Note: Descriptions are shown in the official language in which they were submitted.
CA 02258625 1998-12-17
WO 98/00351 PCT/SE97101130
BLISTER PACK
The present invention relates to a foldable blister pack, especially for
drugs, an apparatus
and a method for manufacturing such a blister pack, as well as the use of the
same.
Blister packs for drugs in tablet form or in the form of powder or liquid
enclosed in a
capsule normally incorporate at least one blister part, which consists of a
set of
interconnected foils covering each other. One relatively rigid foil is in most
cases referred to
as the base and comprises cavities, so-called open "blisters", for
accommodating a tablet or
lo capsule each, while the other foil, which is flat, is in most cases
referred to as the lid and
seals the opening of the cavities or blisters.
Examples of suitable materials for the lid are hard aluminium, soft aluminium,
paper,
polyester, polypropylene and PVC, and examples of suitable materials for the
base are
is aluminium laminate, polypropylene, PVC, PVC/Aclar and PVC/PVDC. There also
exist
various laminates that may be used as basic material for these foils.
Blister packs can be accidentally damaged when they are being carried around
in pockets,
handbags etc. Such damage occurs frequently, especially if the lid foil is
breakable. As a
2o rule, blister packs are therefore stacked in a separate box or casing,
which protects the
blisters during transport. This package is normally bulky and voluminous owing
to the
construction of the blister packs. Further, the user might unintentionally
lose the casing, or
even throw it away. Thus, the presence of a casing does not in practical use
guarantee that
the drug is adequately protected.
To remedy this inconvenience, German Patent Application 44 29 503 discloses a
compact
blister pack comprising a foldable blister assembly. The blister assembly
consists of two
blister parts, each having a set of blisters, and an intermediate part free of
blisters, which is
located between the blister parts and is defined by two folding lines. The
blister parts are
3o foldable towards each other along said folding lines. The blisters of one
blister part are so
offset relative to the blisters of the other blister part that, after folding,
the blisters of the
two blister parts engage between each other. To protect the lid foil of the
folded blister
assembly, there is provided a protective unit which includes two closure
panels that are
interconnected by means of an intermediate panel, which is defined by two
folding lines.
3s This intermediate panel is joined to the intermediate part of the blister
assembly such that a
CA 02258625 1998-12-17
WO 98/00351 PCT/SE99/01130
2
foldable blister pack is formed, in which the closure panels cover the lid
foils after folding
the blister pack.
One disadvantage of this compact blister pack is that the user has little
space available for
handling the blisters, in particular the blisters in the row adjacent to the
intermediate part. A
drug is removed by the user pressing one of the blisters with one of his
fingers, thereby
breaking the lid foil. Due to the lack of space, there is a risk that a
blister part is torn away
from the intermediate part, which is fixed to the protective casing. In such
event, the blister
part is no longer protected by the casing and is also separated from the user
instructions that
~o are printed on or attached to the protective casing.
Also, when a drug is being removed from the known blister pack, the blister
parts have a
tendency to bend and become dented. After some use, it might therefore be
difficult, or
even impossible, to fold the blister pack, since the uneven and dented blister
parts no longer
is fit together.
Further, frequent use of the known blister pack might also lead to
unintentional separation
of a blister part from the casing, since the folding lines of the blister
assembly are weakened
each time the pack is folded or unfolded. This problem is more pronounced when
the blister
2o assembly is made of thin and/or flexible material.
Moreover, it is difficult to combine different drugs in the known blister
pack. This blister
pack requires the use of a foldable blister assembly, which is formed in one
piece. Thus, in
order to combine different drugs, these drugs must be combined when
manufacturing the
2s blister assembly. If different sets of drugs are to be used in the known
blister pack, it is
therefore necessary to keep a variety of blister assemblies in stock, each
blur assembly
containing a specific combination of drugs.
The prior art also comprises GB-B-1 133 947, GB-A-2 266 880, US-A-3 743 084
and US-
so A-4 340 141, disclosing other types of foldable packages containing blister
parts.
The object of the invention is to solve or alleviate at least some of the
problems described
above. More specifically, the blister pack according to the invention should
be compact and
obviate the need for a separate, protective casing. Further, the blister pack
should be
3s durable and minimise the risk of the blister pack being accidentally
damaged during use.
Also, the blister pack should be capable of permanently carrying instructions
for use, and
CA 02258625 2005-O1-27
23940-1276
3
preferably facilitate the provision of different drug
combinations. Preferably, the blister pack should also
provide for simple recycling of the materials used.
