Note: Descriptions are shown in the official language in which they were submitted.
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
METHOD FOR THE PRODUCTION OF PVC PARTICLES
After polyethylene, polyvinyl chloride (PVC) is the most widespread plastic
raw
material in the world. In Western Europe alone, more than 5 million tonnes are
used annually. The area of application is very wide, ranging from hard
products
such as pipes, profiles and calendered sheeting to plasticised products such
as
electrical cables, hoses and films. Both compact and foamed articles are
common.
S-PVC, which is the dominant product variant, is produced by suspension
polymerisation to form particles in the range 100 to 200 pm.
Paste PVC is another product type which accounts for approximately 10% of
total
PVC consumption. It is produced by means of various variants of emulsion
polymerisation and the latexes produced are dried to form fine-grained polymer
particles. The primary particles in latex may typically be from 0.1 to 2 pm,
depending on the technique used. Very special processes can produce particles
right up to 5 Nm. The most common drying technique is spray drying, which also
produces a number of secondary particles of various sizes. This powder is
dispersed in a solvent, the plasticiser, to form a liquid plastisol or paste.
Processing takes place in liquid form in processes such as coating (reverse
roll-coating, knife coating, screen coating}, gravure and screen printing,
rotation
casting, shell casting and dipping. The most important product areas are
floorings,
wallpaper, tarpaulins, rainwear, plating and gloves.
Filler PVC (extender resin) is a special product which is mixed with standard
paste
PVC in plastisol formulations to produce the best possible flow, at both high
and
low shear rates during the processing process. The admixture of filler PVC
will
CA 02252514 2004-11-25
26625-271
2
are achieved by means of special measures such as the use of
surfactants and changed agitation conditions.
Products with an average diameter in the range 35-45 ~m are
the market leaders. However, it is very difficult, with
this technique, to produce narrow size distributions and the
products therefore usually contain a certain fraction of
particles over 60 ~m as well. This represents a major
restriction in the area of application because processes
such as thin film coating, coil-coating, can-coating,
wallpaper production and printing processes require very
fine-particle media. With a particle size of 40 to 50 Vim,
it is not possible to produce films thinner than 100 Vim.
Known techniques within this area are described thoroughly
by M.J. Bunten in the Encyclopedia of Polymer Science and
Technology; Vinyl Chloride Polymers, Polymerization, 2nd ed.,
Vol. 17, 1982.
An article by R.D. Sudduth (J. of Applied Polymer Science,
48, 37-55 (1993)) states that when the ratio between the
diameters of large and small particles exceeds 10, the
maximum degree of packing is achieved. This means that a
mixture of 20 and 2 micron particles will be equally as
favourable as a mixture of 40 and 2 micron particles and
equally good flow conditions in the plastisol can be
expected. Therefore, it is unnecessary to have large
particles which restrict the area of application.
The present invention produces PVC particles with a narrow
size distribution in the range 10 to 50 microns. The
invention also produces spherical, almost monodisperse PVC
particles in the range 10 to 50 microns. The invention also
provides for the use of such particles in formulations and
CA 02252514 2004-11-25
26625-271
2a
processes for the production of products based on paste PVC
techniques.
These and other aspects of the present invention are
achieved with a procedure, product and application as
described below. The present invention is also described
and characterised by the attached claims.
The present invention concerns a procedure for producing PVC
particles with a narrow size distribution in the range
10-50 Vim, preferably 10-30 Vim, in which, in a first stage, a
vinyl monomer or a mixture of monomers is polymerised to
form a polymer/oligomer seed
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
3
to use such particles in formulations and processes for the production of
products
based on paste PVC techniques.
These and other purposes of the present invention are achieved with a
procedure,
product and application as described below. The present invention is also
described and characterised by the attached claims.
The present invention concerns a procedure for producing PVC particles with a
narrow size distribution in the range 10-50 pm, preferably 10-30 pm, in which,
in a
first stage, a vinyl monomer or a mixture of monomers is polymerised to form a
polymer/oligomer seed particle in the range 1-10 Nm. In a second stage,
another
vinyl monomer or mixture of monomers is swelled into polymer/oligomer seed
particles and the polymerisation takes place in such a way that they grow into
polymer particles of the desired size. The use of aromatic vinyl monomers or
acrylates as the monomers in the seed particles is preferred. The seed
particles in
the first stage can be produced by means of a two-stage swelling process or by
dispersion polymerisation. If dispersion polymerisation is used, it is
preferable to
initiate the polymerisation by dosing a pre-heated mixture of initiator and
solvent
so that the temperature in the medium does not change considerably during the
period in which the particles are nucleated. It is particularly favourable to
separate
the particles from the reaction medium and clean them of accessory agents
before
they are transferred to stage two of the process.
