Note: Descriptions are shown in the official language in which they were submitted.
CA 022~3617 1998-11-0~
WO 97143973 PCT/US97/09048
METHon FOR ARLATING .~NTF.RIOR SF,CTIONS OF T~F, TONGUF,
Cross-Reference to Related ~,nplir~tions
This application is a continuation-in-part of U.S. Patent Application No.
08/651,800, entitled "Method and Apparatus for Treatment of Air Way
Obstructions", filed May 22, 1996, which is a continuation-in-part application of
U.S. Patent Application No. 08/642,053, entitled "Method for Treatment of
Airway Obstructions", filed May 3, 1996, which is a continuation-in-part
application of U.S. Patent Application No. 08/606,195, filed February 23, 19967
entitled "Method for Treatment of Airway Obstructions", which cross-references
U.S. Patent Application No. 08/516,781 filed August 18, 1995, entitled
''Ablation Apparatus and System for Removal of So~ Palate Tissue", having
named inventors Stuart D. Edwards, Edward J. Gough and David L. Douglass,
which is a continuation-in-part of U.S. Application No 08/239,658, filed May 9,
1994 entitled "Method for Reducing Snoring by RF Ablation of the Uvula", all
incorporated by reference herein.
BACKGROUND OF T~F INVENTION
Field of the Tnvention
This invention relates to a method for the treatment of air way
obstructions, and more particularly to a method for ablating selected tissue sites
in an interior of the tongue without ~l~m~ging the hypoglossal nerve using
ablation energy and/or an ablative agent.
Description of RPl~tP-l Art
Sleep-apnea syndrome is a medical condition characterized by daytime
hypersomnomulence, morning arm aches, intellectual deterioration, cardiac
arrhythmias, snoring and thrashing during sleep. It is caused by frequent
episodes of apnea during the patient's sleep. The syndrome is classically
subdivided into two types. One type, termed "central sleep apnea syndrome", is
characterized by repeated loss of respiratory effort. The second type, termed
..... .. . .. ..
. .
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
obstructive sleep apnea syndrome, is characterized by repeated apneic episodes
during sleep resulting from obstruction of the patient's upper airway or that
portion of the patient's respiratory tract which is cephalad to, and does not
include, the larynx.
Treatment thus far includes various medical, surgical and physical
measures. Medical measures include the use of medications such as
protriptyline, medroxyprogesterone, acetazolamide, theophylline, nicotine and
other medications in addition to avoidance of central nervous system depressantssuch as sedatives or alcohol. The medical measures above are sometimes helpful
but are rarely completely effective. Further, the medications frequently have
undesirable side effects.
Surgical interventions have included uvulopalatopharyngoplasty,
tonsillectomy, surgery to correct severe retrognathia and tracheostomy. In one
procedure the jaw is dislodged and pulled forward, in order to gain access to the
base of the tongue. These procedures may be effective but the risk of surgery inthese patients can be prohibitive and the procedures are often unacceptable to
the patients.
Physical measures have included weight loss, nasopharyngeal airways,
nasal CPAP and various tongue retaining devices used nocturnally. These
measures may be partially effective but are cumbersome, uncomfortàble and
patients often will not continue to use these for prolonged periods of time.
Weight loss may be effective but is rarely achieved by these patients.
In patients with central sleep apnea syndrome, phrenic nerve or
diaphragmatic pacing has been used. Phrenic nerve or diaphragmatic pacing
includes the use of electrical stimulation to regulate and control the patient'sdiaphragm which is innervated bilaterally by the phrenic nerves to assist or
support ventilation. This pacing is disclosed in Direct Diaphragnt Stintula~ion
by J. Mugica et al. PACE vol. 10 Jan-Feb. 1987, Part II, Preliminary ~es~ of a
Muscular Diaphragn1 Pacing System on H2mtan Pa~ients by J Mugica et al.
from Neurostim~ tion: An Overview 1985 pp. 263-279 and Electrical
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
Activatio~l of Respiration by Nochomovitez IEEE Eng. in Medicine and Biology,
June, 1993.
However, it was found that many ofthese patients also have some degree
of obstructive sleep apnea which worsens when the inspiratory force is
augmented by the pacer. The ventilation induced by the activation of the
diaphragm also collapses the upper airway upon inspiration and draws the
patient's tongue inferiorly down the throat choking the patient. These patients
then require tracheostomies for adequate treatment.
A physiological laryngeal p~cem~ker as described in Physiological
La~yngeal Pacemaker by F. Kaneko et al. from Trans Am Soc Artif Intern
Organs 1985 senses volume displaced by the lungs and stim~ tes the
appropriate nerve to open the patient's glottis to treat dyspnea. This apparatus is
not effective for tre~tm~nt of sleep apnea. The apparatus produces a signal
proportional in the displaced air volume of the lungs and thereby the signal
produced is too late to be used as an indicator for the treatment of sleep apnea.
There is often no displaced air volume in sleep apnea due to obstruction.
One measure that is effective in obstructive sleep apnea is tracheostomy.
However, this surgical intervention carries considerable morbidity and is
aesthetically unacceptable to many patients. Other surgical procedures include
pulling the tongue as forward as possible and surgically cutting and removing
sections of the tongue and other structures which can close offthe upper airway
passage.
