Note: Descriptions are shown in the official language in which they were submitted.
CA 02255287 1998-12-11
3
BACKGROUND OF THE INVENTION
This invention relates generally to the abatement and mitigation of carbon
dioxide emissions from
power generation, incineration, heating and transportation sectors which use
fossil fuels as the
energy source and during the process emit COZ in natural atmosphere. Tn
particular, it relates
to a novel and cost-effective method of capturing carbon dioxide at source
from flue gas streams
and of utilizing the captured carbon dioxide by transforming it into
economically viable and
environmentally friendly commodity.
Anthropogenic emissions of carbon dioxide arising mainly due to combustion of
fossil fuels,
resulting in COz emissions of about 22 billion tons per year, and
deforestation (about 7 billion
tons per year) is causing a steady increase in global atmospheric
concentration of carbon dioxide.
It is predicted that this increase of carbon dioxide in the atmosphere will
cause the earth's
temperature to rise by an amount larger than that occurred over the past 9,000
years. This global
warming effect is likely to bring about significant changes in local climate
conditions leading to
possible loss of arable land, rise in sea level with associated coastal
flooding, and increase in
ground-water salinity. It may also exacerbate the photochemical smog problem.
Thus, there is
a need for early action to develop methods for abatement and mitigation of
emissions of carbon
dioxide, which is the principal Green House Gas (GHG) contributing to global
warming. This
need has been recognized in the recent global conference on climate change
held in Kyoto
(December, 1997) in which various countries have agreed to significant
reduction of greenhouse
gas emissions in the very near future. The U.S. alone is responsible for
almost quarter of global
carbon dioxide emissions amounting to about 20 tons per year per person. At
the present time,
it is of paramount importance to develop technology for net COZ mitigration in
order to combat
emissions of Green House Gases (GHG).
Possible Strategies for Mitigation of COZ Emissions
Many possible strategies to reduce the build-up of COZ levels in the
atmosphere are discussed
in the literature (P. Freund, Waste Management, 17, 281 (1997). These include:
i) improvements
in energy efficiency and fuel switching; ii) introduction of renewable sources
of energy or nuclear
power to displace fossil fuels; iii) COZ capture and storage; and iv) COZ
capture and utilization
by making chemicals.
CA 02255287 1998-12-11
4
Improvements in Energy Efficiency and Fuel Switching
The increase of energy efficiency of primary energy generating system and/or
switching to a fuel
with lower carbon to hydrogen ratio (for example, coal to natural gas) can
provide useful
reductions in COZ emissions by producing lesser amounts of COz for the same
energy output.
Current power generating systems operate at 30-40% efficiency. Increasing
energy efficiency,
however, requires substantial capital investment. Also there are practical
limits to achievable
efficiencies at reasonable costs. Restricting the usage to a specific type of
fossil fuel is also not
a viable option on a long-term basis due to availability considerations. In
any case, energy
efficiency improvements or fuel switching to low carbon fuels can only offer
short-term solutions
because COz reductions by these means although would initially be substantial,
they will have
diminishing impact over long-range.
Introduction of renewable enemy or nuclear power
Switching to renewable energy or nuclear power can provide deep reductions.
However, it is to
be understood that the world is so much dependent on fossil fuels that it is
important that there
should also be a technology option which would permit continued use of fossil
fuels with much
less emissions of COZ..
Capture and Storage of COZ
The possibility of reduction of COZ emission by capture and storage of COZ
from flue gases is
also receiving considerable attention. According to this process, after the
combustion of fossil
fuels (for power generation, etc.), COZ is to be separated and recovered, and
then be stored for
a long time.
There is a wide range of possible COZ separation and recovery techniques: (i)
gas/liquid
scrubbing (adsorption) ; (ii) gas-solid adsorption ; (iii) cryogenic
fractionation ; and (iv)
membrane separation. Each of these capturing techniques has its own advantages
and
disadvantages. However, the most important point to take into account is that
the COZ capture,
regardless of the technique used, is an expensive process. Actual costs are
source and fuel
dependent. For example, in case of power generation, capturing is projected to
increase the cost
of electricity generation by at least 40 - 50% above current levels.
