Note: Descriptions are shown in the official language in which they were submitted.
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97/02683
1
BAR COMPOSITION COMPRT_SING COPOLYMER MILDNESS ACTIVES
The present invention relates to fatty acid soap bar
compositions (i.e., bars in which fatty acid soaps are used
as the primary detergent, and synthetic surfactants, such as
anionic surfactants and amphoteric surfactants, are used as
co-surfactants).
BACKGROUND
Soap has traditionally been used as a skin cleanser. It
has many advantages (e. g., inexpensive, easy to manufacture
into bars, having good lathering properties), but it can
irritate the skin due to its harsh nature. A number of
strategies have been developed in the art to amelioriate the
harshness of soap cleansing bars.
One approach is to replace some or all of the soap with
a synthetic surfactant. The u.se of synthetic surfactants can
introduce other problems. For example, anionic surfactants
may still be harsh. Non-ionic surfactants generally do not
generate creamy thick lather as do soap or anionic
surfactants. Both non-Tonics and amphoterics can be sticky
and lead to difficulty in standard processing steps such as
extrusion or stamping.
Another approach to reduce the harshness of personal
cleansing bars is to dilute the cleansing agents of the bar
formulation with a filler or inert ingredient, e.g. starches
or fatty acids. Incorporation of some filler materials can
° also lead to processing difficulties, and this approach only
provides a modest improvement in mildness at best.
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97/02683
2
Unexpectedly, applicants have found that the use of
relatively low levels of specific nonionic polymeric
surfactants can be used to obtain these goals. That is, at
levels no higher than 25% by wt. of the bar composition, the
polymers provide enhanced mildness without sacrificing
processability or lather, and have the added benefit of
reducing mushing. While not wishing to be bound by theory,
it is believed that the copolymers may be interacting with
fatty acid soap and anionic surfactant (if present) to form
polymer-surfactant complexes.
The use of polyoxyethylene polyoxypropylene (EO-PO)
nonionic polymeric surfactants in bar compositions per se is
not new.
U.S. Patent No. 3,312,627 to Hooker, for example,
teaches bars substantially free of anionic detergents
comprising 0 to 70o by weight EO-PO polymer, polyethylene
glycol (PEG) or derivatives of these compounds as base; and
10 to 70~ of a nonionic lathering component. In order to
give these bars more "soap-like" characteristics, the
reference contemplates use of 100-800 lithium soap. It is
clear that use of lithium soap is unique to the invention
(column 8, lines 20-23) and that use of other soaps or
anionic (other than fatty acid lithium soap) is not
contemplated. Thus, this reference clearly differs from the
composition of the present invention which comprise 30o to
85o by wt. of a surfactant system of which at least 50o is
general fatty acid soaps other than the special lithium soap
claimed in the reference. Additionally, the use of lithium
soap is excluded from the subject invention.
U.S. Patent No. 3,766,097 to Rosmarin discloses the use
of 300-50% of a specified EO-PO copolymer (Pluronic F-127) in
a bar using sodium cocoyl isethionate (a synthetic
CA 02257903 1998-12-04
C8350
... "~ -".
3
surfactant) as primary anionic surfactant. Here again, the
polymer is being used as a bar structurant at levels well
above the 25~ upper limit of the subject invention. There
is no teaching or suggestion that the polymers can be used
in combination with anionic at much lower levels to
unexpectedly and remarkably enhance mildness (e. g., reduce
irritation) at these low levels.
EP-A-689584 (Unilever) teaches that certain solid EO-PO
polymers can be used as alternatives to solid polyethylene
glycols (PEGS) as bar structurants for synthetic bar
formulations. Once more, the polymers are contemplated for
use as structurants. There is again no teaching or
suggestion that the polymers can be used at much lower
levels (both as total percentage of compositions and as
ratio to total level of anionics) to provide enhanced
mildness (i.e., reduced skin irritation).