Accordingly, in a first aspect of the invention
there is provided a blister pack comprising at least one
blister assembly including two blister parts, each having a
set of blisters and being of the type in which a base foil
formed with blisters is connected to a substantially flat
lid foil, the blister parts being interconnected and
foldable towards each other, the blisters of one blister
part being so offset relative to the blisters of the other
blister part that, after folding, the blisters of the two
blister parts engage between each other, a protective unit
including two closure panels characterised in that a
supporting unit including at least one base panel, which has
at least one hole, is connected to said blister assembly
such that the blisters of at least one blister part are
aligned with said at least one hole, said protective unit
includes a tab, which is connected to one closure panel via
at least one folding line, and said supporting unit is
fixedly joined to said tab such that the closure panels
cover said lid foils after folding of the blister assembly
and the protective unit.
In a second aspect, there is provided a blister
pack comprising at least one blister assembly including two
blister parts, each having a set of blisters and being of
the type in which a base foil formed with blisters is
connected to a substantially flat lid foil, the blister
parts being interconnected and foldable towards each other,
the blisters of one blister part being so offset relative to
the blisters of the other blister part that, after folding,
the blisters of the two blister parts engage between each
other, a protective unit including two closure panels
CA 02258625 2005-O1-27
23940-1276
3a
characterised in that there is provided a supporting unit
including one base panel, which has at least one hole, one
blister part of the blister assembly is joined to said base
panel such that the blisters of said blister part are
aligned with said at least one hole, and the protective unit
is fixedly joined to the supporting unit such that the
closure panels cover said lid foils after folding of the
blister assembly and the protective unit.
In a third aspect, there is provided an apparatus
for manufacturing a blister pack according to the first and
second aspects, characterised by a device for producing a
protective unit and a supporting unit from at least one
blank and for providing folding lines therein; a device for
applying an adhesive to the supporting unit; a device for
aligning and combining a blister assembly with the
supporting unit; and a device for folding the blister pack
along the folding lines.
In a fourth aspect, there is provided a method for
manufacturing a blister pack according to the first and
second aspects, characterised by the steps of producing a
protective unit and a supporting unit from at least one
blank; providing folding lines in said protective unit and
said supporting unit; applying an adhesive to the supporting
unit; aligning and combining a blister assembly with the
supporting unit; and folding the blister pack along the
folding lines.
In a fifth aspect, there is provided the use of a
blister pack according to the first and second aspects for
one or more pharmaceutically active drugs.
CA 02258625 2005-O1-27
23940-1276
3b
The blister pack according to the invention has the advantage that the
supporting unit will
stabilise and protect the blister assembly. This is especially advantageous
when the blister
assembly is made of thin and/or flexible material. Further, separate blister
parts, each
carrying a different drug, can be combined to form a foldable unit by joining
the blister parts
to the supporting snit. In addition, the provision of a supporting unit will
prevent accidental
separation of a blister part from the blister assembly.
Further, since the supporting unit is joined to a tab on the protective unit,
the blisocr pack
has large continuos areas that can be printed with instructions for use or
that can carry
is separate leaflets. Thus, the drugs always are accompanied by adequate
instructions for use.
The invention will now be described in more detail with reference to the
accompanying
drawings, in which
Fig. 1 illustrates a first preferred embodiment and shows in Fig. la the
blister
is . assembly, in Fig. 1b the supporting unit, in Fig. lathe protective unit,
in Fig. Id the
unfolded blister pack, and in Fig. ie an end view of the folded blister pack;
Fig. 2 illustrates a second preferred embodiment and shows in Fig. 2a the
blister
assembly, in Fig. 2b the supporting and protective units, in Fig. 2c the
unfolded blister pack,
and in Fig. 2d an end view the folded blisoer pack; and
lo Fig. 3 illustrates a third pref~d embodiment, wherein Fig. 3a is a
perspective view
of the blister pack in unfolded condition, and Fig. 3b is an opposite
perspective view of the
blister pack in Fig. 3a.
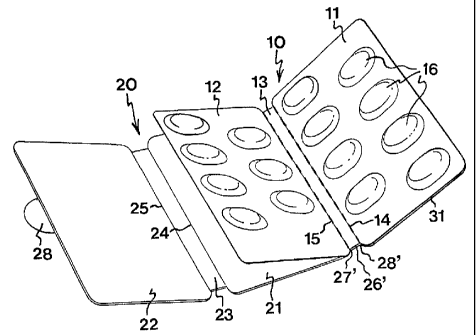
The blister pack in Figs I a-a has a blister assembly 10, which consists of a
first and a second
a blister part 11, I2. Between the blisur parts 11, 12, there is formed an
intermediate part 13
defined by two parallel, longtudinal folding lines 14, 15. Consequently, the
blister parts 1 l,
12 can be folded towards each other along said folding lines 14, 15. The
blister assembly l0
is composed of a base foil, in which blisters 16 are formed, and a flat fid
foil, which is
attached to said base foil. Thus, the lid foil seals the openings of the
blisters 16, each blister
30 16 containing one piece of medicine, e.g. a tablet or a capsule.