A need exists for a method to treat obstructive sleep apnea without major
surgical intervention. A further need exists for a method to ablate selected
interior sections of the tongue without ~l~m~gin~ the hypoglossal nerve with theuse of ablative energy and/or an ablative agent.
.. ...
.
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
SUl~MARY OF THE rl~VFNTION
Accordingly, an object of the invention is to provide a method to reduce
a volume of a selected site in an interior of the tongue without d~m~ginE the
hypoglossal nerve.
Another object of the invention is to provide a method for ablating
selected sections of the interior of the interior of the tongue without ~l~m~ging
the hypoglossal nerve by the delivery of ablation energy or an ablative agent tothe selected tissue site.
These and other objects of the invention are achieved in a method for
reducing a volume of a tongue. An ablation apparatus is provided that includes asource of ablation energy and an ablation energy delivery device. At least a
portion of the ablation energy delivery device is advanced into an interior of the
tongue. A sufficient amount of energy is delivered from the energy delivery
device into the interior of the tongue to debulk a section of the tongue withoutd~m~gin~?; a hypoglossal nerve. Thereafter, the ablation energy delivery device is
retracted from the interior of the tongue.
In another embodiment, an ablative agent source is provided. The
ablative agent source is coupled to an ablative agent delivery device. At least a
portion of the ablative agent delivery device is advanced into an interior of the
tongue. A sufficient amount of an ablative agent is delivered from the ablative
agent delivery device into the interior of the tongue to debulk a section of thetongue without d~m~ging a hypoglossal nerve. Thereafter, the ablative agent
delivery device is retracted from the interior of the tongue.
BR~FF T)ESCRTPTION OF THF FI~'JllRES
Figure 1 is a cross-sectional view of an debulking apparatus used with
the present invention.
Figure 2 is cross-sectional view illustrating the catheter and connector of
the debunking apparatus shown in Figure 1.
Figure 3 is a perspective view ofthe connector illustrated in Figure 1.
_ . . . ... . .
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
Figure 4 is a perspective view of an ablation source delivery device
associated with the debulking apparatus illustrated in Figure 1.
Figure 5 is a perspective view of a flexible ablation source delivery device
utilized with the methods of the present invention.
S Figure 6 illustrates the creation of ablation zones with the debulking apparatus shown in Figure 1.
Figure 7 is a cross-sectional view of the tongue with the mouth closed.
Figure 8 is a cross-sectional view of the tongue with the mouth open.
Figure 9 is a perspective view of the tongue.
Figure 10 is a perspective view of the dorsum of the tongue.
Figure 1 1 is a cross-sectional view of the tongue.
Figure 12 is a cross-sectional view of the tongue illustrating the location
of the hypoglossal nerves and the creation of an ablation zone.
Figure 13 is a cross-sectional view of the tongue illustrating a plurality of
ablation zones.
Figure 14 is a perspective view of the ventral surface of the tongue.
Figure 15 is a cross-sectional view of the tongue.
Figure 16 is a block diagram of a feedback control system useful with the
methods of the present invention.
Figure 17 is a block diagram illustrating an analog amplifier, analog
multiplexer and microprocessor used with the feedback control system of Figure
17.
Figure 18 is a block diagram of a temperature/impedance feedback
system that can be used to control cooling medium flow rate through the
catheter of Figure 1.
Figure 19 is a three dimensional graph illustrating the percent shrinkage
of the tongue following RF ablation.
Figure 20 is a graph illustrating two-dimensional shrinkage of bovine
tongue tissue with RF ablation.
Figure 21 is a graph illustrating three-dimensional shrinkage of bovine
tongue tissue due to RF ablation.
. .
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
Figure 22 is a graph illustrating percent volume change in a tongue
following RF ablation.
DF,T~lT,F,n nF,SCRTPTIQN
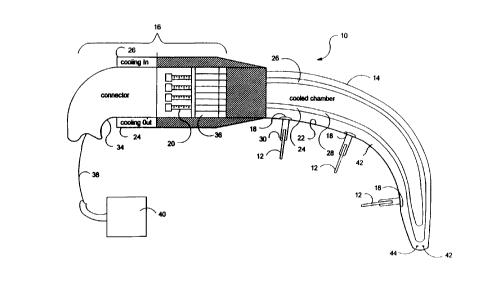
Referring to Figures 1 and 2, a debunking apparatus 10, creating
controlled cell necrosis and a reduction of a volume of a selected tissue site
including but not limited to the tongue, lingual tonsils, and/or soft palate tissue,
including but not limited to the uvula, is illustrated. Debulking apparatus 10 can
be positioned so that one or more ablation source delivery devices 12, includingbut not limited to devices that deliver ablation energy and/or an ablative agentwith chemical ablation with any number of dirrel el" compositions and mixtures
to create an ablation, alcohol ablation, diode laser ablation, laser fiber (defused)
ablation, chemotherapy coupled with ablation, microwave (915 MHz and 2.45
GHz), ultrasound, thermal ablation or cyro ablation using a hot or very cold
solution, solid or gas delivered by infusion such as through a needle, and RF atall relevant frequencies, deliver the ablation energy and/or ablative agent to aselected tissue site and create a desired ablation. Each ablation source delivery
source 12 is introduced into an interior of the tongue through a surface of the
tongue. Debulking apparatus 10 may include traumatic intubation with or
without visualization, provide for the delivery of oxygen or anesthetics, and can
be capable of suctioning blood or other secretions. It will be appreciated that
debunking apparatus 10 is used to treat a variety of di~elenl obstructions in the
body where passage of gas is restricted. One embodiment is the treatment of
sleep apnea using ablation source delivery device 12 to ablate (create cell
necrosis) at selected portions of the tongue, lingual tonsils and/or adenoids bythe use of a variety of different energy sources including but not limited to
resistive heating, RF, microwave, ultrasound and licluid thermal jet. The
preferred energy source is an RF source. ln this regard, debulking apparatus 10
can be used to ablate targeted masses including but not limited to the tongue,
tonsils, turbinates, soft palate tissues, hard tissue and mucosal tissue. In oneembodiment, debulking apparatus 10 is used to ablate an interior region ofthe
CA 022~3617 1998-11-0~
WO 97t43973 PCT/US97/09048
tongue, causing it to become debulked in order to increase the cross-sectional
area of the airway passage. A disinfectant medium introduction member
introduces a disinfectant medium in the oral cavity in order to reduce infection of
the ablated body member.