WO 97/34992 (Unilever) teaches the use of EO-PO
copolymers at levels of 10o by weight and below in a bar
composition containing 10 to 70~ of synthetic surfactants,
which resulted in significant mildness enhancement without
sacrificing user properties and processability. This
invention did not appreciate that EO-PO copolymers can also
be incorporated into bar formulations in which the major
surfactant is fatty acid soap to reduce the skin irritation
potential without affecting user properties and
processability.
In the past, fatty acid soaps have been processed by a
technique involving melting-mixing, chipping, and extruding.
Often, addition of mildness additives cause adverse
processing problems, such as stickiness in extrusion. The
applicants have found that the use of levels of EO-PO
AMEND E
E~
CA 02257903 2005-02-25
4
copolymers up to a level of 25o by weight of the formulation
in fatty acid soap based personal cleansing bar formulations ,.
(i.e. surfactant is greater than or equal to 50% fatty acid
soap) does not cause processing difficulties and can ,
significantly reduce the skin irritation potential.
$$I~~~L~A_RY OF THE INVENTION
Applicants have now found that the use of relatively
small amounts (e.g. less than or equal to 25%) of
specifically defined polyoxyethylene-polyoxypropylene
nonionic polymer surfactants in bar compositions comprising
primarily fatty acid soap systems remarkably and unexpectedly
enhances the mildness of these bars.
More specifically, applicants' invention relates to bar
compositions comprising: _ . __
(a) 30% to 85% by weight of total composition of fatty acid
soaps, preferably 35% to 70% by weight of total
composition;
(b) 0 to 30%, preferably 0 to 20% by wt. total composition
of a synthetic surfactant, preferably selected from the
group consisting of anionic surfactant, nonionic
surfactant (other than the nonionic polymer surfactant
of item (d) below), cationic surfactant, or amphoteric
surfactant, and mixtures thereof;
(c) 0% to 40%, preferably 5% to 35% by wt. total composition
selected from the group consisting of alkylene oxide '
components having a molecular weight of from about 2;000
to about 25,000, preferably from about 3000 to about '
10000; and Ce-CZZ free fatty acids; CZ to Czo alkanols,
paraffin waxes: water-soluble starches (e. g.
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97/OZ683
maltodextrin); and
!d) 1~ to 25~, preferably 3~ to 25~ by wt. total composition
5 of a polyoxyethylene polyoxypropylene nonionic polymer
surfactant !EO-PO polymer) wherein ratio by weight total
composition of fatty acid soaps and anionic surfactants
to EO-PO polymer is between 1.2:1 to 15:1, preferably
1.5 :1 to 9:1;
This range of anionic-soap to EO-PO weight ratio is a
criticality because, above this range, the irritation
potential of the fatty acid soap can not be effectively
mitigated, and below this range, bar user properties, such as
lather performance can be negatively affected.
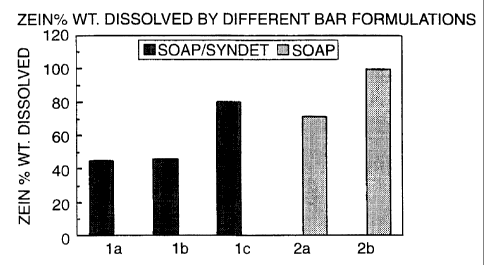
BRIEF DESCRIPTION OF THE FIGURE
Figure 1 shows the Zein % dissolved by bars shown in
Examples 1a, 1b, and 1c. Bars 1a and 1b, which include EO-PO
copolymer, dissolve a significantly smaller quantity of Zein
than Bar 1c, which does not contain EO-PO copolymer.
Therefore the irritation potential of a fatty acid soap
personal washing bar is reduced by including relatively low
levels li.e. 25a wt, and under in a full bar composition) of
Pluronics in the bar formulation.
DETAILED DESCRIPTION OF THE INVENTION
Applicants have now found that the use of relatively
small amounts of specifically defined polyoxyethylene-
polyoxypropylene nonionic polymer surfactants in bar
compositions comprising primarily fatty acid soap systems
remarkably and unexpectedly enhances the mildness of these
bars.
