Note: Descriptions are shown in the official language in which they were submitted.
CA 02258481 2003-10-08
1
ELECTROPIPETTOR AND
COMPENSATION MEANS F'OR ELECTROPHORETIC BIAS
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims priority from U.S. Patent
Application Serial No. 08/760,446, filed December 6, 1996,
which issued on March 9, 1999 as U.S. Patent No. 5,880,071,
and from U.S. Patent Application Serial No. 08/671,986,
filed June 28, 1996, which issued on July 14, 1998 as U.S.
Pa ent No. 5,779,868.
BACKGROUND OF THE INVENTION
There has been a growing interest in the manufacture
and use of microfluidic systems for the acquisition of
chemical and biochemical information. Techniques commonly
associated with the semiconductor electronics industry, such
as photolithography, wet chemical etching, etc., are being
used in the fabrication of these microfluidic systems. The
term, "microfluidic", refers to a system or device having
channels and chambers which are generally fabricated at the
micron or submicron scale, e.g., having at least one cross-
sectional dimension in the range of from about 0.1 ~m to about
500 Vim. Early discussions of the use of planar chip
technology for the fabrication of microfluidic systems are
provided in Mane et al., Trends i_n Anal. Chem. (1990)
10(5):144-149 and Manz et al., Avd. in Chrom ta-oa. (1993) 33:1-
66, which describe the fabrication of such fluidic devices and
particularly microcapillary devices, in silicon and glass
substrates.
Applications of microfluidic systems are myriad.
For example, International Patent Appln. WO 96/04547,
published February 15, 1996, describes the use of microf luidic
systems for capillary electrophoresis, liquid chromatography,
flow injection analyais, and chemical reaction and synthesis.
U.S. Patent No. 5,912,443, issued August 24, 1999,
CA 02258481 2003-10-08
2
discloses wide ranging
applications of microfluidic systems in rapidly assaying large
number of compounds for their effects on chemical, and
preferably, biochemical systems. The phrase, "biochemical
system," generally refers to a chemical interaction which
involves molecules of the type generally found within living
organisms. Such interactions include the full range of
catabolic and anabolic reactions which occur in living systems
including enzymatic, binding, signaling and other reactions.
Biochemical systems of particular interest include, e.g.,
receptor-ligand interactions, enzyme-substrate interactions,
cellular signaling pathways, transport reactions involving
model barrier systems (e.g., cells or membrane fractions) for
bioavailability screening, and a variety of other general
systems.
Many methods have been described for the transport
and direction of fluids, e.g., samples, analytes, buffers and
reagents, within these microfluidic systems or devices. One
method moves fluids within microfabricated devices by
mechanical micropumps and valves within the device. See,
Published U.K. Patent Application No. 2 248 891 (10/18/90),
Published European Patent Application No. 568 902 (5/2/92),
U.S. Patent Nos. 5,271,724 (8/21/91) and 5,277,556 (7/3/91).
See also, U.S'. Patent No. 5,171,132 (12/21/90) to Miyazaki et
al. Another method uses acoustic energy to move fluid samples
within devices by the effects of acoustic streaming. See,
Published PCT Application No. 94/05414 to Northrup and White.
A straightforward method applies external pressure to move
fluids within the device. See, e.g., the discussion in U.S.
Patent No. 5,304,487 to Wilding et al.
Still another method uses electric fields to move
fluid materials through the channels of the microfluidic
system. See, e.g., Published European Patent Application No.
376 611 (12/30/88) to Kovacs, Harrison et al., Ana?. them.
(1992) 64:1926-1932 and Manz et al. J. Chromatoa. (1992)
593:253-258, U.S. Patent No. 5,126,022 to Soane.
Electrokinetic forces have the advantages of direct control,
fast response and simplicity. However, there are still some
CA 02258481 1998-12-15
WO 98/00705 PCT/ITS97/I0963
3
disadvantages. For maximum efficiency, it is desirable that
the subject materials be transported as closely together as
possible. Nonetheless, the materials should be transported
without cross-contamination from other transported materials.
Further, the materials in one state at one location in a
microfluidic system should remain in the same state after
being moved to another location in the microfluidic system.
These conditions permit the testing, analysis and reaction of
the compound materials to be controlled, when and where as
to desired.
In a microfluidic system in which the materials are
moved by electrokinetic forces, the charged molecules and ions
in the subject material regions and in the regions separating
these subject material regions are subjected to various
electric fields to effect fluid flow.
Upon application of these electric fields, however;
differently charged species within the subject material will
exhibit different electrophoretic mobilities, i.e., positively
charged species will move at a different rate than negatively
charged species. In the past, the separation of different
species within a sample that was subjected to an electric
field was not considered a problem, but was, in fact, the
desired result, e.g., in capillary electrophoresis. However,
where simple fluid transport is desired, these varied
mobilities can result in an undesirable alteration or
"electrophoretic bias" in the subject material.
Without consideration and measures to avoid cross-
contamination, the microfluidic system must either widely
separate the subject materials, or, in the worst case, move
the materials one at a time through the system. In either
case, efficiency of the microfluidic system is markedly
reduced. Furthermore, if the state of the transported
materials cannot be maintained in transport, then many
applications which require the materials to arrive at a
location unchanged must be avoided.
The present invention solves or substantially
mitigates these problems of electrokinetic transport. With
the present invention, microfluidic systems can move materials
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
4
efficiently and without undesired change in the transported
materials. The present invention presents a high throughput
microfluidic system having direct, fast and straightforward
control over the movement of materials through the channels of
the microfluidic system with a wide range of applications,
such as in the fields of chemistry, biochemistry,
biotechnology, molecular biology and numerous other fields.
SUMMARY OF THE INVENTION
l0 The present invention provides for a microfluidic
system which electroosmotically moves subject material along
channels in fluid slugs, also termed "subject material
regions," from a first point to a second point in the
microfluidic system. A first spacer region of high ionic
concentration contacts each subject material region on at
least one side and.second spacer regions of low ionic
concentration are arranged with the subject material regions
of subject material and first or high ionic concentration
spacer regions so that at least one low ionic concentration
region is always between the first and second points to ensure
that most of the voltage drop and resulting electric field
between the two points is across the low ionic concentration
region.
The present invention also provides for a
electropipettor which is compatible with a microfluidic system
which moves subject materials with electroosmotic forces. The
electropipettor has a capillary having a channel. An
electrode is attached along the outside length of the
capillary and terminates in a electrode ring at the end of the
capillary. By manipulating the voltages on the electrode and
the electrode at a target reservoir to which the channel is
fluidly connected when the end of the capillary is placed into
a material source, materials are electrokinetically introduced
into the channel. A train of subject material regions, high
and low ionic concentration buffer or spacer regions can be
created in the channel for easy introduction into the
microfluidic system.
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
The present invention further compensates for
electrophoretic bias as the subject materials are
electrokinetically transported along the channels of a
microfluidic system. In one embodiment a channel between two
5 points of the microfluidic system has two portions with
sidewalls of opposite surface charges. An electrode is placed
between the two portions. With the voltages at the two points
substantially equal and the middle electrode between the two
portions set differently, electrophoretic forces are in
opposite directions in the two portions, while electroosmotic
forces are in the same direction. As subject material is
transported from one paint to the other, electrophoretic bias
is compensated for, while electroosmotic forces move the fluid
materials through the channel.
In another embodiment a chamber is formed at the
intersection of channels of a microfluidic system. The
chamber has sidewalls connecting the sidewalls of the
intersecting channels. When a subject material region is
diverted from one channel into another channel at the
intersection, the chamber sidewalls funnel the subject
material region into the second channel. The width of the
second channel is such that diffusion mixes any subject
material which had been electrophoretically biased in the
subject material region as it traveled along the first
channel.