Prior to debulking the tongue, a presurgical evaluation may be performed
- including a physical e~min~tion, fiber optic pharyngoscopy, cephalometric
analysis and polygraphic monitoring. The physical e~min~tion emphasizes the
evaluation of the head and neck. It also includes a close e~min~tion of the nasal
cavity to identify obstructing deformities of the septum and turbinate;
oropharyngeal obstruction from a long, redllnd~nt soft palate or hypertrophic
tonsils, and hypopharyngeal obstruction from a prominent base of the tongue.
Debulking apparatus 10 includes a catheter 14, an optional handle 16 and
one or more ablation source delivery device 12 extending from different ports 18formed along a lon~itu-lin~l surface of catheter 14, or from a distal portion ofablation source delivery device 12 Catheter 14 can be a handpiece. An ablation
source delivery device advancement device 20 may be provided. Ablation
source delivery device advancement device 20 can include guide tracks or tubes
23 positioned in the interior of catheter 14. Ablation source delivery device 12may be positioned in guide tracks 23 and advanced from the guide tracks into theinterior of the tongue. Cabling is coupled to ablation source delivery device 12.
Controlled volumetric reduction of the tongue, under feedback control is
used to achieve an effective opening in the airway passage. A variety of different
pain killing medic~m~nt.c, inçl~l-ling but not limited to Xylocaine, may be used. A
digital ultrasonic measurement system can be used. The ultrasound measurement
quantifies biological shape changes, provides ultrasonic tr~n.~mi.c~ion and
reception, uses piezoelectric transducers (crystals) and provides time of flightdata.
A disinfectant medium introduction member 21 may be included and
introduced into the oral cavity. Disinfectant medium introduction member 21
can be introduced before, after or during the introduction of debulking apparatus
10 into the oral cavity. Additionally, disinfectant medium hltroduction member
... . . . ... . . .
CA 022~36l7 l998-ll-0~
W O 97/43973 PCTAUS97/09048
21 can be removed at the same time or at a di~rel~ time that debulking
apparatus 10 is removed from the oral cavity. Disinfectant medium introduction
member 21 can be included in debunking appa~ s 10, in an interior of catheter
14 or at an exterior of catheter 14, and may be an introducer with a lumen
configured to introduce a disinfectant agent from a disinfectant agent source 23into all or a selected portion of the oral cavity. Disinfectant medium introduction
member 21 can be capable of movement within the oral cavity in order to
provide for disinfection of all or only a portion of the oral cavity. For purposes
of this disclosure, the oral cavity is that body internal environment where
infectious germs may be introduced into the ablated tongue, soft tissue structure,
and the like. Disinfectant medium introduction member 21 may be slideably
positioned in catheter 14 or at its exterior. Alternatively, disinfectant mediumintroduction member 21 can be an optical fiber coupled to a light energy source,including but not limited to a UV source 25. The optical fiber can also be
slideably be positioned in the oral cavity. The optical fiber is configured to
provide for the selective disinfection of all or only a portion of the oral cavity
and can have a variety of di~el ell~ distal ends to achieve this purpose.
Suitable disinfectant agents include but are not limited to Peridex, an oral
rinse cont~ining 0.12% chlorhexidine glucinate ~1, 1'-hexanethylenebis[5-(p-
chlorophenyl) biganide} di-D-gluconate in a base cont~inin~ water, 11.6%
alcohol, glycerin, PEG 40 sorbitan arisoterate, flavor, dosium saccharin, and
FD&C Blue No. 1.
It will be appreciated that a variety of different disinfectants can be
employed, including other electromagnetic wavelengths, and various chemica3
compositions. The disinfectant medium can be introduced prior to ablation,
during ablation and/or after the ablation. It can be delivered continuously. Thelevel of disinfection of the oral cavity is selectable as is the volume of the oral
cavity that is disinfected. The degree of disinfection varies. Disinfection is
provided to reduce infection of the ablated body structure.
