Note: Descriptions are shown in the official language in which they were submitted.
CA 022~8~36 1998-12-16
WO 97/48682 PCT/US97/09744
HETEROCYCLYLlvlETHYLAMTNO DERTVATIVES OF ~YCLOBUTENE-3,~DIONES AS POTAS-
SIUM CHANNEL MODULATORS
Back~round of Invention
The present invention relates to novel heterocyclylmethylamino derivatives of
cyclobutene 3-4-diones having pharmacological activity, to a process for their
preparation, to pharmaceutical compositions containing them and to their use in the
treatment of disorders associated with smooth muscle contraction via potassium
10 channel modulation. Such disorders include, but are not limited to: urinary
incontinence, ~cthm:~, premature labor, irritable bowel syndrome, congestive heart
failure, angina, cerebral vascular disease and hypertension.
Stemp et al. (EP-426379) disclose a class of amino substituted
15 cyclobutenedione derivatives of chromans described as having blood pressure
lowering activity and bronchodilatory activity. Takeno et al. report a series ofminncyclobuten-3,4-diones in Public Patent Disclosure Bulletin No. 6-92915. Our
own efforts in this area have been disclosed in the following US Patents: 5,464,867;
5,466,712; 5,403,853; 5,403,854; 5,397,790 and 5,401,753. Several series of 1-
20 amino-2-phenylalkylamino-cyclobutene-3,4-diones are reported as H-2 receptor
antagonists by Algieri et al. in US Patent 4,390,701. Several related l-amino-2-phenoxyalkylamino derivatives are disclosed by Nohara et al. in US Patent 4,673,747.
Additionally, several related l-amino-2-pyridyloxyalkylamino derivatives are
disclosed by Nohara et al. in EP-177016. The compounds of Nohara et al. are
25 reported as H-2 receptor antagonists.
A 4-pyridinylmethylamino derivative of cyclobutendione was disclosed by
Chandrakumar et al. in US Patent 5,354,746 to possess analgesic activity. The
compounds of the Chandrakumar series require the presence of a tricyclic
30 dibenzoxazepine moiety. A 3-pyridinylmethylamino derivative of cyclobutendione
was disclosed by Ife in EP-112704 and was reported to be an H-2 antagonist. The
compounds of the Ife series require the presence of an N'-pyridyl-diamino moiety.
CA 022~8~36 1998-12-16
WO 97/48682 PCT/US97/09744
-- 2 --
The syntheses of variously substituted 1,2-diamino-cyclobutene-3,4-diones
are described in the following publications: Tietze et al., Chem Ber. 1991, 124, 1215;
Tietze et al., Bioconjugate Chem. 1991, 2, 148; Ehrhardt et al., Chem. Ber. 1977,
110, 2506, and Neuse et al., Liebigs Ann. Chem. 1973, 619.
T~scriDtion of The ~nvention
In accordance with the present invention, there is provided a group of
compounds represented by the for,-nula (I):
A~N ~ Rl
R~ R2 (I)
wherein:
Rl and R2 are, independently, hydrogen, straight chain alkyl of 1 to 10 carbon
atoms, branched chain alkyl of 3 to lQ carbon atoms, cycloalkyl of 3 to
8 carbon atoms, bicycloalkyl of 4 to 10 carbon atoms or aralkyl of 7 to
20 carbon atoms, wherein the aromatic moiety of the aralkyl group
may be optionally substituted with one to three straight chain alkyl of
2Q 1 to 10 carbon atoms, branched chain alkyl of 1 to 10 carbon atoms,halogen, nitro, cyano, alkoxy of 1 to 6 carbon atoms, alkoxycarbonyl
of 2 to 7 carbon atoms, t~;ifluoromethyl or trifluc l~JIlle~hoxy groups;
R3 is hydrogen, formyl, alkanoyl of 2 to 7 carbon atoms, alkenoyl of 3 to 7
carbon atoms, alkylsulfonyl of 1 to 7 carbon atoms, aroyl of 7 to 12
carbon atoms, arylalkenoyl of g to 20 carbon atoms, arylsulfonyl of 6
to 12 carbon atoms, arylalkanoyl of 8 to 12 carbon atoms or arylalkyl-
sulfonyl of 7 to 12 carbon atoms;
CA 02258536 1998-12-16
WO 97148682 PCT/US97/09744
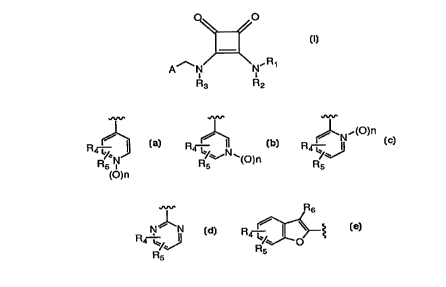
A is selected from the group consisting of:
R~3 4 ~N~o R4 ~D R4--
(O)n
R6
R4--
wherein:
nisOor 1;
R4, Rs and R6 are, independently, cyano, nitro, amino, alkyl of 1 to 6
carbon atoms, perfluoroalkyl of 1 to 6 carbon atoms, alkoxy, of
1 to 6 carbon atoms, perfluoroalkoxy of 1 to 6 carbon atoms,
aLkylamino of 1 to 6 carbon atoms, dialkylamino of 2 to 12
carbon atoms, sulfamyl, alkylsulfonamido of 1 to 6 carbon
atoms, arylsulfonamido of 6 to 12 carbon atoms, alkyl-
carboxamido of 2 to 7 carbon atoms, arylcarboxamido of 7 to
13 carbon atoms, alkanoyl of 2 to 6 carbon atoms, alkyl-
sulfonyl of 1 to 6 carbon atoms, perfluoroalkylsulfonyl of 1 to
6 carbon atoms, arylsulfonyl of 6 to 12 carbon atoms, chloro,
bromo, fluoro, iodo, 1-imi<1~7.~1yl, carboxyl or hydrogen;
or a pharrnaceutically acceptable salt thereof.
A preferred aspect of this invention includes compounds of formula (I)
wherein:
Rl and R2 are as stated above;
R3 is hydrogen;
CA 02258536 1998-12-16
WO 97/48682 PCTIUS97/09744
A is selected from the following:
~r ~ ~r
R~3 R4--~N R4--
4 ~
wherein R4, Rs and R6 are as defined above;
or a pharmaceutically acceptable salt thereof.
Preferred values of Rl and R2 are, independently, hydrogen, methyl, ethyl,
n- or iso-propyl, n-, iso, sec- or tert-butyl, straight or branched chain pentyl, or
phenylalkyl in which the alkyl is methylene or ethylene and in which the phenyl
moiety may be optionally substituted with one to three substituents selected from
methyl, ethyl, fluorine, chlorine, bromine, nitro, cyano, methoxy, ethoxy,
methoxyoxycarbonyl, ethoxyoxycarbonyl, trifluoromethyl and trifluoromethoxy.
1~ R3 is preferably hydrogen. T'referably n is 0.