Several possible methods of long-term storage of the separated and recovered
COZ from flue gas
CA 02255287 1998-12-11
are being discussed. These methods include: i) ocean storage ; ii) underground
(depleted oil and
gas reservoirs) storage; and iii) growth of forest.
In nature the oceans and seas constitute important COZ sink and have large
storage capacities.
However, there are a number of uncertainties associated with this option such
as ecological
impact on the ocean environment, final fate of injected COZ or how long COZ
would remain in
the deep ocean. In other words, a full understanding of the complex chemical,
biological and
oceanographic factors has to be acquired before proposing such an option. Also
not all countries
have suitable access to deep oceans.
It is also possible to store large quantities of COZ in underground reservoirs
consisting of
exhausted oil or gas fields. The possible implications of this type of storage
(for example, due
to possible reaction between COZ and host rock in the underground reservoir)
are unknown.
Also, it remains to be seen if this storage can provide a long-term fixation
of COz.
Ocean and underground storage as discussed above, deals with possible means of
end-of pipe
removal of COz. As an alternative, removal of COZ from the atmosphere by
enhancing the take-
up by natural sinks, typically by growing forests, is also being discussed.
However, availability
of massive amount of land for such afforestation and reforestation and their
possible impact are
in question and delays in rate of utilization is inadmissibly long.
Utilization of COZ (Chemical Fixation)
The most preferred route to mitigate COZ emissions into the atmosphere is to
utilize the captured
COz to make chemical products in which there is a net reduction of COZ during
the product
formation and utilization. This approach is receiving considerable attention.
Various available
methods to recycle COz to produce fuels, fuel additives, or fuel precursors
such as syngas (CO/Hz
mixture), methanol, MTBE (methyl tert-butyl ether), DMC (dimethyl carbonate),
or DME
(dimethyl ether) do not necessarily result in COZ reduction. Some of these
processes are
commercially practiced and the technology is mature. In the context of
mitigating COZ
emissions, it should be noted that these processes are highly endothermic
requiring large energy
input in the product formation. If this energy is supplied by the combustion
of a carbon based
fossil fuel, then COZ would again be generated and emitted to the atmosphere,
which has to be
taken into consideration in the overall COZ mass balance. Edwards (see J.H.
Edwards, Catalysis
Today, 23,59 ( 1995)) critically analyzed overall COZ mass balance of many COz
based fuel
producing reactions and concluded that unless the energy (and, in some cases,
co-reactant
CA 02255287 1998-12-11
6
hydrogen) required for the COZ conversion process is derived from non-fossil
fuel sources, there
is little or no net reduction of CO, emissions in recycling COZ from existing
sources.
1t is evident that currently perceived strategies for abatement and mitigation
of carbon dioxide
as known to the art have numerous uncertainties and difficulties. One prospect
that could offer
significant advantages is the capture process provided it could be performed
economically without
additional fossil fuel consumption (i.e. net COZ reduction) and provided the
captured COZ could
be utilized for making environmentally friendly value added products so as to
provide an
economic incentive to industries world-wide to act voluntarily towards
reducing GHG (Green
House Gas).
It is therefore an object of this invention to provide a novel and alternative
cost-effective process
for capturing carbon dioxide emissions from stationary or mobile sources.
It is another object of this invention to provide a method of transforming
captured COZ to
valuable product. The captured COZ remains permanently fixed in this
transformed product, which
is thermally stable, mechanically strong, non-flammable and does not release
toxic gases.
SUMMARY OF THE INVENTION
According to this invention, the emission streams containing COZ are
chemically interacted with
the capturing agent of this invention forming a material hereinafter referred
to as "CHEM-COZ"
(chemically captured COz). CHEM-COZ is then directly transformed into articles
or bodies
having different potential applications, without producing COZ in the
transformation process.
These articles or bodies obtained by transforming the chemically captured COZ
(CHEM-COZ) into
value added products are, for convenience, hereinafter referred to as "CHEM-
COZ-P". The
method of this invention is superior to other currently considered methods of
reducing COZ
emissions involving chemical utilization for fuel because these other methods,
unlike the method
of this invention, generate COz in the product formation and utilization.
The process is given a trade name "CARBO-FIX".