CA 02257903 1998-12-04
WO 97/47722 PCTlEP97/02683
6
More specifically, applicants' invention relates to bar
compositions comprising:
(a) 30~ to 85o by weight of total composition of fatty acid
soaps, preferably 35~ to 70~ by weight of total
composition;
(b) 0 to 30~, preferably 0 to 20o by wt. total composition
of a synthetic surfactant selected from the group
consisting of anionic surfactant, nonionic surfactant
(other than the nonionic polymer surfactant of item (d)
below), cationic surfactant, or amphoteric surfactant,
and mixtures thereof;
(c) 0~ to 400, preferably 5o to 35o by wt. total composition
selected from the group consisting of alkylene oxide
components having a molecular weight of from about 2,000
to about 25,000, preferably from about 3000 to about
10000; and C8-Cz2 free fatty acids; CZ to Coo alkanols,
paraffin waxes; water-soluble starches (e. g.
maltodextrin); and
(d) 1o to 250, preferably 3o to 25o by wt. total composition
of a polyoxyethylene polyoxypropylene nonionic polymer
surfactant (EO-PO polymer) wherein ratio by weight total
composition of fatty acid soaps and anionic surfactants
to EO-PO polymer is between 1.2 . 1 to 15 . 1,
preferably 1.5 . 1 to 9 . 1;
where the range of the anionic-soap to EO-PO weight
ratio is a criticality because, above this range, the
irritation potential of the fatty acid soap can not be
effectively mitigated, and below this range, bar user
properties, such as lather performance can be negatively
affected.
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97I02683
7
Soaps represent the primary detergent component in the
bar compositions of interest. The soaps may have hydrocarbon
chain lengths from 10 to 22 and are preferably saturated.
The preferred soap is a sodium salt, but other soluble soaps
can be used included potassium, ammonium, triethanolammonium,
and mixtures thereof. The soaps may be added neat or made in
situ by adding a base, e.g., NaOH, to convert free fatty
acids. The soaps are preferably prepared by saponification
of the corresponding fatty acids.
Svnthetic Surfactants
The anionic detergent active which may be used may be
aliphatic sulfonates, such as a primary alkane (e. g., C8-Czz)
sulfonate, primary alkane (e.g. , C8-Czz) disulfonate, Ce-Czz
alkene sulfonate, C8-Czz hydroxyalkane sulfonate or alkyl
glycerol ether sulfonate (AGS); or aromatic sulfonates such
as alkyl benzene sulfonate.
The anionic may also be an alkyl sulfate (e.g., C1~-C18
alkyl sulfate) or alkyl ether sulfate (including alkyl
glycerol ether sulfates). among the alkyl ether sulfates are
those having the formula:
RO ( CHzCH20 ) ~S03M
wherein R is an alkyl or alkenyl having 8 to 18 carbons,
preferably 12 to 18 carbons, n has an average value of
greater than 1.0, preferably greater than 3; and M is a
solubilizing cation such as sodium, potassium ammonium or
substituted ammonium. Ammonium and sodium lauryl ether
sulfates are preferred.
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97I02683
8
The anionic may also be alkyl sulfosuccinates (including
mono- and dialkyl, e.g., C6-Czz sulfosuccinates); alkyl and
acyl taurates, alkyl and acyl sarcosinates, sulfoacetates,
C8-Czz alkyl phosphates and phosphates, alkyl phosphate esters
and alkoxyl alkyl phosphate esters, acyl lactates, C8-Czz
monoalkyl succinates and maleates, sulphoacetates, alkyl
glucosides and acyl isethionates.
Sulfosuccinates may be monoalkyl sulfosuccinates having
the formula:
R902CCHzCH ( SO,M) COZM; and
amide-MEA sulfosuccinates of the formula:
R4CONHCH2CHZO2CCHzCH ( SO,M) CO~M
wherein R4 ranges from C8-Czz alkyl and M is a
solubilizing cation.
Sarcosinates are generally indicated by the formula:
R' CON ( CH3 ) CH7COZM,
wherein R ranges from C8-Czo alkyl and M is a
solubilizing cation.
