Note: Descriptions are shown in the official language in which they were submitted.
CA 022~284 1998-12-17
Unicellular or multicellular organisms for preparing
riboflavin
The present invention relates to a unicellular
or multicellular organism for preparing riboflavin
uslng mlcroorganlsms.
Vitamin B2, also termed riboflavin, is essential
for humans and animals. Inflammations of the oral and
pharyngeal mucous membranes, cracks in the corners of
the mouth and pruritus and inflammations in the skin
folds, among other damage to the skin, conjunctival
inflammations, diminished visual acuity and clouding of
the cornea appear in association with vitamin B2
deficiency. Cessation of growth and decrease in weight
can occur in infants and children. Vitamin B2 therefore
is of importance economically, in particular as a
vitamin preparation in association with vitamin
deficiency and as a feed'additive. In addition to this,
it is also employed as a foodstuff colorant, for
example in mayonnaise, ice cream, blancmange, etc.
Riboflavin is prepared either chemically or
microbially. In the chemical methods of preparation,
the riboflavin is as a rule isolated in multi-step
processes as a pure end product, with, however,
relatively expensive starting compounds, such as D-
ribose, having to be employed. For this reason, the
chemical synthesis of riboflavin is only suitable for
those applications for which pure riboflavin is
required, for example in human medicine.
Using microorganisms to prepare riboflavin
offers an ~alternative to preparing this substance
chemically. Preparing riboflavin microbially is
particularly suitable in those instances in which this
substance is not required to be of high purity. This is
the case, for example, when the riboflavin is to be
employed as an additive to feed products. In such
CA 022~284 1998-12-17
cases, the microbial preparation of riboflavin has the
advantage that the riboflavin can be obtained in a one-
step process. In addition, renewable raw materials,
such as vegetable oils, can be employed as starting
compounds for the microbial synthesis.
It is known to prepare riboflavin by fermenting
fungi such as Ashbya gossypii or Eremothecium ashbyi
(The Merck Index, Windholz et al., eds. Merck & Co.),
page 1183, 1983, A. Bacher, F. Lingens, Augen. Chem.
1969, p. 393); however, yeasts, such as Candida or
Saccharomyces, and bacteria, such as Clostridium, are
also suitable for producing riboflavin.
Methods using the yeast Candida famata are also
described, for example in US 05231007.
Bacterial strains which overproduce riboflavin
are described, for example, in EP 405370, GB 1434299,
DE 3420310 and EP 0821063, where the strains were
obtained by transforming the riboflavin biosynthesis
genes from Bacillus subtilis. However, these
prokaryotic genes were unsuitable for a method of
preparing riboflavin recombinantly which used
eukaryotes such as Saccharomyces cerevisiae or Ashbya
gossypii. For this reason, the specific genes for
riboflavin biosynthesis were, as described in
WO 93/03183, isolated from a eukaryote, namely from
Saccharomyces cerevisiae, in order thereby to provide a
recombinant method for preparing riboflavin in a
eukaryotic production organism. However, recombinant
preparation methods of this nature are either
unsuccessful, or only enjoy limited success, in
producing riboflavin if there is inadequate provision
of substrate for the enzymes which are specifically
involved in the riboflavin biosynthesis.
In 1967, Hanson (Hanson AM, 1967, in Microbial
Technology, Peppler, HJ, pp. 222-250, New York) found
that adding the amino acid glycine increases the
formation of riboflavin in Ashbya gossypii. However,
such a method is disadvantageous because glycine is a
CA 022~284 1998-12-17
very expensive raw material. For this reason, efforts
were made to optimize riboflavin production by
preparing mutants.
German Patent Specification 19525281 discloses
a method for preparing riboflavin which involves
culturing microorganisms which are resistant to sub-
stances which have an inhibitory effect on isocitrate
lyase.
German Laid-Open Specification 19545468.5-41
discloses another method for preparing riboflavin
microbially in which the isocitrate lyase activity or
the expression of the isocitrate lyase gene of a
riboflavin-producing microorganism is increased.
However, even in comparison with these methods, there
is still a need for a further optimization of the
riboflavin preparation.
The object of the present invention is
consequently that of making available a unicellular or
multicellular organism, preferably a microorganism, for
the biotechnological preparation of riboflavin, which
microorganism enables formation of the riboflavin to be
further optimized. In particular, an organism should be
made available which is suitable for preparing
riboflavin while economizing on raw materials and which
consequently makes possible a production which is more
economical than that of the previous state of the art.
In particular, the organism should permit an increased
formation of riboflavin, without any addition of
glycine, as compared with the previous organisms.
This object is achieved by means of a uni-
cellular or multicellular organism which exhibits a
glycine metabolism which is altered such that its
synthetic output of riboflavin without any external
supply of glycine is at least equal to that of a wild
type of the Ashbya gossypii species ATCC10895, which is
cultured under standard conditions with the addition of
6 g of external glycine/l.
CA 022~284 1998-12-17
Culturing under standard conditions means
culturing, at 30~C and 120 rpm, in 500 ml shaker flasks
possessing two baffles. 50 ml of a solution of 10 g of
yeast extract/l containing either 10 g of glucose/l or
10 g of soybean oil/l are employed per flask as the
medium. The media are inoculated with 1~ of a 16 h
culture carried out under the same conditions.
The objective of this sought-after alteration of the
intracellular metabolism of glycine can be achieved
using the known methods for improving organism strains.