BRIEF DESCRIPTION OF THE DRAWINGS
The objects, features and advantages of the present invention will become
apparent from the
following description reference being made to the following figures in which:
CA 02255287 1998-12-11
7
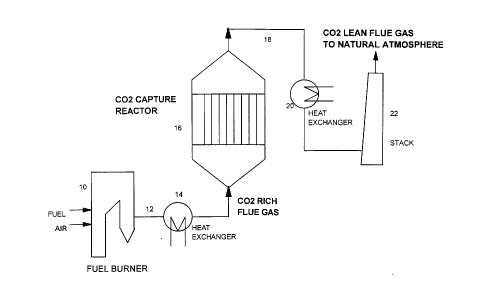
FIG. 1. is an illustration of a method of insitu capture of COz for
utilization in
accordance with one embodiment of the invention.
FIG. 2. is an illustration of a variation of the method of capture of COz.
FIG. 3. is an illustration of the preferred embodiment on the utilization of
the
captured COZ. ,
DESCRIPTION OF THE INVENTION
This invention relates to a novel process of capturing and utilization of COZ
from flue gas
streams in order to reduce COz emissions in the atmosphere. The process steps
include:
a) passing the flue gas through a reactor packed with a bed of COZ capturing
agent
of this invention; and
b) transforming chemically captured COZ (CHEM-COZ) formed in the capturing
step
(a) above into value added articles (CHEM-COZ P) by mixing it with a suitable
binding material and molding the mix in a pelleting press or in an extruder.
Preferred embodiment on capture of CO,
A first embodiment of the invention relates to a novel method of capturing COZ
from flue gas
stream by allowing the flue gas to pass through a reactor containing a bed of
capturing agent.
The said capturing agent according to this invention is selected from the
group consisting of
calcium hydroxide, magnesium hydroxide, iron hydroxide and combinations
thereof. The
capturing reaction is carried out at a temperature ranging from about 150 to
about 450° C. The
reaction is conveniently carried out at atmospheric pressure, although
elevated pressure may also
be used. The flue gas stream is passed through the bed of capturing agent at a
gas hourly space
velocity (GHSV) ranging from about 1,000 to 10,000 h-'. The capturing agent is
charged in the
reactor in the granular form, although pellet or honeycomb monolith forms may
also be used.
Applicants have found that the treatment of flue gas with the capturing agents
specified above
and according to the procedure described above can lead to substantial
reductions in carbon
dioxide emissions from the flue gas. The capturing process of this invention
can be applied to
the abatement and mitigation of the emission of carbon dioxide from different
types of power
generation power plants including those of pulverised fuel and flue gas
desulphurisation (PF-
FGD), natural gas fired combined cycle /gas turbine combined cycle (GTCC) and
integrated
gasification combined cycle (IGCC). It is also applicable to industrial
boilers, municipal waste
CA 02255287 1998-12-11
g
incinerators, cogenerators, heating and transportation sectors, and
miscellaneous industrial sources
of C02 emissions including mining, metallurgical, cement and fertilizer
production processes.
The following reactions represents the COZ capturing/removal pathways from the
flue gas when
Ca(OH)2 is used as the capturing agent:
Ca(OH)Z + COz CaCO, + Hz0
Ca(OH)z ~ Ca0 + H20
Ca0 + COz CaC03
According to one preferred aspect of the first embodiment, enormous supply of
calcium
hydroxide and magnesium hydroxide essential to accomplish large-scale capture
of COz from flue
gases can be obtained from the following naturally occurring sources: i)
chloride salts of calcium
and magnesium present in abundance in sea water; ii) fluorite/fluorspar (CaFZ)
mineral; and iii)
apatite/rock phosphate (impure Ca3(P04)Z mineral. Because these sources are
not carbonate salts
or minerals, synthesis of hydroxides of calcium or magnesium do not cause
emissions of COz.
The soluble chloride salts of calcium and magnesium are converted to
corresponding insoluble
hydroxides by hydrolysis reactions under alkaline conditions according to
known wet chemical
methods, which are then easily recovered from the aqueous medium by
filtration. Fluorite and
apatite minerals can also be converted to calcium hydroxide by conventional
wet chemical
methods.
According to another preferred aspect of the first embodiment, dissolved
chloride salts of calcium
and magnesium from the sea water are recovered by evaporation using solar
energy. The use of
natural solar energy avoids the use of fossil fuel based energy sources and
therefore not only
avoids generation of carbon dioxide but also offers substantial cost savings.