CA 02258625 1998-12-17
WO 98/00351 PCT/SE97/01130
4
Each blister part 1 l, 12 has two parallel rows of blisters 16, the blisters
16 of one part 11
being so offset relative to the blisters 16 of the other part 12 that, when
the blister parts 11,
12 are mated in face-to-face relationship, the blisters 16 engage between each
other to form
a single blister layer. To this end, the height of the blisters 16 essentially
corresponds to the
distance between the folding lines 14, 15.
The protective unit 20 consists of first and second closure panels 21, 22 and
an intermediate
panel 23 therebetween. The intermediate panel 23 is defined by two parallel,
longitudinal
folding lines 24, 25, and the protective unit 20 is foldable along these
folding lines 24, 25.
io Further, the protective unit 20 has a tab 26, which is connected to one
longitudinal edge of
the first closure panel 21 via a folding line 27.
Further, a separate supporting unit 30 is provided, which includes first and
second base
panels 31, 32, each having two parallel rows of holes 33. Between the base
panels 31, 32,
is there is formed a linking panel 34 defined by two parallel, longitudinal
folding lines 35, 36,
along which the base panels 31, 32 can be folded towards each other.
The blister assembly 10 is attached to the supporting unit 30 in such a manner
that the
blisters 16 are aligned with the holes 33 and the lid foil of the blister
assembly 10 is facing
2o the supporting unit 30.
The protective and supporting units 20, 30 are so interconnected that the
folding line 36
between the second base panel 32 and the linking panel 34 coincides with one
edge of the
first closure panel 21. To this end, the linking panel 34 of the supporting
unit 30 is fixedly
is joined to the tab 26 on the protective unit 20. Consequently, the folding
lines 24, 25, 27 of
the protective unit 20 are parallel to the folding lines 14, 15 of the blister
assembly 10 and
folding lines 35, 36 of the supporting unit 30.
The folding of the blister pack is simple, since only two folding operations
are necessary to
so close the pack, namely folding the first base panel 31 onto the second base
panel 32 and,
finally, folding the second closure panel 22 onto the first base panel 31. In
the folded
condition shown in Fig. 1e, the blister pack is protected by the closure
panels 21, 22
abutting against the base panels 3I, 32 and thereby covering the holes 33.
ss Preferably, the width of the intermediate panel 23 essentially corresponds
to the thickness of
the folded supporting unit 30, and the first closure panel 21 has essentially
the same
CA 02258625 1998-12-17
WO 98/00351 PCT/SE97/01130
dimensions as the second closure panel 22, thereby creating a folded package
in the form of
a rectangular parallelepiped. The blister pack is maintained in its folded
condition by
fastening means 28, e.g. a piece of reclosable adhesive tape. Obviously, the
folded blister
pack is very stable and protected on all longitudinal sides.
5
One longitudinal side of the folded blister pack is formed by the tab 26,
which is further
stabilised by the supporting unit 30 and the blister assembly 10 being joined
thereto. This
improves the stability of the bliser pack, in particular with respect to shear
forces.
io It should also be noted that the supporting unit 30 will stabilise and
protect the blister
assembly 10. There is no risk of a blister part 11, 12 being accidentally torn
away from the
blister assembly 10.
In the blister pack according to the invention, instructions can be printed on
the closure
is . panels 21, 22 and/or on a separate leaflet that is fixed to one closure
panel side facing the
blister assembly 10. Thus, it is ensured that the drugs always are accompanied
by adequate
instructions for use.
In Figs 2a-d, a second preferred embodiment is shown, which differs from the
first
2o embodiment in that the supporting unit 30 is formed integral with the
pr~ective unit 20. All
embodiments employ a similar blister assembly 10, which therefore need not be
described in
more detail here. The units 20, 30, having already been described with
reference to Figs l,
need no further description either.
2s One edge of the second base panel 32 is connected to a tab 26' of the first
closure panel 21
via a folding line 28'. The tab 26' is connected to the first closure panel 21
via a folding line
27'. Evidently, all folding lines 24, 25, 27', 28', 35, 36 of the protective
and supporting
units 20, 30 are parallel to each other.