This means that, in the simplest case, appropriate
strains can be prepared by means of screening after the
selection which is customary in microbiology. It is
also possible to use mutation in conjunction with
subsequent selection. In this case, the mutation can be
carried out either by means of chemical mutagenesis or
by means of physical mutagenesis. A further method is
that of selection and mutation together with subsequent
recombination. Finally, the organisms according to the
invention can be prepared by means of genetic
manipulation.
According to the invention, the organism is
altered such that it produces glycine intracellularly
in a quantity which is greater than its requirement for
maintaining its metabolism. According to the invention,
this increase in intracellular glycine production can
be achieved by preparing an organism in which the
activity of the enzyme threonine aldolase is increased.
This can be achieved, for example, by increasing
substrate turnover by means of altering the catalytic
center or by abolishing the effect of enzyme inhibitors.
An increase in the activity of the threonine aldolase
enzyme can also be elicited by increasing the synthesis
of the enzyme, for example by means of gene
amplification or by eliminating factors which repress
the biosynthesis of the-enzyme.
According to the invention, the endogenous
threonine aldolase activity can preferably be increased
CA 022~284 1998-12-17
by mutating the endogenous threonine aldolase gene.
Such mutations can either be produced randomly by means
of classical methods, such as using W irradiation or
mutation-provoking chemicals, or in a targeted manner
using genetic engineering methods such as deletion,
insertion and/or nucleotide exchange.
Increased expression of the threonine aldolase
gene can be achieved by incorporating copies of the
threonine aldolase gene and/or by enhancing regulatory
factors which exert a positive effect on threonine
aldolase gene expression. For example, regulatory
elements can preferentially be enhanced at the
transcriptional level by, in particular, increasing the
transcription signals. In addition to this, however, it
is also possible to enhance translation by, for
example, improving the stability of the mRNA.
In order to increase the gene copy number, the
threonine aldolase gene can, for example, be
incorporated into a gene construct or a vector which
preferably contains regulatory gene sequences which are
assigned to the threonine aldolase gene, in particular
those sequences which enhance gene expression. A
riboflavin-producing microorganism is then transformed
with the gene construct containing the threonine
aldolase gene.
According to the invention, the threonine
aldolase can also be overexpressed by exchanging the
promoter. In this context, it is also possible to
achieve the higher enzymic activity in an alternative
manner by incorporating gene copies or by exchanging
the promoter. However, it is equally also possible to
achieve the desired alteration in the enzymic activity
by simulta~eously exchanging the promoter and incor-
porating gene copies.
Since threonine is limiting in an organism
which has been altered in this way, it is necessary to
feed in threonine when the cell according to the
invention is employed. The improved uptake of the
CA 022~284 1998-12-17
threonine and its virtually quantitative conversion
into glycine lead to a surprisingly large increase in
riboflavin formation such as was not previously
achievable by feeding in glycine. Alternatively, the
endogenous formation of threonine in the organism can
be increased, for example, by eliminating the feedback
resistance of the aspartate kinase.
The threonine aldolase gene is preferably
isolated from microorganisms, particularly preferably
from fungi. Fungi of the genus Ashbya are once again
preferred in this context. The species Ashbya gossypii
is highly preferred.
However, all other organisms whose cells
contain the sequence for forming threonine aldolase,
that is animal and plant cells as well, are also
suitable for isolating the gene. The gene can be
isolated by means of homologous or heterologous comple-
mentation of a mutant which is defective in the
threonine aldolase gene or by means of heterologous
probing or PCR using heterologous primers. For
subcloning, the size of the insert in the complementing
plasmid can subsequently be reduced to a minimum by
means of suitable restriction enzyme steps. After the
putative gene has been sequenced and identified,
subcloning which gives an accurate fit is effected by
means of fusion PCR. Plasmids which carry the resulting
fragments as inserts are introduced into the threonine
aldolase gene-defective mutant, which is then tested
for the functionality of the threonine aldolase gene.
Functional constructs are finally used to transform a
riboflavin producer.
Following isolation and sequencing, the
threonine aldolase genes can be obtained with nucleo-
tide sequences which encode the given amino acid
sequence or its allelic variation. Allelic variations
include, in particular, derivatives which can be
obtained by deleting, inserting or substituting
nucleotides from appropriate sequences while at the
CA 022~284 1998-12-17
same time retaining the threonine aldolase activity. A
corresponding sequence, from nucleotide 1 to nucleotide
1149, is shown in Figure 2b.
A promoter having the nucleotide sequence from
nucleotide -1231 to nucleotide -1 as depicted in the
abovementioned sequence, or a DNA sequence which has
essentially the same effect, is, in particular, placed
upstream of the threonine aldolase genes. Thus, a
promoter which differs by one or more nucleotide
substitutions, by insertion and/or by deletion from the
promoter which possesses the nucleotide sequence shown
without, however, the functionality or the activity of
the promoter being impaired, can, for example, be
placed upstream of the gene. In addition, the activity
of the promoter can be increased by altering its
sequence, or the promoter can be completely replaced by
active promoters.
Moreover, regulatory gene sequences or
regulatory genes which, in particular, increase the
activity of the threonine aldolase gene can be assigned
to the threonine aldolase gene. Thus, enhancers, which
increase threonine aldolase gene expression by
improving the interaction between the RNA polymerase
and the DNA, can, for example, be assigned to the
threonine aldolase gene.