According to another preferred aspect of the first embodiment, enormous supply
of iron
hydroxide (one of the capturing agent of this invention) essential to
accomplish large-scale
capture of COZ from the flue gas according to the method of this invention can
be obtained from
haematite (Fe20~) which is the most common ore of iron, the fourth most
abundant element (by
weight) making up the crust of the earth. Haematite ore can be converted to
iron hydroxide by
conventional wet chemical methods.
In the insitu capture process which is schematically illustrated in Fig. 1,
the COz rich flue gas
produced in the fuel burner 10 by combustion of coal or natural gas fuel and
discharged via line
CA 02255287 1998-12-11
9
12 is passed through a heat exchanger 14 for recovering most of the heat
generated by the fuel
combustion and also for lowering the temperature of flue gas stream to about
150 - 450° C, and
then sent to the COz capture reactor 16 containing a fiixed bed of capturing
agent chosen from
the group consisting of Ca(OH)z, Mg(OH)2 and Fe(OH)z or a combination thereof.
As COz rich
flue gas passes through the capturing agent bed maintained at a temperature of
150 - 450° C, COZ
of the flue gas reacts with the capturing agent forming CHEM-COZ and remains
permanently
fixed to it. Thus the flue-gas exiting the capturing reactor contains
substantially lesser amount
of COz than the flue gas entering the reactor. The effluent stream from the
capturing reactor
discharged via line 18 is passed through a heat exchanger 20 for recovering
useful heat and then
through a stack 22 before being vented at a regulatory height to the natural
atmosphere. When
the bed of capturing agent becomes fully saturated with C02, it is removed
from the reactor and
a fresh batch of capturing agent is loaded into the reactor.
According to a preferred variation of the first embodiment, if desired, COZ
from the flue gas
stream can first be separated by a membrane process, and then captured with
the capturing agents
of this invention. For use in the process of this invention, the package of
membranes (fabricated
from suitable glassy and rubbery polymers) is preferrably placed in the
membrane separator in
a plate-and-frame configuration, as illustrated by way of example in Figure 2.
For gas
separations, traditionally, membranes are used in tubular, spiral-wound and
hollow-fiber forms.
All of these forms, however, have drawbacks. The tubular form has a very low
membrane
surface area per unit packing volume and consequently would produce a flux
(flux is the rate of
permeation through the membrane expressed, for example, in cubic metres of gas
per hour) which
is far too low to be of practical significance. The hollow-fiber and spiral-
wound forms of
membrane offer the desired high membrane surface areas, but create large
pressure drops in the
bulk flow directions, thus requiring high inlet pressure and low flow velocity
for their operation.
Consequently, membranes of hollow-fiber and spiral-wound configurations are
not suitable for
low pressure and high velocity flue gases produced by power plants, industrial
boilers, etc. On
the other hand, the conceptual plate-and-frame membrane module of this
invention would offer
several advantages: it is simple and compact; pressure drop would be
relatively low; there would
be no need for flue gas pressurization; it would allow treatment of flue gases
of high flow
velocities; it would maintain structural integrity against high pressure; and
it would allow easy
replacement of fouled membrane elements.
As illustrated in Fig. 2, the conceptual membrane separator consists of
housing 1 having inlet 2
for introducing feed gas mixture (flue gas). The membrane package consisting
of several flat
symmetric or composite gas-separation membranes 3 (a symmetric or composite
membrane
CA 02255287 1998-12-11
consists of a thin separating layer supported on thick porous substrate)
having holes 4 in the
centre are placed in the housing 1. The central holes 4 coincide with the
collector channel 5
which, in turn, is connected with outlet 6. Carbon dioxide of flue gas
preferentially permeating
through gas-separation membranes 3 is collected in collector channel 5 and
exits via outlet 6.
Between each pair of membranes 3 are gas-impervious diaphragms 7 with the
central holes 8 that
coincide with collector channel 5. Alternating membranes and diaphragms are
packed with strips
9 in the housing 1. The retentate exits via second outlet 10.