3o As is apparent from Fig. 2c, the blister assembly 10 is joined to the
supporting unit 30 in
such a manner that the blisters 16 are aligned with the holes 33 and the lid
foil of the blister
assembly 10 is facing the supporting unit 30.
The blister pack is folded from left to right, as seen in Fig. 2c, the first
base panel 31 being
ss first folded onto the second base panel 32. These parallel panels 31, 32
are then folded onto
the first closure panel 21 and, finally, folded onto the second closure panel
22. In the folded
CA 02258625 1998-12-17
WO 98/00351 PCT/SE97/01130
6
condition of the blister pack, the first closure panel 21 will cover the first
base panel 31, and
the second closure panel 22 will cover the second base panel 32, thereby
protecting that
part of the lid foil which is accessible through the holes 33.
s The second embodiment, apart from having the same advantages, is also easier
to
manufacture than the first embodiment, since it contains only two separate
parts. However,
the second embodiment requires a more complicated folding operation and might
also be
more difficult to handle for the patient because of the greater length of the
blister pack in
unfolded condition.
lo
Figs 3a-b show a third embodiment, which differs from the second embodiment in
that the
supporting unit has only one base panel 31, which is formed integral with the
protective unit
20. The base panel 31 is connected to a tab 26' of the first closure panel 21
via a folding
line 28'. The tab 26' is connected to the first closure panel 2I via a folding
line 27'.
is
One and only one blister part 11 of the blister assembly 10 is joined to the
base panel 31 in
such a manner that the blisters 16 are aligned with the holes 33 and the lid
foil faces the base
panel 31.
2o Folding the blister pack is easy, and only two folding operations are
required to close the
pack, namely folding the base panel 31 onto the second blister part 12 and,
finally, folding
the second closure panel 22 onto the base panel 31. In folded condition, the
blister pack is
protected by the closure panels 21, 22 covering the holes 33 and is thereby
protected on al/
its longitudinal sides.
2s
T-he folded blister pack is very stable and shear resistant. One reason for
this is that one
longitudinal side of the folded blister pack is formed by the tab 26', which
is stabilised by
the base panel 31 being joined thereto. Since the base panel 31 is placed
inside the folded
pack, between the blister assembly 10 and the closure panel 22, the blister.
pack is locked in
3o a stable configuration when folded. This stability is achieved with minimum
use of raw
material in the protective and supporting units 20, 30.
Further, since the base panel 31 is joined to the tab 26' on the protective
unit 20, the blister
pack has large continuos areas that can be printed with instructions for use.
Thus, the drugs
3s always are accompanied by adequate instructions for use.
CA 02258625 1998-12-17
WO 98/00351 PCT/SE97/01130
7
This third embodiment enables the user to remove the second blister part 12,
when emptied,
from the blister pack by simply tearing along the folding line 15, which might
be perforated
to facilitate separation.
s In another conceivable embodiment, the intermediate part 13 is also joined
to the
intermediate panel 26'. In the preferred third embodiment, the intermediate
part 13 is,
however, not joined to the intermediate panel 26', thereby providing the
additional
advantage of facilitating the removal of the drugs from the blisters, since
the user has more
space available for handling the blisters 16 on the second blister part 12, in
particular the
~o blisters 16 in the row adjacent to the intermediate part 13. A drug could
be removed from
the blister pack by the user pressing one of the blisters 16 with one of his
fingers, thereby
breaking the lid foil, and this preferred embodiment allows the user more
liberty of action
when applying pressure on the blisters. Thus, the risk of accidentally
separating the blister
part 12 from the blister pack is less than in a conventional blister pack.
is
This embodiment also has a cutout 36', which is formed at one of the corners
of the base
panel 31 and which uncovers part of the blister assembly 10. This feature
facilitates the
separation of the blister assembly 10 from the supporting and protective units
20, 30, since
the blister assembly 10 can readily be gripped at the cutout 36' and be torn
away from said
2o units 20, 30. In view of the recycling of the materials used, this is an
attractive feature,
which can be incorporated in any of the embodiments of the invention.
In all embodiments shown, the folding lines are arranged in parallel to each
other. This
parallelism is preferred, since it facilitates the folding of the blister
pack.
2s
Evidently, the blister assembly of the first and second embodiments of the
inventive blister
pack could consist of two separate blister parts, which are joined in any
suitable manner,
e.g. by being glued to a supporting unit.
3o Further, it is appreciated that the blister assembly could consist of
several blister parts,
which are interconnected by intermediate parts free of blisters, said blister
parts being folded
in pairs in a meandering manner. Also, the blister pack can include more than
one blister
assembly, for example by one blister part of each blister assembly being
joined to a
respective supporting unit on the protective unit.
CA 02258625 1998-12-17
WO 98!00351 PCT/SE97/01130
Further, it should be noted that a combined blister pack could be formed from
two blister
packs according to the invention, preferably by joining a closure panel of one
blister pack
with a closure panel of the other blister pack. Referring to the embodiment of
Fig. 3, the
first closure panel 21 of one blister pack could, on the side facing away from
the blister
assembly 10, be joined to a corresponding closure panel 21 on another blister
pack. This
combined blister pack has the same advantages as the included, individual
blister packs.
According to the invention, the blister assembly can be fixedly joined to the
protective unit
by any suitable means, e.g. an adhesive. This also applies to the attachment
of the blister
io assembly to the supporting unit as well as the attachment of the supporting
unit to the
protective unit.
Further, the shape of the holes in the supporting unit must not necessarily
correspond to the
shape of the blisters and could have any form uncovering the lid foil in front
of the blisters.
~s
In a preferred embodiment, the blister pack according to the invention is used
for a
pharmaceutically active drug, such as a proton pump inhibitor, e.g.
omeprazole. The blister
pack could have at least two differently shaped sets of blisters, each set
containing a
different drug. This type of blister pack is especially useful for packing, in
one blister pack,
2o two drugs e.g. a proton pump inhibitor and at least one antibiotic that
should be
administered in combination, such as omeprazole and an antibiotic. Another
embodiment of
the invention is to use the blister pack for packing tablets which contain a
combination of
drugs.
2s A wide variety of antibiotics may be used in combination with a suitable
proton pump
inhibitor. Such antibiotics include for example nitroimidazole antibiotics,
tetracyclines,
penicillins, cephalosporins, carbopenems, aminoglycosides, macrolide
antibiotics,
lincosamide antibiotics, 4-quinolones, rifamycins and nitrofurantoin. In the
following
examples of such antibiotics are listed: ampicillin, amoxicillin,
benzylpenicillin,
3o phenoxymethylpenicillin, bacampicillin, pivampicillin, carbenicillin,
cloxacillin, cyclacillin,
dicloxacillin, methicillin, oxacillin, piperacillin, ticarcillin,
flucloxacillin, cefuroxime,
cefetamet, cefetrame, cefixime, cefoxitin, ceftazidime, ceftizoxime,
latamoxef,
cefoperazone, ceftriaxone, cefsulodin, cefotaxime, cephalexin, cefaclor,
cefadroxil,
cefalothin, cefazolin, cefpodoxime, ceftibuten, aztreonam, tigemonam,
erythromycin,
3s dirithromycin, roxithromycin, azithromycin, clarithromycin, clindamycin,
paldimycin,
lincomycin, vancomycin, spectinomycin, tobramycin, paromomycin, metronidazole,
CA 02258625 1998-12-17
WO 98/00351 PCT/SE97101130
9
tinidazole, ornidazole, amifloxacin, cinoxacin, ciprofloxacin, difloxacin,
enoxacin,
fleroxacin, norfloxacin, ofloxacin, temafloxacin, doxycycline, minocycline,
tetracycline,
chlortetracycline, oxytetracycline, methacycline, rolitetracyclin,
nitrofurantoin, nalidixic
acid, gentamicin, rifampicin, amikacin, netilmicin, imipenem, cilastatin,
chloramphenicol,
s furazolidone, nifuroxazide, sulfadiazin, sulfametoxazol, bismuth
subsalicylate, colloidal
bismuth subcitrate, gramicidin, mecillinam, cloxiquine, chlorhexidine,
dichlorobenzylalcohol,
methyl-2-pentylphenol. The active antibiotics could be in standard forms or
used as salts,
hydrates, esters etc. A combination of two or more of the above listed drugs
may be used.
Preferable antibiotics are clarithromycin, erythromycin, roxithromycin,
azithromycin,
lo amoxicillin, metronidazole, tinidazole and tetracycline. Clarithromycin and
metronidazole
alone or in combination are especially suitable.
An apparatus (not shown) for manufacturing any of the embodiments having a
supporting
~s unit, comprises a device, such as a punching machine, for producing a
pr~ective unit and a
supporting unit from one or two blanks and for providing folding Iines
therein, a device for
applying an adhesive to the supporting unit, a device for aligning and
combining a blister
assembly with the supporting unit, and a device for folding the blister pack
along the folding
lines. In the case of a blister pack with separate supporting and protective
units, the
2o apparatus could comprise a device for combining these units before folding
the blister pack.