One or more DNA sequences can be placed
upstream and/or downstream of the threonine aldolase
gene, which does or does not possess an upstream
promoter or does or does not possess a regulatory gene,
such that the threonine aldolase gene is contained in a
gene structure. Plasmids or vectors which contain the
threonine aldolase gene and are suitable for trans-
forming a . riboflavin producer can be obtained by
cloning the threonine aldolase gene. The cells which
can be obtained by transformation contain the gene in
replicatable form, i.e-. in additional copies in the
chromosome, with the gene copies being integrated at
- CA 022~284 1998-12-17
arbitrary sites in the genome by means of homologous
recombination.
The objective, according to the invention, of partial
or complete intracellular formation of glycine can also
be achieved by preparing organisms in which the intra-
cellular degradation of glycine is at least partially
blocked. Mutations of this nature can, as already
described above, either be generated in a random manner
by means of classical methods using physical or
chemical mutagenesis, for example using W irradiation
or mutation-provoking chemicals, or in a targeted
manner by means of genetic engineering methods.
According to the invention, the objective of
the increased intracellular formation of glycine can
preferably be achieved by altering the gene for serine
hydroxymethyltransferase. Such alterations can, for
example, be achieved by mutations, such as insertions,
deletions or substitutions, in the structural gene or
the regulatory elements, such as promoters and trans-
cription factors, which are associated with this gene.
According to the invention, it was observed,surprisingly, that these mutants include mutants which
are resistant to glycine antimetabolites. The glycine
antimetabolite-resistant mutants which are preferred
are those unicellular or multicellular organisms which
are resistant to alpha-aminomethylphosphonic acid
and/or alpha-aminosulfonic acid.
This can, for example, be achieved in exactly
the same way by selecting mutants which are replaced by
the threonine structural analog ~-hydroxynorvaline
and/or which are substituted at the threonine and/or
lysine analogs.
Consequently, mutants which can be employed in
accordance with the invention can also be prepared by
appropriate selection. Such resistant unicellular or
multicellular organisms can therefore be prepared using
the classical screening methods which are in general
use in microbiology.
CA 022~284 1998-12-17
In the organisms described, riboflavin produc-
tion can be further increased if the export into the
medium of the glycine which is formed intracellularly
is at least partially blocked. In the simplest case, it
is sufficient to supplement with glycine in order to
achieve this. As an alternative, the carrier which is
responsible for the export can be switched off by
disrupting the gene.
In addition, an increase in intracellular
glycine concentration can be achieved by altering the
glyoxylate metabolism, e.g. by increasing the activity
of glyoxylate aminotransferase. Another option is to
optimize the synthesis of intracellular glycine from
carbon dioxide and ammonia.
In summary, it can be stated that the object
according to the invention can preferably be solved by
increasing intracellular synthesis of the glycine, at
least partially blocking degradation of the glycine, at
least partially inhibiting transport of the glycine out
of the cell, altering the glyoxylate metabolism and
optimizing glycine synthesis from ammonia and carbon
dioxide. These solutions can be used as alternatives,
or cumulatively or in any arbitrary combination.
An additional increase in riboflavin formation
can be achieved by adding glycine to the nutrient
medium.
The unicellular or multicellular organisms
which are obtained in accordance with the invention may
be any arbitrary cells which can be employed for
biotechnological processes. Examples of these cells are
fungi, yeasts, bacteria and plant and animal cells. In
accordance with the invention, the cells are preferably
transformed. fungal cells, particularly preferably
fungal cells of the genus Ashbya. The species Ashbya
gossypii is particularly preferred in this context.
In that which follows, the invention is
explained in more detail with the aid of examples,
CA 022~284 1998-12-17
- 10 -
without this being associated with any restriction of
the invention to the subject matter of the examples:
Example 1
- Selecting a mutant which is resistant to alpha-amino-
methylphosphonic acid (AMPS).
Ashbya gossypii spores were mutagenized with W
light. The spores were then added to plates treated
with 70 mM alpha-aminomethylphosphonic acid. Inhibition
of riboflavin formation can be recognized by the fungus
forming yellow colonies without inhibition and white
colonies with inhibition. Accordingly, the yellow
organisms, i.e. those which were resistant to the
inhibitor, were isolated. This method was used to
obtain the resistant strain AMPS-NM-01, inter alia.
Experiments carried out on plates containing
200 mM AMPS demonstrated that this strain still
exhibited a yellow colony color, in contrast to the
starting strain, which remained completely white. In
submerged culture, the mutant exhibited the same
formation of riboflavin in the absence of glycine as
did the wild type in the presence of glycine (cf.
Figure 1).
Investigations of the specific enzymic activities of
the wild type and mutant showed that serine
hydroxymethyltransferase activity was reduced by 50~
(~ig. 7). Since it was possible to demonstrate by
feeding l3C-labeled threonine that formation of serine,
which is presumably catalyzed by serine
hydroxymethyltransferase, takes place from glycine
(Table 1), the increase in riboflavin formation can be
explained by a reduction in the quantity of glycine
draining off to form serine.
The composition of the minimal medium used in
Table 1 is as follows:
CA 022~284 1998-12-17
Solution A: KH2PO4200 g/l pH 6.7 with KOH
(100 times)
Solution B: NH4Cl15 g/l
5 (10 times) Asparagine5 g/l
NaCl 2 g/l
MgSO4 x 7H2O 4 g/l
MnSO4 x H2O 0.5 g/l
ClCl2 x 2H2O 0.4 g/l
Myoinositol 1.0 g/l
Nicotinamide 2.5 g/l
Yeast extract 2 g/l
C source: Glucose or
soybean oil 2.5 g/l
In order to prepare the medium, the C source
was added to one-times concentrated solution B and the
mixture was sterilized by autoclaving. After the medium
had cooled down, 1/100 of the volume of separately
autoclaved solution A was added.