Taurates are generally identified by formula:
3 0 RlCONR3CH~CH~S03M
wherein Rz ranges from CH-C,~ alkyl, R3 ranges from C1-C9
a alkyl and M is a solubilizing cation.
Particularly preferred are the CR-C,R acyl isethionates.
CA 02257903 2005-02-25
9
These esters are prepared by reaction between alkali metal
isethionate with mixed aliphatic fatty acids having from 6 to
18 carbon atoms and an iodine value of less than 20. At
least 75~ of the mixed fatty acids have from 12 to 18 carbon
atoms and up to 25~ have from 6 to 10 carbon atoms.
Acyl isethionates, when present, will generally range
from about 0~ to about 30o by weight of the total
composition. Preferably, this component is present from
about loo to about 25~.
- The acyl isethionate may be an alkoxylated isethionate
such as is described in Ilardi et al., U.S. Patent No.
5,393,466. This compound has the general formula:
o x y --
R C-O-C~H-CHI-(O~H-CH2~-SO-3M+
wherein R is an alkyl group having 8 to 18 carbons, m is
an integer from 1 to 4, X and Y are hydrogen or an alkyl
group having 1 to 4 carbons and M' is a monovalent cation
f such as, for example, sodium, potassium or ammonium.
The anionic surfactant comprises 0~ to 30$ of total
surfactant system and must comprise no more than 50% of total
surfactant system.
Amphoteric detergents which may be used as synthetic
' surfactants in this invention include at least one acid
group. This may be a carboxylic or a sulphonic acid group.
They include quaternary nitrogen and therefore are quaternary
amido acids. They should generally include an alkyl or
alkenyl group of 7 to 18 carbon atoms. They will usually
i
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97/02683
comply with an overall structural formula.
2
O R
5 R~ C.,_NH (CH2 n N3 X_Y
R
where R1 is alkyl or alkenyl of 7 to 18 carbon atoms;
10 RZ and R3 are each independently alkyl, hydroxyalkyl or
carboxyalkyl of 1 to 3 carbon atoms;
m is 2 to 4;
n is 0 to 1;
X is alkylene of 1 to 3 carbon atoms optionally
substituted with hydroxyl, and
Y is -COZ - or -S03-
Suitable amphoteric detergents within the above general
formula include simple betaines of formula:
R
CH~C02
13
R
and amido betaines of formula:
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97/02683
11
R2
R CONH(CH ~ ~~ CH S02
2 2
~3
wherein m is 2 or 3.
In both formulae Rl, R2, and R' are as defined
previously. R1 may in particular be a mixture of C12 and C14
alkyl groups derived from coconut so that at least half,
preferably at least three quarters of the groups R1 are
preferably methyl.
A further possibility is that the amphoteric detergent
is a sulphobetaine of formula
R
2 0 R1 -N3 (CH2~3 S03_
R
or
30
R
R CONH(CH~ m ~~ CH~ S02
_) _
R3
wherein m is 2 or 3, or variants of these in which -(CH~)~
S03- is replaced by
. CA 02257903 2005-02-25
12
OH
-CH2 CHCH2 S03 :>
in these formulae R1, RZ and R' are as discussed
previously.
The nonionic which may be used as synthetic surfactants
includes in particular the reaction products of compounds
having a hydrophobic group and a reactive hydrogen atom, for
example aliphatic alcohols, acids, amides or alkyl phenols
with alkylene oxides, especially ethylene oxide either alone
or with propylene oxide. Specific nonionic detergent
compounds are alkyl (C6-C2Z) phenols-ethylene oxide
condensates, the condensation products of aliphatic (CeCle)
primary or secondary linear or branched alcohols with __ ,_
ethylene oxide, and products made by condensation of ethylene
oxide with the reaction products of propylene oxide and
ethyienediamene Other so-called nonionic detergent compounds
include long chain tertiary amine oxides, long chain tertiary
phosphine oxides and dialkyl sulphoxides.
The nonionic may also be a sugar amide, such as a
polysaccharide amide. Specifically, the surfactant may be
one of the lactobionamides described in U.S. Patent No.