Preferred values of R4, Rs and R6 are, independently, hydrogen, cyano, nitro,
amino, methyl, methoxy, chloro, bromo, fluoro, iodo, trifluoromethyl,
trifluoromethoxy or carboxyl.
Compounds of formula (T) of particular interest are:
3-(1,1-dimethylpropylamino)-4-[(pyridin-4-ylmethyl)-amino]-cyclobut-3-ene- 1,2-
dione or a pharmaceutically acceptable salt thereof;
3-(1,1 -dimethylpropylamino)-4-[pyridin-3-ylmethyl)amino]cyclobut-3-ene- 1 ,2-dione
25 or a pharmaceutically acceptable salt thereof;
CA 022~8~36 1998-12-16
WO 97/48682 PCT/US97/09744
3-(1,1-dimethylpropylamino)-4-[(pyridin-2-ylmethyl)amino]-cyclobut-3-ene- 1,2-dione
or a pharmaceutically acceptable salt thereof;
3-tert-butylamino-4-[(pyridin4-ylmethyl)amino]-cyclobut-3-ene-1,2-dione or a
pharmaceutically acceptable salt thereof;
3-tert-butylamino-4-~(pyridin-3-ylmethyl)arnino]-cyclobut-3-ene-1,2-dione or a
pharm~reutically acceptable salt thereof;
3-tert-butylamino-4-,r(pyridin-2-ylmethyl)amino]-cyclobut-3-ene-1,2-dione or a
pharmaceutically acceptable salt thereof;
3-(isopropyl-methyl-amino)-4-[(pyridin-4-ylmethyl)-amino] -cyclobut-3-ene- 1 ,2-dione
or a pharmaceutically acceptable salt thereof;
3-[(5-nitro-benzofuran-2-ylmethyl)-amino-4-( 1 ,2,2-t;imethylpropylamino)-cyclobut-3-
ene-1,2-dione or a pharmaceutically acceptable salt thereof;
3-(1, 1 -dimethyl-propylamino)-4-[(S-nitro-benzofuran-2-ylmethyl)-amino~-cyclobut-3-
ene-1,2-dione or a ph~ eutically acceptable salt thereof;
3-tert-butylamino-4-[~5-nitro-benzofuran-2-ylmethyl)-amino]-cyclobut-3-ene-1,2-dione
or a pharm~,reutically acceptable salt thereof;
2- { [2-(1,1 -dimethyl-propylamino)-3,4-dioxo-cyclobut- 1 -enylamino] -methyl } -
ben~ofu~dn-5-carbonitrile or a pharm~ce~ltically acceptable salt thereof;
2- { [3,4-dioxo-2-(1 ,2,2-trimethyl-propylamino)-cyclobut- l -enylamino]-methyl } -
benzofuran-5-ca bol iLlile or a pharm:~relltically acceptable salt thereof;
2-[(2-tert-butylamino-3,4-dioxo-cyclobut- 1 -enylamino)-methyl]-benzofuran-5-
carbonitrile or a pharm:~reutic~lly acceptable salt thereof;
2- { [2-(1,1-dimethyl-2-phenyl-ethylamino)-3,4-dioxo-cyclobut- 1 -enylamino] -
methyl}-benzoruldn-5-carbonitrile or a pharm~reutir~lly acceptable salt thereof;3-[(pyridin-4-ylmethyl)-amino]-4-(1,2,2-trimethyl-propylamino)-cyclobut-3-ene-1,2-
dione or a pharmaceutically acceptable salt thereof;
2- { [2-(1,1 -dimethyl-propylamino)-3,4-dioxo-cyclobut- 1 -enylamino] -methyl } -3-
chloro-benzuruldn-5-carbonitrile or a pharmaceutically acceptable salt thereof.
It is understood that the definition of the compounds of formula (I), when Rl,
R2, R3, R4, Rs or R6 contain asymmetric chiral centers, encompasses all possible
stereoisomers and mixtures thereof which possess the activity discussed below. In
particular, it encompasses racemic modifications and any optical isomers which
possess the indicated activity. Optical isomers may be obtained in pure forrn by
CA 022~8~36 1998-12-16
WO 97148682 PCT/US97/09744
standard separation techniques or enantiomer specific synthesis. It is understood that
this invention encomp~ses all crystalline forms of compounds of formula (I). Thecompounds of this invention, throughout this specification, are equivalently named as
3,4-diones or 1 ,2-diones. The pharmaceutically acceptable salts of the basic
5 compounds of this invention are those derived from such organic and inorganic acids
as: lactic, citric, acetic, tartaric, succinic, maleic, malonic, hydrochloric, hydrobromic,
phosphoric, nitric, sulfuric, methanesulfonic, and similarly known acceptable acids.
Where R4, Rs or R6 contains a carboxyl group, salts of the compounds of this
invention may be forrned with bases such as alkali metals (Na, K, Li) or the alkaline
10 earth metals (~a or Mg).
The present invention also provides a process for the preparation of a
compound of formula (I). More particularly, the compounds of forrnula (I) may bep~GL)~d by reacting a compound of formula (II):
0~0
X (II)
wherein X is a suitable leaving group, for example, methoxy, ethoxy, isopropoxy,butoxy, halogen or a similar leaving group with a compound of formula (III):
~Ra1
HN~
(III)
wherein Ra1 and Ra2 are Rl and R2, respectively, as defined hereinbefore or a group
of atoms convertible thereto. The subsequent intertne~ t/ may then be allowed toreact with a compound of formula (IV):
A1--CH2NH2 (IV)
wherein A1 is A as defined hereinbefore or a group of atoms convertible thereto. The
reactions mentioned above may be carried out in a solvent such as acetonitrile,
CA 022~8~36 1998-12-16
WO 97/48682 PCT/US97/09744
methanol, ethanol, tetrahydrofuran or dioxane at elevated or ambient temperatures.
Furthermore, the order of reaction may be reversed, that is, a compound of formula
~I) may be allowed to react initially with a compound of formula (IV), The
subsequent interrn~ e may then be allowed to react with a compound of formula
S (III) as described hereinbefore, to give the compounds of formula (I).
The compounds of formula ~I) and their pharmaceutically acceptable salts are
smooth muscle relaxants functioning via potassium channel activation. They are
therefore useful in the treatment of disorders associated with smooth muscle
contraction, disorders involving excessive smooth muscle contraction of the urinary
tract (such as incontinence), or of the gastro-intestinal tract (such as irritable bowel
syndrome), asthma and hair loss. Furthermore, the compounds of formula (I) are, as
potassium channel activators, useful for treatment of peripheral vascular disease,
hypertension, congestive heart failure, stroke, anxiety, cerebral anoxia and other
neurodegenerative disorders.
The present invention accordingly provides a pharrnaceutical composition
which comprises a compound of this invention in combination or association with a
pharmaceutically acceptable carrier. In particular, the present invention provides a
pharmaceutical composition which comprises an effective amount of a compound of
this invention and a pharmaceutically acceptable carrier.