Example 2
Isolation of the GLY1 gene from Ashbya gossypii
In order to isolate the gene for threonine
aldolase, the glycine-auxotrophic Saccharomyces
cerevisiae mutant YM 13F (SHM1 : : HIS3 shm2 : : LEU2
glyl : : URA3) was transformed, after selection for
resistance to fluoroorotic acid, with an Ashbya
gossypii gene library. The gene library consisted of
genomic DNA which had been partially digested with
Sau3A and from which fragments of 8 - 16 kb in size had
been isolated by density gradient centrifugation and
ligated into the BamHI-cut vector Yep352. The trans-
formants were first of all selected for uracil proto-
trophy. Selection for glycine prototrophy was carried
out in a second step after replica plating. 25 glycine-
prototrophic clones were isolated from about 70,000
.
CA 022~284 1998-12-17
uracil-prototrophic clones. Curing of the transformants
and retransformation with the isolated plasmids
demonstrated that the complementation was plasmid-
encoded. Whereas there was no measurable threonine
aldolase activity (~ 0.1 mU/mg of protein) in the
glycine-auxotrophic Saccharomyces strain, it was possi-
ble to measure significant enzyme activity (25 mU/mg of
protein) in the strains which were transformed with the
isolated gene library plasmids. A sub-cloned 3.7 kb
Hind III fragment which exhibited complementation was
sequenced (Figure 2). A threonine aldolase-encoding
gene which was homologous to Saccharomyces cerevisiae
GLYl was found.
Example 3
Overexpressing the GLYl gene in Ashbya gossypii
In order to overexpress the GLYl gene, it was
cloned into the expression vector pAG203 (cf.
W09200379). In this plasmid, the gene is under the
control of the TEF promoter and the TEF terminator
(Figure 3). A gene for resistance to G418 functions as
a selective marker in Ashbya gossypii. After Ashbya
gossypii had been transformed with this plasmid and
single-spore clones had subsequently been isolated,
because the spores are mononuclear and haploid, the
threonine aldolase activity in the crude extract was
then measured. Both when growing on glucose and when
growing on soybean oil, at least ten-fold overexpression
was measured in A.g.p.AG203GLYl as compared with a
strain which had been transformed with the empty
plasmid pAG203 (Figure 4).
CA 022~284 1998-12-17
Example 4
Increasing riboflavin formation by overexpressing GLY1
and feeding threonine
Threonine was added to the medium in order to
check whether the threonine which is formed in the cell
limits the formation of glycine by the overexpressed
threonine aldolase. When 6 grams of threonine were
added per liter when A.g.pAG203GLYl was growing on
glucose as the carbon source, the strain formed
approximately twice as much riboflavin as it did when
6 grams of glycine were added per liter (Figure 5).
This effect was not observed when a wild type and a
control strain which was transformed with the empty
plasmid were tested. Analysis of the amino acids in the
medium showed that only about 6 mM of the fed-in 52 mM
of threonine remained in the case of the GLY1
overexpresser and that, surprisingly, the concentration
of glycine had increased from 2 mM to 42 mM. These
results demonstrated that glycine formation was limited
by threonine, that the overexpressed threonine aldolase
was capable of functioning, that glycine which was
formed intracellularly was more effective than glycine
which was fed extracellularly, and that the fungal
cells exported glycine massively.
Example 5
Inhibiting glycine export
If the threonine aldolase-overexpressing strain
A.g.pAG203GLY1 was cultured on soybean oil instead of
glucose, as in Example 4, the increase in riboflavin
formation Qbtained when feeding threonine did not
exceed that when feeding glycine (Fig. 6). However,
analysis of the medium showed that the threonine had
been degraded down to about 13 mM. There cannot, there-
fore, have been any limitation in the threonine. At the
same time, it was found that the extracellular glycine
CA 022~284 1998-12-17
had increased from 2 to about 44 mM. All the glycine
which had been formed by the fungus had therefore been
exported into the medium. It was possible to inhibit
this export by introducing glycine into the medium, a
measure which then resulted in the riboflavin formation
being substantially increased in association with the
same uptake of threonine (Table 2). In order to rule
out the possibility that it was only the glycine which
had been introduced which was responsible for the
increased production, as much glycine was introduced,
in a control, as was ultimately formed, as glycine, in
the experiment using glycine and threonine. This
finding underlines the fact that glycine which is
formed intracellularly is much more effective than
glycine which is added extracellularly.
Example 6
Increasing the formation of riboflavin by selecting
~-hydroxynorvaline-resistant mutants
Since it was not the conversion of threonine
into glycine but the synthesis of threonine which first
of all limited glycine formation, the threonine analog
~-hydroxynorvaline was used to search for resistant
mutants. Radial growth was significantly inhibited on
agar plates filled with minimum medium containing
2.5 mM ~-hydroxynorvaline. Mutants which grew more
vigorously formed spontaneously at the edges of the
colonies. Stable mutants which grew significantly more
vigorously on the ~-hydroxynorvaline minimal medium
than did the parental strains (Fig. 8) were produced by
isolating spores and selecting once again. Investigation
of riboflavin formation indicated a marked increase in
productivity. First, in minimal medium containing
soybean oil, the strain HNV-TB-25 formed 41 + 11 mg of
riboflavin/l whereas its parental strain only produced
18 + 3 mg/l. The progeny strain HNV-TB-29 also exhibits
a marked increase, with a formation of 116 + 4 mg/l, as
CA 022~284 1998-12-17
compared with its strain of origin, i.e. Ita-GS-O1,
which only formed 62 i 10 mg/l.