In still a further embodiment, the present invention
provides a microfluidic system and method of using that system
for controllably delivering a fluid stream within a
microfluidic device having at least two intersecting channels.
The system includes a substrate having the at least two
intersecting channels disposed therein. In this aspect, the
one of the channels is deeper than the other channel. The
system also includes an electroosmotic fluid direction system.
The system is particularly useful where the fluid stream
comprises at least two fluid regions having different ionic
strengths.
The present invention also provides a sampling
system using the electropipettor of the invention. The
CA 02258481 2004-04-28
6
sampling system includes a sample substrate, which has a
plurality of different samples immobilized thereon. Also
included is a translation system for moving the
electropipettor relative to the sample substrate.
In accordance with another aspect of the invention,
there is provided a microfluidic system with compensation
for electrophoretic bias, including a capillary channel
having sides, a first end and a second end. The
capillary channel is further divided into first and
second portions, the sides of the first and second
portions having surface charges of opposite polarities.
The sides of at least one of the portions of the
capillary channel are defined by a film, the film having
the surface charge of the one portion. The system
further includes a first electrode at the first end, a
second electrode between the first and second portions of
the capillary channel, and a third electrode at the
second end. The first, second and third electrodes are
set at voltages such that a fluid is electroosmotically
pumped through the first and second portions from the
first end to the second end, and electrophoretic movement
in the second portion is opposite to electrophoretic
movement in the first portion.
In accordance with another aspect of the invention,
there is provided a microfluidic system with compensation
for electrophoretic bias, including a first capillary
channel, and a second capillary channel intersecting the
first capillary channel. The system further includes a
chamber at the intersection of the first and second
capillary channels defined by sides along a first
capillary channel length funneling into the second
capillary channel such that a region containing subject
material moving from the first capillary channel to the
second capillary channel is mixed to compensate for
electrophoretic bias in moving along the first capillary
channel.
In accordance with another aspect of the invention,
there is provided a microfluidic system, including a
substrate having at least a first channel and at least a
second channel disposed in the substrate. The at least
CA 02258481 2004-04-28
6A
second channel intersects the first channel, and the
first channel is deeper than the second channel. The
system further includes an electroosmotic fluid direction
system.
In accordance with another aspect of the invention,
there is provided a microfluidic substrate having at
least a first and a second microfluidic fluid channel
which intersect. The first channel has at least twice
the depth of the second channel.
In accordance with another aspect of the invention,
there is provided a microfluidic system with compensation
for electrophoretic bias. The system includes a first
capillary channel, and a second capillary channel
intersecting the first capillary channel. The system
further includes a chamber at the intersection of the
first and second capillary channels shaped such that a
region containing subject material moving from the first
capillary channel to the second capillary channel is
mixed to compensate for electrophoretic bias in moving
along the first capillary channel. The subject material
has a diffusion constant and the second capillary channel
has a width such that the subject material diffuses
across the second channel width before being diverted
from the second channel.
The invention as hereinbefore described may be put
into a plurality of different uses, which are themselves,
inventive, for example, as follows:
The use of a substrate having a channel, in
transporting at least a first subject material from at
least a first location to a second location along the
channel, utilizing at least one region of low ionic
concentration which is transported along the channel due
to an applied voltage.
A use of the aforementioned invention, in which the
ionic concentration of the one region is substantially
lower than that of the subject material.
A use of the aforementioned invention, wherein a
plurality of subject materials are transported, separated
by high ionic concentration spacer regions.
CA 02258481 2003-10-08
The use o-f a substrate having a channel along which
at least a first subject material may be transported, in
electrophoretic bias compensation, the channel being
divided into a first and a second portion, in which the
wall or walls of the channel are oppositely charged, such
that electrophoretic bias on the at least first subject
material due to transportation in the first portion is
substantially compensated for by electrophoretic bias due
to transport in the second portion.
A use of the aforementioned invention in which a
first electrode is located at a remote end of the first
portion, a second electrode is located at the
intersection between the portions and a third electrode
is located at a remote end of the second portion.
A use of the aforementioned invention, in which the
substrate is a microfluidic system.
A use of the aforementioned invention in which the
substrate is an electropipettor.
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
7
A use of the aforementioned invention, in which the
electropipettor has a main channel for transportation of
the subject material and at least one further channel
fluidly connected to the main channel from which a
further material to be transported along the main channel
is obtained.
A use of the aforementioned invention, in which the
further material is drawn into the main channel as a
buffer region between each of a plurality of separate
subject materials.
The use of a microfluidic system having at least a
first and a second fluid channel which intersect, in
optimizing flow conditions, the channels having different
depths.
A use of the aforementioned invention in which one
channel is between 2 to 10 times deeper than the other
channel.
The use of a microfluidic system having a first
channel and a second channel intersecting the first
channel, in electrophoretic compensation, the
intersection between the channels being shaped such that
a fluid being transported along the first channel towards
the second channel is mixed at the intersection and any
electrophoretic bias in the fluid is dissipated.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows a schematic illustration of one
embodiment of a microfluidic system;
Figure 2A illustrates an arrangement of fluid
regions traveling in a channel of the microfluidic system of
Figure 1, according to one embodiment of the present
invention; Figure B is a scaled drawing of another arrangement
of different fluid regions traveling in a channel of the
microfluidic system according to the present invention;.
Figure 3A is another arrangement with high ionic
concentration spacer regions before a subject material region
traveling within a channel of the microfluidic system; Figure
3B shows an arrangement with high ionic concentration spacer
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
8
regions after a subject material region traveling in a channel
of the microfluidic system;
Figure 4A is a schematic diagram of one embodiment
of a electropipettor according to the present invention;
Figure 4B is a schematic diagram of another electropipettor
according to the present invention;
Figure 5 is a schematic diagram of a channel having
portions with oppositely charged sidewalls in a microfluidic
system, according to the present invention; and
Figures 6A-6D illustrate the mixing action of
funneling sidewalls at the intersection of channels in a
microfluidic system, according to the present invention.
Figure 7A shows the results of three injections of a
sample fluid made up of two oppositely charged chemical
species in a low salt buffer, into a capillary filled with low
salt buffer. Figure 7B shows the results of three sample
injections where the sample is in high salt buffer, high salt
buffer fluids were injected at either end of the sample region
to function as guard bands, and the sample/guard bands were
run in a low salt buffer filled capillary. Figure 7C shows
the results of three sample injections similar to that of
Figure 7B except that the size of the low salt spacer region
between the sample/high salt spacers (guard bands) is reduced,
allowing partial resolution of the species within the sample,
without allowing the sample elements to compromise subsequent
or previous samples.
Figure 8 shows a schematic illustration of an
electropipettor for use with a sampling system using samples
immobilized, e.g., dried, on a substrate sheet or matrix.
Figure 9A is a plot of fluorescence versus time
which illustrates the movement of a sample fluid made up of
test chemical species which is periodically injected into, and
moved through, an electropipettor, according to the present
invention. Figure 9B is another plot which shows the movement
of the sample fluid with the chemical species through a
microfluidic substrate which is connected to the
electropipettor, under different parameters. Figure 9C is a
plot which illustrates the movement of the sample fluid and
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
9
chemical species through an electropipettor formed from an air
abraded substrate.
Figure 10 is a plot which again illustrates the
movement of a chemical species in a sample fluid which has
been periodically injected into an electropipettor, according
to the present invention. In this experiment, the species is
a small molecule compound.