The compositions are preferably adapted for oral ~fimini~tration. However,
they may be adapted for other modes of 7l~iministration~ for example, parenteral~r1mini~ation for patients suffering from heart failure.
In order to obtain consistency of ~lmini~tration, it is preferred that a
composition of the invention is in the form of a unit dose. Suitable unit dose forms
include tablets, capsules and powders in sachets or vials. Such unit dose forms may
contain from 0.1 to 100 mg of a compound of the invention and preferably from 2 to
50 mg. Still further preferred unit dosage forms contain 5 to 25 mg of a compound of
the present invention. The compounds of the present invention can be ~-iministered
orally at a dose range of about O.nl to 100 mg/kg or preferably at a dose range of 0.1
CA 022~8~36 1998-12-16
WO 97148682 PCT/US97/09744
to 10 mg/kg. Such compositions may be ~1mini~tered from 1 to 6 times a day, moreusually from 1 to 4 times a day.
The compositions of the invention may be formulated with conventional
5 excipients, such as a filler, a disintegrating agent, a binder, a lubricant, a flavoring
agent and the like. They are formulated in conventional manner, for example, in a
manner similar to that used for known antihypertensive agents, diuretics and ,B-blocking agents.
The present invention further provides a compound of the invention for use as
an active pharm~reu~ l or therapeutic substance. Compounds of formula (I) are ofparticular use in the induction of smooth muscle relaxation.
The present invention further provides a method of treating smooth muscle
disorders in m~3mm~ including man, which comprises ~tlmini~tering to the afflicted
"~"~ l an effective amount of a compound or a pharmaceutical composition of the
invention.
The following examples are presented to illustrate rather than limit the
methods for production of representative compounds of the invention.
~,Y~mDIe 1
3~ imethylvroDvlamino~-4-r~Dvridin-4-YI~nethvl)-aminol-
cvclobut-3-ene-1.2-dione
A solution of 3,4-dibutoxycyclobut-3-ene-1,2-dione (4.526 g, 20 mmol) and
l,1-dimethylpropylamine (1.743 g, 20 mmol) in tetrahydrofuran (20 mL) was stirred
at room temperature for 19.5 hours. The solvent was removed and the residue was
30 chromatographed (gravity, chloroform-hexane) on neutral, activity III silica (150 g).
The white solid isolated from the apprvpl iate eluates was recrystallized from hexane to
give 4.105 g (86%) of a white product: mp 56.5-57.5~C (softens 55.5~C), One gramof this material was recrystallized twice from hexane to provide 0.794 g of 3-butoxy-4-
(1,1-dimethylpropylamino)-cyclobut-3-ene-1,2-dione as a white solid: mp 56-57 C
CA 022~8~36 1998-12-16
WO 97/48682 PCT/US97/09744
(softens 55~C); IH NMR (DMSO-d6): ~ 8.63 and 8.48 (two br s lH, rotomers), 4.67
(m, br, 2H), 1.67 (m, br, 4H), 1.39 (m, 2H), 1.26 (m, br, 6H), 0.91 (t, 3H), 0.78 (t, 3H).
IR (KBr): 3170, 1790, 1700 cm~l; MS (m/z) 239 (M+).
A solution of the above 3-butoxy-4-(1,1-dimethylpropylamino)-cyclobut-3-
ene-1,2-dione (1.197 g, 5.0 mmol) and 4-aminomethylpyridine (0.541 g) in
tetrahydrofuran (10 mL) was stirred at room temperature for 23 hours. The ~ u~
was freed of solvent and the residue was triturated with diethyl ether and dried to
provide 1.154 g of a buff solid. Recrystallization (twice) of the crude product from
methanol gave 0.832 g (61 %) of 3-(1,1-dimethylpropylamino)-4-[(pyridin-4-
ylmethyl)-amino]cyclobut-3-ene-1,2-dione as a white compound: mp 258.0-259.5~C
dec (softens 257.5~C); lH NMR (DMSO-d6): ~ 8.56 (m, 2H), 7.86 (m, br, lH), 7.46 (s,
br, lH), 7.32 (m, 2H), 4.77 (d, 2H), 1.67 (~, 2H), 1.32 (s, 6H), 0.83 (t, 3H). IR (KBr):
3270, 1790, 1650 cm~l; MS (m/z) 273 (M+).
Elemental Analysis for ClsHl9N3o2
Calcd: C, 65.91; H, 7.01; N, 15.37
Found: C, 65.55; H, 6.88; N, 15.12
Ex~mDle 2
3-(1.1-l)imethvl,~roDvlamino)-4-rDyridin-3-vlmethyl)aminol-
cyclobut-3-ene-1.2-dione
Tetrahydrofuran (10 mL), 3-butoxy-4-~1,1-dimethylpropylamino)cyclobut-3-
ene-1,2-dione (1.197 g, 5.0 mmol, as prepared in Example 1), and 3-aminomethyl-
pyridine (0.541 g) were stirred together at room temperature for 24 hours. The
~ reaction mass was freed of solvent and the residue was triturated with diethyl ether and
dried to afford 1.228 g of a cream-colored solid. Two recryst~lli7~ions of this
material from methanol provided 0.887 g (65%) of 3-~1,1-dimethylpropylamino)-4-
[pyridin-3-ylmethyl)amino]cyclobut-3-ene-1,2-dione as a white solid: mp 263.5-
264.5~ C (softens 260.0~C); lH NMR (DMSO-d6): ~i 8.57 (m, lH), 8.52 (m, lH), 7.80
(m, br, lH), 7.76 (m, lH), 7.41 (m, lH), 7.39 (s, br, lH), 4.72 (d, 2H), 1.66 (q, 2H),
1.30 (s, 6h), 0.82 (t, 3H). IR (KBr): 3230, 1790, 1650 cm~i; MS (m/z): 274 (M+H)+.
CA 02258536 1998-12-16
WO 97/48682 PCT/US97109744
Elemental Analysis for Cl5Hl4N3O2
Calcd: C, 65.91; H, 7.01; N, 15.37
Found: C, 66.23; H, 7.08; N, 15.38
~xamDle 3
3-(l .l -Dimethvll~roDvlamino)-4-r(pvridin-2-vlmethvl~aminol-
cvclobu~-3-ene-l .~-dione
A solution of 3-butoxy-4-(1,1-dimethylpropylamino)-cyclobut-3-ene-1,2-dione
(0.622 g, 2.6 mmol, as prepared in Example 1) and 2-aminomethylpyridine (0.281 g,
2.6 mmol) in tetrahydrofuran (5 mL) was stirred at room temperature for 22 hours.