Table 1: 13C-enrichment in the C atoms of serine, threonine and
glycine following growth of A. gossypii ATC10895 on the given
media and subsequent total hydrolysis of the resulting biomass
(MM: minimal medium; YE: yeast extract; YNB: yeast nitrogen
base; n.d.: not determined)
Medium MM + 0.2 g of YE/l MM + 0.2 g of YNB/l
+ 1 g of ethanol/l + 1 g of ethanol/l
+ 2.7 mg of 13C2-serine/l + 2.6-mg of 13C1-serine/l
Serine C1 1.1 4.9
C2 5.9 1.1
C3 1.1 1.1
Threonine C1 n.d. 39.0
C2 1.1
C3 1.1
C4 1.1
Glycine C1 1.1 7.1
C2 4.3 1.1
CA 022~284 1998-12-17
Table 2: Effect of supplementation with threonine and glycine
on riboflavin formation when GLY1 is simultaneously being
overexpressed
Carbon t = 0 t = 72 h t = 72 h t = 72 h
Strain source Supple- Riboflavin Gly Thr
ment [mg/l] [mM] [mM]
80 mM Gly 80 + 2
Soybean oil 50 mM Thr 22 i 1 42 + 0
WT 130 mM Gly 18 ~ 3 129 + 2 n.d.
80 mM Gly 80 + 0
Glucose 50 mM Thr 5 + 1 35 + 0
130 mM Gly 7 + 1 126 + 2 n.d.
80 mM Gly 117 + 2
Soybean oil 50 mM Thr 31 + 0 11 + 1
Ag pAG 130 mM Gly 20 + 3 129 + 1 n.d.
203 GLY1 80 mM Gly 113 + 2
Glucose 50 mM Thr 40 + 1 12 + 0.7
130 mM Gly 9 + 1 129 + 3 n.d.
n.d. = not detectable
CA 022~284 1998-12-17
Comments on the figures
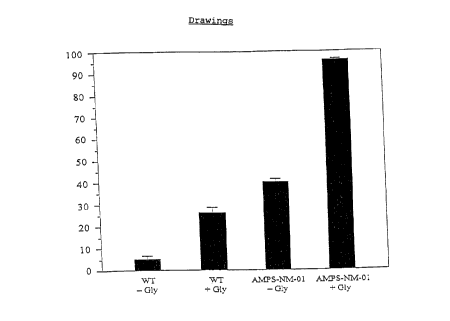
Figure 1: Formation of riboflavin by the Ashbya
gossypii strains ATCC 10895 (wild type, WT)
and the AMPS-resistant mutant AMPS-MN-01 in
the presence or absence of 6 g of glycine/l
following growth on complete medium contain-
ing 10 g of soybean oil/l as the carbon
source. The measured values were obtained
from three independent experiments.
Figure 2a: Gly 1 locus in the Ashbya gossypii genome.
The clones GB 7-1 and GB 26-9, and also the
3.7 kb Hind III subclone GB-26-9-6, comple-
ment the S. cerevisiae mutant. GB-26-9-6
was sequenced entirely while GB 7-1 was
sequenced in order to complete the C
terminus of GLYl.
~0 Figure 2b: Nucleotide sequence, and deduced amino acid
sequence, of the A. gossypii GLYl gene
together with the flanking nucleotide
sequence.
~5 Figure 3: Diagrammatic depiction of the construction
of the vector pAG203GLYl for overexpressing
the GLYl gene in A. gossypii.
Figure 4: Comparison of Ashbya gossypii wild type
(solid symbols) and A.g.pAG203GLYl (open
symbols) with regard to growth, riboflavin
formation and specific threonine aldolase
--activity when cultured on complete medium
containing 10 g of soybean oil/l.
Figure 5: Growth and riboflavin formation of Ashbya
gossypii strains ATCC 10895 (wild type),
pAG203 and pAG203GLYl when cultured on YE
CA 022~284 1998-12-17
complete medium containing 10 g of glucose/l
as the carbon source and in association
with glycine or threonine supplementation.
The Table shows the glycine and threonine
concentrations in the medium in each case
before and after culture. The mean values
and standard deviations shown represent the
results from three independent experiments.
Figure 6: Growth and riboflavin formation of Ashbya
gossypii strains ATCC 10895 (wild type),
pAG203 and pAG203GLY1 when cultured on
complete medium containing 10 g of glucose/l
as the C source and in association with
glycine or threonine supplementation. The
Table shows the glycine and threonine con-
centrations in the medium in each case
before and after culture. The mean values
and standard deviations shown represent the
results from three independent experiments.
Figure 7: Comparison of Ashbya gossypii wild type
(solid symbols) and the AMPS-resistant
mutant AMPS-NM-01 with regard to growth,
riboflavin formation and the specific
activities of threonine aldolase, serine
hydroxymethyltransferase and glutamate gly-
oxylate aminotransferase when cultured on
complete medium containing 10 g of soybean
oil/l. The measured values were obtained
from three independent experiments.