DETAILED DESCRIPTION OF THE INVENTION
I . U~neZ<a1 Organization of a M' crnfl a l d l c- System
Fig. 1 discloses a representative diagram of an
exemplary microfluidic system 100 according to the present
invention. As shown, the overall device 100 is fabricated in
a planar substrate 102. Suitable substrate materials are
generally selected based upon their compatibility with the
conditions present in the particular operation to be performed
by the device. Such conditions can include extremes of pH,
temperature, ionic concentration, and application of
electrical fields. Additionally, substrate materials are also
selected for their inertness to critical components of an
analysis or synthesis to be carried out by the system.
Useful substrate materials include, e.g., glass,
quartz and silicon, as well as polymeric substrates, e.g.,
plastics. In the case of conductive or semiconductive
substrates, there should be an insulating layer on the
substrate. This is particularly important where the device
incorporates electrical elements, e.g., electrical fluid
direction systems, sensors and the like, or uses
electroosmotic forces to move materials about the system, as
discussed below. In the case of polymeric substrates, the
substrate materials may be rigid, semi-rigid, or non-rigid,
opaque, semi-opaque or transparent, depending upon the use for
which they are intended. For example, devices which include
an optical or visual detection element, will generally be
fabricated, at least in part, from transparent materials to
allow, or at least, facilitate that detection. Alternatively,
transparent windows of, e.g., glass or quartz, may be
incorporated into the device for these types of detection
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
elements. Additionally, the polymeric materials may have
linear or branched backbones, and may be crosslinked or non-
crosslinked. Examples of particularly preferred polymeric
materials include, e.g., polydimethylsiloxanes (PDMS),
5 polyurethane, polyvinylchloride (PVC), polystyrene,
polysulfone, polycarbonate and the like.
The system shown in Figure 1 includes a series of
channels 110, 112, 114 and 116 fabricated into the surface of
the substrate 102. As discussed in the definition of
10 "microfluidic," these channels typically have very small cross
sectional dimensions, preferably in the range of from about
0.1 ~,m to about 100 Vim. For the particular applications
discussed below, channels with depths of about 10 ~Cm and
widths of about 60 ~,m work effectively, though deviations from
these dimensions are also possible.
Manufacturing of these channels and other microscale
elements into the surface of the substrate 102 may be carried
out by any number of microfabrication techniques that are well
known in the art. For example, lithographic techniques may be
employed in fabricating glass, quartz or silicon substrates,
for example, with methods well known in the semiconductor
manufacturing industries. Photolithographic masking, plasma
or wet etching and other semiconductor processing technologies
define microscale elements in and on substrate surfaces.
Alternatively, micromachining methods, such as laser drilling,
micromilling and the like, may be employed. Similarly, for
polymeric substrates, well known manufacturing techniques may
also be used. These techniques include injection molding
techniques or stamp molding methods where large numbers of
substrates may be produced using, e.g., rolling stamps to
produce large sheets of microscale substrates, or polymer
microcasting techniques where the substrate is polymerized
within a microfabricated mold.
Besides the substrate 102, the microfluidic system
includes an additional planar element (not shown) which
overlays the channeled substrate 102 to enclose and fluidly
seal the various channels to form conduits. The planar cover
element may be attached to the substrate by a variety of
.. _.....
CA 02258481 2003-10-08
11
means, including, e.g., thermal bonding, adhesives or, in the
case of glass, or semi-rigid and non-rigid polymeric
substrates, a natural adhesion between the two components.
The planar cover element may additionally be provided with
access ports and/or reservoirs for introducing the various
fluid elements needed for a particular screen.
The system 100 shown in Figure 1 also includes
reservoirs 104, 106 and 108, which are disposed and fluidly
connected at the ends of the channels 114, 116 and 110
respectively. As shown, sample channel 112, is used to
introduce a plurality of different subject materials 120
into the device. As such, the channel 112 is fluidly
connected to a source of large numbers of separate subject
materials 120 which are individually introduced into the
sample channel 112 and subsequently into another channel
110. As shown, the channel 110 is used for analyzing the
subject materials 120 by electrophoresis to separate the
subject materials into their individual components 123
based on the different electrophoretic mobilities of the
components 123 through channel 110. It should be noted
that the term, "subject materials," simply refers to the
material, such as a chemical or biological compounds, of
interest. Subject compounds may include a wide variety of
different compounds, including chemical compounds, mixtures
of chemical compounds, e.g., polysaccharides, small organic
or inorganic molecules, biological macromolecules, e.g.,
peptides, proteins, nucleic acids, or extracts made from
biological materials, such as bacteria, plants, fungi, or
animal cells or tissues, naturally occurring or synthetic
3o compositions.
The system 100 moves materials through the channels
110, 112, 114 and 116 by electrokinetic forces which are
provided by a voltage controller that is capable of applying
selectable voltage levels, simultaneously, to each of the
reservoirs, including ground. Such a voltage controller can,
be implemented using multiple voltage dividers and multiple
CA 02258481 2003-10-08
11A
relays to obtain the selectable voltage levels.
Alternatively, multiple independent voltage sources may be
used.- The voltage controller is electrically connected to
each of the reservoirs via an electrode positioned or
fabricated within each of the plurality of reservoirs. See,
for example, published International Patent Application No. WO
CA 02258481 2003-10-08
12
96/04547 to Ramsey.
II. Elegtrokinetic Transyort
A.
The electrokinetic forces on the fluid materials in
the channels of the system 100 may be separated into
electroosmotic forces and electrophoretic forces. The fluid
control systems used in the system of the present invention
l0 employ electroosmotic force to move, direct and mix fluids in
the various channels and reaction chambers present on the
surface of the substrate 102. In brief, when an appropriate
fluid is placed in a channel or other fluid conduit having
functional groups present at the surface, those groups can
ionize. Where the surface of the channel includes hydroxyl
functional groups at the surface, for example, protons can
leave the surface of the channel and enter the fluid. Under
such conditions, the surface possesses a net negative charge,
whereas the fluid possesses an excess of protons or positive
charge, particularly localized near the interface between the
channel surface and the fluid.
By applying an electric field across the length of
the channel, cations flow toward the negative electrode.
Movement of the positively charged species in the fluid pulls
the solvent with them. The steady state velocity of this
fluid movement is generally given by the equation:
v = E E
4nn
where v is the solvent velocity, a is the dielectric constant
of the fluid, ~ is the zeta potential of the surface, E is the
electric field strength, and n is the solvent viscosity.
Thus, as can be easily seen from this equation, the solvent
velocity is directly proportional to the zeta potential'~and
the applied field.
Resides electroosmotic forces, there are also
electrophoretic forces which affect charged molecules as they
move through the channels of the system 100. In the transport
of subject materials from one point to another point in the
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
13
system 100, it is often desirable for the composition of the
subject materials to remain. unaffected in the transport, i.e.,
that the subject materials are not electrophoretically
differentiated in the transport.
In accordance with the present invention, the
subject materials are moved about the channels as slugs of
fluid (hereafter termed "subject material regions"), which
have a high ionic concentration to minimize electrophoretic
forces on the subject materials within these particular
regions. To minimize the effect of electrophoretic forces
within the subject material regions, regions of spacer fluids
("first spacer regions") are placed on either side of a slug.
These first spacer regions have a high ionic concentration to
minimize the electric fields in these regions, as explained
below, so that subject materials are essentially unaffected by
the transport from one location to another location in a
microfluidic system. The subject materials are transported
through the representative channels 110, 112, 114, 116 of the
system 100 in regions of certain ionic strengths, together
with other regions of ionic strengths varying from those
regions bearing the subject materials.