It is preferable to activate the seed particles produced by dispersion
polymerisation by swelling them in a mixture of oil-soluble initiator and
monomer
before the initiator decomposes and more monomer is dosed for the
- implementation of the polymerisation. Oil-soluble organic peroxides or azo-
type
initiators are preferred for use in the first stage as polymerisation
initiators. The
polymerisation can also be carried out by using residual initiator in the seed
particles. It is preferable to carry out stage two with continuous monomer
dosing
in order to avoid phase separation problems.
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
4
It is preferable to use dispersion polymerisation in the first stage for
production of
the seed particles by using an oil-soluble organic peroxide as the
polymerisation
initiator and, in the second stage, only to dose the polymerisation medium,
stabilisers, monomer and initiator-activator and for the polymerisation in the
second stage to be carried out by using residual initiator in the seed
particles. The
present invention also comprises a PVC mixture, specially for plastisol
purposes,
which contains spherical PVC particles in the range 10-50 pm, in particular 10-
30
pm, with a narrow size distribution, standard paste PVC, 0-100 parts
plasticiser,
0.1-10 parts thermostabiliser and 0-100 parts other standard additives for
PVC-based products. The spherical particles in the range 10-50 Nm may account
for 0-100% of the total PVC content of the formulation. It is preferable to
use
fine-grained products without large agglomerated secondary particles as the
standard paste PVC in such mixtures. Such products can be used for thin-film
products such as wallpaper, plating and decorating film or for printing ink in
gravure or screen printing processes or for processes for textile coating and
fixing
of cloth.
Spherical PVC particles in the range 10-50 Nm, in particular 10-30 Nm, with a
narrow size distribution, produced according to the present invention, can be
used
as viscosity reducers in other polymer systems or in liquids where other
polymers
are not present, as fillers in columns for separation purposes or as basic
particles
for further chemical modification.
The production of the stated PVC particles with a narrow size distribution
takes
place in a two-stage process. First a vinyl monomer or a mixture of monomers
is
polymerised to form a polymer/oligomer seed particle. In a second stage,
another
vinyl monomer or a mixture of monomers is swelled into the polymer/oligomer
seed particles and polymerisation takes place in such a way that they grow
into
polymer particles of the desired size.
Polymerloligomer particles of aromatic vinyl monomers or acrylates are
advantageous as seed particles for vinyl chloride polymerisation. This is
because
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
such particles are relatively easy to produce using known processes to produce
the desired size and size distribution. Another advantage is that they can be
produced with a given content of oligomer, which produces a dramatic increase
in
swelling capacity for the vinyl chloride monomer in the second stage. Thus it
is
possible to ensure final particles with a very low content of seed particles.
However, one problem with this two-stage seed process is that the mixture of
incompatible systems may lead to phase separation and the production of
perfect
spherical particles is impossible. One reason for this is the difference in
the
interfacial tension between the vinyl chloride, polyvinyl chloride and seed.
If
polystyrene is used as the seed particles, the compatibility between this
phase
and the polyvinyl chloride will be poor and phase separation will occur with
increasing degree of swelling. It is, therefore, more advantageous to use
methyl
methacrylate as the monomer for the seed particles as polymethyl methacrylate
has relatively good compatibility with PVC. PMMA also produces better outdoor
stability for the end products than will be produced with aromatic compounds.
However, a special feature of polyvinyl chloride is that the polymer is not
soluble
in its own monomer. Furthermore, there is also a very great difference in
density
between the monomer and the polymer (0.91 and 1.39 g/cm3). This has the result
that pertectly spherical particles are not formed for PVC above a certain size
(approximately 1 Nm) when traditional production techniques are used. One aim
of
the present invention has been to avoid this problem so that it is possible to
produce spherical particles of 10-50 Nm.
The seed particles can be produced in various processes. One possibility is to
use the two-stage swelling technique described by Ugelstad (Advances in
Colloid
and Interface Science, Vol. 13, 101-149, 1980). Such a seed will have a large
swelling capacity and the particle size can be controlled easily. At the same
time,
this method produces a very narrow particle size distribution because it
starts with
seed particles with a narrow size distribution. However, one restriction
exists in
that polymerisation in several stages is required to achieve particles of a
given
size.
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
6
Dispersion polymerisation is another method for producing seed particles. This
is
a relatively new technique described in the literature, see, for example, Shen
et al.
{Journal of Polymer Science; Part A: Polymer Chemistry, Vol. 31, 1393-1402,
1993) and Paine et al. (Journal of Polymer Science; Part A: Polymer Chemistry,
Vol. 28, 2485-2500, 1990). The technique makes it possible directly to produce
particles with diameters up to 10 m and a narrow size distribution.
According to the present invention, it turned out to be possible to separate
the
particles from the dispersion medium and transfer them to a new polymerisation
medium. They can thus be used as seed in conventional polymerisation
processes. This is thus a simple process for producing seed particles with
good
swelling properties. A further improvement in swelling properties can be
produced
by using a chain transfer agent during polymerisation so that the molecular
weight
is reduced to a more favourable level. In many cases, this can be
advantageous.