The separated COz exiting the membrane separator of preferred plate-and-frame
configuration is
sent to the capture reactor where it reacts with the capturing agent of this
invention forming
CHEM-COz, which is then utilized to produce valuable chemical commodities
(CHEM-COZ-P)
as described below under second main embodiment of this invention.
Preferred embodiment on utilization of captured COZ
A second main embodiment of the invention relates to a method of utilization
of the captured
COz. It involves the transformation of the CHEM-COZ (chemically captured COZ),
as defined
earlier, into CHEM-COZ-P, which as defined earlier, are mechanically strong
articles of different
shapes and sizes, having numerous potential applications. The method, as
illustrated in
accompanying Fig. 3, consists of following steps:
(a) grinding CHEM-COZ into a fine powder;
(b) mixing the powder of step (a), without loss of CO2, with an aqueous
dispersion
or aqueous solution of a suitable inorganic or organic binding material;
(c) partially drying the powder-binder mix to yield a plastic-like material of
desired
consistency;
(d) fabricating mechanically strong shaped bodies either by compression
molding of
the material of step (c) in a pelleting press or by extrusion molding with a
suitable
extruder; and
(e) totally drying the pellets or extrudates at a temperature of about
80° C to about
140° C in air atmosphere.
Applicants have found quite unexpectedly that binding and consolidation of
CHEM-COZ
materials, according to the procedure described above, produce articles of
different shapes and
sizes (CHEM-COZ-P), which exhibit a number of desirable properties: good
mechanical strength,
CA 02255287 1998-12-11
non-flammability and fire resistibility, good thermal stability, no tendency
to re-release COZ upon
storage, and no emissions of toxic gases or vapours upon storage. These
attributes make CHEM-
COz-P suitable for application in diverse areas such as household decorations,
appliances,
business and office machines, scientific instruments, electronic equipments,
etc.
In one preferred aspect of the second main embodiment of the invention, the
binding material
used in step (b) above is selected from the group consisting of inorganic
binders such as
bentonite clay, attapulgite clay, kaolin clay, sepiolite clay, silica sol and
combinations thereof,
or from the group consisting of thermoplastic or thermosetting organic
polymers/resins such as
polyvinyl alcohol, polypropylene, polyethylene and combinations thereof, or
from the group
consisting of gellable organic binders such as methylcellulose,
ethylcellulose,
hydroxymethylcellulse, hydroxyethylcellulose, hydroxybutylcellulose,
carboxymethylcellulose and
mixtures thereof. The binding material used in step (b) can also be an
admixture of an inorganic
binder chosen from the substances specified above and an organic binder chosen
from the
substances specified above.
The preferred binders to use in the practice of the present invention are clay
materials such as
bentonite, kaolin, attapulgite and sepiolite. They are chosen because they
offer a number of
advantages: i) they exhibit excellent binding and agglomerating property; ii)
they are naturally
occuring substances available in abundance worldwide; iii) they exhibit high
thermal stability
being stable even at a temperature of 500° C; and iv) they do not
release toxic gases or vapours
upon storage or exposure to elevated temperature.
In another aspect of the second main embodiment, in making value-added
articles with CHEM-
COZ (e.g. CaCO, resulting from capturing COZ with Ca(OH)2), the binder is used
in the amount
ranging from about 5% to about 30% based on the weight of CHEM-COZ. The higher
the binder
content, the greater is the mechanical strength of the formed shaped article
(CHEM-COz P).
The following non-limiting examples are provided to further illustrate the
invention.
FYAMP1.F 1
Capturing of Carbon Dioxide
A sample of Ca(OH)Z, in the form of a fine powder, was moistened with water
and a paste made
of. The paste was dried in an oven at a temperature of about 100° C to
form hardened lumps,
which were then broken to form granules of Ca(OH)2.
CA 02255287 1998-12-11
12
A glass microreactor was packed with 1.0 gram of the granular Ca(OH)2 prepared
as above and
placed in a continuous flow system. A feed gas mixture consisting of, by
volume, 30% CO2, 5%
OZ and 65% NZ was continuously passed at a flow rate of 27.5 mL/min (STP)
through the
Ca(OH)Z bed maintained at the desired reaction temperature. The reactor was
maintained at 1
atmosphere. As the feed mixture passes over the bed, Ca(OH)Z captures COz from
the mixture
by chemically reacting with it and is converted to CHEM-CO2, which in the
present instance is
CaC03. The reaction was carried out at 200, 300, 400 and 450° C,
starting with a new sample
of the capturing agent, Ca(OH)2, at each temperature. At each temperature, the
capturing reaction
was allowed to proceed for a definite period of time.