5,389,279 to Au et al. or it may be one of the sugar amides
described in Patent No. 5,009,814 to Kelkenberg.
Other surfactants which may be used are described in
U.S. Patent No. 3,723,325 to Parran Jr.
CA 02257903 2005-02-25
13
Nonionic and cationic surfactants which may be used
include any one of those described in U.S. Patent No.
3,761,418 to Parran, Jr.
Those included are the
aldobionamides taught in U.S. Patent No: 5,389,279 to Au et
al. and the polyhydroxy fatty acid amides as taught in U.S.
Patent No. 5,312,934 to Letton.
The synthetic surfactants generally comprise 10 to 300
of the total composition except, as noted that total
synthetic surfactant comprises 50~ or less of the surfactant
system and no more than 30~ total.
A preferred surfactant system is one comprising, in
addition to fatty acid soap, aryl isethionate.
The structurant of the invention, if used, can be a
water soluble or water insoluble structurant.
Water soluble structurants include moderately high
molecular weight polyalkylene oxides of appropriate melting
~25 , point (e.g. , 40° to 100°C, preferably 50° to
90°) and in
particular polyethylene glycols or mixtures thereof.
Polyethylene glycols (PEG's) which are used may have a
molecular weight in the range 2,000 to 25,000, preferably
3,000 to 10,000. However, in some embodiments of this
' invention it is preferred to include a fairly small quantity
of polyethylene glycol with a molecular weight in the range
from 50,000 to 500,000, especially molecular weights of
around 100,000. Such polyethylene- glycols have been found to
improve the wear rate of the bars. It is believed that this
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97/02683
14
is because their long polymer chains remain entangled even
when the bar composition is wetted during use.
If such high molecular weight polyethylene glycols for
any other water soluble high molecular weight polyalkylene
oxides? are used, the quantity is preferably from 1~ to 5%,
more preferably from 1g or 1.5~ to 40 or 4.5o by weight of
the composition. These materials will generally be used
jointly with a large quantity of other water soluble
structurant such as the above mentioned polyethylene glycol
of molecular weight 2,000 to 25,000, preferably 3,000 to
10,000.
Water insoluble structurants also have a melting point
in the range 40-100°C, more preferably at least 50°C, notably
50°C to 90°C. Suitable materials which are particularly
envisaged are fatty acids, particularly those having a carbon
chain of 12 to 24 carbon atoms. Examples are lauric,
myristic, palmitic, stearic, arachidic and behenic acids and
mixtures thereof. Sources of these fatty acids are coconut,
topped coconut, palm, palm kernel, babassu and tallow fatty
acids and partially or fully hardened fatty acids or
distilled fatty acids. Other suitable water insoluble
structurants include alkanols of 8 to 20 carbon atoms,
particularly cetyl alcohol. These materials generally have a
water solubility of less than 5 g/litre at 20°C.
The relative proportions of the water soluble
structurants and water insoluble structurants govern the rate
at which the bar wears during use. The presence of the
water-insoluble structurant tends to delay dissolution of the
bar when exposed to water during use and hence retard the
rate of wear.
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97/02683
The structurant is used in the bar in an amount of 0% to
40~, preferably 5~ to 35~.
5
The polyoxyethylene polyoxypropylene nonionic copolymers
(EO-PO copolymers) of the subject invention are generally
commercially available polymers having a broad molecular
weight range and EO/PO ratio and a melting temperature of
10 from about 25° to 85°C, preferably 40° to 65°C.
Generally, the polymers will be selected from one of two
classes of polymers, i.e., (1) (EO)m(PO)"(EO)m type copolymers
or (PO)~(EO)m(PO)n type copolymers of defined m/n ratio and
15 optional hydrophobic moieties (e. g., decyltetradecanol ether)
attached to either EO or PO compounds (such products are
commercially available for example, from BASF under the
Trademark Pluronic'R' or Pluronic-R'R' , respectively) ; or ( 2 )
EO-PO polymers with amine constituents such as
NZC2H4 (PO) 4~ (EO) 4m or N2C~H9 (EO) 9m (PO) 9~ with defined values of m
and n and optional hydrophobic moieties [for example?]
attached to either EO or PO components (such products are
commercially available, for example from BASF as Tetronic'P'
and Tetronic-R'R', respectively).