Figure 8: Effect of ~-hydroxynorvaline on Ashbya
gossypii; growth of wild type (W) and
HNV-TB-25 (H) on an agar plate which is
filled with minimal medium containing 2.5 g
of glucose/l and 2.5 mM ~-hydroxynorvaline.
2255284.seq
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANTS:
(A) NAME: Forschungszentrum Juelich GmbH
(B) STREET: Leo-Brandt-Str.
(C) CITY: Juelich
(E) COUNTRY: Germany
(F) POSTAL CODE (ZIP): D-52428
(G) TELEPHONE: 02461 61-2843
(H) TELEFAX: 02461 61-2710
(A) NAME: BASF Aktiengesellschaft
(B) STREET: ZDX/A C6
(C) CITY: Ludwigshafen
(E) COUNTRY: Germany
(F) POSTAL CODE (ZIP): D-67056
(ii) TITLE OF INVENTION: UNICELLULAR OR MULTICELLULAR ORGANISMS
FOR PREPARING RIBOFLAVIN
(iii) NUMBER OF SEQUENCES: 2
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: Robic
(B) STREET: 55 St-Jacques
(C) CITY: Montréal
(D) STATE: QC
(E) COUNTRY: Canada
(F) ZIP: H2Y 3X2
(G) TELEPHONE: 514-987-6242
(H) TELEFAX: 514-845-7874
(v) COM~Ul~ READABLE FORM:
(A) MEDIUM TYPE: Disk 3.5" / 1.44 MB
(B) COM~U'1'~K: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: TXT ASCII
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER: 2,255,284
(B) FILING DATE: December 17, 1998
Page 1
CA 022~284 1999-03-22
....
2255284.seq
(vii) PRIOR APPLICATION DATA:
(A) APPLICATION NUMBER: DE 197 57 180.8
(B) FILING DATE: December 22, 1997
(A) APPLICATION NUMBER: DE 198 40 709.2
(B) FILING DATE: September 09, 1998
(2) INFORMATION FOR SEQ ID NO: 1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 2744 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: double
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iv) ANTI-SENSE: NO
(v) FRAGMENT TYPE: N-terminal
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Ashbya gossypii
(ix) FEATURE:
(A) NAME/KEY: CDS
(B) LOCATION:1232..2377
(C) IDENTIFICATION METHOD: experimental
(D) OTHER INFORMATION:/codon_start= 1232
/product= "Threonin-Aldolase"
/evidence- EXPERIMENTAL
/number= 1
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 1:
TTGCCATTAA TGACCGGGAG CCTGAAGGTG TGTGATGAAC AAGCCAGTCT TCCCCGCGCG 60
TCGCCAACTG CTCGTCATAT AATCCCGGAA AAGCTCGCAT TAGGTGAAAT TTTTCTTAGG 120
AATTACATCT GCTACTGACA AAACTAAGTA AAAGCTCCGA TAGGTAGCCG TGCTGCCGAG 180
CACCTGCCTA ATACACGCAG GCGCCATACA CTATTTAAGC ACAATGTTAT CGCCCCGCAG 240
CTTGAGGTAT TCCTGGTCGA TGCCAGGTGT CATAGGCTTG ATCACCAGCG AGTAGACCTC 300
Page 2
CA 022~284 1999-03-22
2255284.seq
ACTATTGTAG AAGCGCAGCC CGTTGCTGGG GGACTTGTAG CGCGCCTTGA GCCCCGTGAT 360
GTCGCAGTAG CGTTTCACGG GATACTGCGA TGGTGGCGCC TGAATGTTGA AGTATGTCAG 420
CTTCGTGCGC CCTGCGTCAC GCCCGGCTTC CGACTGTGCC TCTGTCGTGA GCCGTTTCCA 480
CTCGTCTGTC AGAAGCTGAC GTGTCGGCTT GTGGCGGCGC GTGGGTTTCT TCCACGTGGG 540
CGACTTGAAG TCGCTACGAC TGGTATCATT ACGTGCTGCA ATCGCTCGGA GGTTCTCCAT 600
CTGGGGTCCA CGGTCGCTCG TTGATCTGTC TATCTCGAAA TCCCTGCCCA GATGTACTCC 660
CATGTTATCA CGTGACCACA CGCCGTTTTC GTGTGTAGTG ATGCAGATGG TTCTAGAGCA 720
TCACGTGGCT TACATAGCTT TGTTACATAA TCGATTTTCC GCAGGAGCGT TACGTCCAAC 780
GGTCGTTCTG TGCCAAAAGC AACAACTGAG CGTCAGGCGG CCGTCTCCCC AGACACGCTC 840
CGCCCCAAAC TGAGCTCCAC GCGGCCTTCT GTCCGAGTTA AGTTCCTCCC CGCTCGTCAG 900
CACGGGGTCT TTCGTCGCCT ATCCTCCTGC AGCGTTCGCT ACTGCAGATC GTGAGCAGTG 960
GCACCCGCGA CCAAAAAAAG AAATTATGTT CCTTACGCAA GGAATATGCC TCGCGCCATG 1020
CCATCGCAAA GAGTGATGCC GCAGAGGTTG CTTCTGCGAG GCAACTCCTG GGCAATAGGG 1080
TGGAAAATTC AGCTTGGGCT TATATAAAAG AAACCGTTCG AGCTCGTCGG AGCCAGGTGG 1140
AAAATTTTTC GTAACGTAGG TAGAGGTTAT AGTTAGCGTC AGTCTCTTTT CTGCCAAGCT 1200
GCTACAGTTG ACTACAAGTA ACAAACCCAG G ATG AAT CAG GAT ATG GAA CTA 1252
Met Asn Gln Asp Met Glu Leu
CCA GAG GCG TAC ACG TCG GCT TCG AAC GAC TTC CGT TCG GAC ACG TTC 1300
Pro Glu Ala Tyr Thr Ser Ala Ser Asn Asp Phe Arg Ser Asp Thr Phe
10 15 20
ACC ACT CCA ACG CGC GAA ATG ATC GAG GCT GCG CTA ACG GCG ACC ATC 1348
Thr Thr Pro Thr Arg Glu Met Ile Glu Ala Ala Leu Thr Ala Thr Ile
25 30 35
GGT GAC GCC GTC TAC CAA GAG GAC ATC GAC ACG TTG AAG CTA GAA CAG 1396
Gly Asp Ala Val Tyr Gln Glu Asp Ile Asp Thr Leu Lys Leu Glu Gln
Page 3
CA 022~284 1999-03-22
,.. ~. ~,. .. .