A specific arrangement is illustrated in Figure 2A,
which illustrates subject material regions~200 being
transported from point A to point B along a channel of the
microfluidic system 100. In either side of the subject
material regions 200 are first spacer regions 201 of high
ionic strength fluid. Additionally, second spacer regions 202
of low ionic concentration fluid periodically separate
arrangements of subject material regions 200 and first spacer
regions 201. Being of low ionic concentration, most of the
voltage drop between points A and B occurs across these second
spacer regions 202. The second or low concentration spacer
regions 202 are interspersed between the arrangements of
subject material region 200 and first spacer region 201 such
that, as the subject material regions 200 and the first spacer
regions 201 are electroosmotically pumped through the channel,
there is always at least one second or low ionic concentration
spacer region 202 between the points A and B. This ensures
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
14
that most of the voltage drop occurs in the second spacer
region 202, rather than across the subject material region 200
and first spacer regions 201. Stated differently, the
electric field between points A and B is concentrated in the
second spacer region 202 and the subject material regions 200
and first spacer regions 201 experience low electric fields
(and low electrophoretic forces). Thus, depending upon the
relative ionic concentrations in the subject material regions
200, first spacer regions 201 and second or low ionic
l0 concentration spacer regions 202, other arrangements of these
subject material regions 200, and first and second spacer
regions 201 and 202 can be made.
Far example, Figure 2B illustrates an arrangement in
which a second or low ionic concentration spacer region 202 is
regularly spaced between each combination of first spacer
region 201/subject material region 200/first spacer region
201. Such an arrangement ensures that there is always at
least one second or low concentration spacer region 202
between points A and B. Furthermore, the drawings are drawn
to scale to illustrate the relative lengths of a possible
combination of subject material region 200, first or high
concentration spacer region 201 and second or low
concentration spacer region 202. In the example of Figure 2B,
the subject material region 200 holds the subject material in
a high ionic concentration of 150 mM of NaCl. The subject
material region 200 is 1 mm long in the channel. The two
first spacer regions 201 have ionic concentrations of 150 mM
of NaCl. Each first spacer region 201 is 1 mm long. The
second spacer region 202 is 2 mm and has an ionic
concentration of 5 mM of borate buffer. This particular
configuration is designed to maintain a rapidly
electrophoresing compound in the subject material region 200
and buffer regions 201 while the compound travels through the
channels of the microfluidic system. For example, using these
methods, a subject material region containing, e.g., benzoic
acid, can be flowed through a microfluidic system for upwards
of 72 seconds without suffering excessive electrophoretic
bias.
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
Stated more generally, the velocity of fluid flow,
vEoe. through the channels of the microfluidic system can be
determined and, by measurement, it is possible to determine
the total distance, 1;, which a subject matter molecule is to
5 travel through the channels. Thus the transit time, tTr, for
the subject matter molecule to travel the total distance is:
tTr - 1T~VEoF
To contain a subject matter molecule x within the first spacer
region 201 next to the subject material region 200, the length
10 of the first spacer region 201, 1g, should be greater than the
electrophoretic velocity of the subject matter molecule x in
the first spacer region 201, vgX, multiplied by the transit
t ime
19 ~ (V9x) (tTr)
15 Since the electrophoretic velocity is proportional to the
electric field in the first spacer region 201, the present
invention allows control over v9X so that the subject materials
can be contained in transport through the microfluidic system
channels.
In the arrangements in Figures 2A and 2B, the first
or high ionic concentration spacer regions 201 help maintain
the position of the subject materials in the vicinity of its
subject material region 200. No matter what the polarity of
the charges of the subject material, the first spacer regions
201 on either side of the subject material region 200 ensures
that any subject material leaving the subject material region
200 is only subject to a low electric field due to the
relative high ionic concentrations in the first spacer regions
201. If the polarity of the subject material is known, then
the direction of the electrophoretic force on the molecules of
the subject material is also known.
Figure 3A illustrates an example where charges of
the subject material in all the subject material regions 200
are such that the electrophoretic force on the subject
material molecules are in the same direction as the direction
of the electroosmotic flow. Hence the first spacer regions
201 precede the subject material regions 200 in the direction
of flow. There,are no first spacer regions 201 following the
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
16
subject material regions 200 because the electrophoretic force
keeps the subject material from escaping the subject material
region 200 in that direction. By eliminating one-half of the
ffirst spacer regions 201, more subject material regions 200
with their subject material can be carried per channel length.
This enhances the transportation efficiency of the
microfluidic system. The second or low ionic concentration
spacer regions 202 are arranged with respect to the subject
material regions 200 and the first or high ionic concentration
spacer regions 201 so that high electric fields fall in the
second spacer regions 202 and the electric fields (and
electrophoretic forces) in the subject material regions 200
and first spacer regions 201 are kept low.
In Figure 3B the first spacer regions 201 follow
the subject material regions 200 in the direction of the
electroosmotic flow. In this example, the charges of the
subject material in all the subject material regions 200 are
such that the electrophoretic force on the subject matter
molecules are in the opposite direction as the direction of
the electroosmotic flow. Hence the subject material may
escape the confines of its subject material region, in effect,
being left behind by its subject material region 200. The
first spacer regions 201 following the subject material
regions 200 keep the subject material from migrating too far
from its subject material region 200. Likewise, the second or
low ionic concentration spacer regions 202 are arranged with
the subject material regions 200 and the first or high ionic
concentration spacer regions 201 so that high electric fields
fall in the second spacer regions 202 and the electric fields
in the subject material regions 200 and first spacer regions
201 are kept low.
Various high and low ionic strength solutions are
selected to produce a solution having a desired electrical
conductivity for the first and second spacer regions 201 and
202. The specific ions that impart electrical conductivity to
the solution maybe derived from inorganic salts (such as NaCl,
KI, CaClz, FeF3, (NH4) ~SO4 and so forth) , organic salts (such as
pyridinium benzoate, benzalkonium laurate), or mixed
.... T
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
17
inorganic/organic salts (such as sodium benzoate, sodium
deoxylsulfate, benzylaminehydrochloride). These ions are also
selected to be compatible with the chemical reactions,
separations, etc. to be carried out in the microfluidic
system. In addition to aqueous solvents, mixtures of
aqueous/organic solvents, such as low concentrations of DMSO
in water, may be used to assist in the solubilization of the
subject matter molecules. Mixtures of organic solvents, such
as CHCI3:MeOH, may be also used for the purpose of
l0 accelerating assays for phospholipase activity, for example.
Generally, when aqueous solvents are used, solution
conductivity is adjusted using inorganic ions. When less
polar solvents are used, organic or mixed inorganic/organic
ions are typically used. In cases where two immiscible
solvents may be simultaneously present (e.g., water and a
hydrocarbon such as decane) such that electrical current must
flow from one into the other, ionophores (e. g., valinomycin,
nonactin, various crown ethers, etc.) and their appropriate
ions may be used to conduct current through the non-polar
solvent.
B. E7ectrokinetic Control o Pressure Ba~P~ Fl~w
In the electrokinetic flow systems described herein,
the presence of differentially mobile fluids (e.g., having a
different electrokinetic mobility in the particular system) in
a channel may result in multiple different pressures being
present along the length of a channel in the system. For
example, these electrokinetic flow systems typically employ a
series of regions of low and high ionic concentration fluids
(e. g., first and second spacer regions and subject material
regions of subject material) in a given channel to effect
electroosmotic flow, while at the same time, preventing
effects of electrophoretic bias within a subject material
containing subject material region. As the low ionic
concentration regions within the channel tend to drop the most
applied voltage across their length, they will tend to push
the fluids through a channel. Conversely, high ionic
concentration fluid regions within the channel provide
CA 02258481 2003-10-08
18
relatively little voltage drop across their lengths, and tend
to slow down fluid flow due to viscous drag.