Specifically, examples of various Pluronic and Tetronic EO-
PO polymers are set forth in Table 1 below wherein Tm (°C) and
Ross Miles foam height data (measured at 0.1o and 50°C) were
digested from literature from BASF.
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97/02683
16
Polymer Tm(C) Foam Heights EO and PO Number
(ml)
Pluronic: (EO)m-(PO)"-(EO)m m/n
F38 48 35 46/16
F68 52 35 75/30
F77 48 47 52/35
F87 49 44 62/39
]-0 F88 54 48 97/39
F98 58 43 122/47
F108 57 41 128/54
F127 56 41 98/67
Pluronic-R:(PO~-(EO)m-(PO)~
1088 46 20 90/9
1788 53 2 155/15
2588 54 15 227/21
Tetronic N2CzH4- ( PO)
: a~ (EO) Qm
707 46 60 35/12
1107 51 50 64/20
908 58 40 85/16
1307 54 90 78/25
1508 60 40 159/30
Tetronic-R:NZC.,Ha-(EO,m(PO)Qn
2 5 9088 47 0 90/17
11087 47 0 64/21
15088 53 0 12/29
In general, the molecular weight of the copolymers used
ranges from 2,000 to 25,000 (preferably 3,000 to 10,000). The
EO-terminated polymers (Pluronic and Tetronic) are preferred to
the PO-terminated ones (Pluronic-R and Tetronic-R) for the
advantages of mildness enhancement and lather generation. To
ensure water solubility, we prefer that the portion of ethylene
oxide moiety per mole is between 50o to 90o wt., more
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97/02683
17
preferably 60-85~ wt. In other words, 2m:n (for Pluronic) or
m:n (for Tetronic) ranges from 1.32 to 11.9, preferably 2.0 to
7.5.
As noted, melting temperature of the compounds must be
about 25°-85°, preferably 40° to 65°C, the latter
being more
favorable for processing (e. g., chips form more easily and logs
plod more readily).
Other Inaredients
Bars of the invention may comprise Oo to 250, preferably
2~ to 15o by wt. of an emollient such as ethylene glycol,
propylene glycol and/or glycerine.
Bar compositions of this invention will usually contain
water, but the amount of water is only a fairly small
proportion of the bar. Larger quantities of water reduce the
hardness of the bars. Preferred is that the quantity of water
is not over 15o by weight of the bars, preferably 1o to about
10~, more preferably 3o to 90, most preferably 3% to 80.
Bars of this invention may optionally include so-called
benefit agents - materials included in relatively small
proportions which confer some benefit additional to the basic
cleansing action of the bars. Examples of such agents are:
skin conditioning agents, including emollients such as fatty
alcohols and vegetable oils, essential oils, waxes,
phospholipids, lanolin, anti-bacterial agents and sanitizers,
opacifiers, pearlescers, electrolytes, perfumes, sunscreens,
fluorescers and coloring agents. Preferred skin conditioning
agents comprise silicone oils, mineral oils and/or glycerol.
The examples below are intended to better illustrate the
invention, but are not intended to be limiting in any way.
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97/02683
18
All percentages, unless otherwise noted, are intended to
be percentages by weight.
10 Zein dissolution test was used to preliminarily screen the
irritation potential of the formulations studied. In an 8 oz.
jar, 30 mLS of an aqueous dispersion of a formulation were
prepared. The dispersions sat in a 45°C bath until fully
dissolved. Upon equilibration at room temperature, 1.5 gms of
zero powder were added to each solution with rapid stirring for
one hour. The solutions were then transferred to centrifuge
tubes and centrifuged for 30 minutes at approximately 3,000
rpms. The undissolved zero was isolated, rinsed and allowed to
dry in a 60°C vacuum oven to a constant weight. The percent
zero solubilized, which is proportional to irritation
potential, was determined gravimetrically.