2255284.seq
CAC GTC GCC AAG CTG GCC GGC ATG GAG GCC GGT ATG TTC TGC GTA TCT 1444
His Val Ala Lys Leu Ala Gly Met Glu Ala Gly Met Phe Cys Val Ser
60 65 70
GGT ACT TTG TCC AAC CAG ATT GCT TTG CGG ACC CAC CTA ACT CAG CCA 1492
Gly Thr Leu Ser Asn Gln Ile Ala Leu Arg Thr His Leu Thr Gln Pro
75 80 85
CCA TAT TCG ATT CTT TGC GAC TAC CGT GCG CAT GTG TAC ACG CAC GAG 1540
Pro Tyr Ser Ile Leu Cys Asp Tyr Arg Ala His Val Tyr Thr His Glu
9O 95 100
GCT GCG GGG TTG GCA ATT TTG TCC CAG GCC ATG GTG ACA CCT GTC ATT 1588
Ala Ala Gly Leu Ala Ile Leu Ser Gln Ala Met Val Thr Pro Val Ile
105 110 115
CCT TCC AAC GGC AAC TAC TTG ACT TTG GAA GAC ATC AAG AAG CAC TAC 1636
Pro Ser Asn Gly Asn Tyr Leu Thr Leu Glu Asp Ile Lys Lys His Tyr
120 125 130 135
ATT CCT GAT GAT GGC GAC ATC CAC GGT GCT CCA ACA AAG GTT ATC TCG 1684
Ile Pro Asp Asp Gly Asp Ile His Gly Ala Pro Thr Lys Val Ile Ser
140 145 150
TTG GAA AAC ACC TTG CAC GGT ATC ATT CAC CCA CTA GAG GAG CTT GTT 1732
Leu Glu Asn Thr Leu His Gly Ile Ile His Pro Leu Glu Glu Leu Val
155 160 165
CGG ATC AAG GCT TGG TGT ATG GAG AAC GAC CTC AGA CTA CAC TGC GAT 1780
Arg Ile Lys Ala Trp Cys Met Glu Asn Asp Leu Arg Leu His Cys Asp
170 175 180
GGT GCG AGA ATC TGG AAC GCG TCC GCA GAA TCC GGT GTG CCT CTA AAA 1828
Gly Ala Arg Ile Trp Asn Ala Ser Ala Glu Ser Gly Val Pro Leu Lys
185 190 195
CAG TAC GGA GAG CTA TTC GAC TCC ATT TCC ATC TGC TTG TCC AAG TCC 1876
Gln Tyr Gly Glu Leu Phe Asp Ser Ile Ser Ile Cys Leu Ser Lys Ser
200 205 210 215
ATG GGT GCC CCA ATG GGC TCC ATT CTC GTC GGG TCG CAC AAG TTC ATA 1924
Met Gly Ala Pro Met Gly Ser Ile Leu Val Gly Ser His Lys Phe Ile
220 225 230
Page 4
CA 022~284 1999-03-22
2255284.seq
AAG AAG GCG AAC CAC TTC AGA AAG CAG CAA GGT GGT GGT GTC AGA CAG 1972
Lys Lys Ala Asn His Phe Arg Lys Gln Gln Gly Gly Gly Val Arg Gln
235 240 245
TCT GGT ATG ATG TGC AAG ATG GCG ATG GTG GCT ATC CAG GGT GAC TGG 2020
Ser Gly Met Met Cys Lys Met Ala Met Val Ala Ile Gln Gly Asp Trp
250 255 260
AAG GGC AAG ATG AGG CGT TCG CAC AGA ATG GCT CAC GAG CTG GCC AGA 2068
Lys Gly Lys Met Arg Arg Ser His Arg Met Ala His Glu Leu Ala Arg
265 270 275
TTT TGC GCA GAG CAC GGC ATC CCA TTG GAG TCG CCT GCT GAC ACC AAC 2116
Phe Cys Ala Glu His Gly Ile Pro Leu Glu Ser Pro Ala Asp Thr Asn
280 285 290 295
TTT GTC TTT TTG GAC TTG CAG AAG AGC AAG ATG AAC CCT GAC GTG CTC 2164
Phe Val Phe Leu Asp Leu Gln Lys Ser Lys Met Asn Pro Asp Val Leu
300 305 310
GTC AAG AAG AGT TTG AAG TAC GGC TGC AAG CTA ATG GGC GGG CGT GTC 2212
Val Lys Lys Ser Leu Lys Tyr Gly Cys Lys Leu Met Gly Gly Arg Val
315 320 325
TCC TTC CAC TAC CAG ATA TCT GAG GAG TCC CTT GAG AAG ATC AAG CAG 2260
Ser Phe His Tyr Gln Ile Ser Glu Glu Ser Leu Glu Lys Ile Lys Gln
330 335 340
GCC ATC CTA GAG GCG TTC GAG TAC TCG AAG AAG AAC CCT TAC GAT GAA 2308
Ala Ile Leu Glu Ala Phe Glu Tyr Ser Lys Lys Asn Pro Tyr Asp Glu
345 350 355
AAC GGC CCC ACG AAG ATC TAC AGA AGT GAG TCC GCT GAC GCT GTG GGT 2356
Asn Gly Pro Thr Lys Ile Tyr Arg Ser Glu Ser Ala Asp Ala Val Gly
360 365 370 375
GAG ATC AAG ACC TAC AAG TAT TAAGGGATTT CGATGATGAC ATGAAAAATT 2407
Glu Ile Lys Thr Tyr Lys Tyr
380
ACATATTGGC ACGGCATAGG CATTGGGTAA TATTAAGCAT ATGGTTGAGA TGAATTACTG 2467
TTCGGGTACC GGTATTTCCA AAGTGCTGTC GACTTTTGCA AGAGATGGCT ATGAATGGGG 2527
Page 5
CA 022~284 1999-03-22
. .