Ablation source delivery device 12 may be least partially positioned in an
interior of catheter 14. In one embodiment, ablation source delivery device 12is
t
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
advanced and retracted through a port 18 formed in an exterior surface of
catheter 14. Ablation source delivery device advancement and retraction device
20 advances ablation source delivery device 12 out of catheter 14, into an
interior of a body structure and can also provide a retraction of ablation source
delivery device 12 from the interior of the body structure. Although the body
structure can be any number of different structures, the body structure will
hereafter be referred to as the tongue. Ablation source delivery device 12 pierce
an exterior surface of the tongue and are directed to an interior region of the
tongue. Sufficient ablation energy is delivered by ablation source 12 to the
interior of the tongue to cause the tongue to become sufficiently ablated and
debulked. Ablation source delivery device 12 can be a hollow structure that is,
(i) adapted to deliver different chemicals to a selected tongue interior ablation
site (for chemical ablation) (ii) deliver alcohol or other liquids or semi~ uids to
achieve ablation as well as a variety of different infusion mediums, including but
not limited to saline, chemotherapy and the like. Different modalities can be
combined to achieved a desired ablation including but not limited to RF and
chemotherapy, chemical and chemotherapy. Ablation source delivery device 12
may have a limited travel distance in the tongue. ln one embodiment with RF
electrodes, this is achieved with an insulation sleeve that is in a surrounding
relationship to an exterior of an electrode. Other devices can include a structure
located on ablation source delivery device 12 which limits their advancement, ora structure coupled to a catheter which limits the advancement of ablation source
delivery devices 12, such as a stop and the like.
Ablation source delivery device 12 can include a central lumen for
receiving a variety of fluids that can be introduced into the interior of the tongue,
as well as a plurality of fluid delivery ports. In one embodiment, the disinfectant
agent is introduced through ablation source delivery device 12 into the interior of
the selected body structure. One suitable fluid is an electrolytic solution.
Instead of direct contact with tissue and ablation source delivery source 12 forthe delivery of ablation energy and/or ablative agent, a cooled electrolytic
solution can be used to deliver the ablation energy and/or ablative agent to the
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
tissue. The electrolytic solution may be cooled in the range of about 30 to 55
degrees C.
Catheter 14 includes a catheter tissue interface surface 22, a cooling
medium inlet conduit 24 and a cooling medium exit conduit 26 extending
through an interior of catheter 14. Ports 18 are formed in the exterior of
catheter 14, and are preferably formed on catheter tissue interface surface 22.
Ports 18 are isolated from a cooling medium flowing in inlet and outlet conduits24 and 26. Cooling medium inlet and exit conduits 24 and 26 are configured to
provide a cooled section of catheter tissue interface surface 22 of at least 1 to 2
cm~. In one embodiment, the cooled section of catheter tissue interface surface
22 is at least equal to the cross-sectional diameter of the underlying zone of
ablation. In another embodiment, the cooled section of catheter tissue interfacesurface 22 only provides cooling to an area associated with each deployed
ablation source delivery device.
The size of the cooled section of catheter tissue interface surface 22
varies for each patient. The size is sufficient enough to minimi7e swelling of the
tongue following the delivery of the ablation creation source. The reduction of
swelling can be 50% or greater, 75% or greater, and 90% and greater. The
amount of cooling provided is sufficient to enable the patient to return home
shortly after the debulking procedure is performed, and not run the risk of
choking on the tongue. lt has been found that by providing a sufficient level ofcooling over a relatively large area, the amount of ablation in an interior region
of the tongue is enhanced. By providing a sufficiently large enough cooled
section of catheter tissue interface surface 22, an adenomas response is
minimi7ed.
An ablation delivery surface 30 of ablation source delivery device 12 can
be adjusted by inclusion of an adjustable or non-adjustable insulation sleeve 32(Figures 3, 4, and 5). Insulation sleeve 32 can be advanced and retracted along
the exterior surface of ablation source delivery device 12 in order to increase or
decrease the length of the ablation delivery surface 30. Insulation sleeve 32 can
be made of a variety of materials including but not limited to nylon, polyimides,
CA 022~3617 1998-11-0~
WO 97143973 PCT/US97tO9048
other thermoplastics and the like. The size of ablation delivery surface 30 can be
varied by other methods inch1~1ing but not limited to creating a segmented
ablation source delivery device 12 with a plurality of sections that are capable of
- being multiplexed and individually activated, and the like.
Referring specifically to Figure 4, ablation source delivery device 12 has
an advancement length 33 that extends from an exterior surface of catheter 14
and is directed into the interior ofthe tongue. Advancement length 33 is
sufficient to position ablation delivery surface 30 at a selected tissue site in the
interior of the tongue. Ablation delivery surface 30 is of sufficient length so that
the ablation energy is delivered to the selected tissue site, create a desired level
of ablation (cell necrosis) at the selected tissue site without causing damage to
the hypoglossal nerve. Ablation deiivery surface 30 is not always at the distal
end of ablation source delivery device 12. Insulation 32 can also be positioned at
the distal end of ablation source delivery device 12. In this embodiment, ablation
delivery surface 30 does not extend to the distal end of ablation source delivery
device 12. However, ablation delivery surface 30 still delivers sufficient ablation
energy to create a desired level of cell necrosis in the interior of the tongue at the
selected tissue site without d~m~ging the hypoglossal nerve and/or damage to
the surface of the tongue. Additionally, only one side or a portion of a side ofablation source delivery device 12 can be insulated. This also provides for an
ablation source delivery device 12 which can be positioned throughout the
tongue, including adjacent to a hypoglossal nerve. Where ablation source
delivery device 12 is adjacent to the hypoglossal nerve, ablation source delivery
device 12 is in~ terl
In one embodiment, advancement length 33 is 1.2 to 1.5 cm, and the
length of ablation delivery surface 30 is 5 to 10 mm7 more preferably about 8
mm.