Large quantities of solvents and accessory agents are used in the dispersion
polymerisation process. A process which involves reusing the reaction medium
will therefore be of great advantage. Shen et al. also describe further
polymerisation in which polystyrene particles produced by dispersion
polymerisation are used as seed, but in this case, the medium is not changed
continuously.
The reuse of reaction medium is possible using a direct method in which the
particles are separated and the medium used for a new reaction, possibly in a
mixture with fresh solution. Another method is to filter or centrifuge out the
particles and purify the solvent using known techniques such as distillation.
The
consumption of solution is thus kept to a minimum. Another advantage of such a
separation process is that the particles can, at the same time, be washed and
thus
transferred to the next polymerisation stage in a completely clean form
without the
stabilisers from the dispersion process contaminating the seed polymerisation.
This is also favourable for the swelling properties of the particles as
emulsifier
and dispersants on the surface will prevent diffusion into the particles. A
further
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
7
advantage is that there are no restrictions in the choice of stabilisers in
the
decisive polymerisation and good control of the particle formation is
achieved.
In order to avoid the new formation of particles outside the swelled seed
particles
during polymerisation in the second stage, it is an advantage to have
continuous
dosing of monomer. With vinyl chloride, therefore, polymerisation takes place
at a
pressure below the saturation pressure of the monomer at a given temperature.
Under such conditions, the monomer will not exist as a separate phase in the
reaction medium and the likelihood of secondary nucleation processes is thus
very much reduced. Continuous dosing of monomer can, for vinyl chloride, be
assured by dosing with a pump which is regulated against the pressure in the
reactor. The pressure is kept constant and as the monomer is consumed,
monomer is dosed. Another method is to have two reactors connected in series
in
such a way that the VCM pressure in the reactor with the polymerisation
reaction
is regulated by regulating the temperature in the other reactor used as the
VCM
reservoir. In both these methods for continuous dosing, the monomer is dosed
in
proportion to the reaction speed. Another advantage of such continuous dosing
of
monomer is that the reaction will take place more rapidly than for standard
polymerisation as the work always takes place in the so-called Trommsdort
range.
In order for the properties of the finished material to be as good as
possible, it is,
however, advantageous to keep the pressure as close to the saturation pressure
at the polymerisation temperature as possible. Polymerisation which takes
place
at a pressure considerably below the saturation pressure produces reduced
thermal stability in the end product. Another surprising result of this method
of
carrying out the polymerisation reaction was that the end particles could
easily be
made completely spherical. Without continuous monomer dosing, the form of the
particles was very irregular.
If other monomers with a much lower vapour pressure than vinyl chloride are
used, any continuous dosing will take place using another principle such as
dosing the monomer as a function of the calculated conversion and a
calculation
of the composition of the polymer in the existing particles in the reaction
medium.
CA 02252514 2004-11-25
26625-271
8
Another important element in order to achieve a successful result for particle
production
is the choice of stabiliser systems which keep the particles freely dispersed
in the
reaction medium. The present invention has no restrictions in the choice of
such
substances. Both standard emulsifiers of anionic or non-ionic types known from
the
emulsion polymerisation area and polymer suspension agents of the types
polyvinyl
alcohol, polyvinyl pyrrolidone, styrene malelc anhydride copolymers and
various types of
cellulose such ss methylhydroxyethyl cellulose and methylhydroxypropyl
cellulose are
-used:=Onewparticulariy advantageous possibility which is provided bytlje
uswolwPVC
particles according to the present invention is the use of polymer suspension
agents
instead of low-molecular and ionic emulsifiers during particle produetlon_
4n account of the oomptetely spedal particle size and the particle size
distribution, the
particles according to the present invention can account for the majority and
also the full
proportion of PVC in the formulations for the ~nd products. Thus finished PVC
products
avoid disadvantages such as high water absorption and vontent of volatile
substances
which usually accompany standard pastry PVC products produced by means of
traditional emulsifiers for the production of paste PVC.
The present invention is illustrated in further detail by the following
examples and the
attached figures 1-4, where
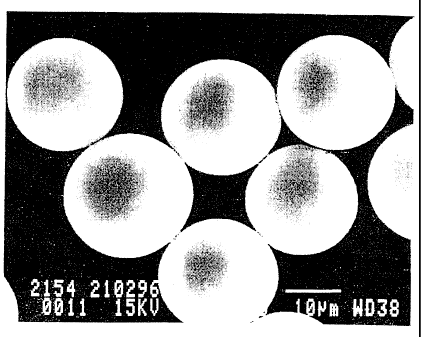
Figure 1 shows the particles produced as in example B-1.
Fgure 2 shows the particles produced as in example B-11.
Figure 3 shows the particles produced as in example B-13.
TM
Fgure 4 shows commercial ~Iler PVC, Vinnot C65 V.