Bar Mush Assessment
Bar mush is determined by placing a bar in a small dish;
adding 30 grams of water to the dish; letting the bar soak for
24 hours; and gently scraping the bar with a blunt blade to
remove the mush layer. The weight of the mush layer is
measured and divided by the initial weight of the bar prior to
soaking to obtain a mush weight fraction, xm = Wm/Wi. The final
weight of the bar, Wf, after the mush layer has been scraped
off is also measured. The water uptake weight fraction, x°,
can be calculated as
x° _ (Wm + Wf - Wi) /Wi
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97/02683
19
Three bar samples of a formulation are evaluated in this
manner, and the average xm and x" are reported here.
Bar formulations were prepared in a 5 lb Patterson mixer
with a sigma blade. The components were mixed together at
110°C. The batch was mixed with a cover on to prevent
moisture loss for about 20 minutes after all the components had
melted, then it was mixed uncovered to dry down to the desired
moisture. Total mixing time was approximately 40 minutes. At
the final moisture level, the formulation was dropped onto a
heated applicator roll and then was chipped over a chill roll.
The chill roll chips were plodded under vacuum in a Weber
Seelander duplex refiner with screw speed at ~20 rpm. The nose
cone of the plodder was heated to 45-50°C. The cut billets
were stamped into bars using a 4Veber Seelander L4 hydraulic
press with a standard bar-shaped die in place.
Exambles
Three example formulations, 1a, 1b, and 2a, are provided
in Table 2, along with two comparative formulations, 1c and 2b.
The comparatives 1c and 2b are essentially representative of a
commercial soap/syndet bar and a fatty acid soap bar,
respectively. The examples 1a, 1b, and 2a provided rich,
creamy and slippery lather; the skin-feel of the bars were
found to be smooth and non-tacky; and the processing behavior
of the example formulations was acceptable with the similar
equipment used to produce the comparatives 1c and 2b.
Mildness assessments of the examples and comparatives were
carried out as discussed above by zero solubilization
experiments. The results are summarized in Figure 1. Examples
la and lb show greater than 40% reduction in zero
CA 02257903 1998-12-04
WO 97/47722 PCT/EP97/02683
solubilization compared to 1c indicating that these
formulations are much milder than the comparative. Zein
solubilization is also reduced in the fatty soap bar with the
EO-PO copolymer, Example 2a, by comparison to 2b.
5
Mushing behavior of Examples la, 1b, and Comparative 1c is
presented in Table 3. The soap/syndet comparative has about
40a more mush than the soap/syndet examples which incorporate
the EO-PO copolymers.
TABLE 2
Formulation 1a 1b Comp- 2a Comp-
(expressed in wtg) arative arative
1c 2b
Sodium Tallowate 21.3 21.3 37.3 40.1 56.1
Sodium Cocoate 12.0 12.0 21.0 22.5 31.5
Sodium acyl 14.0 14.0 14.0 0.0 0.0
isethionate
Stearic-palmitic 8.6 8.6 8.6 0.0 0.0
acid
2 0 Coconut Fatty Acid 1.4 1.4 1.4 3.9 3.9
Pluronic F88" 25.0 5.0 0.0 25.0 0.0
PEG 8000** 0.0 20.0 0.0 0.0 0.0
Misc. Salts 5.9 5.4 5.4 0.0 0.0
Other Minor 0.5 0.5 0.5 0.2 0.2
2 5 Components**
Water 11.2 11.2 11.2 8.3 8.3
Total 100.0 100.0 100.0 100.0 100.0
30 # Pluronic F88: see definition in Table 1.
* PEG 8000: polyoxyethylene glycol with mean molecular
weigh at 8000.
**Other minor components include preservatives, perfume,
Ti02.
CA 02257903 1998-12-04
WO 97!47722 PCT/EP97/02683
21
~ mush g water uptake
1a 2.8 g,0
lb 3.2 6.1
1c 4.2 10.9