As a result of these pushing and dragging effects,
pressure variations can generally be produced along the length
of a.fluid filled channel. The highest pressure is typically
found at the front or leading edge of the low ionic
concentration regions (e. g., the second spacer regions), while
the lowest pressure is typically found at the trailing or back
edge of these low ionic strength fluid regions.
While these pressure differentials are largely
irrelevant in straight channel systems, their effects can
result in reduced control over fluid direction and
manipulation in microfluidic devices that employ intersecting
channel arrangements, i.e., the systems described in U:S.
Paverit ~o. 5,942,443, is ued August 24, 1999. For example,
where a second
channel is configured to intersect a first channel which
contains fluid regions of varying ionic strength, the pressure
fluctuations described above can cause fluid to f low in and
out of the intersecting second channel as these different
fluid regions move past the intersection. This fluctuating
flow could potentially, significantly disturb the quantitative
electroosmotically driven flow of fluids from the second
chancel, and/or perturb the various fluid regions within the
channel.
By reducing the depth of the intersecting channel,
e.g., the second channel, relative to the first or main
channel, the fluctuations in fluid flow can be substantially
eliminated. In particular, in electroosmotic fluid propulsion
or direction, for a given voltage gradient, the rate of flow
(volume/time) generally varies as the reciprocal of the depth
of the channel for channels having an aspect ratio of >10
(width:depth). With some minor, inconsequential error for the
calculation, this general ratio also holds true for lower
aspect ratios, e.g., aspect ratios >5. Conversely, the
pressure induced flow for the same channel will vary as the
third power of the reciprocal of the channel depth. Thus, the
pressure build-up in a channel due to the simultaneous
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
19
presence of fluid regions of differing ionic strength will
vary as the square of the reciprocal of the channel depth.
Accordingly, by decreasing the depth of the
intersecting second channel relative to the depth of the first
or main channel by a factor of X, one can significantly reduce
the pressure induced flow, e.g., by a factor of X3, while only
slightly reducing the electroosmotically induced flow, e.g.,
by a factor of X. For example, where the second channel is
reduced in depth relative to the first channel by one order of
magnitude, the pressure induced flow will be reduced 1000
times while the electroosmotically induced flow will be
reduced by only a factor of ten. Accordingly, in some
aspects, the present invention provides microfluidic devices
as generally described herein, e.g., having at least first and
second intersecting channels disposed therein, but where the
first channel is deeper than the second channel. Generally,
the depths of the channels may be varied to obtain optimal
flow conditions for a desired application. As such, depending
upon the application, the first channel may be greater than
about two times as deep as the second channel, greater than
about 5 times as deep as the second channel, and even greater
than about ten times as deep as the second channel.
In addition to their use in mitigating pressure
effects, varied channel depths may also be used to
differentially flow fluids within different channels of the
same device, e.g., to mix different proportions of fluids from
different sources, and the like.
III. F1_ectro~ or
As described above, any subject material can be
transported efficiently through the microfluidic system 100 in
or near the subject material regions 200. With the first and
second spacer regions 201 and 202, the subject materials are
localized as they travel through the channels of the system.
For efficient introduction of subject matter into a
microfluidic system, the present invention also provides an
electropipettor which introduces subject material into a
microfluidic system in the same serial stream of combinations
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
of subject material region 200, first and second spacer
regions 201 and 202.
A. StructLre and Operation
As illustrated in Figure 4A, an electropipettor 250
5 is formed by a hollow capillary tube 251. The capillary tube
251 has a channel 254 with the dimensions of the channels of
the microfluidic system 100 to which the channel 254 is
fluidly connected. As shown in Figure 4A, the channel 254 is,
a cylinder having a cross-sectional diameter in the range of
10 1-100 ~cm, with a diameter of approximately 30 ~cm being
preferable. An electrode 252 runs down the outside wall of
the capillary tube 251 and terminates in a ring electrode 253
around the end of the tube 251. To draw the subject materials
in the subject material regions 200 with the buffer regions
15 201 and 202 into the electropipettor channel 254, the
electrode 252 is driven to a voltage with respect to the
voltage of a target reservoir (not shown) which is fluidly
connected to the channel 254. The target reservoir is in the
microfluidic system 100 so that the subject material regions
20 200 and the buffer regions 201 and 202 already in the channel
254 are transported serially from the electropipettor into the
system 100.
Procedurally, the capillary channel end of the
electropipettor 250 is placed into a source of subject
material. A voltage is applied to the electrode 252 with
respect to an electrode in the target reservoir. The ring
electrode 253, being placed in contact with the subject
material source, electrically biases the source to create a
voltage drop between the subject material source and the
target reservoir. In effect, the subject material source and
the target reservoir become Point A and B in a microfluidic
system, i.e., as shown in Figure 2A. The subject material is
electrokinetically introduced into the capillary channel 254
to create a subject material region 200. The voltage on the
electrode 252 is then turned off and the capillary channel end
is placed into a source of buffer material of high ionic
concentration. A voltage is again applied to the electrode
252 with respect to the target reservoir electrode such that
CA 02258481 1998-12-15
WO 98/00705 PCTIUS97/10963
21
the first spacer region 201 is electrokinetically introduced
into the capillary channel 254 next to the subject material
region 200. If a second or low ionic concentration spacer
region 202 is then desirable in the electropipettor channel
254, the end of the capillary channel 254 is inserted into a
source of low ionic concentration buffer material and a
voltage applied to the electrode 252. The electropipettor 250
can then move to another source of subject material to create
another subject material region 200 in the channel 254.
By repeating the steps above, a plurality of subject
material regions 200 with different subject materials, which
are separated by first and second spacer regions 201 and 202,
are electrokinetically introduced into the capillary channel
254 and into the microfluidic system 100.
Note that if the sources of the subject material and
the buffer materials (of low and high ionic concentration)
have their own electrode, the electrode 252 is not required.
Voltages between the target reservoir and the source
electrodes operate the electropipettor. Alternatively, the
electrode 252 might be in fixed relationship with, but
separated from, the capillary tube 251 so that when the end of
the tube 251 contacts a reservoir, the electrode 252 also
contacts the reservoir. operation is the same as that
described for the Fig. 4A electropipettor.
Figure 4B illustrates a variation of the
electropipettor 250 of Figure 4A. In this variation the
electropipettor 270 is not required to travel between a
subject material source and buffer material sources to create
the first and second spacer regions 201 and 202 within the
pipettor. The electropipettor 270 has a body 271 with three
capillary channels 274, 275 and 276. The main channel 274
operates identically to the channel 254 of the previously
described electropipettor 250. However, the two auxiliary
capillary channels 275 and 276 at one end are fluidly
connected to buffer source reservoirs (not shown) and the
other end of the channels 275 and 276 is fluidly connected to
the main channel 274. One reservoir (i.e., connected to
auxiliary channel 275) holds buffer material of high ionic
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
22
concentration, and the other reservoir (i.e., connected to the
channel 276) holds buffer material of low ionic concentration.
All of the reservoirs are connected to electrodes
for electrically biasing these reservoirs for operation of the
electropipettor 270. The electropipettor 270 may also have an
electrode 272 along the walls of its body 271 which terminates
in a ring electrode 273 at the end of the main channel 274.
By applying voltages on the electrode 272 (and ring electrode
273) to create voltage drops along the channels 274, 275, 276,
not only can subject material be drawn into the main channel
274 from subject material sources, but buffer material of high
and low ionic concentrations can also be drawn from the
auxiliary channels 275 and 276 into the main channel 274.