At the end of each experiment, the reactor was cooled to room temperature, the
resultant
CHEM-COZ removed and analyzed for its carbon content using a CHN elemental
micro-analysis
apparatus. From the carbon content of the product and the total volume of COZ
passed through
the solid bed over the reaction period, and assuming that the capture reaction
stoichiometry is
Ca(OH)z + COZ -~ CaCO, + HzO, the percentage of COz captured (or removed) from
the feed gas
mixture and the percentage of Ca(OH)Z converted to CaC03 due to the capturing
reaction were
calculated. The results are summarized in Table 1.
TABLE 1
CO~capturin~ by Ca(OH),
Reaction Contact Carbon in Ca(OH)z COz removed
temperature Time the converted from feed
(C) Product (%) mixture (%)
(%)
200 2 sec 3.573 29.8 1.7
300 2 sec 4.808 40.0 2.6
400 2 sec 8.232 69.0 19.3
450 2 sec 9.940 82.8 24.2
It is apparent from Table 1 that at temperatures of 400° C and above,
significant removal of COZ
from feed gas mixture is achieved. By increasing the contact time, the COz
removal can be
further increased and maximized. It may be noted that in real situations the
total removal of COZ
is not necessary in combating GHG emissions, nor is it desirable from
ecological considerations.
CA 02255287 1998-12-11
13
EXAMPLE 2
Capturing of Carbon Dioxide
The capturing agent for the cases included in this example was granular
Ca(OH)2 prepared as
described in Example 1.
The reaction was carried out in a stainless steel batch reactor, having an
internal volume of 75
mL. The reactor had provisions for filling it with the feed gas mixture and
for withdrawal of the
gas sample for analysis by gas chromatography. A weighed amount of sample
(0.75 - 1.5 g) of
granular Ca(OH)Z was loaded in the reactor. The reactor was purged and then
filled with a feed
gas mixture, consisting of 30% COZ, 5% Oz, and 65% N2, to a pressure of 25
psi. A sample of
the feed gas mixture (0.2 mL) was withdrawn from the reactor with a gas-tight
syringe and
analyzed by gas chromatography. The reactor was rapidly heated to the desired
reaction
temperature and the capturing of COZ from the feed gas mixture by the
capturing agent, Ca(OH)2,
was allowed to proceed. Samples of reaction gas mixture (0.2 mL of sample each
time) were
withdrawn at different reaction times and analyzed by gas chromatography. The
percentage of
COz removed from the feed mixture due to the capturing reaction, as a function
of reaction time,
was calculated from gas chromatographic responses of the feed gas mixture and
reaction gas
mixture using Nz present in the feed as internal standard. The reaction was
earned out at 200
and 250" C, starting with a new charge of Ca(OH)2 at each temperature. The
results are
summarized in Table 2.
TABLE 2
Capturing of Carbon dioxide
(CHEM-COZ Formation)
Reaction temperatureReaction time, t Percent COz
(C) (min) Removal from feed
250 10 21.3
60 99.5
90 99.7
200 20 49.7
40 73.5
75 97.5
note 0.75 g of Ca(OH)Z was used at 250° C tests and 1.5 g of Ca(OH)z
was used at
200° C tests.
It is apparent from Table 2 that the treatment of COZ - containing gas mixture
with Ca(OH)2 as
capturing agent in a batch reactor can over longer reaction times accomplish
nearly 100% COz
CA 02255287 1998-12-11
14
removal at temperatures of 200° C and above.
