Note: Descriptions are shown in the official language in which they were submitted.
CA 022~7937 1998-12-lo
W O 98/00144 PCT~US97/11037
TITLE O~ THE INVENTION
FIBRINOGEN RECEPTOR ANTAGONIST PRODRUGS
-
BACKGROUND OF THE INVENTION
The invention relates generally to mod~ ting cell adhesion
and to inhibiting the binding of fibrinogen and other proteins to blood
platelets, and inhibiting the aggregation of blood platelets specifically to
the gp IIb/llIa fibrinogen receptor site. Fibrinogen is a glycoprotein
present in blood plasma that participates in platelet aggregation and in
fibrin formation. Platelets are cell-like anucleated fragments, found in
the blood of all m~mm~ls, that also participate in blood coagulation.
Interaction of fibrinogen with the IIb/IIIa receptor site is known to be
essential for normal platelet function.
When a blood vessel is damaged by an injury or other
causative factor, platelets adhere to the disrupted subendothethial
surface. The adherent platelets subsequently release biologically active
constituents and aggregate. Aggregation is initiated by the binding of
agonists, such as thrombin, epinephrine, or ADP to specific platelet
membrane receptors. Stimulation by agonists results in exposure of
latent fibrinogen receptors on the platelet surface, and binding of
fibrinogen to the glycoprotein IIb/IIIa receptor complex.
Attempts have been made to use natural products and
synthetic peptides to determine the mech~ni~m of adhesion and platelet
aggregation. For example, Rouslahti and Pierschbacher in Science, 238,
491-497 (1987), describe adhesive proteins such as fibronectin,
vitronectin, osteopontin, collagens, thrombospondin, fibrinogen, and
von Willebrand factor that are present in extracellular matrices and in
blood. The proteins contain the tripeptide arginine-glycine-aspartic acid
(RGD) as their glycoprotein IIb/IIIa recognition site. These arginine-
glycine-aspartic acid cont~ining tripeptides are recognized by at least
one member of a family of structurally related receptors, integrins,
which are heterodimeric proteins with two membrane-spanning
subunits. The authors state that the conformation of the tripeptide
CA 022~7937 1998-12-lo
W O 98/00144 PCT~US97/11037
sequence in the individual proteins may be critical to recognition
specificity.
Cheresh in Proc. Nat'l Acad. Sci. U.S.A., 84, 6471-6475,
(1987), describes an Arg-Gly-Asp directed adhesion receptor expressed
by human endothethial cells that is structurally similar to the IIb/~lIa
complex on platelets but is antigenically and functionally distinct. This
receptor is directly involved in endothelial cell attachment to fibrinogen,
von Willebrand factor, and vitronectin.
Pierschbacher and Rouslahti, in J. of Biol. Chem., 262,
(36), 17294- 17298 (1987) hypothesized that the Arg-Gly-Asp sequence
alone would be a sufficient signal for receptor recognition and binding
and that, therefore, the conforrnation of the tri-peptide se~uence would
be determinative. Various synthetic peptides were produced and the
authors concluded that the stereochemical conformation of Arg-Gly-Asp
as influenced by enantiomeric substitutions or additions to this sequence
significantly influenced receptor-ligand interaction. The authors further
showed that cyclization of a decapeptide by forrning a disulfide bridge
between non-terminal residues Pen and Cys, rendered the peptide much
less effective at inhibiting attachment to fibronectin.
~n Proc. Nat'l Acad. Sci. U.S.A., 81, 5985-5988 (1984), the
same authors describe tetrapeptide variants of the cell recognition site of
fibronectin that retain attachment-promoting activity. Peptides having a
tetrapeptide recognition site are described in U.S. Pat. Nos. 4,589,881
and 4,614,517. A number of large polypeptide fragments in the cell-
binding domain of fibronectin have cell-attachment activity. For
example, see U.S. Pat. Nos. 4,517,686, 4,661,111 and U.S. Pat. No.
4,578,079.
Ruggeri et al., Proc. Nat'l Acad. Sci. U.S.A., 83, 5708-
5712 (1986) explore a series of synthetic peptides designed in lengths to
16 residues, that contain RGD and a valine attached to the aspartic acid
residue of RGD that inhibit fibrinogen binding to platelets. See also
Koczewiak et al., Biochem. 23, 1767-1774 (1984); Ginsberg et al.,
CA 022~7937 1998-12-lO
W O 98/00144 PCT~US97/11037
J. Biol. Chem. 260(7), 3931-3936 (1985); and Haverstick et al., Blood
66(4), 946-952 (1985). Other inhibitors are disclosed in Eur. Pat. App.
Nos. 275,748 and 298,820.
A number of low molecular weight polypeptide factors
5 have been isolated from snake venom. These factors apparently have
high affinity for the gp IIb/IIIa complex. For example, Huang el al., J.
Biol Chem., 262, 16157-16163 (1987); Huang et al., Biochemistry, 28,
661-666 (1989) describe the primary structure of the venom trigramin
which is a 72 amino acid polypeptide that contains the RGD subunit.
10 Echistatin is another compound which has high affinity for the gp
IIb/~Ia complex. This polypeptide contains 49 amino acids and has the
RGD subunit and various disulfide bridges. Gan et al., J. Biol. Chem.,
263, 19827-19832 (1988). See also, Dermi.s et al., Proc. Nat'l Acad. Sci.
USA, 87, 2471-2475 (1989). However, these snake venom factors also
15 have high affinity for other members of the adhesive protein receptor
family including the vitronectin and fibronectin receptors so are not
selective for the gp IIb/IIIa complex.
While it is known that the tripeptide sequence Arg-Gly-Asp
is present in certain polypeptides that can duplicate or inhibit the cell
20 attachment-promoting effects of fibronectin and vitronectin, the
tripeptide Arg-Gly-Asp has low activity. At present, there is little
understanding of how other amino acids coupled to this sequence
influence binding specificity. U.S. Pat. No 5,023,233 discloses small
cyclic hexapeptides which contain the sequence Arg-Gly-Asp and are
25 useful platelet aggregation inhibitors. U.S. Pat. No. 5,037,808 discloses
the use of indolyl platelet-aggregation inhibitors which are believed to
act by antagonizing interactions between fibrinogen and/or extracellular
matrix proteins and the platelet gp IIb/IIIa receptor. U.S. Pat. No.
5,037,808 discloses guanidino peptide mimetic compounds that retain an
30 Asp residue which inhibit platelet aggregation. WO9014103 describes
the use of antibody-polypeptide conjugates wherein said polypeptides
contain the Arg-Gly-Asp (RGD) sequence.
WO9111458 discloses the use of large cyclic peptides
containing RGD flanked by proline residues which are platelet
CA 022~7937 1998-12-10
WO 98/00144 PCT/US97/11037
- 4 -
aggregation inhibitors. WO9101331 discloses small cyclic platelet
aggregation inhibitors which are synthetic cyclic pentapeptides
containing the tripeptide sequence Arg-Gly-Asp and a thioether linkage
in the cycle. U.S. Patent No. 5,051,405 also discloses the use of peptides
5 and pseudopeptides such as N-amidino-piperidine-3-carboxylglycyl-L-
aspartyl-L-valine that inhibit platelet aggregation and thrombus
formation in m~mm~lian blood. EP 445 796 discloses linear compounds
which can include internal piperazinyl or piperidinyl derivatives.
EP437 367 discloses linear polypeptide fibrinogen receptor antagonists.
10 U.S. Patent No. 5,256,812 discloses compounds of the R1-A-(W)a-X-
(CH2)b-(Y)C-B-zcooR wherein Rl is a guandidino or amidino moiety
and A and B are chosen from specific monosubstituted aryl or
heterocyclic moieties.
While a multitude of compounds or peptide analogs
15 believed to inhibit platelet aggregation by inhibiting binding to a blood
platelet by fibrinogen are known, the present invention provides novel
fibrinogen receptor antagonist prodrugs of antagonists that have
significant binding activity and are, therefore, useful for the reasons
stated herein.
SUMMARY OF THE INVENTION
The invention relates to compounds having the formula
X'-A-B
and pharmaceutically acceptable salts, wherein
X' is a moiety, comprising between 8 and 11 contiguous atoms selected
from carbon and nitrogen, terrninating at the non-A bond end in an
30 amino, aliphatic amino, aromatic amino, amidino, or guanidino
substituent having a pKa of between about 5-14, wherein the atom
attached to A is selected from carbon and nitrogen;
CA 022~7937 l998-l2-lO
W O 98/00144 PCTAUS97/11037
A is
a 5 or 6 membered aromatic ring, having 0, l, 2 or 3 heteroatoms
selected from N, O, and S, and either unsubstituted or
monosubstituted on carbon and nitrogen atoms with R5,
disubstituted on carbon and nitrogen atoms with R5 and R6, or
trisubstituted on carbon and nitrogen with R5, R6, and R9, where
R5, R6, and R9 are independently selected from the group
consisting of
hydrogen,
l 0 halogen,
C 1 1 o alkyl,
C3-8 cycloalkyl,
aryl,
aryl Cl 8 alkyl,
amino,
amino C1 8 alkyl,
C l 3 acylamino,
Cl 3 acylamino C1 8 alkyl,
C 1 6 alkylamino,
C1 6 alkylamino C1 8 alkyl,
C l 6 dialkylamino,
C1 6 dialkylamino Cl ~ alkyl,
Cl 6 alkoxy,
Cl 6 alkoxy Cl 6 alkyl,
aryl C1 6 alkyloxy,
aryl C1 6 alkyloxy C1 6 alkyl,
carboxy Cl 6 alkyl,
Cl 3 alkoxycarbonyl,
C1 3 alkoxycarbonyl Cl-6 alkyl,
carboxy,
carboxy Cl 6 alkyloxy,
hydroxy, and
- hydroxy Cl 6 alkyl, or
CA 022~7937 1998-12-10
WO 98/00144 PCT/US97/11037
a 9 or 10 membered fused aromatic ring, having 0, l, 2 or 3
heteroatoms selected from N, O, and S, and either unsubstituted
or monosubstituted on carbon and nitrogen atoms with R5,
disubstituted on carbon and nitrogen atoms with R5 and R6, or
trisubstituted on carbon and nitrogen with R5, R6, and R9, where
RS, R6, and R9 are independently selected from the group
consisting of
hydrogen,
halogen,
Cl lo alkyl,
C3 ~ cycloalkyl,
aryl,
aryl Cl-~s alkyl,
amino,
amino Cl p~ alkyl,
C1-3 acylamino,
C1 3 acylamino Cl ~ alkyl,
C l 6 alkylamino,
Cl 6 alkylamino Cl ~ alkyl,
Cl -6 dialkylamino,
Cl 6 dialkylamino Cl ~ alkyl,
C l 6 alkoxy,
C1 6 alkoxy C1 6 alkyl,
aryl C1 6 alkyloxy,
aryl Cl 6 alkyloxy Cl 6 alkyl,
carboxy C1 6 alkyl,
C1 3 alkoxycarbonyl,
Cl 3 alkoxycarbonyl C1 6 alkyl,
carboxy,
carboxy Cl 6 alkyloxy,
hydroxy, and
hydroxy Cl 6 alkyl; and
CA 02257937 1998-12-10
WO 98/00144 PCTtUS97/11037
B is
--O (CH2)nCH20R8 ~
--CH2(CH2)mCH20R, or
--CH(CH2)mCH20R8
R7
wherein n is l or 2, and m is 0, l, or 2;
R7 is selected from the group consisting of
hydrogen,
halogen,
Cl 1o alkyl,
C3-8 cycloalkyl,
aryl,
aryl C 1-8 alkyl,
ammo,
amino C1 g alkyl,
C 1 3 acylamino,
Cl 3 acylamino Cl 8 alkyl,
C l -6 alkylamino,
Cl 6 alkylamino Cl 8 alkyl,
C l 6 dialkylamino,
Cl 6 dialkylamino Cl 8 alkyl,
C l 6 alkoxy,
Cl 6 alkoxy Cl 6 alkyl,
aryl Cl 6 alkyloxy,
aryl Cl 6 alkyloxy Cl 6 alkyl,
carboxy,
carboxy Cl 6 alkyl,
Cl 3 alkoxycarbonyl,
C1-3 alkoxycarbonyl Cl-6 alkyl,
carboxy,
carboxy C1 6 alkyloxy,
hydroxy, and
hydroxy C1 6 alkyl;
CA 022~7937 1998-12-10
WO 98/00144 PCT/US97/11037
R~ is selected from the group consi,sting of
hydrogen,
-C(O)-Cl -8alkyl,
-C(O)-C3 -8cycloalkY
-C(O)-aryl, and
-C(O)-C1 3alkylaryl.
The compounds are useful as prodrugs of fibrinogen
receptor antagonists.
The invention also includes the use of a compound of the
invention, or a pharmaceutically acceptable salt thereof, in the
manufacture of a medicament for inhibiting the aggregation of blood
platelets, preventing platelet thrombosis, preventing thromboembolism
or preventing reocclusion, in a m~mm~l.
DETAILED DESCRIPTION OF THE INVENTION
The invention relates to compounds having the formula
X'-A-B
and pharmaceutically acceptable salts, wherein
X' is a moiety, comprising between 8 and 11 contiguous atoms selected
from carbon and nitrogen, termin~ting at the non-A bond end in an
amino, aliphatic amino, aromatic amino, amidino, or guanidino
substituent having a pKa of between about 5-14, wherein the atom
attached to A is selected from carbon and nitrogen;
Ais
a 5 or 6 membered aromatic ring, having 0, 1, 2 or 3 heteroatoms
selected from N, O, and S, and either unsubstituted or
monosubstituted on carbon and nitrogen atoms with R5,
disubstituted on carbon and nitrogen atoms with R5 and R6, or
trisub.stituted on carbon and nitrogen with R5, R6, and R9, where
CA 022~7937 1998-12-10
WO 98/00144 PCT/US97/11037
R5, R6, and R9 are independently selected from the group
consisting of
hydrogen,
halogen,
Cl -10 alkyl,
C3-8 cycloalkyl,
aryl,
aryl C1 ~ alkyl,
amino,
amino C1 8 alkyl,
C1 3 acylamino,
C 1 3 acylamino C 1 ~ alkyl,
C1 6 alkylamino,
C1 6 alkylamino C1 8 alkyl,
C 1-6 dialkylamino,
C1 6 dialkylamino C1 8 alkyl,
C 1 6 alkoxy,
C1 6 alkoxy C1 6 alkyl,
aryl C1 6 alkyloxy,
aryl C1 6 alkyloxy C1 6 alkyl,
carboxy C1 6 alkyl,
Cl 3 alkoxycarbonyl,
Cl 3 alkoxycarbonyl Cl 6 alkyl,
carboxy,
carboxy C1 6 alkyloxy,
hydroxy, and
hydroxy C1 6 alkyl, or
a 9 or 10 membered fused aromatic ring, having 0, 1, 2 or 3
heteroatoms selected from N, O, and S, and either unsubstituted
or monosubstituted on carbon and nitrogen atoms with R5,
disubstituted on carbon and nitrogen atoms with R5 and R6, or
trisubstituted on carbon and nitrogen with RS, R6, and R9, where
. .