To operate the electropipettor 270 with the
electrode 272, the end of the main capillary channel 274 is
placed into a source 280 of subject material. A voltage is
applied to the electrode 272 with respect to an electrode in
the target reservoir to create a voltage drop between the
subject material source 280 and the target reservoir. The
subject material is electrokinetically drawn into the
capillary channel 274. The capillary channel end is then
removed from the subject material source 280 and a voltage
drop is created between the target reservoir connected to the
channel 274 and the reservoir connected to the channel 275. A
first or high ionic strength spacer region 201 is formed in
the channel 274. Capillary action inhibits the introduction
of air into the channel 274 as the buffer material is drawn
from the auxiliary channel 275. If a second or low ionic
concentration spacer region 202 is then desired in the main
channel 274, a voltage is applied to the electrodes in the
target reservoir and in the reservoir of low ionic
concentration buffer material. A second spacer region 202 is
electrokinetically introduced into the capillary channel 274
from the second auxiliary channel 276. The electropipettor
270 can then move to another source of subject material to
create another subject material region 200 in the channel 274.
By repeating the steps above, a plurality of subject
material regions 200 with different subject materials, which
_.___. ... I
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
23
are separated by first and second spacer regions 201 and 202,
are electrokinetically introduced into the capillary channel
274 and into the microfluidic system 100.
If it is undesirable to expose the subject material
source to oxidation/reduction reactions from the ring
electrode 273, the electropipettor may be operated without the
electrode 272. Because electroosmotic flow is slower in
solutions of higher ionic strength, the application of a
potential (- to +) from the reservoir connecting the channel
l0 274 to the reservoir connecting the channel 275 results in the
formation of a vacuum at the point where the channels 274 and
275 intersect. This vacuum draws samples from the subject
material source into the channel 274. When operated in this
mode, the subject material is somewhat diluted with the
solutions in the channels 275 and 276. This dilution can be
mitigated by reducing the relative dimensions of the channels
276 and 275 with respect to the channel 274.
To introduce first and second spacer regions 201 and
202 into the capillary channel 274, the electropipettor 270 is
operated as described above. The capillary channel end is
removed from the subject material source 280 and a voltage
drop is created between the target reservoir for the channel
274 and the reservoir connected to the selected channels 275
or 276.
Although generally described in terms of having two
auxiliary channels and a main channel, it will be appreciated
that additional auxiliary channels may also be provided to
introduce additional fluids, buffers, diluents, reagents and
the like, into the main channel.
As described above for intersecting channels within
a microfluidic device, e.g., chip, pressure differentials
resulting from differentially mobile fluids within the
different pipettor channels also can affect the control of
fluid flow within the pipettor channel. Accordingly, as
described above, the various pipettor channels may also be
provided having varied channel depths relative to each other,
in order to optimize fluid control.
B. Method of Electro~t~et-t~r Manufacture
CA 02258481 2003-10-08
24
The electropipettor might be created from a hollow
capillary tube, such as described with respect to Fig. 4A.
For more complex structures, however, the electropipettor is
optimally formed from the same substrate material as that of
the microchannel system discussed above. The electropipettor
channels (and reservoirs are formed in the substrate in the
same manner as the microchannels for a microfluidic system and
the channeled substrate is coverad by a planar cover element,
also described above. The edges of the substrate and cover
element may then be shaped to the proper horizontal dimensions
of the pipettor, especially its end, as required. Techniques,
such as etching, air abrasion (blasting a surface with
particles and forced air), grinding and cutting, may be used.
Electrodes are then created on the surface of the substrate
and possibly cover, as required. Alternatively, the edges of
the substrate and the cover element may be shaped prior to
being attached together. This method of manufacture is
particularly suited for multichannel electropipettors, such as
described immediately above with respect to Fig. 4B and
described below with respect to Fig. 8.
IV. Wing Sv~ste~m
As described above, the methods, systems and
apparatuses described above will generally find widespread
applicability in a variety of disciplines. For example, as
noted previously, these methods and systems may be
particularly well suited to the task of high throughput
chemical screening in, e.g., drug discovery applications. such
as is described in copending U.S. Patent No. 5,942,443.
A. .amn1_e Matrices
The pipetting and fluid transport systems of the
invention are generally described in terms of sampling numbers
of liquid samples, 1.e., from multi-well plates. In many
instances, however, the number or nature of the liquid based
samples to be sampled may generate sample handling problems.
For example, in chemical screening or drug discovery
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
applications, libraries of compounds for screening may number
in the thousands or even the hundreds of thousands. As a
result, such libraries would require extremely large numbers
of sample plates, which, even with the aid of robotic systems,
5 would create myriad difficulties in sample storage,
manipulation and identification. Further, in some cases,
specific sample compounds may degrade, complex or otherwise
possess relatively short active half-lives when stored in
liquid form. This can potentially result in suspect results
10 where samples are stored in liquid form for long periods prior
to screening.
Accordingly, the present invention provides sampling
systems which address these further problems, by providing the
compounds to be sampled in an immobilized format. By
15 "immobilized format" is meant that the sample material is
provided in a fixed position, either by incorporation within a
fixed matrix, i.e., porous matrix, charged matrix, hydrophobic
or hydrophilic matrix, which maintains the sample in a given
location. Alternatively, such immobilized samples include
20 samples spotted and dried upon a given sample matrix.
In preferred aspects, the compounds to be screened are
provided on a sample matrix in dried form. Typically, such
sample matrices will include any of a number of materials that
can be used in the spotting or immobilization of materials,
25 including, e.g., membranes, such as cellulose, nitrocellulose,
PVDF, nylon, polysulfone and the like. Typically, flexible
sample matrices are preferred, to permit folding or rolling of
the sample matrices which have large numbers of different
sample compounds immobilized thereon, for easy storage and
handling.
Generally, samples may be applied to the sample
matrix by any of a number of well known methods. For example,
sample libraries may be spotted on sheets of a sample matrix
using robotic pipetting systems which allow for spotting of
large numbers of compounds. Alternatively, the sample matrix
may be treated to provide predefined areas for sample
localization, e.g., indented wells, or hydrophilic regions
surrounded by hydrophobic barriers, or hydrophobic regions
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
26
surrounded by hydrophilic barriers (e.g., where samples are
originally in a hydrophobic solution), where spotted materials
will be retained during the drying process. Such treatments
then allow the use of more advanced sample application
methods, such as those described in U.S. Patent No. 5,474,796,
wherein a piezoelectric pump and nozzle system is used to
direct liquid samples to a surface. Generally, however, the
methods described in the '796 patent are concerned with the
application of liquid samples on a surface for subsequent
reaction with additional liquid samples. However, these
methods could be readily modified to provide dry spotted
samples on a substrate.
Other immobilization or spotting methods may be
similarly employed. For example, where samples are stable in
liquid form, sample matrices may include a porous layer, gel
or other polymer material which retain a liquid sample without
allowing excess diffusion, evaporation or the like, but permit
withdrawal of at least a portion of the sample material, as
desired. In order to draw a sample into the pipettor, the
pipettor will free a portion of the sample from the matrix,
e.g., by dissolving the matrix, ion exchange, dilution of the
sample, and the like.
H. Resolubilizina Pigettor
As noted, the sampling and fluid transport methods
and systems of the present invention are readily applicable to
screening, assaying or otherwise processing samples
immobilized in these sample formats. For example, where
sample materials are provided in a dried form on a sample
matrix, the electropipetting system may be applied to the
surface of the matrix. The electropipettor is then operated
to expel a small volume of liquid which solubilizes the
previously dried sample on the matrix surface (dissolves a
retaining matrix, or elutes a sample from an immobilizing
support), e.g., by reversing the polarity of the field .applied
to the pipettor, or by applying a potential from the low ionic
concentration buffer reservoir to the high ionic concentration
buffer reservoir, as described above. Once the sample is
resolubilized, the pipettor is then operated in its typical
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
27
forward format to draw the solubilized sample into the
pipettor channel as previously described.