EXAMPLE 3
Transformation of CHEM-CO~ into Value-added Commodity
(a) 2.5 grams of polyvinyl alcohol (PVA) binder was dissolved in about 50 mL
water. 50 grams
of ground CaCO, (CHEM-COZ obtained by capturing of COZ with Ca(OH)Z as
capturing agent)
was added to the PVA solution and thoroughly mixed to form a slurry. The
slurry was then
partially dried by heating at about 70 - 85° C with occasional stirnng
to form a plastic-like mass
of desired consistency (corresponding to a moisture content of about 10-20%
based on the weight
of CaC03). The material was thereafter formed into several pieces of
cylindrical shaped pellet
of 20 mm diameter and 1 S-25 mm length by compression molding in a cylindrical
die by means
of a hydraulic press. Pellets were then dried at about 150° C until
constant weight. The final
product (CHEM-COz-P) was mechanically strong blocks of white appearance. For
convenience,
this type of CHEM-COz-P product is referred to as "CaC03-PVA bodies" in
Example 5 and
Example 7 below.
(b) An aqueous suspension of 10 grams of bentonite clay in about 100 mL water
was prepared
by adding the clay to the water in steps while continuously stirring. 40 grams
of CaC03 (CHEM-
COZ obtained by the capturing of COz with Ca(OH)z as capturing agent) was
added to the clay
dispersion and blended to form a slurry. The slurry was then partially dried
by heating at about
70-85" C with occasional stirring to form a plastic-like mass of desired
consistency
(corresponding to a moisture content of about 10-20% based on the weight of
CaC03). The
material was thereafter formed into several pieces of cylindrical shaped
pellet of 20 mm diameter
and 10-20 mm length by compression molding in a cylindrical die by means of a
hydraluic press.
Pellets were then dried at about 150" C until constant weight. The final
product (CHEM-COz P)
was mechanically strong blocks of white appearance. For convenience, this type
of CHEM-COz-
P product is referred to as "CaCO; - bentonite bodies" in Example 4 and
Example 6 below. The
appearance could be changed by adding varying colouring agents as desired for
decorative
articles of commercial value (see example (c) below).
(c) 5 grams of polyvinyl alcohol binder was dissolved in about 50 mL water. To
this solution
were added 40 grams of CaC03 (CHEM-COz obtained by the capturing of COZ with
Ca(OH)2 as
capturing agent) and 5 grams of Fez03, and thoroughly mixed to form a slurry.
The slurry was
then partially dried by heating at about 70-80° C with occasional
stirnng to form a plastic-like
mass of desired consistency (corresponding to a moisture content of about 10 -
20% based on
CA 02255287 1998-12-11
the weight of CaC03). The material was thereafter formed into several pieces
of cylindrical
shaped pellet of 20 mm diameter and 10-20 mm length by compressing molding in
a cylindrical
die by means of a hydraulic press. Pellets were then dried at about
150° C until constant weight.
The final product (CHEM-COz P) was red coloured mechanically strong blocks.
EXAMPLE 4
Agin Test of CHEM CO; P (obtained with bentonite binder)
To test whether the CaC03 - bentonite bodies would retain the captured COZ
over extended
periods (as is desirable for any meaningful mitigation and utilization
process), these bodies were
subjected to accelerated aging by heat treatment at various temperatures.
The dried pellet sample of known weight was heat treated in a muffle furnace
at the desired
aging temperature in air for 3 hours. It was then cooled to room temperature,
weighed and
analyzed for carbon content (using CHN elemental microanalyzer). The aging or
thermal stability
tests were performed at 300, 400 and 500° C, using a separate pellet
sample for each temperature.
The results are reported in Table 3.
Table 3
A~in~ Tests for CHEM-CO,-P (obtained with bentonite binder)
Aging Carbon Pellet weight % Weight loss,
W-W
Temperature content [
y,C) (%~ Initial, After ~ * 100
Wo Wo
(g) ag~ng~W
(g)
Drying at 150"C9.800 - - -
300 9.705 10.715 10.626 0.83
400 9.611 9.776 9.695 0.82
500 9.780 9.046 8.967 0.87
As can be seen in Table 3, carbon content and weight of the CaC03 - bentonite
bodies are
virtually unaffected by aging at different temperatures, which indicate that
these bodies would
not re-release the captured COz.
CA 02255287 1998-12-11
16
FXAMP1.F G
A~in~ Test of CHEM-CO; P (obtained with PVA binder)
To test whether the CaC03 -PVA bodies would retain the captured COZ over
extended periods
(as is desirable for a meaningful COZ mitigation and utilization process),
these bodies were
subjected to accelerated aging by heat treatment at various temperatures. ,
The dried pellet sample of known weight was heat treated in a muffle furnace
at the desired
aging temperature for 3 hours. It was then cooled, weighed and analyzed for
carbon content
(using a CHN elemental microanalyzer). The aging or thermal stability tests
were performed at
300, 400 and 500" C, using a separate pellet sample for each temperature. The
results are
reported in Table 4.
