 which may additionally include conventional
circuitry for
maintaining the potential at a selected voltage, typically between about -S00
to +800 mV.
Voltage source 78 is connected to counter electrode 76 through a current
measuring device
for measuring current between the two electrodes during biosensor operation.
The'reference and counter electrodes are Pt, Ag, AgIAgCI, or other suitable
electrodes. The
reference and working electrodes, and the circuitry connecting them to the
working electrode,
are also referred to herein, collectively, as detector means for measuring ion-
mediated current
across the working-electrode monolayer, in response to ligand-receptor binding
events
. occurring at the monolayer surface.
CA 02252474 1998-10-22
WO 97/41424 PCT/CA97/00275
In operation, the chamber is filled with a solution containing analyte and
ionic species
capable of undergoing a redox reaction, i.e., losing or gaining an electron,
at a suitably
charged electrode. Exemplary redox species are Fe(CN)6~''', as a negatively
charged species,
and Ru(NH3)62+"+ as a positively charged species. Other probes which can be
used include
5 Mo{CN)6~ (Eo = +800 mV), W(CN)6'- (F.~=+580 mV), Fe(CN); (Fro=+580 mV),
Ce'+"'
(Eo= + 1.4V), and Fe+'~2+ (F~= +666mV). Typical redox ion concentrations are
between 0.01
and 10 mM. The redox solution is contained in chamber and is in contact with
reference and
counter electrodes.
The voltage potential placed on the electrode, i.e., between the electrode and
reference
10 electrode, is typically at least 90 mV above the electrochemical potential
(Eo) value of the redox
species, for oxidation, and at least 90 mV below the electrochemical
potential, for reduction
of the species. Consider, for example, Fe(CN)6~'ø, with an En of 450 mV (vs.
NHE). Above
about 550 mV electrode potential, any Fe2+ species is oxidized to Fe3+, and at
an electrode
potential below about 350 mV, and Fe+3 is reduced to Fe+2. Similarly,
Ru(NH~bz+~3+ h~
15 an Eo of +50 mV (vs. NAE), so oxidation is achieved at an electrode
potential above about
+ 150 mV, and reduction, below about -50 mV.
The ability of heterodimer formation in the monolayer to enhance the close
packed
structure of the monolayer, as evidenced by monolayer conductance, is
illustrated in Fig. 7.
The figure shows the drop in conductance, as measured by ion-mediated current
flow, after
addition to a monolayer containing a K-coil peptide alone, of an oppositely
charged E-coil
peptide. The pairing of the two peptides to form charge-neutral heterodimers
in the monolayer
is effective to reduce monolayer conductance substantially, as evidenced by
the time-dependent
fall in measured oxidation or reduction current in the presence of Fe(CN)b~
ions.
In one exemplary biosensor, the biosensor surface includes (l) a monolayer
with embedded
K-coil peptides (HSP1) covalently attached to the electrode surface, (ii)
oppositely charged E-
coil peptides (HSP2) forming heterodimers with the K-coil peptides in the
monolayer, and (iii)
surface disaccharide ligands derivatized to the E-coil peptides and disposed
therefore at the
monolayer surface. As seen in Fig. 8, addition of the anti-ligand receptor (a
PAK protein
receptor) produces an increase in both oxidation and reduction currents, with
the current
increase over time reflecting the kinetics of receptor binding to the surface
ligands. A similar
biosensor having a trisaccharide, rather than disaccharide, ligand attached to
the E-coil peptide
subunit in the electrode monolayer was tested with a Verotoxin receptor, with
the results seen
- in Fig. 9. The solid lines in the figure show the increase in oxidation and
reduction current
observed, as a function of time, after addition of Verotoxin.
- CA 02252474 2005-O1-13
16
In the absence of receptor bmdmg to the ngand, me monolayer recams its sense
ordered
packing, forming an effective barrier to current across the monolayer mediated
by the redox
ion species, when a suitable oxidizing or reducing potential is placed across
the monolayer.
This is reflected by a low or zero measured current across the membrane. The
dielectric
constant of the monolayer in this condition is typically about 1-2.
The triggering event in the biosensor is the binding of a ligand-binding agent
to the
surface-bound ligand. This binding perturbs the ordered structure of the
monolayer sufficiently
to allow the movement of redox species through the monolayer, producing
current through the
electrode. Measurements performed in support of the invention indicate that
one triggering
event leads to 102 to 10° ionic and electron transfer events per
second, and thus is highly
multiplicative. The biosensor records this binding event as an increase in
current across the
electrode, t.e., between the working and counter electrodes.
By analogy to a transistor, the redox solution serves as the "source", the
monolayer as the
"gate", and the underlying electrode as the "drain". Current in a transistor
is initiated by
applying a threshold voltage to the gate. In the biosensor of the invention,
current is initiated
by a stimulus- in this case, a ligand-receptor binding event- to the monolayer
"gate".