A schematic illustration of one embodiment of an
electropipettor useful in performing this function, and its
operation, is shown in Figure 8. Briefly, the top end 802 of
the pipettor (as shown) 800 is generally connected to the
assay system, e.g., a microfluidic chip, such that voltages
can be supplied independently to the three channels of the
pipettor 804, 806 and 808. Channels 804 and 808 are typically
fluidly connected to buffer reservoirs containing low and high
ionic concentration fluids, respectively. In operation, the
tip of the pipettor 810 is contacted to the surface of a
sample matrix 812 where an immobilized(e.g., dried) sample 814
is located. A voltage is applied from the low ionic
concentration buffer channel 804 to the high ionic
concentration buffer channel 808, such that buffer is forced
out of the end of the pipettor tip to contact and dissolve the
sample. As shown, the pipettor tip 816 may include a recessed
region or "sample cup" 818 in order to maintain the expelled
solution between the pipettor tip and the matrix surface. In
some cases, e.g., where organic samples are being screened, in
order to ensure dissolution of the sample, an appropriate
concentration of an acceptable solvent, e.g., DMSO, may be
included with the low ionic concentration buffer. Voltage is
then applied from the high ionic concentration buffer channel
to the sample channel 806 to draw the sample into the pipettor
in the form of a sample plug 820. Once the sample is
completely withdrawn from the sample cup into the pipettor,
the high surface tension resulting from air entering the
sample channel will terminate aspiration of the sample, and
high ionic concentration buffer solution will begin flowing
into the sample channel to form a first spacer region 822,
following the sample. Low ionic concentration buffer solution
may then be injected into the sample channel, i.e., as a
second spacer region 824, by applying the voltage from the low
ionic concentration buffer channel 804 to the sample channel
806. Prior to or during presentation of the next sample
position on the matrix, a first or high ionic concentration
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
28
spacer region 822 may be introduced into the sample channel by
applying the voltage between the high ionic concentration
buffer channel and the sample channel. As noted previously, a
roll, sheet, plate, or numerous rolls, sheets or plates, of
sample matrix having thousands or hundreds of thousands of
different compounds to be screened, may be presented in this
manner, allowing their serial screening in an appropriate
apparatus or system.
V.
As explained above, electrokinetic forces are used
to transport the subject material through the channels of the
microfluidic system 100. If the subject material is charged
in solution, then it is subject to not only electroosmotic
forces, but also to electrophoretic'forces. Thus the subject
material is likely to have undergone electrophoresis in
traveling from one point to another point along a channel of
the microfluidic system. Hence the mixture of the subject
material or the localization of differently charged species in
a subject material region 200 at the starting point is likely
to be different than the mixture or localization at the
arrival point. Furthermore, there is the possibility that the
subject material might not even be in the subject material
region 200 at the arrival point, despite the first spacer
regions 201.
Therefore, another aspect of the present invention
compensates for electrophoretic bias as the subject materials
are transported through the microfluidic system 100. One way
to compensate for electrophoretic bias is illustrated in
Figure 5. In the microfluidic system 100 described above',
each of the channels 110, 112, 114 and 116 was considered as a
unitary structure along its length. In Figure 5, an exemplary
channel 140 is divided into two portions 142 and 144. The
sidewalls of each channel portion 142 and 144 have surface
charges which are of opposite polarity. The two channel
portions 142 and 144 are physically connected together by a
salt bridge 133, such as a glass frit or gel layer. While the
salt bridge 133 separates the fluids in the channel 140 from
1
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
29
an ionic fluid in a reservoir 135 which is partially defined
by the salt bridge 133, the salt bridge 133 permits ions to
pass through. Hence the reservoir 135 is in electrical, but
not fluid, communication with the channel 140.
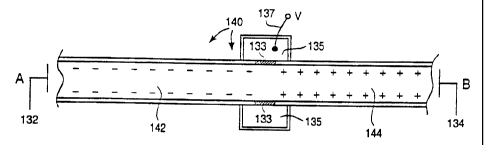
To impart electroosmotic and electrophoretic forces
along the channel 140 between points A and B, electrodes 132
and 134 are arranged at points A and B respectively.
Additionally, a third electrode 137 is placed in the reservoir
135 at the junction of the two portions 142 and 144. The
electrodes 132 and 134 are maintained at the same voltage and
the electrode 137 at another voltage. In the example
illustrated in Figure 5, the two electrodes 132 and 134 are at
a negative voltage, while the electrode 137 and hence the
junction of the two portions 142 and 144 are at zero voltage,
i.e., ground. Thus the voltage drops, and hence the electric
fields, in the portions 142 and 144 are directed in opposite
directions. Specifically, the electric fields point away from
each other. Thus the electrophoretic force on a particularly
charged molecule is in one direction in the channel portion
142 and in the opposite direction in the channel portion 144.
Any electrophoretic bias on a subject material is compensated
for after traveling through the two portions 142 and 144.
The electroosmotic force in both portions 142 and
144 are still in the same direction, however. For example,
assuming that the sidewalls of the channel portion 142 have
positive surface charges, which attract negative ions in
solution, and the sidewalls of the channel portion 144 have
negative surface charges, which attract positive ions in
solution, as shown in Figure 5, the electroosmotic force in
both portions 142 and 144 is to the right of the drawing.
Thus the subject material is transported from point A to point
B under electroosmotic force, while the electrophoretic force
is in one direction in one portion 142 and in the opposite
direction in the other portion 144.
To create a channel with sidewalls having positive
or negative surface charges, one or both portions of the
channel is coated with insulating film materials with surface
charges, such as a polymer. For example, in the microfluidic
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
system 100 the substrate 102 and the channels may be formed of
glass. A portion of each channel is coated with a polymer of
opposite surface charge, such as polylysine, for example, or
is chemically modified with a silanizing agent containing an
5 amino function, such as aminopropyltrichlorosilane, for
example. Furthermore, the surface charge densities and
volumes of both channel portions should be approximately the
same to compensate for electrophoretic bias.
Rather than being formed in a solid planar
10 substrate, the channel can also be formed by two capillary
tubes which are butted together with a salt bridge which
separates an ionic fluid reservoir from fluids in the
capillary tubes. An electrode is also placed in the ionic
fluid reservoir. One capillary tube has a negative surface
15 charge and the other capillary tube a positive surface charge.
The resulting capillary channel operates as described above.
Figures 6A-6D illustrates another embodiment of the
present invention for which the effects of electrophoretic
bias induced upon the subject material is moving from point A
20 to point B are compensated. In this embodiment the subject
material is mixed at point B, an intersection between two
channels, such as illustrated in Figure 1.
Figures 6A-6D show a chamber 160 is formed at the
intersection of the channels 150, 152, 154 and 156. The
25 chamber 160 has four sidewalls 162, 164, 166 and 168. The
sidewall 162 connects a sidewall of the channel 152 to a
sidewall of the channel 150; the sidewall 164 connects a
sidewall of the channel 154 to the other sidewall of the
channel 152; the sidewall 166 connects a sidewall of the
30 channel 156 to the other sidewall of the channel 154; and the
sidewall 168 connects the opposite sidewall of the channel 156
to the opposite sidewall of the channel 150. Assuming a flow
of materials through the channel 152 toward the channel 156,
the sidewalls 162 and 168 form as a funnel if the materials
are diverted into the channel 150.
The dimensions of the sidewalls 162 and 168
accommodate the length of a subject material plug 200
traveling along the channel 152. The sidewalls 162 and 168
T
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
31
funnel down the plug 200 into the width of the channel 150.