In another embodiment, advancement length 33 is insufficient to reach
the hypoglossal nerve when introduced through any of the tongue surfaces,
- 30 particularly the dorsum ofthe tongue.
.
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
Ablation source delivery device advancement device 20 is configured to
advance at least a portion of each ablation source delivery device 12 to a
placement position in the interior of the tongue. Ablation source delivery device
advancement device 20 can also be configured to retract each ablation source
S delivery device 12. At the placement position, ablation delivery surface delivers
sufficient ablation energy and/or effect to reduce a volume of the selected sitewithout d~ ing a hypoglossal nerve and/or a surface of the tongue. In one
embodiment, ablation source delivery device advancement and retraction device
20, with or without guide tracks 23, directs the delivery of ablation source
delivery device 12 from catheter 14 into the interior ofthe tongue at an angle of
60 to 90 degrees relative to a longitudinal axis of catheter 14, and preferably
about 70 degrees.
In certain embodiments, ablation source delivery device 12 has a
geometric shape, including but not limited to a curved configuration that includes
one or more insulated surfaces, either partially insulated on one side, at a
proximal end, at a distal end, and the like, that is configured to reduce the
volume of the selected tissue site without d~m~ging a hypoglossal nerve. In one
embodiment, ablation source delivery device 12 is introduced through any
tongue surface and is configured so that a section of ablation source delivery
device 12 which may be positioned close to the hypoglossal nerve is provided
with insulation 32. As previously noted, insulation 32 can be positioned at
different sites of ablation source delivery device 12.
Handle 16 is preferably made of an ins~ ting material. Ablation source
delivery device 12 may be made of a conductive material such as stainless steel.Additionally, ablation source delivery device 12 can be made of a shaped
memory metal, such as nickel tit~nillm, commercially available from Raychem
Corporation, Menlo Park, California. In one embodiment, only a distal end of
ablation source delivery device 12 is made of the shaped memory metal in order
to effect a desired deflection. When introduced into the oral cavity, catheter 14
can be advanced until a patient's gag response is initiated. Catheter 14 is thenretracted back to prevent patient's g~gging The distal end of ablation source
CA 022~3617 1998-ll-0
W O 97/43973 PCT~US9710904
delivery device 12 can be semi-curved. The distal end can have a geometry to
conform to an exterior of the tongue.
In one embodiment of the invention catheter 14 is a handpiece and shall
- for purposes of this invention catheter 14 shall be referred to as ("handpiece
14"). In this embodiment, a separate handle 16 is not necessary. Debulking
apparatus 10 is used to treat an interior region ofthe tongue. ~andpiece 14 has
a distal end that is sized to be positioned within an oral cavity. Ablation source
delivery device 12is at least partially positioned within an interior of handpiece
14. Ablation source delivery device 12 includes an ablation delivery surface 30.Ablation source delivery device advancement member 20is coupled to ablation
source delive~ device 12 and calibrated to advance ablation source delivery
device 12 from handpiece 20, including but not limited to a distal end of
handpiece 20, into the interior of the tongue when handpiece 20 is positioned
adjacent to a surface ofthe tongue. Ablation source delivery device 12 is
advanced an advancement distance 33 from handpiece 20 of sufficient length to
treat the interior region of the tongue with ablation energy and/or an ablative
agent without d~m~ging the hypoglossal nerve or the surface of the tongue.
Catheter 14 can be malleable in order to conform to the surface of the
tongue when a selected ablation target site is selected An encapsulated soft
metal, such as copper, or an annealed metal/plastic material can be ùsed to formmalleable catheter 14. All or a portion of catheter 14 may be malleable or made
of a shaped memory metal.
For many applications it is desirable for a distal end 14' of catheter 14 to
be deflectable. This can be achieved mechanically or with the use of memory
metals. A steering wire, or other mechanical structure, can be ~tt~ched to either
the exterior or interior of distal end 14'. In one embodiment, a deflection knoblocated on handle 16 is activated by the physician causing a steering wire to
tighten. This imparts a retraction of distal end 14', resulting in its deflection. It
will be appreciated that other mechanical devices can be used in place of the
- 30 steering wire. The deflection may be desirable for tissue sites with difficult
access.
CA 022~3617 1998-11-0~
WO 97143973 PCT/US97109048
Handle 6 can comprise a connector 34 coupled to retraction and
advancement device 20. Connector 34 provides a coupling of a ablation source
delivery device to power, feedback control, temperature and/or im~ging systems.
An RF/temperature control block 36 can be included.
In one embodiment, the physician moves retraction and advancement
device 20 in a direction toward a distal end of connector 34. Ablation source
delivery device 12 can be spring loaded. When ablation source delivery device
advancement device 20 is moved back, springs cause selected ablation source
delivery devices 12 to advance out of catheter 14.