2255284.seq
CACGCTCCAT CACCTCTCTG CGAGCCGGAC TCAGCATTAT ATCCATCTCA AAACCTAATA 2587
TCA~ATGGGA TTGTGGTGCG CAGTACATGC GCAGTGCTGC ACATTTGAGG ATCAATGGGT 2647
TTTTCCAGGC ACTGCCTGGG TCACTCACCC TATTGCGGAG GGACTAGTAG CTCTACCATT 2707
CTGAGCTGAC TAAAATGTTT GATTCTTTTG GTACTTA 2744
(2) INFORMATION FOR SEQ ID NO: 2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 382 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 2:
Met Asn Gln Asp Met Glu Leu Pro Glu Ala Tyr Thr Ser Ala Ser Asn
1 5 10 15
~sp Phe Arg Ser Asp Thr Phe Thr Thr Pro Thr Arg Glu Met Ile Glu
Ala Ala Leu Thr Ala Thr Ile Gly Asp Ala Val Tyr Gln Glu Asp Ile
Asp Thr Leu Lys Leu Glu Gln His Val Ala Lys Leu Ala Gly Met Glu
Ala Gly Met Phe Cys Val Ser Gly Thr Leu Ser Asn Gln Ile Ala Leu
~rg Thr His Leu Thr Gln Pro Pro Tyr Ser Ile Leu Cys Asp Tyr Arg
~la His Val Tyr Thr His Glu Ala Ala Gly Leu Ala Ile Leu Ser Gln
100 105 110
Ala Met Val Thr Pro Val Ile Pro Ser Asn Gly Asn Tyr Leu Thr Leu
115 120 125
Glu Asp Ile Lys Lys His Tyr Ile Pro Asp Asp Gly Asp Ile His Gly
Page 6
CA 022~284 1999-03-22
2255284. seq
-
130 135 140
Ala Pro Thr Lys Val Ile Ser Leu Glu Asn Thr Leu HiS Gly Ile Ile
145 150 155 160
~iS Pro Leu Glu Glu Leu Val Arg Ile Lys Ala Trp Cys Met Glu Asn
165 170 175
~sp Leu Arg Leu HiS Cys Asp Gly Ala Arg Ile Trp Asn Ala Ser Ala
180 185 190
Glu Ser Gly Val Pro Leu Lys Gln Tyr Gly Glu Leu Phe Asp Ser Ile
195 200 205
Ser Ile Cys Leu Ser Lys Ser Met Gly Ala Pro Met Gly Ser Ile Leu
210 215 220
Val Gly Ser HiS Lys Phe Ile Lys Lys Ala Asn His Phe Arg Lys Gln
225 230 235 240
~ln Gly Gly Gly Val Arg Gln Ser Gly Met Met Cys Lys Met Ala Met
245 250 255
~al Ala Ile Gln Gly Asp Trp Lys Gly Lys Met Arg Arg Ser His Arg
260 265 270
Met Ala His Glu Leu Ala Arg Phe Cys Ala Glu His Gly Ile Pro Leu
275 280 285
Glu Ser Pro Ala Asp Thr Asn Phe Val Phe Leu Asp Leu Gln Lys Ser
290 295 300
Lys Met Asn Pro Asp Val Leu Val Lys Lys Ser Leu Lys Tyr Gly Cys
305 310 315 320
~ys Leu Met Gly Gly Arg Val Ser Phe His Tyr Gln Ile Ser Glu Glu
325 330 335
~er Leu Glu Lys Ile Lys Gln Ala Ile Leu Glu Ala Phe Glu Tyr Ser
340 345 350
Lys Lys Asn Pro Tyr Asp Glu Asn Gly Pro Thr Lys Ile Tyr Arg Ser
355 360 365
Glu Ser Ala Asp Ala Val Gly Glu Ile Lys Thr Tyr Lys Tyr
Page 7
CA 022~284 1999-03-22
2255284 . seq
370 375 380
Page 8
CA 02255284 1999-03-22