Removal of solvent, trituration of the residue with diethyl ether and drying gave 0.656
g white solid. Recrystzl11i7~tion (twice) of the crude product from acetonitrile afforded
0.447 g (63%) of 3-(1,1-dimethylpropylamino)-4-[(pyridin-2-ylmethyl)amino~-
cyclobut-3-ene-1,2-dione as a white solid: mp 192.0-192.5~ C (softens 188.5~); lH
NMR (DMSO-d6): ~ 8.58 (m, lH), 8.00 (m, br~ lH), 7.82 (m, 1~), 7.58 (s, br, lH),7.37 (m, lH), 7.33 (m, lH), 4.85 (d, 2H), 1.67 ~q, 2H), 1.31 (s, 6H), 0.83 (t, 3H). IR
(KBr): 3210, 1790, 1660 cm~l; MS (rn/z): 273 (M+).
Elemental Analysis for Cl5Hl9N3O2
Calcd:C, 65.91; H, 7.01; N, 15.37
Found:C, 65.78; H, 6.94; N, 15.48
FxamDIe 4
3-tert-Butvlamino-4-r(r)vridin-4-vlnnethvl)arninol-
cvclobut-3-ene- 1.2-dione
In a procedure similar to that described in Example 1 utilizing the appropriate
starting materials, 3-tert-butylamino-4-[(pyridin-4-ylmethyl)amino]-cyclobut-3-ene-
1,2-dione was ~e~ ,d as a white solid: mp 271.0-271.5~C (softens at 269.5 C).
CA 02258536 1998-12-16
WO 97148682 PCT/US97/09744
~,xamDIe 5
3-tert-Butvlamino-4-rf~yridin-3-vlmethvl)aminol-
cvclobut-3-ene-1 ~2-dione
s
In a procedure similar to that described in Example 1 utilizing the ~pl~liate
starting materials, 3-tert-butylamino-4-[(pyridin-3-ylmethyl)amino]-cyclobut-3-ene-
1,2-dione was prepared as a white solid: mp 296.0~C dec. (softens at 290.5~C).
Examvle 6
3-tert-Butvlamino-4-r(~yridin-2-ylmethvl~aminol-
cvclobut-3-ene- 1.2-dione
In a procedure similar to that described in Example 1 utilizing the appropriate
starting materials, 3-tert-butylamino-4-[(pyridin-2-ylmethyl)amino]-cyclobut-3-ene-
1,2-dione was ~ ,d as a white solid: mp 236.0-236.5~C dec. (softens at 233.5 C).
F.x~m~le 7
3-(Isopro~yl-methvl-amino)-4-r~Dyridin-4-ylmethyl~-aminol-
cvclol)ut-3-ene- 1.2-dione
In a procedure similar to that described in Example 1 utilizing the appl~liate
25 starting materials, 3-(isopropyl-methyl-amino)-4-[(pyridin-4-ylmethyl)-amino]-
cyclobut-3-ene-1,2-dione was prepared as a white solid: mp 214.5-215.0~C dec.
(softens at 211.5-C).
CA 022~8~36 1998-12-16
WO 97/48682 PCT/US97/09744
Fxa~Dle ~
3-r(5-N;tro-b~1-7.oruran-2-vlmethvl)-amino-4-~l.2.2-lr;~ ylDro~ylamino)-
cvclobut-3-ene-1.2-dione
To a suspension of NaH (3.41 g, 80%, 113.6 mmol) in dimethylformami~le (400
mL) at 0~C was added acetone oxime (7.61 g, 104.1 mmol). After stirring for 1 hour at
0~C, 4-nitrofluorobenzene (10.00 mL, 94.6 mmol) was introduced via syringe and the
resulting mixture was stirred for 1 hour. Brine (400 mL~ was added and the resulting
10 precipitate was collected by filtration. The product was washed with water and dried in
vacuo to afford 18 g (100%) of white solid: IH NMR (DMSO-d6): ~ 8.20 (d, 2H), 7.25
(d, 2H), 2.06 (s 3H), 2.11 (s, 3H).
The above oxime adduct (10.39 g, 53.56 mmol) was heated in saturated ethanolic
15 HCl (200 mL) at reflux. After 3 hours, the reaction l~ ule was cooled and concentrated
to 1/4 volume. Water was added and the l~G~ ated cyclization product was collected
by filtration to afford 9.0 g (95%) of 2-methyl-5-nitrobenzofuran: IH NMR (DMSO-d6):
~ 8.39 (d, lH), 8.15 (dd, lH), 7.45 (d, lH), 6.49 (s, lH), 2.48 (s, 3H).
To a stirring solution of the above benzofuran (5.00 g, 28.25 mmol) and benzoyl
peroxide (0.68 g, 2.83 mmol) in carbon tetrachloride (200 mL) was added 1,3-dibromo-
S,S-dimethylhydantoin (4.04 g, 14.12 mmol). The mixture was irradiated with a 200 watt
lamp while stirring for 1 hour, cooled and partitioned between dichloromethane/water.
The organic phase was washed with water (2 x 100 mL) and brine (2 x 100 mL), dried
(MgS04), decolorized (charcoal), and concentrated in vacuo to afford 7.12 g of crude
product. Recry~t~11i7~tion from ethyl acetate/hexane afforded 4.38 g (61%) of 2-bromomethyl-S-nitro-benzofuran as an off-white solid: IH NMR (CDCl3): ~ 8.49 (d,lH), 8.25 (dd, lH), 7.58 (d, lH), 6.91 (s, lH), 4.59 (s, 2H~.
A ~ Lure of the above 2-bromomethyl-5-nitrobenzofuran (1.57 g, 6.13 mmol),
potassium phthalimifle (1.70 g, 9.19 mmol), and 18-crown-6 (0.161 g, 0.61 mmol) was
stirred in acetonitrile (15 mL) overnight at room temperature. The solvent was removed
by vacuum, and the residue was partitioned between ethyl acetate and brine. The organic
phase was washed with O.lN sodium hydroxide (2 x 50 mL) then brine (2 x 50 mL),
CA 022~8~36 1998-12-16
WO 97t48682 PCT/US97/09744
- 13-
dried (MgSO4) and concentrated to afford an off-white solid. The crude product was
triturated with cold ethyl acetate/diethyl ether/hexane to afford 1.47 g (74%) of
phsh~limirle adduct as a white solid: IH NMR (DMSO-d6): ~ 8.55 (d, lH), 8.17 (dd, lH),
7.88 (m, 4H), 7.75 (d, lH), 5.00 (s, 2H).
s
The above phth~limicle adduct (1.45 g, 4.49 mmol) was treated with hydrazine
hydrate (0.38 mL) in refluxing ethanol (15 mL) for 1.5 hours. The reaction was cooled to
0~C and acidified (conc. HCl) to pH=l. The mixture was filtered and the solid was
washed with 6N HCl and water. The filtrate was basified with potassium carbonate and
10 then extracted with ethylacetate. The organic phase was dried (MgSO4) and concentrated
to afford 0.74 g (86%) of 2-methylamino-5-nitrobenzofuran as a light yellow solid: IH
NMR (DMSO-d6): ~ 8.55 (d, lH), 8.11 (dd, lH), 7.72 (d, lH~, 6.85 (s, lH), 3.84 (s, 2H),
2.00 (brs, 2H).