CA 022~7937 1998-12-lo
W O 98/00144 PCTAUS97/11037
- 10 -
R5, R6, and R9 are independently selected from the group
consisting of
hydrogen,
halogen,
S C 1 1 o alkyl,
C3-8 cycloalkyl,
aryl,
aryl C1 ~ alkyl,
amlno,
amino C1 8 alkyl,
Cl 3 acylamino,
Cl 3 acylamino C1 8 alkyl,
C 1 6 alkylamino,
C1 6 alkylamino C1 8 alkyl,
Cl -6 dialkylamino,
C 1 6 dialkylamino C 1 8 alkyl,
C1 6 alkoxy,
C1 6 alkoxy C1 6 alkyl,
aryl C1 6 alkyloxy,
aryl C1 6 alkyloxy C1 6 alkyl,
carboxy C1 6 alkyl,
C1 3 alkoxycarbonyl,
C1 3 alkoxycarbonyl Cl 6 alkyl,
carboxy,
carboxy C1 6 alkyloxy,
hydroxy, and
hydroxy C1 6 alkyl; and
B is
--O (C H2)nC H2OR8,
--CH2(CH2)mcH2OR, or
- --CH(CH2)mCH20R8
R7
CA 022~7937 1998-12-lo
W O 98/00144 PCTrUS97/11037
wherein n is 1 or 2, and m is 0, 1, or 2;
R7 is selected from the group consisting of
hydrogen,
halogen,
Cl -10 alkyl,
C3-8 cycloalkyl,
aryl,
aryl C1 8 alkyl,
amino,
amino C1 8 alkyl,
Cl 3 acylamino,
C l 3 acylamino C 1 8 alkyl,
C1 6 alkylamino,
C1 6 alkylamino Cl 8 alkyl,
Cl -6 dialkylamino,
C1 6 dialkylamino C1 8 alkyl,
C 1 6 alkoxy,
C1 6 alkoxy C1 6 alkyl,
aryl C1 6 alkyloxy,
aryl C1 6 alkyloxy C1 6 alkyl,
carboxy,
carboxy C1 6 alkyl,
C1 3 alkoxycarbonyl,
Cl 3 alkoxycarbonyl C1 6 alkyl,
carboxy,
carboxy Cl 6 alkyloxy,
hydroxy, and
hydroxy C1 6 alkyl;
30 R8 is selected from the group consisting of
hydrogen,
-C(O)-C 1 -8alkYI,
-C(O)-C3 gcycloalkyl,
-C(O)-aryl, and
CA 022~7937 1998-12-10
WO 98/00144 PCT/US97/11037
- 12 -
-C(O)-C1 3alkylaryl.
In one class of the invention, the compound has the formula
X-Y-Z-A-B
and pharmaceutically acceptable salts, wherein
xis
a 5, 6 or 7 membered aromatic or nonaromatic ring, having 1, 2
or 3 heteroatoms selected from N, O, and S, and either
unsubstituted or monosubstituted on carbon and nitrogen atoms
with Rl or disubstituted with Rl and R2, where Rl and R2 are
independently selected from the group consisting of
1 5 hydrogen,
halogen,
Cl -10 alkyl,
C3-8 cycloaLkyl,
aryl,
aryl Cl -8 alkyl,
ammo,
amino C1 8 alkyl,
C1 3 acylamino,
C 1-3 acylamino C ~ -8 alkyl,
C 1-6 alkylamino,
C1 6 alkylamino C1 8 alkyl,
C 1 6 dialkylamino,
C1 6 dialkylamino Cl ~ alkyl,
C 1 6 alkoxy,
C1 6 alkoxy C1 6 alkyl,
aryl C1 6 alkyloxy,
aryl C1 6 alkyloxy C1 6 alkyl,
carboxy C1 6 alkyl,
C1 3 alkoxycarbonyl,
CA 022~7937 1998-12-lo
W O 98/00144 PCTrUS97/11037
C1 3 alkoxycarbonyl Cl 6 alkyl,
carboxy,
carboxy Cl-6 alkyloxy,
hydroxy, and
hydroxy Cl 6 alkyl, or
a 9 or 10 membered fused aromatic or nonaromatic ring, having
1, 2 or 3 heteroatoms selected from N, O, and S, and either
unsubstituted or monosubstituted on carbon and nitrogen atoms
with R 1 or disubstituted with R1 and R2, where R l and R2 are
independently selected from the group consisting of
hydrogen,
halogen,
Cl -10 alkyl,
C3-8 cycloalkyl,
aryl,
aryl C1 8 alkyl,
amino,
amino C1 g alkyl,
C 1-3 acylamino,
C1 3 acylamino C1 8 alkyl,
C1 6 alkylamino,
C1 6 alkylamino C1 8 alkyl,
C1 6 dialkylamino,
C1 6 dialkylamino Cl 8 alkyl,
C 1 6 alkoxy,
C1 6 alkoxy Cl-6 alkyl,
aryl C1 6 alkyloxy,
aryl C1 6 alkyloxy C1 6 alkyl,
carboxy C1 6 alkyl,
C1 3 alkoxycarbonyl,
C l 3 alkoxycarbonyl C1 6 alkyl,
carboxy,
carboxy Cl 6 alkyloxy,
CA 022~7937 1998-12-10
WO 98/00144 PCT/US97/11037
- 14 -
hydroxy, and
hydroxy Cl 6 alkyl;
Y is
a 5 or 6 membered aromatic or nonaromatic ring, having 0, l, 2
or 3 heteroatoms selected from N, O, and S, and either
unsubstituted or substituted on carbon and nitrogen atoms with R3
selected from the group consisting of
halogen,
C1 lo alkyl,
C3-8 cycloalkyl,
aryl,
aryl Cl 8 alkyl,
amino,
amino C l 8 alkyl,
C1 3 acylamino,
Cl 3 acylamino Cl 8 alkyl,
C 1 6 alkylamino,
Cl 6 alkylamino Cl ~ alkyl,
C1 6 dialkylamino,
Cl 6 dialkylamino Cl ~ alkyl,
C1 6 alkoxy,
C1 6 alkoxy C1 6 alkyl,
aryl C1 6 alkyloxy,
aryl C1 6 alkyloxy Cl 6 alkyl,
carboxy C1 6 alkyl,
C1 3 alkoxycarbonyl,
Cl 3 alkoxycarbonyl Cl 6 alkyl,
carboxy,
carboxy Cl 6 alkyloxy,
hydroxy, and
hydroxy Cl 6 alkyl;
or
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 15 -
X and Y combined together fo~n the structure
R1N~ ~ R1N~ ~ , ~r
~N--~\55s ;
R1NJ~/
zis
R ~4
--C--N-- ,
~4R
--N-C--
--CH2CH2--
--CH=CH--
--CH2-~--
--O--CH2
CA 02257937 1998-12-lo
W O 98/00144 PCT~US97/11037
- 16 -
--C--CH2--
--CH2-C--
--CH2NR4--
--NR4CH2--
OH
--CH--CH2--
OH
--CH2--CH ' or
Z represents a bond;
5 R4 is ,selected from the group consisting of
hydrogen,
halogen,
C1 1o alkyl,
C3-8 cycloalkyl,
aryl,
aryl Cl 8 alkyl,
amino,
amino C1 8 alkyl,
C1 3 acylamino,
C 1-3 acylamino C 1 -~ aLkyl,
C 1 6 alkylamino,
C 1 6 alkylamino C l ~s alkyl,
C1 6 dialkylamino,
- Cl-6 dialkylamino C1 8 alkyl,
C1 4 alkoxy,
CA 022~7937 1998-12-lo
W O98/00144 PCTrUS97/11037 -.
Cl 4 alkoxy C1 6 alkyl,
carboxy,
carboxy 1-6 alkyl,
Cl 3 alkoxycarbonyl,
Cl 3 alkoxycarbonyl C1 6 alkyl,
carboxy C1 6 alkyloxy,
hydroxy, and
hydroxy C1 6 alkyl;
10 Ais
a 5 or 6 membered aromatic ring, having 0, 1, 2 or 3 heteroatoms
selected from N, O, and S, and either unsubstituted or
monosubstituted on carbon and nitrogen atoms with R5,
disubstituted on carbon and nitrogen atoms with R5 and R6, or
trisubstituted on carbon and nitrogen with R5, R6, and R9, where
R5, R6, and R9 are independently selected from the group
consisting of
hydrogen,
halogen,
C1-10 alkyl,
C3-8 cycloalkyl,
aryl,
aryl C1 8 alkyl,
amino,
amino C~ ~ alkyl,
C1 3 acylamino,
C1 3 acylamino C1 8 alkyl,
C 1 6 alkylamino,
C1 6 alkylamino Cl ~ alkyl,
Cl -6 dialkylamino,
C1 6 dialkylamino Cl ~ alkyl,
C1 6 alkoxy,
C1 6 alkoxy C1 6 alkyl,
aryl C1 6 alkyloxy,
CA 022F,7937 1998- 12- lo
WO 98tO0144 PCT/US97/11037
aryl C1 6 alkyloxy Cl 6 alkyl,
carboxy Cl 6 alkyl,
C1 3 alkoxycarbonyl,
Cl 3 alkoxycarbonyl C1 6 alkyl,
S carboxy,
carboxy C1 6 alkyloxy,
hydroxy, and
hydroxy Cl 6 alkyl, or
a 9 or 10 membered fused aromatic ring, having 0, l, 2 or 3
heteroatoms ,selected from N, O, and S, and either unsubstituted
or monosubstituted on carbon and nitrogen atoms with RS,
disubstituted on carbon and nitrogen atoms with RS and R6, or
trisubstituted on carbon and nitrogen with RS, R6, and R9, where
R5, R6, and R9 are independently selected from the group
consisting of
hydrogen,
halogen,
C1 lo alkyl,
C3-8 cycloalkyl,
aryl,
aryl C1 8 alkyl,
amino,
amino C1 8 alkyl,
C1 3 acylamino,
C ~ 3 acylamino C l 8 alkyl,
Cl 6 alkylamino,
C1 6 alkylamino C1 ~ alkyl,
C1 6 dialkylamino,
C1 6 dialkylamino C1 ~ alkyl,
C l 6 alkoxy,
C1 6 alkoxy Cl 6 alkyl,
aryl Cl-6 alkyloxy,
aryl C1 6 alkyloxy C1 6 alkyl,
CA 02257937 1998-12-lo
W O 98/00144 PCTrUS97/11037
- 19 -
carboxy C1 6 alkyl,
C1 3 alkoxycarbonyl,
Cl 3 alkoxycarbonyl C1 6 alkyl,
carboxy,
S carboxy C1 6 alkyloxy,
hydroxy, and
hydroxy C1 6 alkyl;
B is
--O (CH2)nCH20R8 ~
--CH2(CH2)mCH20R~ or
--CH(CH2)mCH20R8
R7
whereinnis 1 or2,andmisO, 1, or2;
R7 is selected from the group consisting of
hydrogen,
halogen,
C1 1o alkyl,
C3-8 cycloalkyl,
aryl,
aryl C1 8 alkyl,
amino,
amino C1 8 alkyl,
Cl 3 acylamino,
C1 3 acylamino C1 8 alkyl,
C 1 6 alkylamino,
C1 6 alkylamino Cl 8 alkyl,
C 1-6 dialkylamino,
C1 6 dialkylamino C1 8 alkyl,
C1 6 alkoxy,
C1 6 alkoxy C1 6 alkyl,
aryl C1 6 alkyloxy,
aryl Cl 6 aIkyloxy C1 6 alkyl,
CA 022F,7937 1998- 12- lo
WO 98/00144 PCTIUS97/11037
- 20 -
carboxy,
carboxy C1 6 alkyl,
Cl 3 alkoxycarbonyl,
C l 3 alkoxycarbonyl C 1 6 alkyl,
carboxy,
carboxy C1 6 alkyloxy,
hydroxy, and
hydroxy C1 6 alkyl;
10 R8 is selected from the group consisting of
hydrogen,
-C(O)-Cl -8alkYl,
-C(O)-C3 -8cYcloalkyl,
-C(O)-aryl, and
-C(O)-C1 3alkylaryl.
In a subclass of the class, the compounds have the formula
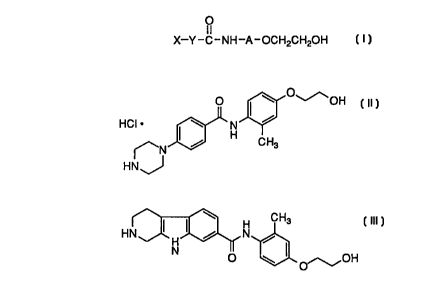
X--Y -C-NH-A-OCH2CH20H
and pharmaceutically acceptable salts, wherein
X is a 6-membered aromatic or nonaromatic ring having 1, 2 or
3 nitrogen atoms;
Y is a 6-membered aromatic or nonaromatic ring having 0, 1, 2
or 3 nitrogen atoms;
A is a 6-membered aromatic ring unsubstituted, mono-
substituted with a moiety selected from the group consisting
of halogen,Cl 3alkyl,andCl 3alkylsulfonylamino,
disubstituted with one or more moieties, same or different,
selected from the group consisting of halogen, Cl 3aLkyl,
and Cl 3alkylsulfonylamino or trisubstituted with one or
CA 022~7937 1998-12-10
WO 98/00144 PCT/US97/11037
- 21 -
more moieties, same or different, selected from the group
consisting of halogen, Cl 3alkyl~ and C
3alkylsulfonylamino,
~ and all other substituents are as previously defined.
In a group of this subclass, the compounds have the formula
1~l
X--Y -C - NH -A - OCH2CH20H
and pharmaceutically acceptable salts, wherein
X is a 6-membered aromatic or nonaromatic ring having 1 or 2
nitrogen atoms;
Y is a 6-membered aromatic or nonaromatic ring having 0 or I
nitrogen atoms;
A is a 6-membered aromatic ring unsubstituted, mono-
substituted with a moiety selected from the group consisting
of Br, CH3, and NHSO2CH3, disubstituted with one or
more moieties, same or different, selected from the group
consisting of Br, CH3, and NHSO2CH3, or trisubstituted
with one or more moieties, same or different, selected from
the group consisting of Br, CH3, and NHSO2CH3; and
and all other substituents are as previously defined.