Table 4
Aging Tests for CHEM-CO~-P (obtained with PVA binder)
Aging Carbon Pellet weight % Weight loss,
T t W
t t W
empera con Initial, After o-
ure en Wo ~ Wo l 100
C) (%~
(g) aging,W
(g)
Drying at 13.744 - - -
150"C
300 12.345 6.666 6.419 3.7
400 11.812 7.240 6.848 5.4
500 11.863 6.314 5.945 5.8
As can be seen in Table 4, carbon contents of 400° C and 500° C
aged samples are the same
within experimental errors, indicating that, once captured, COZ would be
permanently held by
these bodies. Losses of carbon content and sample weight observed upon
300° C and 400° C
aging are most likely due to partial loss of PVA binder at these temperatures.
EXAMPLE 6
Mechanical Strength Test of CHEM-CO=-P (obtained with bentonite binder)
In order to find wide range of applications, it is desirable for CaC03 -
bentonite bodies to have
sufficient mechanical strength. This test consists of measuring the axial
crushing strength of
cylindrical shaped bodies in the dried state as well as after having been
subjected to hot aging
CA 02255287 1998-12-11
17
at different temperatures. Strengths were measured in a screw-thread press
connected to an
electronic balance. The body was subjected to increasing pressure until it
began to disintegrate.
The results are summarized in Table S.
TABLE 5
Mechanical Strength Test of CHEM-COZ-P (obtained with bentonite binder) ,
Aging Temperature Crushing Strength
(C) (kg/cmZ)
As - dried ( 150C) 7.3
300 6.5
400 6.4
500 6.4
As can be seen from Table S, hot aging of bodies does not significantly effect
their mechanical
strength. It may be noted that the crushing strength of CaC03 bodies formed
without any binder
was found to be about 3 kg/cm2. Thus, it is evident that the addition of
bentonite binder
significantly reinforced the bodies so formed.
TiYAMPI.F '1
Mechanical Strength Test of CHEM-CO; P (obtained with PVA binder)
In order to find wide range of application, it is desirable for CaC03-PVA
bodies to have
sufficient mechanical strength. This test consists of measuring the axial
crushing strength of
cylindrical shaped bodies in the dried state as well as after being subjected
to hot aging at
different temperatures. Strengths were measured in a screw-thread press
connected to an
electronic balance. The body was subjected to increasing pressure until it
began to disintegrate.
The results are summarized in Table 6.
CA 02255287 1998-12-11
18,
TABLE 6
Mechanical Strength Test of CHEM-CO,-P (obtained with PVA binder)
Aging Temperature Crushing Strength
(C) (k~cmz)
As - dried ( 150C) 10.2
300 S.0
400 4.8
500 4.8
As can be seen from Table 6, hot aging of bodies significantly lowered their
mechanical strength.
This is due to partial loss of PVA binder from the bodies upon exposure to
high temperatures.
It may be noted that the crushing strength of CaCO, bodies formed without any
binder was found
to be about 3 kg/cmz. On the other hand, the crushing strength of dried CaC03-
PVA bodies is
greater than 10 kg/cm~, indicating that binding with PVA causes a significant
improvement of
mechanical strength of the bodies so formed.
While the invention has been described and illustrated herein by references to
various specific
materials, procedures and examples, it is understood that the invention is not
restricted to the
particular materials and procedures selected for that purpose. Numerous
variations of such details
can be employed, as will be appreciated by those skilled in the art.
We claim:
1. A novel process of c~turing and utilization of COZ from flue gas streams as
a measure
to reduce COZ emissions in~t~e atmosphere, which comprises of the following
steps:
(a) passing the flue gas through a reacte.~packed with a bed of COZ capturing
agent
of this invention; and
(b) transforming the chemically captured COZ material "CH~COz" formed in step
(a) into value-added shaped articles "CHEM-COZ P" by mixinglt~ith a suitable
binding material and molding the mix in a pelleting press or in an