The width of~the channel 150 is such that diffusion of the
subject material occurs across the width of the channel 150,
i.e., mixing occurs and any electrophoretic bias of the
subject material created in the subject material region 200
traveling along the channel 162 is eliminated. For example,
if the channel 150 is 50 ~,m wide, diffusion across the channel
occurs in approximately one second for a molecule having a
diffusion constant of 1 x 10-5 cm2/sec.
In Figure 6A a plug 200 of cationic subject material
is moving along the channel 152 towards the channel 156: By
the time the plug 200 reaches the chamber 160, the subject
material has undergone electrophoresis so that the material is
more concentrated at the forward end of the subject material
region 200. This is illustrated by Figure 6B. Then the
voltage drop impressed along the channels 152 and 156 is
terminated and a voltage drop is created along the channels
154 and 150 to draw the subject material region 200 into the
channel 150. The sidewalls 162 and 168 of the chamber 160
funnel the subject material region 200 with its
electrophoretically biased subject material. This is
illustrated by Figure 6C. By diffusion the subject material
is spread across the width of the channel 150 before the
subject material travels any significant distance along the
channel 150; the subject material in the subject material
region 200 is mixed and ready for the next step of operation
in the microfluidic system 100.
In addition to its use in correcting electrophoretic
bias within a single sample, it will be appreciated that the
structure shown in Fig. 6 will be useful in mixing fluid
elements within these microfluidic devices, e.g., two distinct
subject materials, buffers, reagents, etc.
EXAMPLES
F-xamp~e i- Forced Co-Migration of Differentials Charged
Sneci es,. in E1_ect_ro~oettr,r_T~~be Format
In order to demonstrate the efficacy of methods used
to eliminate or reduce electrophoretic bias, two oppositely
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
32
charged species were electrokinetically pumped in a capillary
channel, and comigrated in a single sample plug. A Beckman
Capillary Electrophoresis system was used to model the
electrophoretic forces in a capillary channel.
In brief, a sample containing benzylamine and
benzoic acid in either low ionic concentration {or "low salt")
(5 mM borate), or high ionic concentration ("high salt") (500
mM borate) buffer, pH 8.5, was used in this experiment. The
benzoic acid was present at approximately 2X the concentration
of the benzylamine. All injections were timed for 0.17
minutes. Injection plug length was determined by the
injection voltage, 8 or 30 kV. The low salt and high salt
buffers were as described above.
In a first experiment, three successive injections
of sample in low salt buffer were introduced into a capillary
ffilled with low salt buffer. Injections were performed at 8
kV and they were spaced by low salt injections at 30 kV. Data
from these injections is shown in Figure 7A. These data show
that benzylamine (identifiable as short peaks, resulting from
its lesser concentration) from the first and second injections
precede the benzoic acid peak (tall peak) from the first
injection. Further, the benzylamine peak from the third
injection is nearly coincident with the first benzoic acid
peak. Thus, this experiment illustrates the effects of
electrophoretic bias, wherein sample peaks may not exit a
capillary channel in the same order they entered the channel.
As can be clearly seen, such separations can substantially
interfere with characterization of a single sample, or worse,
compromise previously or subsequently introduced samples.
In a second experiment, the capillary was filled
with low salt buffer. Samples were injected by first
introducing/injecting high salt buffer into the capillary at 8
kV (first spacer region 1). This was followed by injection of
the sample in high salt buffer at 8 kV, which was followed by
a second injection of high salt buffer at 8 kV (first spacer
region 2). Three samples were injected in this manner, and
spaced apart by injecting a low salt buffer at 30 kV. As can
be seen in Figure 7B, both compounds contained in the sample
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
33
were forced to co-migrate through the capillary channel in the
same sample plug, and are represented by a single peak from
each injection. This demonstrates sample registration,
irrespective of electrophoretic mobility.
By reducing the size of the low salt spacer plug
between the samples, relative to the size of the samples,
partial resolution of the components of each sample injection
can be accomplished. This may be useful where some separation
of a sample is desired during electrokinetic pumping, but
l0 without compromising subsequently or previously injected
samples. This was carried out by injecting the low-salt
spacer plug at 8 kV rather than 30 kV. The data from this
example is shown in Figure 7C.
EX~~le 2 - M~aration of Subi-,eat Materials through
Figs. 9A-9C illustrate the experimental test results
of the introduction of a subject material, i.e., a sample,
into a microfluidic system substrate through an
electropipettor as described above. The sample is rhodamine B
in a phosphate buffered saline solution, pH 7.4. A high ionic
concentration ("high salt") buffer was also, formed from the
phosphate buffered saline solution, pH 7.4. A low ionic
concentration ("low salt") buffer was formed from 5 mM Na
borate, pH 8.6, solution.
In the tests subject material regions containing the
fluorescent rhodamine B were periodically injected into the
capillary channel of an electropipettor which is joined to a
microfluidic system substrate. High salt and low salt buffers
were also injected between the subject material regions, as
described previously. Fig. 9A is a plot of the fluorescence
intensity of the rhodamine B monitored at a point along the
capillary channel near its junction with a channel of the
substrate versus time. (In passing, it should be observed
that the numbers on fluorescent intensity axis of the Figs.
9A-9C and 10 plots are for purposes of comparative reference,
rather than absolute values.) The injection cycle time was 7
seconds and the electric field to move the subject material
regions through the electropipettor was 1000 volts/cm. The
CA 02258481 1998-12-15
WO 98/00705 PCT/US97/10963
34
integration time for the photodiode monitoring light from the
capillary channel was set at 10 msec. From the Fig. 9A plot,
it should be readily evident that light intensity spikes
appear at 7 second intervals, matching the injection cycle
time of the fluorescent rhodamine B.
In another experiment, the same buffers were used
with the rhodamine B samples. The monitoring point was in the
channel of the substrate connected to the electropipettor.
The injection cycle time was set at 13.1 seconds and the
l0 voltage between the source reservoir containing the rhodamine
B and destination reservoir in the substrate set at -3000
volts. The monitoring photodiode integration time was 400
msec. As shown in Fig. 9B, the fluorescent intensity spikes
closely match the rhodamine B injection cycle time.
The results of a third experimental test are
illustrated in Fig. 9C. In this experiment the
electropipettor was formed from a substrate and shaped by air
abrasion. The monitoring point is along the capillary channel
formed in the substrate (and a planar cover). Here the sample
material is 100 uM of rhodamine B in a buffer of PBS, pH 7.4.
The high salt buffer solution of PBS, pH 7.4, and a low salt
buffer solution of 5 mM Na borate, pH 8.6, are also used.
Again periodic spikes of fluorescent intensity match the
cyclical injection of rhodamine B into the electropipettor.
Fig. 10 illustrates the results of the cyclical
injection of another subject material into an electropipettor,
according to the present invention. In this experiment the
sample was a small molecule compound of 100 ~,M with 1% DMSO in
a phosphate buffered saline solution, pH 7.4. A high salt
buffer of the same phosphate buffered saline solution, pH 7.4,
and a low salt buffer of 5 mM Na borate, pH 8.6, was also
used. The applied voltage to move the subject material
regions through the electropipettor was -4000 volts, and the
integration time for the photodiode monitoring light from the
capillary channel was set at 400 msec. The samples were
periodically injected into the electropipettor as described
previously. As in the previous results, the Fig. 10 plot
illustrates that for small molecule compounds, the
_....____. T
CA 02258481 2003-10-08
electropipettor moves the samples at evenly spaced time (and
space) intervals.
While the foregoing invention has been described in
some detail for purposes of clarity and understanding, it will
5 be clear to one skilled in the art from a reading of this
disclosure that various changes in form and detail can be made
without departing from the true scope of the invention. For
example, all the techniques described above may be used in
various combinations.