In a subgroup of this group, the compounds have the
formula
1~l
X--Y-C-NH-A-OCH2CH20H
and pharmaceutically acceptable salts, wherein
CA 02257937 1998-12-lo
W O 98/00144 PCTAUS97tllO37
xis
A /=\
HN~N--~ or N~ ;
yis
~ or ~ N~ d
A is
CH3
Br
NHSO2CH ~ CH ~ Br
and all other substituents are as previously defined.
Examples of this subgroup include
2-(4-(4-( 1 -Piperazinyl)phenylcarbonylamino)phenoxy)ethanol,
2-(3 -Methyl-4-(4-( 1 -piperazinyl)phenylcarbonylamino)phenoxy)-
1 5 ethanol,
2-(4-(4-(4-Piperazin- I -yl)phenylcarbonylamino)-2-methanesulfonyl-
aminophenoxy)ethanol,
20 2-(3-Methyl-4-(~-carbolin-7-yl-carboxamido)phenoxy)ethanol,
CA 022~7937 1998-12-lo
W O 98/00144 PCTAUS97/11037
2-(2,6-Dibromo-3 -methyl-4-(4-(piperizin-4-yl)phenylcarbox-
amide)phenoxy) ethanol,
4-(Pyridyl)(piperidin)-4-carbonylamino-3 -methylphenoxyethanol
and pharmaceutically acceptable salts, e.g. hydrochloride salts.
The active acids of these compounds have been evaluated in
vitro and found to have an IC50 for inhibiting platelet aggregation of
between about 0.008 ,uM and 2 ~M.
The prodrugs may be ~f~mini~tered in low amounts relative
to achieve inhibition of fibrinogen binding to the fibrinogen receptor.
The prodrugs may be ~llmini~tered orally. The prodrugs retain
structural integrity while passing though the gastrointestinal system, and
are effectively delivered to cells. They are subjected to oxidative
15 enzymes such as alcohol and aldehyde dehydrogenase to form the active
acid which then interacts with the platelet receptor site.
A number of very serious diseases and disorders involve
hyperthrombotic complications which lead to intravascular thrombi and
emboli. Myocardial infarction, stroke, phlebitis and a number of other
20 serious conditions create the need for novel and effective fibrinogen
receptor antagonists.
One test which is used to evaluate fibrinogen receptor
antagonist activity is based on evaluation of inhibition of ADP-
stimulated platelets. Aggregation requires that fibrinogen bind to and
25 occupy the platelet fibrinogen receptor site. Inhibitors of fibrinogen
binding inhibit aggregation. In the ADP-stimulated platelet aggregation
assay used to determine inhibition associated with the acids of the
compounds claimed in the instant invention, human platelets are isolated
from fresh blood, collected into acid citrate/dextrose by differential
30 centrifugation followed by gel filtration on Sepharose 2B in divalent
ion-free Tyrode's buffer (pH 7.4) containing 2% bovine serurn albumin.
Platelet aggregation is measured at 37~C in a Chronolog
aggregometer. The reaction mixture contains gel-filtered human
platelets (2 x 10~ per ml), fibrinogen (100 micrograms per ml (ug/ml)),
CA 022~7937 1998-12-lo
W O 98/00144 PCT~US97/11037
- 24 -
Ca2+ (1 mM), and the compound to be tested. The aggregation is
initiated by adding 10 mM ADP 1 minute after the other components
are added. The reaction is then allowed to proceed for at least 2
minutes. The extent of inhibition of aggregation is expressed as the
5 percentage of the rate of aggregation observed in the absence of
inhibitor. The IC50 is the dose of a particular compound inhibiting
aggregation by 50% relative to a control lacking the compound.
Additionally, these compounds are useful for treating
m~mmals suffering from a bone condition caused or mediated by
10 increased bone resorption, who are in need of such therapy.
Pharmacologically effective amounts of the compounds, including
pharamaceutically acceptable salts thereof, are administered to the
m~mm~l, to inhibit the activity of m~mm~ n osteoclasts.
Additionally, these compounds are useful for treating
15 angiogenesis (formation of new blood vessels). It has been postulated
that the growth of tumors depends on an adequate blood supply, which
in turn is dependent on the growth of new vessels into the tumor.
Inhibition of angiogenesis can cause tumor regression in ~nim~ models.
(See, Harrison's Principles of Internal Medicine, 12th ed., 1991). These
20 compounds are therefore useful in the treatment of cancer for inhibiting
tumor growth. (See e.g., Brooks et al., Cell, 79:1 157-1 164 (1994)).
The term "pharmaceutically acceptable salts" shall mean
non-toxic salts of the compounds of this invention which are generally
prepared by reacting the free base with a suitable organic or inorganic
25 acid. Representative salts include the following salts:
acetate, benzenesulfonate, benzoate, bicarbonate, bisulfate, bitartrate,
borate, bromide, calcium edetate, camsylate, carbonate, chloride,
clavulanate, citrate, dihydrochloride, edetate, edisylate, estolate, esylate,
fumarate, gluceptate, gluconate, glutamate, glycollylarsanilate,
30 hexylre,sorcinate, hydrabamine, hydrobromide, hydrochloride,
hydroxynapthoate, iodide, isothionate, lactate, lactobionate, laurate,
malate, maleate, mandelate, mesylate, methylbromide, methylnitrate,
methylsulfate, mucate, napsylate, nitrate, oleate, oxalate, pamaote,
palmitate, panthothenate, phosphate/diphosphate, polygalacturonate,
CA 022~7937 1998-12-lo
W O 98/00144 PCTrUS97/11037
- 25 -
salicylate, stearate, subacetate, succinate, tannate, tartrate, teoclate,
tosylate, triethiodide, valerate.
Some of the compounds of the present invention are chiral;
included within the scope of the present invention are racemic mixtures
5 and separated enantiomers of the general formula. Furthermore, all
diastereomers, including E, Z isomers, of the general formula are
included in the present scope. Furthermore, hydrates as well as
anhydrous compositions and polymorphs of the general formula are
within the present invention.
The compounds of the invention are prodrugs of active
acids which inhibit fibrinogen binding to the gpIrb/IIIa platelet receptor
site. For example~ these acids form in vivo, subsequent to
~lministration to the patient, according to successive alcohol
dehydrogenase and aldehyde dehydrogenase reactions:
alcohol O
dehydrogenase ll
R'-CH20H ' R'-CH
NAD+ NADH
aldehyde
1~l dehydrogenase
R'-CH ~ ~ R'-COOH
NAD+ NADH
Other mech~ni.cms may contribute to the conversion of alcohol to acid.
Compounds of the invention of the general formula R'-CH20R8, where
- 20 R8 is an acyl moiety, may be esters which metabolize into the active
acid.
CA 022~7937 1998-12-lo
W O 98/00144 PCTrUS97/11037
- 26 -
The invention also includes the use of a compound of the
invention, or a pharmaceutically acceptable salt thereof, in the
manufacture of a medicament for inhibiting the binding of fibrinogen to
blood platelets, inhibiting the aggregation of blood platelets, treating
5 thrombus formation or embolus formation, or preventing thrombus or
embolus formation in a m~mm~l.
The term "a moiety comprising between 8 and 11
contiguous atoms" means a series of sequentially bonded atoms,
including a series of atoms that are sequentially bonded in linear
10 relation, wherein none of the atom,s are part of a cyclic moiety, and a
series of atoms that are sequentially bonded in a linear relation, wherein
some of the atoms are part of a cyclic moiety.
For example, the following is an example of 10 continguous
carbon or nitrogen atoms bonded in linear relation, wherein none of the
lS atom,s are part of cyclic moiety:
~ 8 6 4 2
H2N~~
9 7 5 8
- The following is an example of 10 continguous carbon or
nitrogen atoms bonded in linear relation, wherein ~ of the atoms are
20 part of one or more cyclic moieties:
o
H--N N~N
9 8 5 4 H
The following is an example of a structure having 11
continguous carbon or nitrogen atoms (atoms numbered 1'-11') bonded
25 in linear relation, wherein 7 of the atoms are part of one or more cyclic
moieties:
CA 022~7937 1998-12-lo
W O 98/00144 PCT~US97/11037 -.
- 27 -
O
12
11 10
The above structure also has 12 continguous carbon or
nitrogen atoms (atoms numbered 1-12) bonded in linear relation,
wherein 8 of the atoms are part of one or more cyclic moieties. Since
5 the above structure has 11 continguous carbon or nitrogen atoms bonded
in linear relation, wherein 7 of the atoms are part of one or more cyclic
moieties, the structure falls within the definition of X'.
The term "pharmaceutically effective amount" shall mean
that amount of a drug or pharmaceutical agent that will elicit the
10 biological or medical response of a tissue, system or ~nim~l that is being
sought by a researcher or clinician. The term "anti-coagulant" shall
include heparin, and warfarin. The term "thrombolytic agent" shall
include agents such as streptokinase and tissue pl~minogen activator.
The term "platelet anti-aggregation agent" shall include agents such as
15 aspirin and dipyridamole.
The term "alkyl" means straight or branched alkane
containing I to about lO carbon atoms, e.g., methyl, ethyl, n-propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, pentyl, iso-amyl,
hexy, octyl radicals and the like, straight or branched alkene cont~ining
20 2 to about 10 carbon atoms, e.g., propylenyl, buten-l-yl, isobutenyl,
pentenylen-l-yl, 2,2-methylbuten-l-yl, 3-methylbuten-1-yl, hexen-l-yl,
hepten-l-yl, and octen-l-yl radicals and the like, or straight or branched
alkyne containing 2 to about lO carbon atoms, e.g., ethynyl, propynyl,
butyn-l-yl, butyn-2-yl, pentyn-1-yl, pentyn-2-yl, 3-methylbutyn-l-yl,
25 hexyn-l-yl, hexyn-2-yl, hexyn-3-yl, 3,3-dimethylbutyn-l-yl radicals and
the like.
The term "aryl" means a 5- or 6-membered aromatic ring
con~ining 0, 1, or 2 heteroatoms selected from O, N, and S, e.g.
phenyl, pyridine, pyrimidine, imidazole, thiophene, oxazole, isoxazole,
30 thiazole, and amino- and halogen- substituted derivatives thereof.
CA 022~7937 l998-l2-lO
W O 98/00144 PCT~US97/11037
- 28 -
The terms "alkyloxy" or "alkoxy" include an alkyl portion
where alkyl is as defined above, e.g., methyloxy, propyloxy, and
butyloxy.
The terms "arylalkyl" and "alkylaryl" include an alkyl
5 portion where alkyl i,s as defined above and to include an aryl portion
where aryl is as defined above. The C0-n or C1 n designation where n
may be an integer from 1-10 or 2-10 respectively refers to the alkyl
component of the arylalkyl or alkylaryl unit. Examples of arylalkyl
include benzyl, fluorobenzyl, chlorobenzyl, phenylethyl, phenylpropyl,
10 fluorophenylethyl, chlorophenylethyl, thienylmethyl, thienylethyl, and
thienylpropyl. Examples of alkylaryl include toluene, ethylbenzene,
propylbenzene, methylpyridine, ethylpyridine, propylpyridine,
butylpyridine, butenylpyridine, and pentenylpyridine.
The term "halogen" includes fluorine, chlorine, iodine and
1 5 bromine.
The term "oxy" means an oxygen (O) atom. The term
"thio" means a sulfur (S) atom. Under standard nonmenclature used
throughout this disclosure, the terminal portion of the designated side
chain is described first followed by the adjacent functionality toward the
20 point of attachment. For example, a C1 6 alkyl substituted with C1 5
alkyl-carbonylamino is equivalent to
HO
1 11
C1 6-alkyl-N-C-Cl s-alkyl
wherein the Cl 5alkyl moiety attaches to the substituted molecule.
In the schemes and examples below, various reagent
symbols have the following meanings:
BOC
(or Boc): t-butyloxycarbonyl
Pd-C: palladium on activated carbon catalyst
DMF: dimethylformamide
DMSO: dimethylsulfoxide
CBZ: carbobenzyloxy
CA 022~7937 1998-12-10
WO 98/00144 PCT/US97/11037
- 29 -
CH2Cl2: methylene chloride
CHC13: chloroforrn
EtOH: ethanol
MeOH: methanol
5 EtOAc: ethyl acetate
HOAc: acetic acid
BOP: benzotriazol- l -yloxytris(dimethylamino)phosphonium,
hexafluorophosphate
EDC: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
l 0 hydrochloride
Oxone: potassium peroxymonosulfate
LDA: lithium diisopropylamide
PYCLU: Chloro N,N,N'N'-bis(pentamethylene)formamidinium
hexafluorophosphate
The compounds of the present invention can be
~lmini~tered in such oral forms as tablets, capsules (each of which
includes sustained release or timed release formulations), pills, powders,
granules, elixirs, tinctures, suspensions, syrups, and emulsions.
Likewise, they may be ~lmini~tered in intravenous (bolus or infusion),
intraperitoneal, subcutaneous, or intramuscular form, all using forms
well known to those of ordinary skill in the pharmaceutical arts. An
effective but non-toxic amount of the compound desired can be
employed as an anti-aggregation agent.
Compounds of the invention may be ~mini~tered to
patients where prevention of thrombosis by inhibiting binding of
fibrinogen to the platelet membrane glycoprotein complex rIb/IIIa
receptor is desired. They are useful in surgery on peripheral arteries
(arterial grafts, carotid endarterectomy) and in cardiovascular surgery
where manipulation of arteries and organs, and/or the interaction of
platelets with artificial surfaces, leads to platelet aggregation and
consumption. The aggregated platelets may form thrombi and
- thromboemboli. Compounds of this invention may be ~dministered to
CA 022~7937 1998-12-lo
W O 98/00144 PCT~US97/11037
- 30 -
these surgical patients to prevent the formation of thrombi and
thromboemboli.
Extracorporeal circulation is routinely used for
cardiovascular surgery in order to oxygenate blood. Platelets adhere to
5 surfaces of the extracorporeal circuit. Adhesion is dependent on the
interaction between gp IIb/IIIa on the platelet membranes and
fibrinogen adsorbed to the surface of the circuit. (Gluszko et al., Amer.
J. Physiol., 252(H), 615-621 (1987)). Platelets released from artificial
surfaces show impaired hemostatic function. Compounds of the
10 invention may be a-lmini.~tered to prevent adhesion.