One or more cables 38 may be coupled to ablation source delivery device
12 to an energy source 40. A variety of energy sources 40 can be used with the
present invention to including but not limited to RF, microwave, ultrasound,
coherent light, incoherent light, ultrasound, chemical ablation, alcohol ablation,
thermal transfer, thermal jet, chemotherapy combined with RF, and other
combinations of these sources. Preferably, energy source 40 is a RF generator.
When a RF energy source is used, the physician can activate RF energy source
40 by the use of a foot switch (not shown) coupled to RF energy source 40.
One or more sensors 42 may be positioned on an interior or exterior
surface of ablation source delivery device 12, insulation sleeve 32, or be
independently inserted into the interior of the body structure. Sensors 42 permit
accurate measurement of temperature at a tissue site in order to determine, (i)
the extent of ablation, (ii) the amount of ablation, (iii) whether or not further
ablation is needed, and (iv) the boundary or periphery of the ablated geometry.
Further, sensors 42 prevent non-targeted tissue from being destroyed or ablated.Sensors 42 are of conventional design, including but not limited to
thermistors, thermocouples, resistive wires, and the like. Suitable sensors 42
include a T type thermocouple with copper constantene, J type, E type, K type,
f1ber optics, resistive wires, thermocouple IR detectors, and the like. It will be
appreciated that sensors 42 need not be thermal sensors.
Sensors 42 measure temperature and/or impedance to permit ablation
monitoring. This reduces damage to tissue surrounding the targeted ablation
14
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
mass. By monitoring the temperature at various points within the interior of thebody structure the periphery of ablation can be ascertained and it is possible to
determine when the ablation is completed. If at any time sensor 42 determines
- that a desired ablation temperature is exceeded, then an appropriate feedback
signal is received at energy source 40 and the amount of energy delivered is
reg~ tecl
Debulking appa~aLIls 10 can include visll~li7~tion capability including but
not limited to a viewing scope, an expanded eyepiece, fiber optics, video
im~ging, and the like.
Additionally, ultrasound im~ging can be used to position the ablation
source delivery device 12 and/or determine the amount of ablation. One or more
ultrasound transducers 44 can be positioned in or on ablation source delivery
device 12, catheter 14, or on a separate device. An im~ging probe may also be
used internally or externally to the selected tissue site. A suitable im~ging probe
is Model 21362, m~n~lf~ctured and sold by Hewlett Packard Company. Each
ultrasound transducer 44 is coupled to an ultrasound source ~not shown).
With reference now to Figure 6 catheter 14 is shown as being introduced
into the oral cavity and multiple ablation source delivery devices 12 are advanced
into the interior of the tongue creating different ablation zones 46. Using RF,
debulking apparatus 10 can be operated in either bipolar or monopolar modes.
In Figure 6, ablation source delivery device is an RF electrode operated in the
bipolar mode, creating sufficient ablation zones 46 to debulk the tongue withoutaffecting the hypoglossal nerves and creating a larger airway passage. With thisdebulking, the back of the tongue moves in a forward direction away from the airpassageway. The result is an increase in the cross-sectional diameter of the airpassageway.
Using RF, debulking apparatus 10 can also be operated in the monopolar
mode. A groundpad can be positioned in a convenient place such as under the
chin. In this embodiment, a single RF electrode is positioned in the tongue to
create a first ablation zone 46. The RF electrode can then be retracted from theinterior of the tongue, catheter 14 moved, and the RF electrode is then advanced
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
from catheter 14 into another interior section ofthe tongue. A second ablation
zone 46 is created. This procedure can be completed any number of times to
form different ablation regions in the interior of the tongue.
More than one ablation source delivery device 12 can be introduced into
the tongue and operated in the bipolar mode. One or more ablation source
delivery devices 12 are then repositioned in the interior of the tongue any
number of times to create a plurality of connecting or non-connecting ablation
zones 46.
Referring now to Figures 7 through 15, various anatomical views of the
tongue and other structures are illustrated. The different anatomical structuresare as follows: the genioglossus muscle, or body of the tongue is denoted as 48;the geniohyoid muscle is 50; the rnylohyoid muscle is 52; the hyoid bone is 54;
the tip of the tongue is 56; the ventral surface of the tongue is denoted as 58; the
dorsum of the tongue is denoted as 60; the inferior dorsal of the tongue is
denoted as 62; the reflex of the vallecula is 64; the lingual follicles are denoted as
66; the uvula is 68; the adenoid area is 70; the lateral border of the tongue is 72;
the circumvallate papilla is 74, the palatine tonsil is 76; the pharynx is 78; the
redundant pharyngeal tissue is 80; the foramen cecum is 82; the hypoglossal
nerve is 84, and the lingual frenum of the tongue is 86.
Dorsum 60 is divided into an anterior 2/3 and inferior dorsàl 62. The
delineation is determined by circumvallate papilla 74 and foramen cecum 82.
Inferior dorsal 62 is the dorsal surface inferior to circumvallate papilla 74 and
superior reflex of the vallecula 64. Reflex of the vallecula 64 is the deepest
portion of the surface of the tongue contiguous with the epiglottis. Lingual
follicles 66 comprise the lingual tonsil.