To the 2-methylamino-5-nitrobenzofuran (0.74 g, 3.85 mmol) stirring in
tetrahydrofuran (15 mL) at 0~C was added 3,4-dibutoxy-3-cyclobutene-1,2-dione (1.25
mL, 5.78 mmol) via syringe. The Illi~UlC was stirred for S hours at room temperature
and was then concentrated in vacuo. The residue was crystallized from ethyl
acetate/diethyl ether/hexanes to afford 0.83 g of adduct as an off-white solid. A second
crop (0.17 g) was isolated from the mother liquor. Total yield: 75%. IH NMR (DMSO-
d6): ~ 9.40 and 9.20 (2 br m, lH rotameric), 8.60 (d, lH), 8.20 (dd, lH), 7.80 (d, lH),
7.04 (s, lH), 4.88 and 4.67 (2 br m, 2H, rotameric), 4.60 (t, 2H), 1.67 (m, 2H), 1.30 (m,
2H), 0.85 (m, 3H).
3-Butoxy-4-[(5-nitro-benzofuran-2-ylmethyl)-amino]-cyclobut-3-ene- 1,2-dione
(0.250 g, 0.727 mmol) was dissolved in an ethanolic solution of R(+)-2-amino-3,3-
dimethylbutane (0.167 N, 6.0 mL, 1.00 mmol). The mixture was diluted with ethanol (1
mL) and tetrahydrofuran (1 mL). After 3 hours, an additional 6.0 mL of ethanolic amine
(1.00 mmol) was added and the mixture was allowed to stir for 48 hours at room
temperature. The heterogeneous mixture was diluted with 1: 1 diethylether/ethyl aceate
and filtered to afford 0.20 g (74%) of (R) isomer of 3-[(5-nitro-benzofuran-2-ylmethyl)-
amino]-4-(1,2,2-1fil~ }lylpropylamino)-cyclobut-3-ene-1,2-dione: mp ~ 300~C; IH NMR
(DMSO-d6): o 8.61 (d, lH), 8.21 (dd, lH), 7.88 (d and m, 2H), 7.35 (br d, lH), 7.06 (s,
CA 02258536 l998- l2- l6
WO 97/48682 PCT/US97/09744
- 14-
lH), 4.98 (m, 2H), 3.92 (m, lH), 1.10 (d, 3H), 0.86 (s, 9H). IR (KBr): 3180, 2950, 1800,
1650, 1550 cm~l; MS (m/z~ 371 (M+).
Elemental Analysis for C~9H21N3O5
S Calcd: C, 61.45; H, 5.70; N, 11.31
Found: C, 60.52; H, 5.50; N, 11.21
F,xamDle 9
3~ Dimethyl-DroDvlamino)-4-r(5-nitro-ben~ofilran-2-vlmethvl)-aminol-
cvclobut-3-ene-1.2-dione
To 3-butoxy-4-[(5-nitrobenzofuran-2-ylmethyl)-amino]-cyclobut-3-ene-1,2-dione
(0.25 g, 0.727 mmol), as prepared in Example 8, in ethanol (5 mL) was added tert-
15 amylamine (0.53 mL, 4.54 mmol). The reaction was stirred at 70~C for 18 hours and
then at room temperature for 48 hours. The precipitated product was filtered and washed
with ethyl acetate, diethyl ether, and petroleum ether to afford 0.22 g (85%) of 3-(1,1-
dimethyl-propylamino)-4-[(5-nitro-bell~oru.dn-2-ylmethyl)-amino]-cyclobut-3-ene- 1,2-
dione as an off-white solid: mp 283.5-287.5 ~C (dec); lH NMR (DMSO-d6): ~ 8.61 (d,
20 lH), 8.18 (dd, lH), 7.96 (br t, lH), 7.81 (d, 1~), 7.46 (br s, lE~), 7.07 (s, lH), 4.99 (d,
2H), 1.66 (q, 2H), 1.30 (s, 6H), 0.82 (t, 3H). IR (KBr): 3220, 2950, 1800 cm~l; MS (mlz)
357 (M ).
Elemental Analysis for Cl8Hl9N3Os
Calcd: C, 60.5Q; H, 5.36; N, 11.7G
Found: C, 59.31; H, 5.15; N, 11.53
I~.xaml~le 10
3-tert-Butvlamino-4-r~5-nitro-ben~:ofuran-2-vlmethvl)-am;nol-
cvclobut-3-ene-1 2-dione
To 3-butoxy-4-1(5-nitro-benzofuran-2-ylmethyl)amino]-cyclobut-3-ene-1,2-dione
(0.25 g, 0.727 mmol), as prepared in Example 8, in ethanol (5 mL) was added tert-
CA 022~8~36 1998-12-16
WO 97t48682 PCT/US97/09744
- 15 -
butylamine (0.51 mL, 4.85 mmol). The reaction was stirred at 70~C for 18 hours and
then at room temperature for 48 hours. Workup in a manner identical to Example 9afforded 0.20 g (80%) of 3-tert-butylamino-4-[(5-nitro-benzofuran-2-ylmethyl)-amino]-
cyclobut-3-ene-1,2-dione as an off-white solid: mp > 300 ~C; IH NMR (DMSO-d~,): o
8.61 (d, lH), 8.20 (dd, lH), 7.91 ~br t, lH), 7.81 (d, lH), 7.59 (br s, lH), 7.07 (s, lH),
4.98 (d, 2H), 1.36 (s, 9H). IR (KBr): 3220, 2930, 1800, 1675 cm~l; MS (m/z) 344.3
[M+H]+ .
Elemental ~nalysis for Cl7HI7N3Os
Calcd: C, 59.47; H, 4.99; N, 12.24
Found: C, 58.86; Hm 4.78; N, 11.88
FxamDIe 11
2-rr2-~1~1-Dimethvl-nropylamino)-3~4-diQxo-cvclobut-l-enylaminol-methyl~-
ben~ofi~ran-5-carbonitrile
Acetone oxime (6.34 g, 86.64 mmol) was added to a suspension of sodium
hydride (as a 80% dispersion in mineral oil; 2.72 g, 90.82 mmol) in N, N-
dimethylform~mi~le (400 mL) at 0~C. The frothy suspension was stirred for 1 houras the ~ lul~, was warmed to 25 C. p-Fluorobenzonitrile (10.00 g, 82.51 mmol) was
added and the reaction Il~ Lule was stirred at 0~C for 15 minutes and then allowed to
warm to room temperature. After stirring overnight, the reaction ~ ul~ was poured
into brine (300 mL). The resulting white precipitate was collected by filtration,
washed with water, and dried in vacuo. Yield: 13.97 g (98 %): lH NMR (DMSO-
d6): o 7.78 (d, 2H), 7.76 (d, 2H), 2.04 (s, 3H), 2.00 (s, 3H~.
To the product of the proceeding paragraph (13.97 g, 80.28 mmol) was added
ethanolic hydrochloric acid (400 rnL). The Illix.Lul~ was heated at reflux for 4 hours.