A 1.1-titre or 5-litre thermostat-controlled glass reactor with reflex is used
in examples
A-1 to A-7. An isothermic reaction calorimeter (CPA-2, ThermoMetrics,
S~nreden) with a
reactor with a volume of 200 ml or a 1.1-litre or 1.4-litre thermostat-
controlled glass
reactor or a 14-litre steel reactor is used in examples B-1 to 8-17.
CA 02252514 1998-10-22
WO 97140076 PCT/N097/00106
9
Another important element in order to achieve a successful result for particle
production is the choice of stabiliser systems which keep the particles freely
dispersed in the reaction medium. The present invention has no restrictions in
the
choice of such substances. Both standard emulsifiers of anionic or non-ionic
types
known from the emulsion polymerisation area and polymer suspension agents of
the types polyvinyl alcohol, polyvinyl pyrrolidone, styrene malefic anhydride
copolymers and various types of cellulose such as methylhydroxyethyl cellulose
and methylhydroxypropyl cellulose are used. One particularly advantageous
possibility which is provided by the use of PVC particles according to the
present
invention is the use of polymer suspension agents instead of low-molecular and
ionic emulsifiers during particle production.
On account of the completely special particle size and the particle size
distribution, the particles according to the present invention can account for
the
majority and also the full proportion of PVC in the formulations for the end
products. Thus finished PVC products avoid disadvantages such as high water
absorption and content of volatile substances which usually accompany standard
paste PVC products produced by means of traditional emulsifiers for the
production of paste PVC.
The present invention is illustrated in further detail by the following
examples and
the attached figures 1-4, where
Figure 1 shows the particles produced as in example
B-1.
Figure 2 shows the particles produced as in example
B-11.
Figure 3 shows the particles produced as in example
B-13.
Figure 4 shows commercial filler PVC, Vinnol C65
V.
A 1.1-litre or 5-litre thermostat-controlled glass reactor with refiux is used
in
examples A-1 to A-7. An isothermic reaction calorimeter (CPA-2, ThermoMetrics,
Sweden) with a reactor with a volume of 200 ml or a 1.1-litre or 1.4-litre
CA 02252514 2004-11-25
26625-271
Table 1. Recipo far dispersion polymerisation of methyl methacrylate.
Materials Weight %
MMA 10-15
Mslhanot 8Q-85
PVP K 30'x' 2.5-5.0
AIBN 0~-0,~ __
2-ethylhexylthioglycoiate0.0-0.6
Polymerisation temperature :55°C. Polymerisation time: ~8 hours_
Weight 96 is based on the total recipe.
PVP K~o = poly(vinyt pyrroGdone) Mw = 40000 glmol.
AIBN ~ 2.2'-azobis(isobutyronitriie).
PVP and methanol are heated to boiling point and the solution is boned for 1
hour in an
N2 atmosphere. The mixture is tooled to 55°C and MMA is added. When a
stable
temperature of 55 degrees has been reached, a solution of AIBN in methanol is
added.
Polymerisation is carcied out for 48 hours at oonstaM temperature. The chain
transfer
agent can be added at any time during the process.
Depending on the exact redpe used, partides in the range 0.5 to 10 Nm are
produced,
and in ail cases the size distribution was narrow. When the chain transfer
agent was
used, seed particles with a larger diameter and a somewhat wider size
distribution were
produced. The exact size of the dispersion seed of PMMA produced and used in
each
case is stated in the examples of productjon of PVC partiGes.
When the chain transfer agent was used, the procedure was that a solution of
P1/P K.-30
(10.00 g) in methanol (175.75 g) was added to the reactor and the mbdure was
boiled in
an N2-atmosphere for 1 hour. 'fhe mixtur~a was cooled to 55°C before
methyl
methacrylate (25.00 g) was added. A mixture of 2-ethylhexylthioglycolate (0.15
g),
2.2-azobis(isobutyronitrile) (0.30 g) and methanol (39.00 g) was added to the
reactor
when the temperature was stable at 55°C. The polymerisation was carried
out at 5b°C for
48 hours.
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
11
Test A-3 Dispersion seed of polymethyl acrylate.
A dispersion polymerisation of MMA was carried out in methanol with polyvinyl
pyrrolidone) (MW - 40000) as the stabiliser. The initiator was
2.2'-azobis(isobutyronitrile).
2-ethylhexylthioglycoiate was used as the chain transfer agent to reduce the
molecular weight. The recipe for the test is shown in table 1.
Table 1. Recipe for dispersion polymerisation of methyl methacrylate.
Materials Weight
MMA 10-15
Methanol 80-85
PVP K-30 2.5-5.0
AIBN 0.1-0.4
2-ethylhexylthioglycolate0.0-0.6
Polymerisation temperature: 55°C. Polymerisation time: 48 hours.
Weight % is based on the total recipe.