Other applications of these compounds include prevention
of platelet thrombosis, thromboembolism and reocclusion during and
after thrombolytic therapy and prevention of platelet thrombosis,
thromboembolism and reocclusion after angioplasty or coronary artery
15 bypass procedures. They may also be used to prevent myocardial
infarction.
The dosage regimen ~ltili7ing the compounds of the present
invention is selected in accordance with a variety of factors including
type, species, age, weight, sex and medical condition of the patient; the
20 severity of the condition to be treated; the route of ~dministration; the
renal and hepatic function of the patient; and the particular compound
or salt thereof employed. An ordinarily skilled physician or
veterinarian can readily determine and prescribe the effective amount of
the drug required to prevent, counter, or arrest the progress of the
25 condition.
Oral dosages of the present invention, when used for the
indicated effects, will range between about 0.01 mg per kg of body
weight per day (mg/kg/day) to about 100 mg/kg/day and preferably
0.01-100 mg/kg/day and most preferably 0.01-20 mg/kg/day. For
30 example, a typical 90 kg patient would receive oral dosages ranging
between about 0.9 mg/day and about 9 g/day, most preferably between
about 0.9 mg/day and 1.8 g/day. Suitable pharmaceutical oral
compositions such as tablets or capsules may contain 10-500 mg, for
example, 10 mg, 100 mg, 200 mg and 500 mg. Intravenously, the most
CA 022~7937 1998-12-lo
W O 98/00144 PCT~US97/11037
preferred doses will range from about 1 to about 10 mg/kg/minute
during a constant rate infusion.
Advantageously, compounds of the present invention may
be ~lmini~tered in divided doses of two, three, or four times daily.
5 Furthermore, preferred compounds for the present invention can be
~-lministered in intranasal form via topical use of suitable intranasal
vehicles, or via transdermal routes, using those forms of transdermal
skin patches well known to those of ordinary skill in that art. To be
~lmini.stered in the form of a transdermal delivery system, the dosage
10 ~lministration will, or course, be continuous rather that intermittent
throughout the dosage regime.
In the methods of the present invention, the compounds
herein described in detail form the active ingredient of the prodrug, and
are typically ~lmini.stered in admixture with suitable pharmaceutical
15 diluents, excipients or carriers (collectively referred to herein as
"carrier" materials) suitably selected with respect to the intended form
of ~rlminilstration, that is, oral tablets, capsules, elixirs, syrups and the
like, and consistent with convention pharmaceutical practices.
For instance, for oral ~flministration in the form of a tablet
20 or capsule, the active ingredient prodrug component can be combined
with an oral, non-toxic, pharmaceutically acceptable, inert carrier such
as lactose, starch, sucrose, glucose, methyl cellulose, magnesium
stearate, dicalcium phosphate, calcium sulfate, mannitol, sorbitol and the
like; for oral administration in liquid form, the oral prodrug
25 components can be combined with any oral, non-toxic, pharmaceutically
acceptable inert carrier such as ethanol, glycerol, water and the like.
Moreover, when desired or necessary, suitable binders, lubricants,
distintegrating agents and coloring agents can also be incorporated into
the mixture. Suitable binders include starch, gelatin, natural sugars such
30 as glucose or beta-lactose, corn-sweeteners, natural and ,synthetic gums
such as acacia, tragacanth or sodium alginate, carboxymethylcellulose,
polyethylene glycol, waxes and the like. Lubricants used in these dosage
forms include sodium oleate, sodium stearate, magnesium stearate,
sodium benzoate, sodium acetate, sodium chloride and the like.
CA 022~7937 l998-l2-lO
W O98/00144 PCTAUS97/11037
- 32 -
Disintegrators include, without limit~tion, starch methyl cellulose, agar,
bentonite, x~nth~n gum and the like.
Active drug can also be co-~(lmini~tered with the usual
doses of suitable anticoagulation agents, such as heparin or warfarin
5 (typically given in tablet doses between 1 and 20 mg daily during
;l(lmini.~tration of the active drug), or thrombolytic agents such as tissue
plasminogen activator (typically given in i.v. doses of between 20 and
150 mg over two hour period prior to or during ~lmini~tration of the
active drug), to achieve beneficial effects in the treatment of various
10 vascular pathologies. Such co-~clmini~tration also includes
administration if the active drug with doses of anticoagulant agents or
thrombolyric agent~ less than the usual doses of those agents.
In the examples below, field strength for NMR analysis is
either 300 MHz or 400 MHz.
In one general procedure for making compounds of the
invention, 4-aminophenol is reacted with halogenated ethanol to produce
4-aminophenoxy ethanol, which is combined with a piperazinyl benzoic
acid to produce an alcohol prodrug of the invention.
In another general procedure, 4-aminomethylphenol is
20 combined with halogenated ethyl acetate to form ethyl 4-amino-3-
methylphenoxyacetate, which is then reacted with halogenated ethanol to
produce a 3-methyl-4-aminophenoxyethanol. The 3-methyl-4-
aminophenoxyethanol is reacted with piperazinyl benzoic acid to
produce an alcohol prodrug of the invention.
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 33 -
SCHEME I
H2N~ co2CH3
1 -1
n-butanol A ~
+ ~ HN N~ C02CH3
Cl 1-3
BOC20, NEt3
HCI-HN
L~ DMF
1-2 Cl BOC-N~N~C02cH3
1 N NaOH/ 1-4
BOC-N N~ ~EtOH
1 -5
HN N~CO2CH3
Methyl 4-(N-piperazinyl)benzoate (1-3)
- A solution of amine 1 1 (20.0 g, 132 mmol), amine 1 2
(23.6 g, 132 mmol) and n-butanol (500 ml) was refluxed for 168 h.
lO The solution was allowed to cool to ambient temperature. The crystals
- were collected, washed with Et2O and dried in vac~o to give ester 1 3
as a white solid.
CA 02257937 1998-12-lo
W O 98/00144 PCTrUS97/11037
- 34 -
lH NMR (CD30D): ~ 7.86 (d, J=9Hz, 2H), 7.98 (d, J=9Hz, 2H), 3.7X
(s, 3H), 3.53 (m, 4H), 3.31 (m, 4H).
BocN N-~ CO2CH3
1 -4
5 Methyl 4-(4~ 1 -dimethylethoxycarbonyl)piperazin-1 -yl)benzoate (1 -4)
To a stirred solution of amine 1 3 (15.0 g, 61.1 mmol),
NEt3 (7.42 g, 73.4 mmol) and DMF (150 ml) was added Boc2O (14.7
g, 67.2 mrnol). After 1.0 h, the solution was diluted with EtOAc and
then washed with H2O, 10% KHSO4, brine, dried (MgSO4) and
10 concentrated to furnish ester 1 4 as a yellow solid.
TLC Rf = 0.63 (silica, 40% EtOAc/hexanes)
1H NMR (CD30D): ~ 7.91 (d, J=9Hz, 2H), 7.01 (d, J=9Hz, 2H), 3.
(s, 3H), 3.59 (m, 4H), 3.3~ (m, 4H).
BocN N~ CO2H
1-5
4-(4-(1,1 -Dimethylethoxycarbonyl)piperazin- 1 -vl)benzoic acid (1 -5)
A solution of ester 1 4 (21.1 g, 61.1 mmol) 1 N NaOH (100
ml, 100 mmol) and EtOH (200 ml) was heated to 60~C for 2.0 h. The
20 solution was acidifed with 10% KHSO4 and then extracted with EtOAc.
The EtOAc phase was washed with brine, dried (MgSO4) and
concentrated to furnish acid 1 5 as a white solid.
lH NMR (CD30D): ~ 7.~1 (d, J=9Hz, 2H), 6.g~s (d, J=9Hz, 2H), 3.49
(m, 4H), 3.24 (m, 4H), 1.40 (s, 9H).
CA 02257937 l998-l2-lO
W O 98/00144 PCTrUS97/11037
SC~DE~IE 2
OH OH
BOC20 CHCI3 ~b B ~OH
UX ~ AI;qUOt 336
NaOH-H20
NH2 NHBOC PhCH3 RT
2-1 ~
OH O--
HCI (9aS) ~ HCI
EtOAC 00C ~
NHBOC NH2
2-3
COOH Cl PF6
CN l N~
N + 2-4
Pr2NEt, CH2CI2
N
BOC
1 5
BOC N~N~O O OH
CA 022~7937 1998-12-10
WO 98/00144 PCT/US97/11037
- 36-
SCHEME 2 CONT~UED
,HCI
HCI (gas) ~ ~ O
HN N~\ /)~
EtOAc, 0~C \J '~Y N~O OH
2-6
4-(1 ~ 1 -Dimethylethoxycarbonyl)aminophenol (2-2)
To a 500 mL round bottomed flask with a stirring bar,
reflux condenser and an argon inlet was added 4-aminophenol (10.00 g,
91.6 mmol), di-tert-butylpyrocarbonate (20.00 g, 91.6 mmol) and
CHC13 (250 mL). This heterogeneous mixture was heated at reflux for
6 h during which time all of the solids dissolved. The mixture was
10 cooled to room temperature and the solid product was collected by
filtration. The material was triturated with a mixture of EtOAc-hexanes
(1:2), collected on a frit and dried in vacuo to give 4-(1,1-
dimethylethoxycarbonyl)aminophenol (2-2), mp: 143- 144~C.
lH NMR (CDC13): ~ l.Sl (s, 9H), 5.27 (br ,s, lH), 6.34 (br s, lH), 6.73
(d, j=~s.SHz, 2H), 7.16 (d, j=8.5Hz, 2H).
2-(4-(1,1-Dimethylethoxycarbonyl)aminophenoxv)ethanol (2-3)
To a 500 mL round bottomed flask with a stirring bar and
an argon inlet was added aqueous NaOH (50 mL of a SN solution), 4-
20 (1,1-Dimethylethoxycarbonyl)aminophenol (5.00 g, 23.90 mmol),
toluene (100 mL), Aliquot 336 (0.88 g, 2.19 mmol), and 2-
bromoethanol (1.~6 mL, 26.29 mmol). This mixture was stirred
vigorously at ambient temperature for 4 h. An additional 2 mL of 2-
bromoethanol was added, and the reaction was continued for a total of
25 72 h. The mixture was diluted with EtOAc and the layers were
- separated. The organic phase was washed with water and brine. Drying
(MgSO4), filtration and removal of the solvent in vacuo gave 5.2 g of
CA 022~7937 1998-12-lO
W O98/00144 PCTrUS97/11037
- 37 -
an oil. This material was chromatographed on l00 g of silica gel using
35% EtOAc-hexane as eluant. 2-(4-(1,1-
dimethylethoxycarbonyl)aminophenoxy)ethanol wa.s obtained as white
needles.
lH NMR (CDC13): ~ 1.51 (s, 9H), 2.02 (t, j=6.2Hz, lH), 3.93 (m, 2H),
4.05 (m, 2H), 6.35 (br s, lH), 6.86 (d, j=9.OHz, 2H), 7.26 (d, j=9.OHz,
2H).
2-(4-Aminophenoxy)ethanol~ hydrochloride (2-4)
To a 200 mL round bottomed flask with a gas dispersion
tube was added a solution of 2-(4-(1,1-dimethylethoxycarbonyl)amino-
phenoxy)ethanol (2.15 g, 8.49 mmol) in EtOAc (100 mL). This
solution was cooled in an ice bath and dry HCl gas was sparged through
the solution, vigorously, for 5 min. The resulting mixture was aged for
15 min. at 0~C. The excess HCI and solvent were removed in vacuo and
the product was triturated with 50 mL of EtOAc and collected on a frit.
The crystals were washed with additional EtOAc and dried in vacuo to
give 1.26 g of 2-(4-aminophenoxy)ethanol, hydrochloride (76%).
lH NMR (CD30D): ~ 3.88 (t, j=4.2Hz, 2H), 4.08(t, j=4.2Hz, 2H), 7.07
(d, j=9.OHz, 2H), 7.32 (d, j=9.OHz, 2H).
2-(4-(4-(1 -(1 ,1 -Dimethylethoxycarbonyl)piperazinyl)phenylcarbonyl-
amino)phenoxy)ethanol (2-5)
To a 100 mL round bottomed flask with a stirring bar and
an argon inlet was added 4-(4-(1,1-dimethylethoxycarbonyl)piperazin-l-
yl)benzoic acid (1.00 g, 3.26 mmol), 2-(4-aminophenoxy)ethanol
hydrochloride (0.65 g, 3.43 mmol), chloro-N,N,N',N',-bis(penta-
methylene)formamidinium hexafluorophosphate (1.24 g, 3.43 mmol),
and CH2CI2 (20 mL). This mixture was cooled in an ice bath and
diisopropylethylamine (1.5 mL, 8.58 mmol) was added. The ice bath
was allowed to expire and the solution was stirred at ambient
temperature for 18 h. The CH2C12 was removed i~l vac~o and the
residue was dissolved/suspended in 300 mL of EtOAc. This mixture
was washed with 10% a4ueous citric acid, saturated aqueous NaHCO3,
CA 022~7937 1998-12-lo
W O 98/00144 PCTrUS97/11037
- 38 -
and brine. Drying (MgSO4), filtration and removal of the solvent in
vacuo gave a white solid. This material was chromatographed on 100 g
of silica gel using 70% EtOAc-hexane as eluant. There was obtained
1.30 g of 2-(4-(4-(4-(1,1-dimethylethoxycarbonyl)piperazin-1-
5 yl)phenyl)carbonylamino)phenoxy)ethanol as a crystalline solid.lH NMR (CDC13): ~ 1.49 (s, 9H), 2.02 (br s, lH), 3.28 (m, 4H), 3.59
(m, 4H), 3.96 (m, 2H), 4.09 (m, 2H), 6.93 (m, 4H), 7.52 (d, j=9.OHz,
2H), 7.78 (d, j=9.OHz, 2H).
10 2-(4-(4-( l -Piperazinyl)phenylcarbonylamino)phenoxy)ethanol,
hydroch~oride (2-6)
To a lL round bottomed flask equipped with a stirring bar
and a gas dispersion tube was added 2-(4-(4-(4-(1,1-dimethylethoxy-
carbonyl)piperazin- l -yl)phenylcarbonylamino)phenoxy)ethanol (1.30 g,
15 2.94 mmol) and 500 mL of dry EtOAc. This well stirred suspension
was cooled in an ice bath and HCI gas was sparged through the solution
for 15 min. This mixture was aged 30 min. at 0~C then the excess HCI
was removed with a stream of argon and the EtOAc was removed in
vacuo. The product was recrystallized from boiling MeOH/EtOAc (2X)
20 to give 700 mg of 2-(4-(4-(l-piperazinyl)phenylcarbonylamino)phen-
oxy)ethanol, hydrochloride, mp: >260~C.