E. Gravimetri~Biosensor
Fig. 10 shows basic elements of a gravimetric biosensor 86 incorporating the
novel
biosensor surface of the invention. The biosensor has a piezoelectric crystal
90 whose
biosensor surface 92 includes a monolayer 94 with ligand-bearing hexeerodimer
complexes, such
as complex 96, embedded therein.
Surface acoustic waves (SAVE are generated in the crystal by an oscillator.
97. According
to known piezoelectric biosensor principles, the change in mass in the
biosensor surface
resulting from the binding of ligand-binding agent to the surface-bound ligand
alters the
frequency, resonance frequency, and wavelength of the SAW, and at least one of
these wave
characteristics is measured by a detector 98. The oscillator and detector
collectively form
detector means for detecting binding of ligand binding agent to the biosensor
surface. Details
of crystal construction and associated detector means in gravimetric
biosensors are given, for
example, in US Patent Nos. 5,478,756 and 4,789,804, and in PCT application WO
96/02830.
F. ~,~ge Plasmon Resonance ~~ensor
Fig. 11 shows basic elements of a surface plasmon resonance (SPR) biosensor
100
incorporating the novel biosensor surface of the invention. An open-top
chamber 102 in the
CA 02252474 1998-10-22
WO 97/41424 PCT/CA97/00275
17
biosensor contains a waveguide 104 composed of a dielectric film 106 and a
thin evaporated
metal film 108 constructed to support surface plasmon waves at the
dielectric/metal film
interface. The waveguide surface forms a biosensor surface having a monolayer
110 with
ligand-bearing heterodimer complexes, such as complex I12, embedded therein.
A light source 114 direct a divergent light beam onto the biosensor surface
through a lens
116. At some region along the length of the biosensor surface, the beam angle
strikes the
surface at an absorption angle at which absorption from the evanescent wave by
surface
plasmons occurs. The absorption angle will shift with changes in the
composition of the
material near the interface, that is, in response to binding events occuring
on the monolayer
surface.
The intensity of reflected light from each region along the biosensor surface
is monitored
by a photosensor 118 whose photosensing grid is matched to specific detector
surface regions,
and which is operatively connected to an analyzer 120. The light source and
photosensor are
also referred to herein as biosensor means.
In operation, the SPR absorption angle on the biosensor surface is measured
before and
after analyte addition, with the measured shift in angle being proportional to
the extent of
surface ligand binding to ligand-binding agent.
G. Optical Biosensor
A variety of biosensor devices which rely on changes in the optical properties
of a
biosensor surface, in response to ligand/anti-ligand binding events, have been
proposed. Fig.
12 shows basic elements of an optical biosensor apparatus 122 having an open
chamber 124
and a biosensor surface 126 which includes a hydrocarbon-chain monolayer 128
with embedded
heterodimer complexes, such as shown at 130.
The detector means in the apparatus for detecting binding events on the
biosensor surface
includes a source 132 of polarized light and a lens system 134 for directing
the light in a beam
through the region of the monolayer. A photodetector 136 at the opposite side
of the biosensor
surface functions to measure intensity of light at a given polarization angle,
through a
polarization filter 138. Detection of ligand binding events is based on the
change of
polarization angle and intensity of light transmitted by the monolayer in
response to
perturbation of the regular order of the monolayer by surface binding events.
These changes
are recorded by an analyser 140 operatively connected to the photosensor.
- The biosensors described above have single-region biosensor surfaces, i.e.,
biosensor
surfaces containing a single ligand, for use in detecting a single analyte.
These surface are
CA 02252474 2005-07-05
WO 97/41424 PCT/CA97I0027S
18
readlly constructal, as discussed above, by contacting a selected HSP2-ligand
conjugate with
a universal HSPI biosensor surface, then adding the desired HSP2-ligand
conjugate under
conditions of hetemdimer formation.
It will be appreciated that the mahod of the invention can be used to
construct a biosensor
S with multiple sensor surfaces, or to partition a single surface into several
different ligand
regions, for carrying out multl-analyte tests. In the latter embodiment,
different HSPZ-ligand
conjugates are contacted with the different selected regions on a universal
sensor surface. The
present invention allows for flexibility in tertns~of number and types of
liganda attached, after
manufacture of the biosensor surface(s), particularly where the distinct
biosensor regions can
be selectively contacted with different HSP2 ligand conjugates.
The pct section describes a more goal hod in accordance with the invention for
foraning a b~seasor surface with multi-reagent regions, for use particularly
in constructing a
biosensor surface with a high density of different ligand-containing test
regions.
II. ~$y~~g
Figs.13A-13C illustrate the first iteration in a method of constructing an
array of differed
biological reagents in different, sdeaed regions on an assay support surface,
in accordance
with the inv~tioa.
Fig.13A shows a portion of m assay support surface 142-in this case, a
biosensor surface
for use in as amperomettic biosensor device. The surface has been constructed,
e.g., by
comrearional photolithographic m~Ods, to include as array of electrode
regions, such as the
two regions 144, 146. In the embodiment ills, the regions are metal, e.g.,
gold, film
region forasod on a substrate 148. Although not shown hoc, the surfaces are
prepared as
above as hydrocarbon-chain raonolay~s with HSPi peptides, such as shown at
154, embedded
in the monolayer.
According to an important feature of the imeation, the HSPI peptides have one
or more
photo-releasable blocking groups, such as blocking groups i50a on pepdde 150,
that preveait
het~odimer formation in the presence of HSP2 peptide under selected
conditions. In the
present case, the IiSFI peptide is an E-ooil peptide, and the blocking groups
are nitrophmrolate
protecting groups on two or snore of the glutamate side chain carboxyl groups.
Those skilled
is the art wilt recognize that a variety of photo-releasable blocking groups,
e.g., various photo-
deprotectable groups on one or more of amino acid side chains, can be used to
block
heterodimer formation, either by steric imerference or by reducing charge
interactions.
CA 02252474 2005-O1-13
WO 9?/41424 PGT/CA97/00275
19
Following attachment of the HSP1 peptide with blocking groups to the assay
surface, or
as part of a monolayer on the surface, the surface is selectively irradiated
to release blocking
groups in irradiated regions of the surface only. This can be accomplished, as
illustrated in
Fig. 13A, by irradiating the surface through a photomask 152 placed over the
surface, to
selectively irradiate regions of the surface corresponding to photomask
openings. Fig. 13B
shows selective release of blocking groups from the irradiated region 144.
The surface is then reacted with an HSP2 peptide (Fig. 13C) conjugated to
a'selected
ligand, as shown, to form heterodimers selectively in the unblocked regions of
the surface. If
necessary, the heterodimer formation conditions are selected, e.g., in ionic
strength, to
heterodimer .formation with unblocked HSPl peptides only. Thus, if the
released blocking
groups expose ionic groups, e.g., carboxyl groups, it may be useful to lower
the ionic strength
of the reaction medium, to enhance ionic interaction effects leading to
heterodimer formation.
The above steps are repeated for each ligand to be added to the assay surface,
until the desired
array of different ligands at different addressable regions of the surface is
constructed.
Fig. 14 shows a portion of an mufti-analyte assay surface constructed
according to the
above method, and employed in an amperometric biosensor apparatus 156 of the
type described
above. A biosensor surface 158 in a chamber 157 has a plurality of independent
sensor
regions, such as regions 160, 162, each having a separate ligand, such as
indicated at L1, Lt,
attached to the respective sensor region through heterodimers, such as
heterodimers 166 (L,)
and 168 (L.~. The heterodimers are embedded in a monolayer on each region,
such as
monolayer 164, for detection of different analytes in a analyte-containing
sample introduced
into the biosensor chamber.
Current flow in each detector region, such as regions 160, 162 is interrogated
by $
multiplexes 172 which connects each region to the chamber reservoir through a
voltage source
174 and current device 176. As above, binding of ligand-binding agent to any
region will
perturb the monolayer structure of that region, causing a measured current
increase in the
regions) where such binding has occurred.
From the foregoing, it can be seen how various objects and advantages of the
invention
are met. The biosensor surface of the invention can be formed under controlled
manufacturing
conditions consistent with microchip scale and photomask processes, to produce
highly uniform
andlor miniaturized andlor high-density array sensor devices with attached HSP
1 peptides.
After manufacture of a device with a universal surface, the sensor surface can
be readily
adapted to a wide variety of ligand(s), by reacting the sensor surface with
the an HSP2 peptide
CA 02252474 2005-07-05
wo m4iez4 rcr~aos~s
. - 2o
derivatized with the selected ligand. The ligand-attachment reaction can be
carried out under
relatively simple production oonditiobs, and may eve be accomplished by the
end user, thus
combining both marring precision at the initial production stage, and assay
flexibility at
the ligand-addition stage.
The invention is particularly useful in producing biosensor devices which use
or require
close-packed monolayer biosensor surfaces, as in the case of an asnperometric
biosensor. The
studies reported in Figs. 9-9 show that formation of haterodimers in a
h~rocarbon-chain
~nolayer are coible with a cite mwtolayer stn~cdire that forms an ~ecdve
barrier to ion-carrier aaovemeat, sad at the same time, is responsive to
binding by a ligand
binding agent, to increase ion-csurier movement through the monolayer. .
The inv~tion is easily adapts to any of a variety of biosensor devices, such
as those
illustrated above. Further, the imrention can be readily adapted to Pmduci~~g
mufti-ligand
biosensors, by selectively oontuxiag did regipns of a universal biosensor
surface with
different selectod HSP2-ligand cxfajugates.
In another aspect, the invention can be used to create mufti-ligand assay
surfaces by
photomasking techniques that are capable of producing highly reproducible
microarray
bioseasor devices having a plurality of diifera~t-ligaad regions.
Although the invention has been descxibod with respect to particular devices
and methods,
it will be understood that various changes and modifications can be made
without departing
from the invention, as encompassed by the accompanying claims.