PVP K-30 = polyvinyl pyrrolidone) MW = 40000 glmol.
AIBN = 2.2'-azobis(isobutyronitrile).
PVP and methanol are heated to boiling point and the solution is boiled for 1
hour
in an N2 atmosphere. The mixture is cooled to 55°C and MMA is added.
When a
stable temperature of 55 degrees has been reached, a solution of AIBN in
methanol is added. Polymerisation is carried out for 48 hours at constant
temperature. The chain transfer agent can be added at any time during the
process.
Depending on the exact recipe used, particles in the range 0.5 to 10 Nm are
produced, and in all cases the size distribution was narrow. When the chain
transfer agent was used, seed particles with a larger diameter and a somewhat
CA 02252514 1998-10-22
WO 97/4007b PCT/N097/00106
12
wider size distribution were produced. The exact size of the dispersion seed
of
PMMA produced and used in each case is stated in the examples of production of
PVC particles.
When the chain transfer agent was used, the procedure was that a solution of
PVP K-30 (10.00 g) in methanol (175.75 g) was added to the reactor and the
mixture was boiled in an NZ-atmosphere for 1 hour. The mixture was cooled to
55°C before methyl methacrylate (25.00 g) was added. A mixture of
2-ethylhexylthioglycolate (0.15 g), 2.2-azobis(isobutyronitrile) (0.30 g} and
methanol {39.00 g) was added to the reactor when the temperature was stable at
55°C. The polymerisation was carried out at 55°C for 48 hours.
Spherical particles with diameter 12.5 pm were achieved, with a small fraction
which was 4 Nm.
Test A-4 Polystyrene dispersion seed with dioctanoyl peroxide.
A solution of polyvinyl pyrrolidone (PVP K-30) (5.15 g) in ethanol {236.07 g)
was
heated to boiling point and boiled for 1 hour in an Nz-atmosphere. The
temperature was regulated to 70°C and styrene (78.04 g) was added. When
the
temperature was stable at 70 degrees, a mixture of dioctanoyl peroxide (4.08
g)
and ethanol (35.24 g) was added and polymerisation carried out at 70°C
for 24
hours.
The particles produced had a diameter of 5 Nm.
Test A-5 Polymethyl methacrylate dispersion seed with didecanoyl peroxide.
A solution of PVP K-30 (93.75 g) in methanol {2635 g) was added to the reactor
and the mixture was boiled for 1 hour in an Nz-atmosphere. The mixture was
cooled to 55°C before methyl methacrylate (375 g) was added. A mixture
of
didecanoyl peroxide (18.77 g) and methanol (585 g) was preheated to
30°C and
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
13
charged to the reactor. The polymerisations were carried out at 55°C
for 15 to 24
hours.
The particles produced had a diameter of 8 Nm.
Test A-6 Polymethyl methacrylate dispersion seed with didecanoyl peroxide.
A solution of PVP K-30 (18.75 g) in methanol (644.25 g) was added to the
reactor
and the mixture was boiled for 1 hour in an N2-atmosphere. The mixture was
cooled to 55°C before methyl methacrylate (75 g) was added. A mixture
of
didecanoyl peroxide (3.75 g} and methanol (116.87 g) was preheated to
30°C and
charged to the reactor. The polymerisation was carried out at 55°C for
24 hours.
The particles produced had a diameter of 7 Nm.
Test A-7 Activated polystyrene dispersion seed.
A solution of PVP K-30 (5.15 g) in ethanol (240.11 g) was added to the reactor
and the mixture was boiled for 1 hour in an NZ-atmosphere. The mixture was
cooled to 70°C before styrene (78.04 g) was added. A mixture of
2.2-azobis(isobutyronitrile} (2.34 g) and ethanol (31.20 g) was added to the
reactor when the temperature was stable at 70°C. The polymerisation was
carried
out at 70°C for 24 hours.
The particles produced had a diameter of 6.0 Nm.
Some of these seed particles (5.0 g) in distilled water (25.40 g) were further
swelled with an emulsion of dioctanoyl peroxide (0.5 g), sodium lauryl
sulphate
(0.083 g) and distilled water (16.67 g). The swelling took place for
approximately
20 hours at 25°C and the finished, activated seed had a diameter of 6.2
Nm.
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
14
Production of PVC particles in the range 10 to 50 Nm with seed-based
polymerisation.
Test B-1
Methylhydroxypropyl cellulose dissolved in distilled water (2 gll, 66.25 g),
sodium
lauryl sulphate (0.05 g) and activated oligomer seed from example A-1 (0.26 g)
were heated to 25°C. NZ-gas was added at a pressure of 9 bar. The
reactor was
then evacuated in order to remove oxygen. VCM (18.75 ml) was added and was
allowed to swell into the seed particles for 60 minutes. A solution of
methylhydroxypropyl cellulose in distilled water (2 gll, 33.75 g) and
potassium
iodide (0.038 g) was added to the reactor before the temperature was increased
to 55°C. Polymerisation began and continued until a pressure drop of
2.5 bar was
registered in the reactor. The reaction was then stopped by the reactor being
cooled to 20°C and unconverted monomer was vented.
The particles produced had a concave surface as shown in figure 1. The
diameter
was approximately 15 pm.
Test B-2
Methylhydroxypropyl cellulose dissolved in distilled water (10 g/l, 100 g),
sodium
lauryl sulphate (0.05 g), potassium iodide (0.038 g) and oligomer seed from
example A-1 (0.52 g) were heated to 30°C, the reactor was filled with
Nz and the
oxygen was removed as in B-1. VCM (15 ml), vinyl acetate (5 ml) and a solution
of
azobismethylbutyronitrile (0.25 g) and methanol (0.25 g) were added to the
reactor and were allowed to swell into the seed particles for 60 minutes. The
temperature was then increased to 55°C and the same procedure as in B-1
was
followed. The particles produced were spherical with a diameter of
approximately
7 pm.
Test B-3
Precisely the same procedure as in B-2 was followed, with the difference that
the
quantity of VCM was increased from 15 ml to 18 ml and the quantity of vinyl
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
acetate was reduced from 5 ml to 2 ml. The solution of methylhydroxypropyl
cellulose had a concentration of 6 g/l. The particles produced had a concave
surface and a diameter of approximately 10 Nm.
Test B-4
Seed particles from example A-2 (0.25 g), sodium lauryl sulphate (0.05 g),
potassium iodide (0.038 g) and methylhydroxypropyf cellulose in distilled
water {5
g/I, 100 g) were charged to the reactor. The same procedure as in B-1 was
followed. VCM (18.8 ml) was added and was allowed to swell into the seed
particles for 60 minutes at 25°C. Polymerisation was carried out at
60°C until a
pressure drop of 2.5 bar was achieved. Spherical particles with diameter 13 pm
were produced.
Test B-5
The procedure in B-4 was followed with the difference that activated
polystyrene
seed from example A-4 (0.26 g) was used as seed particles. Concave particles
with diameter approximately 16 pm were produced.
Test B-6
Seed particles from example A-3 (6.8 Nm, 1.0 g), sodium lauryl sulphate (0.05
g),
potassium iodide (0.038 g) and methylhydroxypropyl cellulose in distilled
water (2
g/I, 100 g) were charged to the reactor. After removing the oxygen, VCM (17
ml)
and a solution of azobismethylbutyronitrile (0.25 ml) and methanol (0.25 g)
were
added and were allowed to swell into the seed particles for 60 minutes.
Polymerisation was carried out at 60°C until a pressure drop of 2.5
bar was
achieved. Spherical particles with diameter approximately 11 pm were produced.
Test B-7
The procedure from B-7 was followed with the difference that 34 ml VCM was
added instead of 17 ml. Particles with a concave surface and size
approximately
14 Nm were produced.
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
16
Test B-8
Seed particles from example A-3 with 2-ethylhexythioglycolate (0.15 g), (12.5
pm,
2.00 g), sodium lauryl sulphate (0.05 g), potassium iodide (0.038 g) and
methylhydroxypropyl cellulose in distilled water (2 g/E, 100 g) were added to
the
reactor and the oxygen was removed before VCM (20 ml) and a solution of
2.2-azobis(isobutyronitrile) (0.25 g) and methanol (0.25 g) were added. After
swelling into the seed particles for 3 hours, the temperature was increased to
60°C and polymerisation was carried out until a pressure drop of 2.5
bar was
achieved. Spherical particles with diameter 20 pm and a small fraction of
smaller
particles were produced.
Test B-9
Seed particles from example A-3 (6.8 Nm, 1.0 g), sodium lauryl sulphate (0.05
g),
potassium iodide (0.038 g) and methylhydroxypropyl cellulose in distilled
water (2
g/I, 100 g) were charged to the CPA reactor. After the removal of oxygen, VCM
(7
ml) and a solution of azobismethylbutyronitrile (0.35 g) and methanol (0.35 g)
were added and were allowed to swell into the seed particles for 60 minutes.
The
temperature was increased to 60°C and VCM (20 ml) was then dosed
continuously to the reactor using a piston pump. The pressure in the reactor
was
thus kept constant just below saturation pressure at 60°C. The reaction
continued
until a pressure drop of a total of 2.5 bar was achieved.
Spherical particles with diameter approximately 18.5 Nm were produced.
Examination under a scanning electron microscope (SEM) showed that small
particles were adsorbed to the surface of the spherical particles. This was on
account of new particles formed from the aqueous phase as described earlier.
Test B-10
Seed particles from example A-3 (6.8 pm, 2.2 g), sodium lauryl sulphate (0.33
g),
potassium iodide (0.25 g) and methylhydroxypropyl cellulose in distilled water
(2
gll, 650 g) were added to the reactor. After evacuation, VCM (37.5 ml) and a
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
17
solution of azobismethylbutyronitrile (1.0 g) and methanol (1.0 g) were added
and
were allowed to swell into the seed particles for 60 minutes. The temperature
was
increased to 60 degrees and then VCM (94.5 ml) was added by means of
continuous dosing via a piston pump. The dosing was regulated so that the
pressure was constant just below saturation pressure. The polymerisation was
concluded when a pressure drop of 2.5 bar was achieved.
Spherical particles with diameter approximately 18 pm were produced. Again,
small adsorbed particles were observed on the surtace.
Test B-11
Seed particles from example A-3 (7.2 pm, 6.1 g), sodium lauryl sulphate {0.28
g),
potassium iodide (0.21 g) and methylhydroxypropyl cellulose in distilled water
(2
gll, 550 g) were added to the reactor. After evacuation, VCM (20 ml) and a
solution of azobismethylbutyronitrile (1.0 g) and methanol (1.0 g) were added
and
were allowed to swell into the seed particles for 60 minutes. The temperature
was
increased to 60 degrees and VCM (160 ml) was added continuously as in B-9.
Polymerisation was carried out until a pressure drop of 2.5 bar was achieved.
Spherical particles of 18 pm were produced. Small adsorbed particles were
observed on the surface as shown in figure 2.
Test B-12
Seed particles from example A-3 (1.0 Nm, 10.3 g), sodium lauryl sulphate (0.28
g),
potassium iodide (0.21 g) and methylhydroxypropyl cellulose in distilled water
(2
g/l, 550 g) were added to the reactor. After evacuation, VCM (20 ml) and a
solution of azobismethylbutyronitrile (1.0 g) and methanol (1.0 g) were added
and
were allowed to swell into the seed particles for 60 minutes. The temperature
was
increased to 60 degrees and VCM (155 ml) was dosed continuously as in B-9.
Spherical particles of 3 Nm were produced.
CA 02252514 2004-11-25
26625-271
18
The usa of fiVC pnrticias produced using the prosent invention 1s illustrated
in the
toilowing examples:
es
PVC pastes were produced according to the torntuiations stated in table 2. PVC
powder
was m'uced with plasticiser and liquid thermostabiliser in a Waning rapid
mixer. Mbdng
took place for 10 minutes at increasing speAd at a final temperature of
35°C. The
viscosity wof ° ~thsr- pastesww°measured in a rheorneterw of
type Bohiin VOR, C14
measurement system, at incroasing shear rates from 1 to 300 per second. The
particle
size of the dispersed PVC particles wag measured by spreading a thin film of
the pastes
on a grindometer ( rod). The particle size is stated here at the point at
which stripes occur
in the thin film. The films wens plasticised ir> a Wemer-Mathls furnace at
200°C for 3
minutes. Tha films w~ene inspected for any foaming. Tha water absorption in
the films was
measured as weight increase after storage in water at 50C for 48 hours.
Table 2. Formulations far pVC pastes in example C-~.
IngredientsF-1 F F-3 F-4 F-5 F-6 F-7 F-8 F-9
2
PevikonTM ' 70 70 70 70 - - , -
P14 70
Pevikon - - - - - 30 30 30 30
DP1502
Vinnoi 30 - - - - f0 - -
C65V
MM-11 - 30 - - - - 70 -
MM-15 - _ 3p _ - _ _ 7p _
MM-14 - - - 30 - - - - 70
MM-13 - - - - 30 _ _ - -
DQP 40 40 40 40 40 40 40 40 40
LZ616 2 2 2 2 2 2 2 2 2
PSO 2 2 2 2 2 2 2 2 2
Pevikon P14: standard paste PVC, Norsk Hydra a.s
Pevikon DP1502: development product, fine-grained paste PVC,
Norsic Nydro a_a
Vinnol C65V: extender PVC (filler PVC), Vinnolit GmbH
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
19
was constant until the VCM disappeared as a separate phase in the system. The
temperature range for reactor 2 was 57 to 65 degrees.
Spherical particles with diameter 15 Nm with a non-uniform surface consisting
of
precipitated secondary particles were produced.
Test B-15
Seed particles from example A-6 (7.0 Nm, 7.50 g) which contained initiator,
sodium lauryl sulphate (0.25 g), potassium iodide (0.19 g),
methylhydroxypropyl
cellulose in distilled water (2 g/l, 1.00 g), copper sulphate pentahydrate
(2.00 mg)
and water (630.40 g) were added to the reactor. After evacuation, VCM (20 ml)
was added and was allowed to swell into the seed particles for 60 minutes. The
temperature was increased to 60 degrees and VCM (130 ml) was dosed
continuously with a piston pump. In order to control the reaction speed, a
solution
of ascorbic acid (4 g/l, 2.00 ml) was dosed. The reaction was continued until
a
pressure drop of 2.5 bar was achieved.
Spherical particles of 7 3 Nm were produced.
Test B-16
Activated seed particles of polystyrene from example A-7 (6.2 Nm, 1.00 g),
potassium iodide (0.038 g) and methylhydroxypropyl cellulose in distilled
water (2
g/l, 100 g) were added to the reactor. After the removal of oxygen, VCM (7 ml)
was added and was allowed to swell into the seed particles for 60 minutes. The
temperature was increased to 60°C and VCM (22.5 ml) was then dosed
continuously to the reactor using a piston pump. The reaction was continued
until
a pressure drop of 2.5 bar was achieved.
Particles with a diameter of approximately 15 Nm were produced. The particles
had a non-uniform surface and were not spherical.
For comparison, figure 4 shows commercial filler PVC, Vinnol C65V, from
Vinnolit
GmbH.
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
The use of PVC particles produced using the present invention is illustrated
in the following examples:
Test C-1
PVC pastes were produced according to the formulations stated in table 2. PVC
powder was mixed with plasticiser and liquid thermostabiliser in a Warring
rapid
mixer. Mixing took place for 10 minutes at increasing speed at a final
temperature
of 35°C. The viscosity of the pastes was measured in a rheometer of
type Bohlin
VOR, C14 measurement system, at increasing shear rates from 1 to 300 per
second. The particle size of the dispersed PVC particles was measured by
spreading a thin film of the pastes on a grindometer ( rod). The particle size
is
stated here at the point at which stripes occur in the thin film. The films
were
plasticised in a Werner-Mathis furnace at 200°C for 3 minutes. The
films were
inspected for any foaming. The water absorption in the films was measured as
weight increase after storage in water at 50°C for 48 hours.
Table 2. Formulations for PVC pastes in example C-1.
IngredientsF-1 F-2 F-3 F-4 F-5 F-6 F-7 F-8 F-9
Pevikon 70 70 70 70 70 - - - -
P14
Pevikon - - - - - 30 30 30 30
D P 1502
Vinnol 30 - - - - 70 - -
C65V
MM-11 - 30 - - - - 70 -
MM-15 - - 30 - - - - 70 -
MM-14 - - - 30 - - - - 70
MM-13 - - - - 30 - - - -
DOP 40 40 40 40 40 40 40 40 40
LZ616 2 2 2 2 2 2 2 2 2
ESO 2 2 2 2 2 2 2 2 2
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
21
Pevikon P14: standard paste PVC, Norsk Hydro a.s
Pevikon DP1502: development product, fine-grained paste PVC,
Norsk Hydro a.s
Vinnol C65V: extender PVC (filler PVC), Vinnolit GmbH
DOP: diethylhexyl phthalate
LZ616: barium zinc stabiliser, Ackros
ESO: epoxidised soya bean oil
MM-11: particles produced in example B-11
MM-13: particles produced in example B-13
MM-14: particles produced in example B-14
MM-15: particles produced in example B-15
Table 3 shows the viscosities and particles sizes for the 9 formulations. It
can be
clearly seen that the samples with PVC particles produced according to the
present invention offer opportunities for spreading films which are thinner
than 50
microns. Even when as much as 70 phr of the content of the PVC is filler PVC,
very thin films are produced. At the same time, low-viscosity pastes are
produced.
The importance of having perfectly spherical particles with a uniform surface
can
clearly be seen in a comparison of the viscosities for formulations 1, 2, 3
and 4.
Sample MM-14 had irregularities in the form of precipitated particles on the
surface. The viscosity was very high. It can also be seen that, in this case,
it was
not possible to disperse the particles completely down to their primary
particle
level and the smear on the grindometer shawed a particle size of 75 Nm.
CA 02252514 1998-10-22
WO 97/40076 PCT/N097/00106
22
Table 3. Viscosity, particle size and water absorption.
Viscosity (Pas) F-1 F-2 F-3 F-4 F-5 F-6 F-7 F-8 F-9
at 1 per s. 11 10 11 19 12 9 10 11 5
at 10 per s. 12 12 14 23 12 8 9 11 5
at 100 per s. 18 23 29 40 18 8 9 13 5
at 300 per s. - - - - 19 7 9 11 5
Particle size 75 35 45 75 50 75 25 25 40
(Nm}
Appearance of OK foamOK foam- OK foamOK
the
film
Water absorption1.8 2.2 2 1.9 - 0.8 1.5 1
{%)
In combination with very fine-grained standard paste PVC (Pevikon DP1510),
filler
PVC gives particles according to the present invention the unique opportunity
to
produce films down to 50 micrometres at the same time as the viscosity of the
paste is low and the rheology is Newtonian even though only 44 phr liquid
substance are used in the formulation (F-7, 8 and 9). Up to now, this has not
been
possible with known PVC types for plastisol purposes.