1H NMR (DMSO-d6): ~ 3.31 (m, 4H), 3.56 (m, 4H), 3.81 (m, 2H),
4.02 (m, 2H), 4.40 (br s, 3H), 6.94 (d, j=9.OHz, 2H), 7.09 (d, j=9.OHz,
2H), 7.52 (d, j=9.OHz, 2H), 7.90 (d, j=9.OHz, 2H).
CA 02257937 1998-12-10
WO 98/00144 PCT/US97111037
- 39 -
SCHEME 3
OH OH
~b Boc20, CHCI3 ~b Br~COOEt
H3C~ reflux H3C~ Cs2CO37 DMF
NH2 NHBoc
3-1 3-2
o'~f OEt o~OEt
~ O HCl(gas) ~b ~
H3C~ EtOAc, 0~C H C~ ~HCI
NHBoc NH2
3-3 3-4
5 4-(1 ~ 1 -dimethylethoxycarbonyl)amino-3-methylphenol (3-2)
To a 1 L round bottomed flask with a stirring bar, reflux
condenser and an argon inlet was added 4-amino-3-methylphenol (15.00
g, 121.79 mmol), di-tert-butylpyrocarbonate (27.25 g, 124.84 mmol)
and CHC13 (300 mL). This heterogeneous mixture was heated at reflux
10 for 24 h during which time all of the solids dissolved. The mixture was
cooled to room temperature and the solid product was collected by
filtration. The material was triturated with a mixture of Et2O-hexanes
(1:1), collected on a frit and dried in vacuo to give 21.25 g (92%) of 4-
(1,1 -dimethylethoxycarbonyl)amino-3 -methylphenol (3-2), mp: 143-
15 144~C.1H NMR (CDC13): ~ 1.51 (s, 9H), 2.14 (s, 3H), 6.08 (br s, lH), 6.48
(m, 2H), 6.60 (br s, lH), 7.20 (d, j=8.5Hz, lH).
CA 02257937 1998-12-lo
W O 98/00144 PCTAUS97/11037
- 40 -
Ethyl 4-(1,1-dimethylethoxycarbonyl)amino-3-methylphenoxyacetate
(3-3)
To a 200 mL round bottomed flask with a stirring bar, and
an argon inlet was added 4-(1,1-dimethylethoxycarbonyl)amino-3-
5 methylphenol (5.00 g, 22.39 mmol), CS2CO3 (14.59 g, 44.78 mmol)~DMF (50 mL), and ethyl bromoacetate (2.61 mL, 23.51 mmol). This
mixture was stirred vigorously at ambient temperature for 24 h. The
mixture was filtered through a frit and the DMF was removed under
high vacuum. The residue was dissolved in EtOAc (300 mL) and
10 washed with H2O (2x) and brine (lx). Drying (MgSO4), filtration, and
removal of the solvent in vacuo gave a solid. This material was
triturated with 5% Et2o-hexane~ the solid was collected by filtration
and dried in vacuo to give 5.40 g (78%) of ethyJ 4-(1,1-dimethyl-
ethoxycarbonyl)amino-3-methylphenoxyacetate as a white, crystalline
15 solid.
lH NMR (CDC13): ~ 1.29 (t, j=7.2Hz, 3H), 1.51 (s, 9H), 2.22 (s, 3H),
4.26 (q, j=7.2Hz, 2H), 4.57 (s, 2H), 6.08 (br s, lH), 6.72 (m, 2H), 7.56
(s, lH).
20 Ethyl 4-amino-3-methylphenoxyacetate, hydrochloride (3-4)
To a 500 mL round bottomed flask with a gas dispersion
tube was added a solution of ethyl 4-(1,1-dimethylethoxycarbonyl)-
amino-3-methylphenoxyacetate (5.31 g, 17.13 mrnol) in EtOAc (200
mL). This solution was cooled in an ice bath and dry HCI gas was
25 sparged through the solution, vigorously, for 10 min. The resulting
mixture was aged for 15 min. at 0~C. The excess HCl gas was removed
with a stream of argon and the solvent was removed in vacuo. The
product was triturated with 50 mL of EtOAc and collected on a frit.
The crystals were washed with additional EtOAc and dried in vacuo to
30 give 4.21 g (100%) of ethyl 4-amino-3-methylphenoxyacetate,
hydrochloride as white crystals, mp: 198-200~C.
lH NMR (DMSO-d6): ~ 1.21 (t, j=7.1Hz, 3H), 2.33 (s, 3H), 4.17 (q,
j=7.1Hz, 2H), 4.78 (s, 2H), 6.82 (dd, j-3,9Hz, lH), 6.92 (d, j=3Hz, lH),
7.39 (d, j=9Hz, lH), 10.21 (br s, 3H).
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 41 -
SCHEME 4
OH
H CJ~ Aliquot336 H C
3 NaOH-H20 3
NHBoc PhCH3, RT NHBoc
3-2 4-1
o~,OH
HCI (gas) ~ ~HCI
EtOAc, 0~C H C ~
NH2
4-2
COOH Cl ~PF6-
[~ GNJ~N~
N + 4-2
i-Pr2NEt, CH2CI2
IN
Boc
1 -5
, .. .. . . . .
CA 02257937 1998-12-lo
W O 98/00144 PCTrUS97/11037
- 42 -
SCHEME 4 CONTINUED
Boc--N~N~N ~O OH
H3C
4-3
HCI
HCI (gas) /~ /=\ ~~
EtOAc, 0~C HN N~N~ O OH
H3C
4-4
5 2-(3-Methyl-4-(1,1-dimethylethoxycarbonyl)aminophenoxy)ethanol
(4-1)
In a manner similar to that described for compound 2-3, 2-
(3-methyl-4-(1,1-dimethylethoxycarbonyl)aminophenoxy)ethanol was
prepared.
lH NMR (CDCl3): o 1.50 (s, 9H), 2.20 (s, 3H), 2.59 (t, j=6.1Hz, lH),
3.89 (m, 2H), 4.05 (m, 2H), 6.22 (br s, lH), 6.69 (m, 2H), 7.48 (br s,
lH).
2-(3-Methyl-4-aminophenoxy)ethanol, hydrochloride (4-2)
In a manner similar to that described for compound 2-4,
2-(3-methyl-4-aminophenoxy)ethanol hydrochloride was prepared.
lH NMR (DMSO-d6): â 2.33 (s, 3H), 3.69 (t, j=4.2Hz, 2H), 4.04 (t,
j=4.2Hz, 2H), 4.5~S (br s, lH), 6.91 (m, 2H), 7.37 (d, j=9.OHz, lH),
10.18 (br s, 3H).
CA 022~7937 1998-12-10
WO 98/00144 PCT/US97/11037
- 43 -
- 2-(3-Methyl-4-(4-(1 -(1,1 -dimethylethoxycarbonyl)piperazinyl)-
phenylcarbonylamino)phenoxy)ethanol (4-3)
In a manner similar to that described for compound 2-5, 2-
(3-methyl-4-(4-(1 -(1, I -dimethylethoxycarbonyl)piperazinyl)phenyl-
5 carbonyl-amino)phenoxy)ethanol was prepared.
1H NMR (CDC13): ~ 1.49 (s, 9H), 2.04 (br s, lH), 2.28 (s, 3H), 3.2~
(m, 4H), 3.59 (m, 4H), 3.96 (m, 2H), 4.08 (m, 2H), 6.79 (m, 2H), 6.92
(d, j=9.OHz, 2H), 7.46 (s, lH), 7.67 (d, j=8.0Hz, lH), 7.80 (d, j=9.OHz,
2H).
2-(3-Methyl-4-(4-(1 -piperazinyl)phenylcarbonylamino)phenoxy)-
ethanol. hydrochloride (4-4)
In a manner similar to that described for compound 2-6,
2-(3-methyl-4-(4-(1 -piperazinyl)phenylcarbonylamino)phenoxy)ethanol,
15 hydrochloride was prepared, mp: >260~C.
lH NMR (DMSO-d6): ~ 2.17 (s, 3H), 3.21 (m, 4H), 3.53 (m, 4H), 3.71
(t, j=5.2Hz, 2H), 3.97 (t, j=5.2Hz, 2H), 4.86 (br s, lH), 6.76 (m, lH),
6.83 (d, j=2.5Hz, lH), 7.06 (d, j=9.OHz 2H), 7.15 (d, j=8.4Hz, lH), 7.89
(d, j=9.OHz, 2H), 9.40 (br s, 2H), 9.54 (s, lH).
CA 02257937 1998-12-lo
W O 98/00144 PCTAUS97/11037
- 44 -
SCHEME 5
H2N~ N~2
BOC20
J~ ~ NO2
Cs2CO 3/BrCH2CO2Et
O~CO2Et
1. H2 Pd/C
2. CH3SO2CI
o,~O~,CO2Et O ,~ ~
tBuO HN~ NHSO2CH3 H HN O
5 3 HCI/EtOAc 5-3a
,~O~,CO2Et
H2N NHSO2CH3 -5
CA 02257937 1998-12-lo
W O 98/00144 PCTAUS97/11037
- 45 -
SCHEME 5 (CONT'D)
5-4
tBuO,~ r~ ~OH
o,~ N~N ~ ~ 5-5
H ~ CO2Et
NHSO2CH3
1. HCI/ EtOAc
2. LiOH
~N~H NHSO2CH3 LiBH4
5-6
HN
o ~~~ OH
~J~NH~ NHSO2CH3
~N 5-7
tBuO~NJ
. . .
CA o2257937 1998-12 10
PCT/USg7111037
W Q 98100144
- 46 -
SCHE~lE 5 (CO~T'D)
~-7
HcllEtoAG
O~ oH
,~ NHJ~ NHSO2CH3
~--N
HCI- H~IJ
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 47 -
SCHEME 5 (CONT'D)
O ,/~O~co2Et
tBuOJ~ N ~ No2
5-2
BocN N -~ CO2H
1 -5
EtOH, HCI
o~N~N~N~ O~CO2Et
5-9 NO2
LiOH-H20
HN N~ ~ ~
H ~CO2H
5-1 0 NO2
CA 02257937 1998-12-lo
W O 98/00144 PCT~US97111037
- 48 -
SCHEME S (CONT'D)
O ~O~,CO2Et
tBuOJ~ N~ N~2
5-2
1. H2 Pd/C
2. ClS0
O ~O~,CO2Et
tBuO HN ~ N HSO
5-11 N
HCI /EtOAc
,~ O~,CO2Et
HCI- H2N NHSO
5-12 N
tBuO~ ~ ~OH
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 49 -
SCHEME 5 (CONT'D)
O ~/~O~co2Et
~ N~ HN ~ NHS~2~¢~
tBuO~,N~J 5-13 N
1 . HCI/EtOAc
2. LiOH
O ~O~,CO2H
,,¢~ NH ~ N HSO~
HNJ 5-14
CA 02257937 1998-12-lo
W O98/00144 PCTrUS97111037
- 50 -
SCHEME 5 (CONT'D)
OH
,~,,OCH3
(Aldrich)
NO2
CsCO3/BrCH2CO2Et
~0 ~, CO2Et
02N ~OCH3
5-15
Pd/C
H2
~O~,CO2Et
H2N~OCH3
5-16
CA 022~7937 1998-12-lo
W O 98/00144 PCTAUS97/11037
5-1 ~~2
2-Nitro-4-(1.1 -dimethylethoxycarbonylamino)phenol (5- 1)
A solution of 2-nitro-4-amino phenol (Aldrich) (20 g, 130
mmol) in THF (500 mL) was cooled to 0~C and treated with di-tert-
5 butyldicarbonate (64 g, 293 mmol) and triethylamine (37 mL, 265mrnol). After 24 hours the solution was concentrated and the residue
dissolved in EtOAc, washed with 10% KHSO4, saturated NaHCO3, and
brine, dried over Na2SO4, filtered and evaporated. The crude bis-
protected material (~f (40% EtOAc/Hexanes) 0.69) was then dissolved
10 in 400 mL 1:1 THF/H20 and treated with LiOH-H20 (38 g, 1.3 mol).
After stirring at room temperature overnight the solvent was removed
and the residue was dissolved in EtOAc and washed with brine, dried
with Na2SO4, filtered and concentrated to give 5-1 as a reddish, oily
solid.
15 ~f(20% EtOAc/Hexanes)=0.41
lH NMR (400 MHz, CDC13) ~ 10.35 (s, lH), 8.18 (s, lH), 7.58 (d, lH),
7.13 (d, lH), 6.45 (bs, lH), 1.55 (s, 9H).
~. .. ~. . ..
CA 022~7937 1998-12-lo
W O 98/00144 PCTrUS97/11037
O ~O~,CO2Et
tBuO H ~ N~2
5-2
Ethyl 2-(2-nitro-4-(1,1-dimethylethoxycarbonylamino)phenoxy)acetate
(5-2)
A solution of 5-1 (5 g, 19.7 mmol) in DMF (125 mL) was
5 treated with cesium carbonate (3.17 g, 9.73 mmol), stirred for 10
minutes and treated with ethyl bromoacetate (2.2 mL, 19.8 mmol) at
room temperature. After 1.5 hours the solution was concentrated under
high vacuum and the residue was absorbed to silica gel and
chromatographed in a gradient of 20 to 30% EtOAc/hexanes to give
10 5-2 as a bright yellow solid.
Rf(30% EtOAc/hexanes) 0.26
lH NMR (400 MHz, CDC13) ~ 7.95 (s, lH), 7.5 (d, lH), 6.97 (d, lH),
6.62 (bs, lH), 4.72 (s, 2H), 4.25 (q, 2H), 1.5 (s, 9H), 1.2~ (t, 3H).
~O~,CO2Et
HCI- H2N~ NHSO2CH3
5-4
Ethyl (2-methanesulfonylamino-4-aminophenoxy)acetate hydrochloride
(5-4)
A solution of 5-2 (2 g, 5.88 mmol) in EtOAc (25 mL) was
treated with 10% Pd/C (0.67 g), and hydrogenated under balloon
20 pressure for 1.5 hours. The solution was filtered through SolkaFloc,
and the cake rinsed with EtOAc. The filtrate was not concentrated but
was treated directly with methanesulfonyl chloride (3.0 mL, 39 mmol)
and pyridine (5.0 mL, 62 mmol) and stirred overnight. The solution
was concentrated and the residue was dissolved in EtOAc and washed
CA 022~7937 1998-12-lo
W O 98/00144 PCT~US97/11037
- 53 -
with 10% KHSO4, saturated Na2CO3, and brine, dried with Na2SO4,
filtered and concentrated to give a yellow oil that was chromatographed
(40% EtOAc~exanes) to give 5-3 (Rf 30% EtOAc~exanes) 0.19)
cont~min~ted with 5-3a. The mixture (1.7 g) was dissolved in EtOAc
5 (75 mL), cooled to -78~C and saturated with HCI gas, warmed to 0~C
for 1 hour and concentrated. The residue was partitioned between
CH2C12 and saturated NaCO3, the layers separated and the aqueous
layer extracted with CH2C12. The organic layer was dried over
Na2S04, filtered and evaporated and the residue chromatographed
10 (60% EtOAc/hexanes) to give 5-4 as an off-white solid.