Catheter 14 can be introduced through the nose or through the oral
cavity. Ablation source delivery device 12 can be inserted into an interior of the
tongue through dorsum surface 60, inferior dorsal surface 62, ventral surface
58, tip 56 or geniohyoid muscle 50. Additionally, ablation source delivery device
12 may be introduced into an interior of lingual follicles 66 and into adenoid area
70. Once ablation source delivery device 12 is positioned, insulation sleeve 32, if
16
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
included, may be adjusted to provided a desired energy delivery surface 30 for
each ablation source delivery device 12.
Ablation zones 46 are created without ~l~m~ging hypoglossal nerves 84.
This creates a larger air way passage and provides a treatment for sleep apnea
In all instances, the positioning of ablation source delivery device 12, as
well as the creation of ablation zones 46 is such that hypoglossal nerves 84 arenot ablated or damaged. The ability to swallow and speak is not impaired.
Figure 16 illustrates placement of ablation source delivery device 12 on
the dorsum surface 60 of the tongue. The first ablation source delivery device
12 is positioned 0.5 cm proximal to the circumvallate papilla. The other ablation
source delivery devices 12 are spaced 1.6 cm apart and are 1 cm offa central
axis of the tongue. In one embodiment, 465 MHz RF was applied. The
temperature at the distal end of ablation source delivery device 12 was about 100
degrees C. The temperature at the distal end of the insulation sleeve 32 was
about 60 degrees C. In another embodiment, the temperature at the distal end of
insulation sleeve 32 was 43 degrees C and above. RF energy can be applied as
short duration pulses with low frequency RF. Precise targeting of a desired
ablation site is achieved. One or more ablation source delivery devices 12 may
be used to create volumetric three-dimensional ablation. A variety of ablation
geometries are possible, including but not limited to rectilinear, polyhedral,
redetermined shapes, symmetrical and non-symmetrical.
Referring now to Figures 17 and 18 an open or closed loop feedback
system couples sensors 42 to energy source 40. The temperature of the tissue,
or of ablation source delivery device 12 is monitored, and the output power of
energy source 40 adjusted accordingly. Additionally, the level of disinfection in
the oral cavity can be monitored. The physician can, if desired, override the
closed or open loop system. A microprocessor can be included and incorporated
in the closed or open loop system to switch power on and off, as well as
modulate the power. The closed loop system utilizes a microprocessor 88 to
serve as a controller, watch the temperature, adjust the RF power, look.at the
result, refeed the result, and then modulate the power.
. .
CA 022~3617 1998-11-0~
WO 97143973 PCT/US97/09048
With the use of sensors 42 and the feedback control system a tissue
adjacent to ablation source delivery device 12 can be maintained at a desired
temperature for a selected period of time without impeding out. Each ablation
source delivery device 20 may be connected to resources which generate an
independent output for each ablation source delivery device. An output
m~int~in~ a selected energy at ablation source delivery device 12 for a selectedlength of time.
When an RF electrode is used, current delivered through the RF
electrode is measured by current sensor 90. Voltage is measured by voltage
sensor 92. Impedance and power are then calculated at power and impedance
calculation device 94. These values can then be displayed at user interface and
display 96. Signals representative of power and impedance values are received
by a controller 98. Signals representative of energy delivery for the dirrerel1~ablation sources can also be generated, measured and received by controller 98.
A control signal is generated by controller 98 that is proportional to the
difference between an actual measured value, and a desired value. The control
signal is used by power circuits 100 to adjust the power output in an appropriate
amount in order to m~int~in the desired power delivered at respective ablation
source delivery device 12.
In a similar manner, temperatures detected at sensors 42 provide
feedback for m~int~ining a selected power. The actual temperatures are
measured at temperature measurement device 102, and the temperatures are
displayed at user interface and display 96. A control signal is generated by
controller 98 that is proportional to the difference between an actual measured
temperature, and a desired temperature. The control signal is used by power
circuits l O0 to adjust the power output in an appropriate amount in order to
m~int~in the desired temperature delivered at the respective sensor. A
multiplexer can be included to measure current, voltage and temperature, at the
numerous sensors 42.
Controller 98 can be a digital or analog controller, or a computer with
software. When controller 98 is a computer it can include a CPU coupled
18
CA 022~3617 1998-11-0~
WO 97/43973 PCT~US97/09048
through a system bus. On this system can be a keyboard, a disk drive, or other
non-volatile memory systems, a display, and other peripherals, as are known in
the art. Also coupled to the bus is a program memory and a data memory.
User interface and display 96 includes operator controls and a display.
Controller 98 can be coupled to im~eing systems, including but not limited to
- ultrasound, CT scanners, X-ray, MRI, m~mmographic X-ray and the like.
Further, direct vi.cuali7~tion and tactile im~gin~ can be utilized.
The output of current sensor 90 and voltage sensor 92 iS used by
controller 98 to m~int~in a selected power level at the RF electrodes. The
amount of RF energy delivered controls the amount of power. A profile of
power delivered can be incorporated in controller 98, and a preset amount of
energy to be delivered can also be profiled. Other sensors similar to sensors 90and 92 can be used by controller 98 for other ablation source delivery devices 12
to m~int~in a controllable amount of an ablation energy and/or ablative agent.