The reaction mixture was cooled and concentrated to 1/3 the volume. The reactionmixture was diluted with water, resulting in the instantaneous formation of a
precipitate. The precipitate was collected by filtration, washed with water, and dried
in ~vacuo. The crude product was purified by HPLC (10:1, hexane/ ethyl acetate).Yield: 7.26 g (58 %): 1H NMR (CDC13~: ~ 7.79 (d, lH), 7.47 (dd, lH), 7.43 (d, lH),
6.44 (s, lH), 2.47 (s, 3H).
CA 022~8~36 1998-12-16
WO 97/48682 PCT/US97/09744
- 16-
To a solution of the product of the proceeding paragraph (3.00 g, 19.08 mmol)
in carbon tetrachloride (80 mL) was added benzoyl peroxide (0.46 g, 1.91 mmol) and
1, 3-dibromo-S, S-dimethylhydantoin (2.73 g, 9.54 mmol3. The reaction mixture was
irradiated with a 200 watt lamp for 1 hour. The reaction mixture was cooled and
partitioned between ethyl acetate and sodium bicarbonate. The organic layer was
dried over magnesium sul~ate, treated with Norite(g) (activated carbon), filtered and
concentrated to a solid. The crude product was recryst~ erl from ethyl acetate/
hexane. Yield: 2.40 g (59 %): lH NMR (DMSO-d6): o 8.49 (d, lH), 8.25 (dd, lH),
7.58 (d, lH~, 6.91 (s, lH~, 4.59 (s, 2H).
To a solution of the product of the proceeding paragraph (2.63 g, 10.27 mmol)
in ~re~ ile (30 mL) was added potassium phth~limide (2.85 g, 15.41 mmol) and
18-crown-6 (0.27 g, 1.03 mmol). After stirring overnight, the reaction mixture was
concentrated in vacuo. The residue was partitioned between ethyl acetate and brine
and imme li:~tely a l)leci~ilate forrned. The precipitate was collected by filtration,
washed with diethyl ether and dried. The organic phase was washed with 0.1 N
sodium hydroxide (2xS0 mL) and then brine (2xS0 mL). The solution was
concentrated in vacuo to afford another batch of solid. Yield: 2.50 g (76 %): 1HNMR (DMSO-d6): ~ 8.18 (d, lH), 7.91 (m, 4H), 7.77 (dd, lH), 7.74 (d, lH), 7.07 (d,
lH), 5.00 (s, 2H).
To a solution of the product of the proceeding paragraph (2.50 g, 7.74 mmol)
in ethanol (20 mL) was added hydrazine-hydrate (0.66 mL). The mixture was heatedto reflux for 1.5 hours. The reaction mixture was cooled to O C and acidified with
concentrated hydrochloric acid to pH of 1. The mixture was filtered and the filter
cake was washed with 6N hydrochloric acid and then water. The filtrate was basified
with potassium carbonate and extracted with ethyl acetate. The organic phase wasdried over magnesium sulfate, treated with Norite(~), and concentrated to afford an off
white solid. Yield: 0.85 g (62 %): lH NMR (DMSO-d6): ~ 8.17 (d, lH), 7.78 (dd,
lH), 7.74 (d, lH), 6.82 (s, lH), 3.85 (s, 2H), 2.10 (br s, 2~I).
To a soiution of the product of the proceeding paragraph (0.84 g, 4.40 mmol)
in tetrahydrofuran (lS mL) was added 3,4-diethoxy-3-cyclobutene-1,2-dione (1.13 g,
CA 022~8~36 1998-12-16
WO 97/48682 PCT/US97/09744
6.60 mmol) at 0~C. The reaction mixture was warmed to room temperature and
stirred for 4 hours. The reaction mixture was concentrated in vacuo and the crude
product was triturated with ethyl acetate/ diethyl ether/ hexane to yield an off white
solid. The solid was filtered and dried to yield the desired product. Yield: 1.05 g (76
%): 1H NMR (DMSO-d6): ~ 9.41 and 9.21 (br m, lH, rotamers), 8.22 (d, lH), 7.81
(dd, lH), 7.00 (s, lH), 4.88 and 4.68 (2 br m, 2H, rotamers), 4.65 (t, 2H), 1.39 (m,
3H).
To a solution of the product of the procee-ling paragraph (0.25 g, 0.79 mmol)
in ethanol (17 mL) was added tert-amyl amine (0.21 g, 2.37 mmol). The reaction
mixture was heated at 70~C and allowed to stir overnight. The solid which had
formed was filtered and washed with eth yl acetate, diethyl ether, and hexane to afford
0.18 g (67%) of 2-{[2-(1,1-dimethyl-propylamino)-3,4-dioxo-cyclobut-1-enylamino~-
methyl}-benzofuran-S-carbonitrile as an off white solid: mp 171.1-173.2 C; lH
NMR (DMSO-d6): ~ 8.19 (d, lH), 7.96 (t, lH), 7.81 (d, lH), 7.74 (dd, lH), 7.45 (s,
lH), 6.96 (s, lH), 4.97 (d, 2H), 1.67 (~, 2H), 1.29 (s, 6H), 0.83 (t, 3H). IR (KBr):
3230, 2950, 2220, 1800 cm~l; MS (m/z) 337 (M+).
Elemental analysis for ClgHlgN3O3
Calc'd: C, 67.64; H, 5.68; N, 12.46.
Found: C, 66.87; H, 5.32; N, 12.37.
F.xample 12
2-rr3.4-Dioxo-2-~1.2.2-l~ i---etllyl-DroDylamino)-cyclobut-l-envlaminol-methvl~- ben~ofuran-5-carbonitrile
To a solution of the product of paragraph 6 in Example 11 ( 0.250g, 0.79
mmol) in ethanol (2mL) was added ethanolic (R)-2-amino-3,3-dimethylbutane (0.166N in EtOH, 9.50 ml, 1.58 mmol ). The reaction mixture was heated at 70~C and
allowed to stir overnight. The solid which had formed was filtered and washed with
ethyl acetate, diethyl ether, and hexane. The solid was dried in vacuo to 0.22 g(79%) of the (lR) isomer of 2-{[3,4-dioxo-2-(1,2,2-trimethyl-propylamino)-cyclobut-
l-enylamino]-methyl}-benzofuran-5-carbonitrile as an off white solid: mp >300; lH
CA 02258536 1998-12-16
WO 97148682 PCT/US97/09744
- 18 -
NMR (DMSO-d6): ~ 8.18 (d, lH~, 7.80 (dd, lH), 7.74 (dd, lH), 7.31 (br d, lH), 6.95
(s, lH), 4.96 (m, 2H), 3.91 (br m, lH), 1.11 (d, 3H), 0.81 (s, 9H). IR (KBr): 3170,
2950, 2250, 1850, 1650, 1560 cm~l; MS (m/z) 351 (M+).
Elemental analysis for C20H21N3~3
Calc'd: C, 68.36; H, 6.02; N, 11.96.
Found: C, 68.00; H, 5.83; N, 12.00.
li~xamDle 13
2-r(2-tert-~utylamino-3.4-dioxo-cyclo~ut-1-enylamino~-methyll-
benzofi-ran-5-carh~onitrile
To a solution of the product of paragraph 6 in Example 11 (0.250 g, 0.79
mmol ) in ethanol (17 mL) was added tert-butyl amine (0.17g, 2.37 mmol). The
reaction mixture was heated at 70 C and allowed to stir overnight. The solid which
had formed was filtered and washed with ethyl acetate, diethyl ether, and hexane.
The solid was dried invacuo to yield afford 0.12 g (51%) of 2-~(2-tert-butylamino-
3,~dioxo-cyclobut-1-enylamino)-methyl]-benzofuran-5-carbonitrile as a light pinksolid: mp 298.8-300.3 ~C; lH NMR (DMSO-d6): o 8.18 (d, lH), 7.96 (t, lH), 7.81
(dd, lH), 7.74 (dd, lH), 7.57 (s, lH), 6.95 (s, lE~), 4.96 (d, 2H), 1.35 (s, 9H). IR
(KBr): 3300, 2950, 2210, 1800, 1660, 1525 cm~l; MS (m/z) 323 (M+).
Elemental analysis for C18H17N3~3
Calc'd: C, 66.86; H, 5.30; N, 13.00
Found: C, 65.64; H, 4.93; N, 12.57
~,xaml~le 14
2-~r2-(l.l-l)imethvl-2-1lhenvl-ethylamino)-3~4-dioxo-cvclobut-l-enylaminol-
methvl~-ben~ofuran-5-carbonitrile
To a solution of the product of paragraph 6 in Example 11 (0.23g, 0.73 mmol)
in ethanol (20 mL) was added alpha, alpha-dimethylphenethylamine (0.33g, 2.19
CA 02258536 1998-12-16
WO 97/48682 PCT/US97/09744
- 19-
.
mL). The reaction mixture was heated at 70~C and allowed to stir overnight. The
'~ solid which had formed was filtered and washed with ethyl acetate, diethyl ether, and
hexane. The solid was dried in vacuo to afford 0.11 g (41%) of 2-{[2-(1,1-Dimethyl-
2-phenyl-ethylamino)-3,4-dioxo-cyclobut- 1 -enylamino] -methyl ~ -benzofuran-5-
carbonitrile as an off white solid: mp 233.4-235.2~C; lH NMR (DMSO-d6): ~ 8.20
(d, lH), 7.89 (t, lH), 7.81 (dd, lH), 7.74 (dd, lH), 7.36 (s, lH), 7.23 (m, 3H), 7.06 (d,
2H), 6.94 (s, lH), 4.97 (d, 2H), 2.98 (s, 2H), 1.31 (s, 6H). IR (KBr): 3300, 3000,
2200, 1800, 1660, 1590 cm~ 1; MS (m/z) 399 (M~).
F.l~m~.nt~l analysis for C24H21N3~3
Calc'd: C, 72.17; H, 5.30; N, 10.52
Found: C, 71.28; H, 5.20, N, 10.33
F.~mvle 15
3-r(Pvridin-4-ylmethyl)-~minol-4-(1.22-trimethvl-nrol~vlamino)-
cvclobut-3-ene-1.2-dione
.
In a procedure similar to that described in Example 1 utilizing the a~plo~,liatestarting materials, 3-[(pyridin-4-ylmethyl)-amino]-4-(1,2,2-trimethyl-propylamino)-
cyclobut-3-ene-1,2-dione was prepared as a white solid: mp 282.5-283.0~C dec.
(softens at 271.5-C).
~mDIe 16
2-~r2-(1.1-l~imethvl-proDvlamino)-3.4-dioxo-cyclobut-l -envlaminol-methvl~-3-
chloro-benzofuran-5-carbonitrile
The benzofuran adduct prepared in Example 11, paragraph 2 was used to
synthesize 3-chloro-2-methylbenzofuran-5-carbonitrile (Cross, P. E.; Dickinson, R.
P.; Parry, M. J.; Randall, M. J. J. Med. Chem., 1986, 29, 1643-1650). Yield: 23 %:
H NMR (CDC13): o 7.81 (d, lH), 7.55 (dd, lH), 7.47 (dd, lH), 2.49 (s, 3H).
3-Chloro-2-bromomethyl-benzofuran-5-carbonitrile was prepared in a manner
similar to 2-bromomethyl-benzofuran-5-carbonitrile synthesized in ~xample 11,
CA 022~8~36 1998-12-16
WO 97/48682 PCT/US97/09744
- 20 -
paragraph 3. Yield: 53%: lH NMR (DMSO-d6): ~ 8.24 (d, lH), 7.93 (d, 2H), 4.94
(s, 2H).
The product from the proceeding paragraph was reacted with potassium
S pth~ mi~l~ in a manner similar to Example 11, paragraph 4 to yield the pth~limide~
adduct. Yield: 72%: lH NMR (DMSO-d6): â 8.18 (d, lH), 7.89 (m, 6H), 5.03 (s,
2H).
3-Chloro-2-methylamino-benzofuran-5-carbonitrile was prepared in a manner
similar to Example 11, paragraph 5. Yield:63%: lH NMR (DMSO-d6): ~ 8.18 (d
lH), 7.81 (dd, 2H), 3.85 (s, 2H), 3.21 (br s, 2H).
The above amino compound was reacted with 3,4-dibutoxy-3-cyclobutene-
1,2-dione in a manner similar to Example 11, paragraph 6. Yield: 75%: lH NMR
(DMSO-d6): ~ 9.40 and 9.18 (br m, lH, rotamers), 8.21 (d, lH), 7.91 (dd, 2H), 4.98
and 4.75 (2 br m, 2H, rotamers), 4.61 (t, 2H~, 1.63 (m, 2H), 1.35 (m, 2H), 0.87 (m,
3H).
To a solution of the product of the proceeding paragraph (0.14 g, 0.37 mmol)
in ethanol (12 mL) was ?Id~le-ltert-amyl amine (0.065 g, 0.74 mmol). The reaction
Lule was heated at 70-C and allowed to stir for 13 h. The solid which had formedwas ~lltered and washed with ethyl acetate, diethyl ether, and hexane. the solid was
dried in vacuo to yield a light orange solid. Yield: 0.11 g (74 ~o): mp 257.3-258.1
~C; lH NMR (DMSO-d6): o 8.22 (d, lH), 7.93 (t, lH), 7.gO (d, lH), 7.87 (dd, lH),7.40 (s, lH), 5.05 (d, 2H), 1.66 (q, 2~), 1.29 (s, 6H), 0.80 (t, 3H). IR (KBr): 3230,
2950, 2220, 1800 cm~1; MS (m/z) 371 (M~).
Elemental analysis for C19~18CllN3~3
Calc'd: C, 61.38; H, 4.88; N, 11.30
Found: C, 60.75 H, 4.81; N, 11.11
CA 022~8~36 l998- l2- l6
WO 97/48682 PCT/US97/09744
- 21 -
The smooth muscle relaxing activity of the compounds of this invention was
J established in accordance with standard pharmaceutically accepted test procedures
with representative compounds as follows:
Sprague-Dawley rats (150-200 g) are rendered unconscious by CO2
asphyxiation and then c~lth~ni7~d by cervical ~ loc~tion The bladder is removed into
warm (37 deg.C~ physiological salt solution (PSS) of the following composition
(mM): NaCl, 118.4; KCl, 4.7; CaCl2, 2.5; MgSO4, 4.7; H2O, 1.2; NaHCO3, 24.9;
KH2P04, 1.2; glucose, 11.1; EDTA, 0.023; gassed with 95% O~; 2/5% C02; pH 7.4.
The bladder is opened and then cut into strips 1-2 mm in width and 7-10 mm in
length. The strips are subsequently suspended in a 10 mL tissue bath under an initial
resting tension of 1.5 g. The strips are held in place by two surgical clips one of
which is attached to a fixed hook while the other is attached to an isometric force
transducer. The preparations, which usually exhibit small spontaneous contractions,
are allowed to recover for a period of 1 hour prior to a challenge with 0.1 uM
carbachol. The carbachol is then washed out and the tissue allowed to relax to its
resting level of activity. Following a further 30 min period of recovery an additional
15 mM KCl are introduced into the tissue bath. This increase in KCl concentration
results in a large increase in the amplitude of spontaneous contractions (and initiation
of contractions in previously quiescent strips) superimposed upon a small increase in
basal tone. Following stabilization of this enhanced level of contractile activity,
incremental increases in the concentration of test compound or vehicle are introduced
into the tissue bath. Contractile activity is measured for each compound or vehicle
concentration during the last minute of a 30 minute challenge.
The isometric force developed by the bladder strips is measured using a
concentration required to elicit 50% inhibition of pre-drug contractile activity (ICso
concentration) and is calculated from this concentration-response curve. The
maximum percentage inhibition of contractile activity evoked by a test compound is
also recorded for concentrations of test compound less than or equal to 30 ,uM.
The results of this study are shown in Table I.
CA 02258536 1998- 12- 16
wo 97/48682 PCT/US97/09744
Table I
Inhibition of Contractions in Isolated Rat Bladder Strips
Compound nIC50 lLM
Example 1 40.42 + 0.06
Example 2 51.25 + 0.39
Example 3 41.25 + 0.34
Example 4 63.0 + 0.2
Example 5 42.63 +0.22
Example 6 411.45 + 4.3
Example 7 4*I=27.3 + 7%
Example 8 4**C=8.5 i 1.4%
Example 9 3*I=22.2 + 7.4%
Example 10 4 *I=11.5 + 3.2%
Example 11 4 1.56+0.16
Example 12 2 3.75 + 1.44
2*I=5.5 i 4%
Example 13 3 *I=19.94 + 8.5%
Example 14 2 *I=31.9+6.4%
Example 15 4 2.45 + 0.99
Example 16 2 1.3 +0.63
* Percent inhibition at 30 ~M
** Percent contraction at 30 ~M
CA 022~8~36 1998-12-16
WO 97/48682 PCT/US97/09744
- 23 -
In addition, we tested the ability of the compound of Example 1, as
~' representative of the other compounds of this invention, to inhibit the hyperactivity of
hypertrophied bladder (detrussor) smooth muscle in conscious female rats with
hy~elLIo~hied bladders and thereby alleviate urinary incontinence according to the
protocol described by Malmgrem et al., J. Urol. 142:1134, 1989.
Female Sprague-Dawley rats, ranging in weight from 190-210g are used. Up to 25
~nim~ are prepared each time. After development of bladder hypertrophy 4-8 ~nim~lc
are used per test.
Compounds are dissolved in PEG-200 and ,~-lmini~tered by gastric gavage or
intraveneously in a volume of 5 ml/kg. For primary screening all drugs are
zJ~1mini~tçred at the all,iLIdl y dose of 10 mgtkg p.o. to groups of 4 rats.
The ~nim~ls are anesthetized with halothane. Through a midline incision the
bladder and urethra are exposed and a ligature of 4-0 silk is tied around the proximal
urethra in the presence of a stainless steel rod (1 mm rii~meter) to produce a partial
occlusion. The rod is then removed. The abdominal region is closed using surgical
staples and each rat receives 150,000 units of bicillin C-R. The ~nim~ are allowed
six weeks to develop sufficient bladder hypertrophy. After six weeks, the ligature is
removed under halothane anesthesia and a catheter (PE 60) with a cuff is placed in
the dome of the bladder and secured with a purse string suture. The catheter is
tunneled under the skin and exteriorized through an opening in the back of the neck.
The abdominal incision is sutured and the free end of the catheter sealed. In order to
prevent infections the rats receive an injection of bicillin C-R (150000 units/rat).
Two days later the ~3nim~ are used in cystometrical evaluations. The ~nim~l~ areplaced in the metabolic cages and the catheter is attached (using a "T" connector) to a
Statham pressure transducer (Model P23Db) and to a Harvard infusion pump. A
plastic beaker attached to a force displacement transducer (Grass FT03) is placed
under the rat's cage to collect and record urine volume. Animals are allowed 15-30
minutes to rest before the saline infusion (20 ml/hr for 20 minutes) is started for the
first cystometry period. Two hours after the first cystometry period, the rats are
dosed with the vehicle or the test compound and one hour later a second cystometry is
~clrolllled.
CA 02258536 1998-12-16
WO 97/48682 PCT/US97/09744
- 24 -
The following urodynamic variables are recorded: ,
Basal bladder ~IGS~UIti = the lowest bladder pressure
during cystometry
Threshold pressure = bladder pressure imm~ tely
prior to micturition
lQ Micturition volume= volume expelled
Micturition pressure = peak pressure during voiding
Spontaneous activity = mean amplitude of bladder
pressure fluctuations during
filling
Presentation of results:
The mean value of each variable is calculated before and after compound
~r1mini~1Tation. For each compound the changes in the variables measured
are compared to the values obtained before treatment and expressed as
percent inhibition. The data are also subjected to 2-way analysis of
variance to determine significant (p<0.05) changes in the variable
measured.
The results of this study are shown in Table II
Table II
Inhibition of Spontaneous Contractions In Vivo
C~ompound #of animals dose m~ (p.o.) % Red (F)*
Example l 3 10 -8 +4
* percent reduction in the total number of spontaneous contractions in the
hypertrophied rat bladder model
CA 022~8~36 1998-12-16
WO 97148682 PCTIIJS97/09744
- 25 -
Hence, the compounds of this invention have a pronounced effect on smooth
muscle contractility and are useful in the treatment of urinary incontinence, irritable
bladder and bowel disease, a~hm~, hypertension, stroke, and similar diseases as
mentioned above, which are amenable to treatment with potassium channel activating
5 compounds by ~lmini~tration, orally, parenterally, or by aspiration to a patient in
need thereof.