Rf(60% EtOAc/hexanes)=0.3
1H NMR (400 MHz, CDC13) ~ 7.7 (bs, lH), 6.95 (s, lH), 6.74 (d, lH),
6.4 (d, lH), 4.6 (s, 2H), 4.33 (q, 2H), 2.98 (,s, 3H), 1.3 (t, 3H).
o~N~N ? ~N ~0
H ~CO2Et
N HSO2C H3
lS 5-5
Ethyl 2-(4-(4-(4-(1,1-dimethylethoxycarbonyl)piperazin-1-yl)phenyl-
carbonylamino)-2'-methanesulfonylaminophenoxy)acetate (5-5)
A suspension of 5-4 (0.125 g, 0.433 mmol) and 1 5 (0.136
g, 0.444 mmol) in CH2C12 (4 mL) was treated with diisopropylamine
(0.3 mL, 1.7 mmol) and PYCLU (0.173 g, 0.48 mmol) and stirred at
room temperature for three days. The solution was concentrated and
the residue was absorbed to silica gel and chromatographed in a gradient
of 20 to 60% EtOAc~exanes to give 5-5 as a pale yellow oil.
Rf (60% EtOAc~exanes) 0.27
lH NMR (400 MHz, CDC13) ~ 7.82 (dd, lH), 7.79 (s, lH), 7.77 (s, lH),
7.54 (bs, lH), 7.48 (s, lH), 6.90 (m, 3H), 4.7 (s, 2H), 4.25 (q, 2H), 3.6
(m, 4H), 3.3 (m, 4H), 3.03 (s, 3H), 1.5 (s, 9H), 1.3 (t, 3H).
. .
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 54 -
O ~O~,CO2H
,¢~ N ~ NHSO2CH3
~N
HNJ
5-6
2-(4-(4-(Piperazin- 1 -yl)phenylcarbonylamino)-2'-methanesulfonyl-
aminophenoxy)acetic acid (5-6)
A solution of 5-5 (0.093 g, 0.16 mmol) was dissolved in
S EtOAc (10 mL), cooled to -78~C and saturated with HCI gas, warmed to
0~C for 1 hour and concentrated. The resulting white solid was
dissolved in 1:1:1 H2O/THF/MeOH, treated with LiOH-H2O (0.038 g,
0.9 mmol) and stirred at room temperature for 1 hour. The reaction
was concentrated and chromatographed (18:1:1 EtOH/H20/NH40H) to
10 give a yellow oil that was diluted with CH2C12 and evaporated to give
5-6 as white solid.
Rf(9:1:1 EtOH/H20/NH40H)=0.48
H NMR (400 MHz, D2O + NaOD) ~ 7.74 (s, lH), 7.72 (s, lH), 7.21 (s,
lH), 7.07 (s, lH), 7.05 (s, lH), 6.9 (d, lH), 6.74 (d, lH), 4.38 (s, 2H),
3.15 (m, 4H), 2.85 (m, 7H).
~N ~ N J~ NHSO2C H3
tBuO NJ 5-7
o
2-(4-(4-(4-(1,1 -Dimethylethoxycarbonyl)piperazin- 1 -yl)phenyl-
carbonylamino)-2'-methanesulfonylaminophenoxy)ethanol (5-7)
A solution of 5-S (0.196 g, 0.34 mmol) was dissolved in
THF (5 mL), cooled to 0~C and treated with LiBH4 (2M in THF, 0.51
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 55 -
mL, 1.0 mmol) and allowed to warm to room temperature and stir
overnight. The solution was poured into 10% KHSO4 and extracted
with EtOAc. The organic layer was washed with satruated NaHCO3,
brine, and dried over Na2SO4, filtered and evaporated. The residue
5 was absorbed to silica gel and chromatographed in 100% EtOAc to give
5 as a white solid.
Rf(EtOAc)=0.28
lH NMR (400 MHz, CDC13 + CD30D) ~ 8.78 (s, lH), 7.85 (s, lH),
7.83 (s, lH), 7.71 (d, lH), 7.5 (s, lH), 6.93 (m, 3H), 4.1 (m, 2H), 3.9
10 (m, 2H), 3.6 (m, 4H), 3.3 (m, 4H), 3.0 (s, 3H), 1.5 (s, 9H).
O ~O~ OH
~N~H~NHSO2CH3
HCI- HNJ 5-8
2-(4-(4-(4-Piperazin- 1 -yl)phenylcarbonylamino)-2'-methanesulfonyl-
aminophenoxy)ethanol hydrochloride (5-8)
A solution of 5-7 (0.294 g, 0.466 mmol) was dissolved in
EtOAc (10 mL), cooled to -78~C and saturated with HCl gas, warmed to
0~C for 1 hour and concentrated to give 5-8 as a white solid.
Rf(9:1:1 EtOH/H2O/NH40H)=0.67.
lH NMR (400 MHz, D2O + NaOD) ~ 7.71 (s, lH), 7.70 (s, lH), 7.2 (s,
20 lH), 7.06 (s, lH), 7.05 (s, lH), 6.9 (m, 2H), 4.0 (m, 2H), 3.83 (m, 2H),
3.15 (m, 4H), 2.85 (m, 7H).
CA 02257937 1998-12-10
W O 98/00144 PCTrUS97/11037
- 56 -
o,~N~N~N~ ~CO2Et
5-9 NO2
Ethyl 2-(4-(4-(4-(1,1 -dimethylethoxycarbonyl)piperazin- l -
yl)phenylcarbonylamino)-2'-nitrophenoxy)acetate (5-9)
A solution of 5-2 (0.3 g, 0.88 mmol) was dissolved in
EtOAc (10 mL), cooled to -78~C and saturated with HCl gas, warmed to
0~C for l hour and concentrated to give ethyl 2-(2-nitro-4-
aminophenoxy)acetic acid as a white solid that was coupled immediately
(0.26 g, 0.88 mmol) to 1 5 (0.29 g, 0.95 mmol) as described for 5-5 to
10 give 5-9 as a yellow solid after chromatography in a gradient of 40 to
100% EtOAc~exanes.
Rf(50% EtOAc/hexanes)=0.22
HN N~3~H~ ~CO2H
5-10 NO2
2-(4-(4-(Piperazin- l -yl)phenylcarbonylamino)-2'-nitrophenoxy)-
acetic acid (5-10)
A solution of 5-9 (0.186 g, 0.352 mmol) in EtOAc was
treated first with HCl gas then with LiOH-H2O as described for 5-6 to
20 give 5-10 as a yellow solid after chromatography in 18:1:1
EtOH/H20/NH40H).
Rf(l 8: 1: I EtOH/H20/NH40H)=0.47
1H NMR (400 MHz, D2O) ~ 8.0 (s, lH), 7.68 (2s, 2H), 7.52 (d, lH), 7.0
(m, 2H), 3.12 (bs, 4H), 2.85 (bs, 4H).
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
tBuO~ ~
H \=( ~ CO2Et
NHSO
5-11 ll I
N~
Ethyl (3 -pyridylsulfonylamino-4-(1,1 -dimethylethoxycarbonyl)-
5 aminophenoxy)acetate (5- 11)
A solution of 5-2 (2 g, 5.88 mmol) in EtOAc (25 mL) was
treated with 10% Pd/C and 3-pyridylsulfonyl chloride (JOC, 1989, 54,
389-393) as described for 5-3 to give 5-l l after chromatography in a
gradient of 30 to 50% EtOAc/hexanes as a white solid.
10 Rf(40% EtOAc/hexanes) 0.11
lH NMR (400 MHz, CDCl3) ~ 9.02 (s, lH), 8.71 (d, lH), 8.1 (m, 2H),
7.4 (s, lH), 7.33 (m, 2H), 6.69 (d, lH), 6.58 (s, lH), 4.4 (s, 2H), 4.23
(q, 2H), 1.5 (s, 9H), 1.25 (t, 3H).
tBuO~ N~N ~ _~
O H ~ CO2Et
5-1 3 NHSO2~
Ethyl 2-(4-(4-(4-(1,1 -dimethylethoxycarbonyl)piperazin- 1 -yl)phenyl-
carbonylamino)-2'-(3-pyridylsulfonylamino)phenoxy)acetate (5- 13)
A solution of 5- 11 (0.318 g, 0.704 mmol) in EtOAc (10
mL) was treated with HCl gas as described for 5-4 to give 5-12 as a
- 20 white solid that was coupled directly with 1 5 as described for 5-5 to
CA 02257937 1998-12-10
WO 98100144 PCT/US97/11037
- 5~ -
give 5-13 as a oily yellow solid after chromatography in a gradient of
20 to 40% acetone/hexanes.
Rf(50% EtOAc~exanes) 0.41
lH NMR (400 MHz, CDC13)
S
HN~N~ ~
H ~ CO2H
5-1 4 NHSO2
H ~ ~ COOH
NHSO2 ~3
2-(4-(4-(Piperazin- 1 -yl)phenylcarbonylamino)-2'-(2-(3-pyridyl-
sulfonylamino)phenoxy)acetate (5- 14)
10A solution of 5-13 (0.047 g, 0.087 mmol) in EtOAc was
treated first with HCI gas then with LiOH-H2O as described for 5-6 to
give 5-14 as a yellow solid after chromatography in 18:1:1
EtOH/H20/NH40H) .
Rf(18: 1 :1 EtOH/H2O/NH4OH) 0.38
lSlH NMR (400 MHz, D2O + NaOD) ~ 8.76 (s, lH), 8.5 (m, lH), 8.13
(m, lH), 7.7 (m, 2H), 7.45 (m, lH), 7.12 (s, lH), 7.08 (m, 2H), 6.84
(m, lH), 6.67 (d, lH), 4.13 (s, 2H), 3.25 (m, 4H), 2.87 (m, 4H).
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 59 -
,[~~~C~2Et
O2N OCH3
5-15
Ethyl (2-methoxy-4-nitrophenoxy)acetic acid (5-15)
2-Methoxy-4-nitrophenol (Aldrich) (1.0 g, 5.9 mrnol) was
treated with cesium carbonate and ethylbromoacetate as described for 5-
5 2 to give crude 5-15 after removal of DMF. The crude material was
partitioned between water and EtOAc, the organic layer was dried with
brine and MgSO4, filtered and evaporated to give 5-15 as a yellow
solid.
Rf(50% EtOAc/hexanes)=0.54
1H NMR (400 MHz, CDC13) ~ 7.8 (d, lH), 7.76 (s, lH), 6.75 (d, lH),
4.71 (s, 2H), 4.2 (q, 2H), 3.9 (s, 3H), 1.21 (t, 3H).
~,~O~,CO2Et
H2N ~OCH3
5-16
Ethyl (2-methoxy-4-aminophenoxy)acetic acid (5-16)
A solution of 5-15 (0.7 g, 2.7 mmol) in EtOH (10 mL) was
treated with 10% Pd/C (0.14 g) and hydrogenated at balloon pressure.
The solution was filtered through Solka-Floc and evaporated to give 5-
16 as a tan oil.
lH NMR (400 MHz, CDC13) ~ 6.78 (d, lH), 6.33 (s, lH), 6.21 (d, lH),
20 4.59 (s, 2H), 4.21 (q, 2H), 3.8 (s, 3H), 3.45 (bs, 2H), 1.28 (t, 3H).
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 60 -
tBuO~ N N ~4 ~
O H ~ CO2Et
5-1 7 OCH3
Ethyl (4-(4-(4-(1,1 -dimethylethoxycarbonyl)piperazin- 1 -
yl)phenylcarbonylamino)-2'-(2-methoxy)phenoxy)acetate (S- 17)
Acid 1 S and amine 5-16 were coupled as described for 5-5
to give 5-17 as brown solid after chromatography in 50%
EtOAc/hexanes.
Rf(50% EtOAc/hexanes) = 0.13
1H NMR (400 MHz, CDC13) ~ 7.~ (d, 2H), 7.6 (d, lH), 6.92 (d, 2H),
6.~6 (m, 2H), 4.64 (.s, 2H), 4.24 (q, 2H), 3.9 (s, 3H), 3.6 (m, 4H), 3.3
(m, 4H), 1.5 (s, 9H), 1.25 (t, 3H).
HN N 4-~3~H~ ~CO2H
OCH3
2-(4-(4-(4-Piperazin- 1 -yl)phenylcarbonylamino)-2'-(2-methoxy)-
phenoxy)acetate (5- 1 ~)
Compound 5-17 was treated with LiOH and HCI gas as
described for 5-6 to give 5-18 as a white solid after chromatography in
10: 1: 1 EtOH/H2O/NH4OH.
Rf (10:1 :1 EtOH/H2O/NH4OH)=0.15
lH NMR (400 MHz, D2O) ~ 7.78 (d, 2H), 7.15 (s, lH), 7.08 (d, 2H),
6.9 (m, lHj, 6.78 (d, lH), 4.4 (s, 2H), 3.~ (s, 3H), 3.1P~ (bs, 4H), 2.88
(b,s, 4H).
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 61 -
SCHEl\/IE 6
HN~Br
H 6-
BOC20
tBuO~N~ Br
1. CH3MgBr
2. t-BuLi
3. CO2
tBuO~N N~OH
O H 6-3
. ~.. ....
CA 02257937 1998-12-lo
W O98/00144 PCTAUS97/11037
- 62 -
6-3
PYCLU H2N~O~,CO2Et
HCI ~ CH3 3-4
tBuO~f N~O O~CO2Et
O CH3 6-4
1 . HCI/EtOAc
2. LiOH
H ~ O~CO2H 6-5
CH3
CA 02257937 1998-12-lO
W O 98/00144 PCTAUS97/11037
- 63 -
tBuO~N~OH
o~\~oH
PYCLU ,~3 ~HCI
H3C
NH2
4-2
t-BuO~rN~ ~o~
~~
HCI/EtOAc
HCI ~ HN~ N~
6-7
, .
CA 022~7937 1998-12-lo
W O 98/00144 PCTrUS97111037
- 64 -
tBuO~ N H Br 6-2
2-(1,1-Dimethylethoxycarbonyl)-7-bromo 1,2,3,4-tetrahydro-9H-
pyridol3~4-Blindole (6-2)
A suspension of 6-1, prepared by the method of Rinehard et
5 al. (JACS, 1987109, p 337~s-3387) (0.366 g, 1.46 mmol) in CH2C12 (8
mL) was treated with triethylamine (0.61 mL, 4.4 mmol) followed by
di-tert-butyldicarbonate (0.38 g 1.7 mmol) for 1 hour at room
temperature. The solution was concentrated and the residue
chromatographed (20% EtOAc/hexanes) to give 6-2 a~s a white ~solid.
10 Rf (20% EtOAc/hexanes) 0.28.
1H NMR (400 MHz, CDC13) ~ 8.0-7.6 (m, lH), 7.46 (s, lH), 7.33 (d,
lH), 7.2 (d, lH), 4.6 (bs, 2H), 3.78 (bs, 2H), 2.76 (bs, 2H), 1.5 (s, 9H).
~ H ~ 6-3
15 2-(1,1 -Dimethylethoxycarbonyl)- 1,2,3 ,4-tetrahydro-9H-pyrido-
~3~4-Blindol-7-yl carboxylic acid (6-3)
A solution of 6-2 (0.26 g, 0.734 mmol) in THF (10 mL)
was cooled to 0~C and treated with methylmagnesium chloride (3.0 M in
THF, 0.29 mL, 0.87 mmol) to give a pale yellow solution. After 15
20 minutes the solution was cooled to -78~C and treated with tBuLi (1.7M
in pentane, 4.35 mL, 7.39 mmol) to give a bright yellow solution.
After 10 minutes CO2 gas was bubbled vigorously through the solution
for 10 minutes. Saturated NH4Cl, water and enough 6N NaOH to reach
pH12 were added and the solution extracted with EtOAc. The EtOAc
25 layer was back extracted with 0.5 NaOH and the aqueous layers
combined, acidified to pH 7 and extracted with EtOAc, the EtOAc layer
CA 02257937 1998-12-lo
W O 98/00144 PCTAUS97/11037
- 65 -
was dried (Na2S04) filtered and concentrated to give 6-3 as an off-
white solid.
Rf(75:25: 1 CHC13/MeOH/HOAc)=0.48
1 H NMR (400 MHz, DMSO-d6) ~ 12.0 bs, I H), 11.2 (s, lH), 7.93 (s,
lH), 7.6 (d, lH), 7.45 (d, lH), 4.6 (s, 2H), 3.68 (m, 2H), 2.7 (m, 2H),
1.4 (s, 9H).
tBuO~N~O o~CO2Et 6-4
O CH3
Ethyl (3-methyl -4-(9-H-2-(1,1 -dimethylethoxycarbonyl- 1,2,3,4-
10 tetrahydro-9H-pyrido[3,4-B~indol-7-yl-carboxamido)phenoxy)acetic
acid (6-4)
A solution of 6-3 (0.078 g, 0.25 mmol) and 3-4 (0.303 g,
1.23 mmol) in CH2C12 were treated with diisopropylamine and PYCLU
as described for 5-5 to give 6-4 as a white solid after chromatography in
15 a gradient of 40 to 60% EtOAc/hexanes.
Rf (40% EtOAc/hexanes)=0.11
1H NMR (400 MHz, CDC13) ~ 8.5-8.2 (m, lH), 8.0 (s, lH), 7.75 (d,
lH), 7.63 (s, lH), 7.52 (s, 2H), 6.83 (s, lH), 6.80 (d, lH), 4.7 (bs, 2H),
4.6 (s, 2H), 4.28 (q, 2H), 3.8 (bs, 2H), 2.83 (bs, 2H), 2.82 (s, 3H), 1.5
20 (s, 9H), 1.3 (t, 3H).
.. . . ~. ~ .
CA 02257937 1998-12-lo
W O 98100144 PCT~US97/11037
- 66 -
HN ~O~CO2H 6-5
(3-Methyl-4-(1,2,3,4-tetrahydro-9H-pyrido[3,4-B]indol-7-yl-
5 carboxamido)phenoxy)acetic acid (6-5)
A solution of 6-4 (0.082 g, 0.16 mmol) in EtOAc (10 mL)
was treated first with HCI gas, then with LiOH-H2O as described for
5-6 to give 6-5 as a white solid after chromatography in 18:1:1
EtOH/H20/NH40H .
10 Rf (18: 1.1 EtOH/H2O/NH40H)=0.4~S
lH NMR (400 MHz, D2O) ~ 7.9 (s, lH), 7.54 (m, 2H), 7.13 (d, lH),
6.84 (s, lH), 6.75 (d, lH), 4.40 (s, 2H), 3.8 (s, 2H), 3.0 (m, 2H), 2.7
(m, 2H), 2.15 (s, 3H).
tBuO~N~O O~--OH 6-6
0 CH3
(3-Methyl-4-(9-H-2-(1,1 -dimethylethoxycarbonyl)- 1,2,3,4-tetrahydro-
9H-pyrido~3,4-Blindol-7-yl-carboxamido)phenoxy)ethanol (6-6)
A solution of 6-3 (0.068 g, 0.214 mmol) and 4-2 (0.213 g,
1.04 mmol) in CH2C12 (5 mL) were treated with diisopropylamine and
20 PYCLU as described for 2-5 to give 6-6 as a white solid after
chromatography in 100% EtOAc.
Rf (100% EtOAc)=0.33
lH NMR (400 MHz, CD30D) ~ 7.98 (s, lH), 7.65 (d, lH), 7.5 (d, lH),
7.2 (d, lH), 6.9 (s, lH), 6.82 (d, lH), 5.5 (s, lH), 4.66 (s, 2H), 4.05 (m,
25 2H), 3.P~7 (m, 2H), 3.8 (m, 2H), 2.7 (m, 2H) 2.3 (s, 3H), 1.5 (s, 9H).
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 67 -
HN=~O~ ~
2-(3-Methyl-4-( 1 ,2,3 ,4-tetrahydro-9H-pyrido [3 ,4-B ]indol-7-yl-
carboxamido)phenoxy)ethanol (6-7)
S A suspension of 6-6 in dioxane was treated with HCI gas as
described for S-8 to give 6-7 as a yellow solid after chromatography in
18:1:1 EtOH/H2O/NH4OH.
Rf(18: 1:1 EtOH/H2O/NH40H)=0.5
1H NMR (400 MHz, D2O) o 7.95 (s, lH), 7.6 (m, 2H), 7.15 (d, lH),
6.93 (s, lH), 6.84 (d, lH), 4.44 (s, 2H), 4.1 (m, 2H), 3.85 (m, 2H), 3.55
(m, 2H), 3.05 (m, 2H), 2.I8 (s, 3H).
HCI. H~ o~CO2Et 6-8
Ethyl (3 -Methyl-4- 1 ,2,3 ,4-tetrahydro-9H-pyrido [3 ,4-B]indol-7-yl-
l S carboxamido)phenoxy)acetate hydrochloride (6-8)
A suspension of 6-4 in EtOAc was treated with HCI gas as
described for S-8 to give 6-g as a yellow solid.
lH NMR (400 MHz, DMSO-d6) ~ 11.2 (s, lH), 9.85 (s, lH), 9.5 (bs,
lH), 8.05 (s, lH), 7.7 (d, lH), 7.57 (d, lH), 6.85 (s, lH), 6.75 (d, IH),
4.78 (s, 2H), 4.4 (bs, 2H), 4.18 (q, 2H), 3.45 (m, 2H), 2.2 (s, 3H), 1.23
(t, 3H).
CA 02257937 l998-l2-lO
W O98/00144 PCTAUS97/11037
- 68 -
SC~nE~DE 7
t-BuO HN~OH 3-2
H3C
NBS/THF
O Br
t-BuO N~OH 7-1
H3C Br
Cs2CO3/BrCH2CO2Et
~ ,~ Br
t-BuO~< ~
H ~ ~ CO2Et
H3C Br 7-2
HCI/EtOAc
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 69 -
SCHEME 7 (CONT'D)
Br
HCI H2N~O
)=~ ~ CO2Et
H3C Br 7 3
o ~ ~OH 1-5
0~ \ / ~1~0 7~4
)=~ ~CO2Et
H3C Br
+
o~N~N~3~N~0 7-4a
~ N N~ ~ CO2Et
t-BuO ~/ ~ O H3C Br
CA 02257937 1998-12-10
WO 98/00144 PCTtUS97tllO37
- 70 -
SCHEME 7 (CONT'D)
7-4 + 7-4a
LiOH
O,~ N~N~ ~_ 7-5
~ C02H
/ EtOAc H3C Br
~ HCI
HN N~ ~
~CO2H
H3C Br
t-BuO,~ r~ ~S~ ~1
\
H3C Br
HCI- HN N~ Br
HN ~ O~ OH
7-8 H3C Br
CA 02257937 1998-12-lo
W O 98/00144 PCTnJS97/11037
O Br
t-BuO ~ ~OH 7-1
H3C Br
2,6-Dibromo-3 -methyl-4-(1,1 -dimethylethoxycarbonyl)amino-
phenol (7- 1)
A solution of 3-2 (1.0 g, 450 mmol) in 20 mL THF under
S argon was treated with N-Bromosuccinimide (1.6 g, 9 mmol) for 2 hr.
The solution was concentrated and the residue was resuspended in
carbontetrachloride and filtered. The filtrate was concentrated and
chromatographed (15% EtOAc/hexanes) to give 7-1 as a white solid.
Rf(20% EtOAc/hexanes)=0.56
10 1H NMR (400 MHz, CDCl3) ~ 7.79 (bs, lH), 6.08 (bs, lH), 5.8 (s, lH),
2.33 (s, 3H), 1.43 (s, 9H).
O Br
t-BuO~ ~
H >=~ ~ CO2Et
H3C Br 7-2
Ethyl 2-(2,6-dibromo-3-methyl-4-(1,1 -dimethylethoxycarbonyl)-
15 aminophenoxy) acetic acid (7-2)
A solution of 7-1 (0.6 g, 1.57 rnmol) in DMF was treated
with cesium carbonate and ethyl bromo acetate as described for 3-3 to
give 7-2 as a tan solid
Rf(20% EtOAc/hexanes)-0.56
20 1H NMR (400 MHz, CDCl3) o 8.0 (bs, lH), 6.21(bs, lH), 4.56 (s, 2H),
4.3 (q, 2H), 2.35 (s, 3H), 1.5 (s, 9H), 1.33 (t, 3H).
CA 02257937 1998-12-lo
W O 98/00144 PCT~US97/11037
Br
tl2N{~O
~ ~ CO2Et
H3C Br ~ ~
Ethyl (2.6-dibromo-3-methyl-4-aminophenoxy) acetic acid (7-3)
A solution of 7-2 (0.6 g, 1.29 mmol) in EtOAc (10 mL)
was cooled to -78~C, saturated with HCI gas, warmed to 0~C and stilTed
5 for 1 hour, then concentrated at ambient temperature to give 7-3 as a
tan solid.
lH NMR (400 MHz, DMSO) ~ 7.0 (s, lH), 4.8-4.4 (b, 2H), 4.41 (s, 2H),
4.2 (q, 2H), 2.18 (s, 3H), 1.2 (t, 3H).
~N N~
~ CO2Et
7 4 H3C Br
Ethyl (2,6-dibromo-3-methyl-4-~4-(N-(1,1-dimethylethoxycar-
bonyl)piperazin-4-yl)phenylcarboxamide)phenoxy) acetic acid (7-4)
A solution of 7-3 (0.520 g, 1.29 mmol) and 1 5 (0.395 g,
1.29 mmol) in CH2Cl2 was treated with chloro-N,N,N'N',-bis(penta-
15 methylene)fonnamidinium hexafluorophosphate (0.504 g, 0.1.4 mmol)and diisopropylethyl amine (0.9 mL, 5.16 mmol) and stirred at room
temperature for 24 hours. The solution was diluted with EtOAc and
washed with H2O, 10% KHSO4, saturated NaHCO3 and brine, dried
over MgSO4, filtered and evaporated. The residue was
20 chromatographed (silica gel 30% EtOAc~exanes) to give a mixture of
7 and 7-4a
Rf7-4a(50% EtOAc~exanes)=0.45
lHNMR(400MHz,CDC13)~8.0(m,2H),7.8(d,2H),7.5(s, lH),
6.93 (d, 2H), 6.85 (d, lH), 4.6 (s, 2H), 4.3 (q, 2H), 3.6 (bs, 8H), 3.35
25 (m, 8H), 2.4 ~s, 3H), 1.45 (s, 9H), 1.35 (t, 3H).
CA 02257937 l998-l2-lO
W O 98/00144 PCTAUS97/11037
Rf~(50% EtOAc/hexanes)=0.37
1H NMR (400 MHz, CDC13) ~ 7.6 (d, 2H), 6.7 (d, 2H), 4.6 (s, 2H), 4.3
(q, 2H), 3.55 (bs, 4H), 3.3 (bs, 4H), 2.3~ (s, 3H), 1.45 (s, 9H), 1.33 (t,
3H).
,~ N~N~N ~0 7-5
~ CO2H
H3C Br
2-(2,6-Dibromo-3-methyl-4-(4-(N-( 1,1 -dimethylethoxycarbonyl)-
piperazin-4-yl)phenylcarboxamide)phenoxy) acetic acid (7-5)
A solution of 7-4 and 7-4a (0.3 g) in 1:1:1 THF/MeOH/H2O
10 was treated with LiOH (0.084 g, 2 mmol) at 60~C. After 1 hour the
reaction was diluted with EtOAc and 10% KHSO4 and the layers were
separated. The organic layer was washed with H2O, brine, dried with
MgSO4, filtered and evaporated to give 7-5 as a clear oil after
chromatography in 9:0.5:0.5 CH2C12/MeOH/HOAc.
15 Rf(9:0.5:0.5 CHCl3/MeOH/HOAc)=0.6
lH NMR (400 MHz, CD30D) ~ 7.90 (d, 2H), 7.6 (s, 2H), 7.05 (d, 2H),
4.55 (s, 2H), 3.6 (bs, 4H), 3.3 (bs, 4H), 2.35 (s, 3H), 1.5 (s, 9H).
HN N~N ~0 7-6
~ HCI ~ CO2H
H3C Br
20 (2,6-Dibromo-3-methyl-4-(4-piperazin-4-yl)phenylcarbox-
amide)phenoxy) acetic acid hydrochloride (7-6)
A slurry of the intermediate acid (0.4 g, 0.6 mmol) in
EtOAc was cooled to -78~C and saturated with HCl gas. The reaction
CA 022~7937 1998-12-lo
W O98/00144 PCTrUS97/11037
- 74 -
was warmed to 0~C, then concentrated in vacuo to give 7-6 as the HCI
salt.
Rf(10:0.5:0.5 EtOH/H2O/NH4OH)=0. 18
1H NMR (400 MHz, D2O) ~ 7.73 (d, 2H), 7.23 (s, lH), 7.02 (d, 2H),
5 4.3 (s, 2H), 3.1 (bs, 4H), 2.82 (bs, 4H), 2.1 (s, 3H).
o,~N~N~ ~ ~
H3C Br OH
2-(2,6-Dibromo-3-methyl-4-(4-(N-( 1,1 -dimethylethoxycarbonyl)-
piperazin-4-yl)phenylcarboxamide)phenoxv) ethanol (7-7)
A solution of 7-4 (0.2 g, 0.32 mmol) in THF (5 mL) was
cooled to 0~C and treated with Borane (lM in THF, 3.2 mL, 3.2 mmol)
and stirred at room temperature for 4~ hours. An additional 3.2 mL of
Borane solution was added and after 15 minutes the reaction was
quenched with MeOH, stirred for 0.5 hour, concentrate, the residue was
15 dissolved in EtOAc and washed with 10% KHSO4, brine, dried over
MgSO4, filtered and evaporated to give 7-7 as a white solid.
Rf(50% EtOAc/hexanes)-0.42
1H NMR (400 MHz, CDC13) ~ 7.8 (d, 2H), 7.5 (s, lH), 6.94 (d, 2H), 4.2
(m, 2H), 4.0 (m, 2H), 3.6 (m, 4H), 3.3 (m, 4H), 2.4 (s, 3H), 1.45 (s,
20 9H).
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 75 -
HCI ~O~ OH
H3C Br
2-(2,6-Dibromo-3-methyl-4-(4-(piperazin-4-yl)phenylcarbox-
5 amide)phenoxy) ethanol hydrochloride (7-8)
A solution of 7-7 (0.15 g, 0.24 mmol) in dioxane was
treated with HCI gas as described for 7-5 to give 7-8 as a white solid.
Rf(10% MeOH/CHCI3 saturated with NH3)=0.31
1H NMR (400 MHz, CD30D) ~ 7.85 (d, 2H), 7.59 (s, lH), 7.02 (d, lH),
lO 4.1 (m, 2H), 3.95 (m, 2H), 3.0 (m, 4H), 2.54 (s, 3H).
CA 02257937 1998-12-lo
W O 98/00144 PCTrUS97/11037
- 76 -
SCHEME
N~CI
NMM, HN ~~
NMP l 'CO2Et
/=\ r~
N~ N~ >--CO2Et
8-1
N3 N3co2H
8-2
PYCLU, iPr2NEt, H2N~O /~oH
H3C
CA 022~7937 1998-12-lo
W O 98/00144 PCTrUS97/11037
_
SCHEME 8 (CONT'D)
~ ~ O
N~ N~_)~ N ~o ~OH
8-3
Ethyl 4-pyridylpiperidin-4-ylcarboxylate (P~-l)
Ethyl isonipecotate (6.0g, 38.66 mmol), 4-chloropyridine
hydrochloride (5.9g, 38.66 mmol) and N-methylmorpholine (9.3g mL,
85.00 mmol), were dissolved in N-methylpyrrolidine (50 mL) and the
resulting solution was heated at 100oC for 48h. The solution was
concentrated in vacuo and the residue was dissolved in EtOAc and
washed with water and brine (2 x 100 mL), then dried (Na2SO4) and
evaporated. The resulting residue was purified by flash
chromatography (5% MeOH / CHC13) to afford 8-1 as a crystalline
solid.
H NMR (CDC13) ~ 8.21 (d, j= 6.8 Hz, 2H), 6.78 (d, j= 6.8 Hz, 2H),
4.18 (q, j= 7.0 Hz, 2H), 3.85 (m, 2H), 3.10 (m, 2H), 2.61 (m, lH), 2.05
(m, 2H), 1.85 (m, 2H), 1.23 (t, j= 7.0 Hz, 3H).
4-Pyridylpiperidin-4-ylcarboxylic acid (8-2)
A solution of 8-1 (lOg, 42.7 mmol) in THF (50 mL) was
teated with lN LiOH (47 mL, 47.0 mmol) and water (50 mL). The
resulting solution was stirred at ambient temperature for 12h. The
solution was concentrated and the aqueous residue was cooled to 0~C,
then adjusted to pH = 6 with lN HCI. The resulting solid was collected
by filtration and dried in vacuo to afford 8-1 as a white solid.
H NMR (D2O) ~ 7.95 (d, j=6.8 Hz, 2H), 6.73 (d, j=6.8 Hz, 2H), 3.76
(d, j=12.8 Hz, 2H), 2.81 (m, 2H), 2.20 (m, lH), 1.85 (d, j=12.9 Hz, 2H),
1.55 (m, 2H).
CA 022~7937 1998-12-lo
W O 98/00144 PCTrUS97/11037
- 7~ -
N-(4-Pyridyl)piperidin-4-carbonylamino-3-methylphenoxyethanol
(~-3)
A solution of 2-(3-methyl-4-aminophenoxy)ethanol (2-4)
5 (0.29 g, 1.41 mrnol), 4-(pyridyl)(piperidin)-4-carboxylic acid (0.30 g,
1.41 mmol), chloro-N, N, N', N'-bis(pentamethylene)formamidinium
hexafluorophosphate (0.50 g, 1.41 mmol), and diisopropylamine (0.25
mL, 1.41 mmol) in dimethylformamide (15 mL) was stirred at ambient
temperature for 48 h and the solvent removed in v~cuo to give an oil.
10 This material was chromatographed on silica gel using 5:95 methanol-
ammonia saturated chloroform as eluant to give 8-3 as an off-white
solid.
1H NMR (CD30D): ~ 8.10 (d, j=6.6 Hz, 2H), 7.11 (d, j=8.6 Hz, 2H),
6.87 (d, j=6.6 Hz, 2H), 6.83 (s, lH), 6.77 (d, j=8.6 Hz, 2H), 4.10 (d,
15 j=13.4 Hz, 2H), 4.02 (t, j=4.6 Hz, 2H), 3.85 (t, j=4.9 Hz, 2H), 3.01 (t,
j=12.5 Hz, 2H), 2.72 (m, lH), 2.20 (s, 3H), 1.98 (d, j = 13.2 Hz, 2H),
1.84 (m, 2H).
CA 022~7937 1998-12-10
WO 98/00144PCT/US97/11037
- 79 -
EXAMPLE 9
Tablet Preparation
5Tablets cont:~ining 25.0, 50.0, and 100.0 mg., respectively,
of the prodrug 2-(3-Methyl-4-(4-(1-piperazinyl)phenylcarbonyl-
amino)phenoxy)-ethanol hydrochloride are prepared as illustrated
below:
10TABLE FOR DOSES CONTAINING
FROM 25-100MG OF THE PRODRUG
Amount-mg
Prodrug 25.0 50.0 100.0
Microcrystalline cellulose 37.25 100.0 200.0
Modified food corn starch 37.25 4.25 8.5
Magnesium stearate 0.50 0.75 1.5
All of the active compound, cellulose, and a portion of the
15 corn starch are mixed and granulated to 10% corn starch paste. The
resulting granulation is sieved, dried and blended with the remainder of
the corn starch and the magnesium stearate. The resulting granulation is
then compressed into tablets containing 25.0, 50.0, and 100.0 mg,
respectively, of active ingredient per tablet.
~. ..... .
CA 022~7937 1998-12-lo
W O 98100144 PCT~US97111037
- 80 -
EXAMPLE 10
Intravenous formulations
An intravenous dosage form of the above-indicated prodrug
is prepared as follows:
Prodrug 0.5- lO.Omg
Sodium Citrate 5-SOmg
Citric Acid 1-1 Smg
Sodium Chloride 1 -8mg
Water for Injection (USP) q.s. to 1 L
Utilizing the above quantities, the active compound is
10 dissolved at room temperature in a previously prepared solution of
sodium chloride, citric acid, and sodium citrate in Water for Injection
(USP, see page 1636 of United States Pharmacopeia/National Formulary
for 1995, published by United States Pharmacopeial Convention, Inc.
(Rockvil}e, Maryland, copyright 1994).
EXAMPLE 1 1
Intravenous formulation
A pharmaceutical composition was prepared at room
temperature using 2-(3-Methyl-4-(4-(1-piperazinyl)phenylcarbonyl-
amino)phenoxy)-ethanol hydrochloride, a citrate buffer, and sodium
chloride, to obtain a concentration of of 0.25 mg/ml.
800 grams of water was introduced into a standard
pharmaceutical mixing vessel. 0.25 grams of 2-(3-Methyl-4-(4-(1-
piperazinyl)phenylcarbonylamino)phenoxy)-ethanol hydrochloride was
dissolved in the water. 2.7 grams sodium citrate and 0.16 grams citric
- acid were added to obtain a finished citrate concentration of 10 mM. 8grams of sodium chloride was added. 200 grams of water was then
CA 022~7937 1998-12-10
WO 98100144 PCT/US97/11037
added to achieve the desired final concentrations of ingredients. The
resulting aqueous formulation had the following concentrations:
Ingredient Amount
2-(3-Methyl-4-(4-( 1 -piperazinyl)phenylcarbonyl-
amino)phenoxy)-ethanol hydrochloride 0.25 mg/ml
citrate buffer lO mM
sodium chloride ~S mg/ml
The finished concentrated formulation is stored in a
standard USP Type I borosilicate glass container at 30-40 degrees C.
Prior to compound administration, the concentrated formulation is
diluted in a 4:1 ratio resulting in a finished concentration of 0.05 mg/ml
and transfered to an infusion bag.
Diborane Reduction
Additional alcohol prodrugs of the present invention can be
prepared according to the procedure whereby diborane is used to reduce
the acid to the corresponding alcohol:
R~ ~, THF ~O ~OH
A B
According to the procedure, I mmol of acid A and 50 mL
- 25 of distilled dry THF is added to an oven dried round bottomed flask
with a stirring bar, septum, condenser and argon inlet to form a
solution. This solution is cooled in an ice bath and one molar equivalent
of borane-THF comple as a lM solution in THF is added with a syringe
over S min. The cooling bath is allowed to expire and the mixture is
stirred at ambient temperature until the reduction is complete. The
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 82-
reaction is quenched with methanol and the resulting mixture is stirred
one hour. The solvents are removed in vacuo and the residue is
chromatographed on silica gel to provide the desired alcohol B.
Exemplary starting materials (A) for the diborane reaction
5 are shown in the following table:
,
CA 02257937 1998-12-lo
W O 98/00144 PCTrUS97/11037
_
-83-
A Source
NH
H2N ~ CH3 O ~COOH
N~ ~ O EP381033
O COOH
~ N ~ EP381033
H2N NH O COOH
NH
H2N ~ N ~ US5,256,812
O COOH
NH
HzN ~ N ~ EP381D33
O COOH
CA 02257937 1998-12-lO
W O 98/00144
PCT/US97/1 1037
- ~34 -
A Source
O o ,~O~,COOH
H2N~N~NJ~ EP 632 019
NH
/~\ A
N~N N~ rCOOH WO 94/22834
O ,~O~,COOH
H2N~ H~ EP 632 016
NH
,~0~, COOH
~ ~ ~ EP 632 020
H2N~ O O
NH
F\ ~ '
N~N N~ rCOOH WO 94/22835
O~
COOH
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- ~5 -
A Source
HN~NJ~N ~ r COOH EP 503 548
H2N~ {/~ \ ~ rCOOH EP 503 548
H$~_ rCOOH EP 531 883
HN~o N
CA 02257937 1998-12-lo
W O 98/00144 PCT~US97/11037
- 86 -
A
Source
CH30
H3C-N ~OCH3
EP 659743
HO OCH3 ~ r COOH
H3C--N2~
EP 659743
0~ r COOH
C H30
r~ ~
H3C - N ~ OC H3
OCH3~ rCOOH EP 659743
H3C - N~
~ rCOOH EP 659743
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- ~7 -
A Source
H2N~ J~NH~O~COOH JP 7138221
NH
R N~3,o~,CooH
H2N~H JP 7179407
NH
CA 02257937 1998-12-lo
W O 98/00144 PCTAUS97/11037
A Source
O ,~O~COOH
H2N~--CH~ ~ WO 94/14775
CH3 O
N ~,N~J~ O~,COOH
H2N~/ ~ ~'COOH WO 94/14775
NH
H2N ~ ~
N~ ~ COOH
O ~O WO 94/15913
H2N
l O
O N ~O~,COOH
,~ ' ~0 COOH WO 94/22440
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
_
- 89 -
Source
COOH
NH o
H2N J~
y EP 623615
o~ - /
~COOH WO 94/21599
H O~
H2N A N~ ~=~ COOH
\~0 0~ ~COOH WO 94/21599
O~NH N ~ COOH
\{~o~ ~ C O O H
HN
NH2
CA 02257937 1998-12-10
WO 98/00144 PCT/US97/11037
- 90-
A Source
HN
H2N~5~ 1~l EP O 505 868
NHCH2C N30CH2Co2H
NH
H2N~ ~1~ EP O 505 868
NH-CH N~OCH2CO2H
CH3
H N~-- ~ EP O 635492
OcH2co2H
CA 02257937 1998-12-lo
W O 98/00144 PCTAUS97/11037
- 91 -
A Source
HN~}(CH2)2--N~N{~OCH2CO2H EP 0 587134
o
CH3{~,N{~OCH2CO2H EP 0 612 741
H
~ , NH b,N~ OC H2CO2H
H2NCH2 EP 0 632 016
N~ N N C H2C ~OCH2cO2H WO 94/22835
CA 022~7937 1998-12-lo
W O98/00144 PCT~US97/11037
- 92 -
Therapeutic Treatment
Compounds of the invention may be a~lministered to
patients where inhibition of human or m~mmalian platelet aggregation
or adhesion is desired.
Compounds of the invention are useful in inhibiting platelet
aggregation and thus, they may find utility in surgery on peripheral
arteries (arterial graft,s, carotid endaterectomy) and in cardiovascular
surgery where manipulation of arteries and organs, and/or the interation
of platelets with artificial surfaces, leads to platelet aggregation and
10 consumption. The aggregated platelets may form thrombi and
thromboemboli. Compounds of the invention may be a~1mini~tered to
these surgical patients to prevent the formation of thrombi and
thromboemboli.