Circuitry, software and feedback to controller 98 result in process
control, and the m~intç~nce of the selected power that is independent of
changes in voltage or current, and are used to change, (i) the selected power,
(ii) the duty cycle (on-off and wattage), (iii) bipolar or monopolar energy
delivery, and (iv) infusion medium delivery, inclll-iing flow rate and pressure.These process variables are controlled and varied, while m~int~ining the desireddelivery of power independent of changes in voltage or current, based on
temperatures, or other suitable parameters, monitored at sensors 42.
Current sensor 90 and voltage sensor 92 are connected to the input of an
analog amplifier 104. Analog amplifier 104 can be a conventional differential
amplifier circuit for use with sensors 42. The output of analog amplifier 104 issequentially connected by an analog multiplexer 106 to the input of A/D
converter 108. The output of analog amplifier 104 is a voltage which represents
the respective sensed temperatures. Digitized amplifier output voltages are
supplied by A/D converter 108 to microprocessor 88. Microprocessor 88 may
be a type 68HCII available from Motorola. However, it will be appreciated that
19
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
any suitable microprocessor or general purpose digital or analog computer can
be used to calculate impedance or telllpelal~lre.
Microprocessor 88 sequentially receives and stores digital representations
of impedance and temperature. Each digital value received by microprocessor
88 corresponds to di~erelll temperatures and impedances.
Calculated values, including but not limited to power and impedance, can
be indicated on user interface and display 96. Alternatively, or in addition to the
numerical indication of power or impedance, calculated impedance and power
values can be compared by microprocessor 88 with power and impedance limits.
When the values exceed predetermined power or impedance values, a warning
can be given on user interface and display 96, and additionally, the delivery
energy can be reduced, modified or interrupted. A control signal from
microprocessor 88 can modify the power level supplied by energy source 40.
Figure 18 illustrates a block diagram of a temperature/impedance
feedback system that can be used to control cooling medium flow rate through
catheter 14. Energy is delivered to ablation source delivery device 12 by energysource 44, and applied to tissue. A monitor 110 ascertains tissue impedance,
based on the energy delivered to tissue, and compares the measured impedance
value to a set value. If the measured impedance exceeds the set value a disabling
signal 112 is trAn~mitted to energy source 40, ceasing further delivery of energy
to ablation source delivery device 12. If measured impedance, or other
measured parameter, is within acceptable limits, energy continues to be applied
to the tissue. During the application of energy to tissue sensor 42 measures thetemperature of tissue and/or ablation source delivery device 12. A comparator
1 14 receives a signal representative of the measured temperature and compares
this value to a pre-set signal representative of the desired temperature.
Comparator 1 14 sends a signal to a flow regulator 1 16 representing a need for a
higher cooling medium flow rate, if the tissue temperature is too high, or to
maintain the flow rate if the temperature has not exceeded the desired
temperature.
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
EXAMlPLE I
Debulking apparatus 10 was used to determine two-dimensional
shrinkage of a bovine. RF volumetric reduction was achieved using a single
needle electrode. Four mature ultrasonic crystals were positioned to form a
square. Measurements were taken at control and post volumetric reduction at
- 15 watts initially with a 13% volumetric reduction, and 15 watts for ~ hours with
an additional 4% volumetric reduction. A total 17% volumetric reduction was
achieved.
EXAMPLE 2
Debulking apparatus 10 was used to determine three-dimensional
shrinkage of a bovine tongue. RF volumetric reduction was achieved with a
single needle electrode with eight mini~tllre ultrasonic crystals, creating a cube.
Application of 16 watts initially produced a 17% volumetric reduction of the
tongue, 25 watts applied initially produced a 25% volumetric reduction, and 25
watts after hours produced an additional 4% reduction, for a total volumetric
reduction of 29%.
EXAMPLE 3
A 35~/0 volumetric reduction was achieved in porcine in vivo, with three
dimensional gross at 20 watts initial application.
Referring now to Figure 19, ablation volume dimensions were measured
with a multidimensional digital sonomicrometry. An average decrease in the Z
direction was 20%, and volume shrinkage was 26%. Three-dimensional
shrinkage of tongue tissue due to in vivo RF ablation with the needle, ablation
with 20 Watts) is presented in Figure 20. Control volume before ablation is
compared with a post-ablation volume.
Figure 20 illustrates two-dimensional shrinkage of a bovine tongue tissue
due to RF ablation with a needle electrode. The before and after ablation results
are illustrated.
Figure 21 illustrates in graph form ablation at 16 Watts resulted in a 17%
volume shrinkage of the tissue in post-ablation verses control. Ablation at 25
-
.....
CA 022~3617 1998-11-0~
WO 97/43973 PCT/US97/09048
watts resulted in a 25% volume shrinkage after ablation. An additional 4% area
shrinkage was obtained after in long-term post ablation (4 hours) verses post-
ablation.
Figure 22 illustrates a percent volume change after RF ablation. 16
Watts, ablation at 16 Watts for 20 minlltçs; 25 Watts, ablation at 25 Watts for 20
minutes, 25 Watts (4 hours), and long tern post ablation (4 hours after 25 Wattsablation).
The foregoing description of a preferred embodiment of the invention has
been presented for purposes of illustration and description. It is not intended to
be exhaustive or to limit the invention to the precise forms disclosed. Obviously,
many modifications and variations will be apparent to practitioners skilled in this
art. It is intended that the scope of the invention be defined by the following
claims and their equivalents.
What is claimed is: