Note: Descriptions are shown in the official language in which they were submitted.
CA 022~904~ 1999-01-13
This invention relates to a method of constructing a
structure for determining an item of interest in a
sample.
This Application is a Divisional of Canadian Patent
Application S.N. 2,214,125, filed August 28, 1997.
BACKGROUND
Embodiments described herein relate generally to
methods and structures which determine an item of
interest in a sample.
To provide information about a patient's health, a
number of tests can be performed on a patient sample,
such as the patient's bodily fluids. These bodily fluids
may include blood, urine, etc. The tests performed on
the patient's bodily fluids can determine an item of
interest in the bodily fluids. Based on the
determination of the item of interest in the patient's
bodily fluids, information about the patient's health
status can be obtained.
SUMMARY
In accordance with one aspect of the invention there
is provided a method of constructing a structure for
determining an item of interest in a sample, the method
comprising the steps of: (a) determining an effective
length of movement of a container for holding the sample
in a process lane where a process step is selectively
automatically performed on the sample in the container;
(b) constructing the process lane such that the process
lane meets at least one of a predetermined throughput and
a predetermined physical dimension; and (c) arranging
elements along the process lane such that the container
CA 022~904~ 1999-01-13
moves the effective length during performance of a
determination of an item of interest.
In accordance with another aspect of the invention
there is provided a method of constructing a structure
for determining an item of interest in a sample, the
method comprising the steps of: (a) determining an
effective length of movement of a container for holding
the sample in a process lane where a process step is
selectively automatically performed on the sample in the
container; (b) constructing the process lane such that
the process lane meets at least one of a predetermined
throughput and a predetermined physical dimension; and
(c) operatively connecting the process lane with a
controller such that the container moves the effective
length during performance of a determination of an item
of interest irrespective of the at least one of a
predetermined throughput and a predetermined physical
dimension.
In a particular embodiment the controller causes the
container to move along the process lane at least one,
and in another particular embodiment the controller
causes the container to move along the process lane at
least twice.
CA 022~904~ 1999-01-13
2a
EMBODIMENTS
One embodiment discussed herein relates to a structure for
performing a process for determining an item of interest in a
sample. The structure comprises a process path. The process
path comprises a process lane including a process step
performance lane where a process step is performed, and a
process step avoidance lane where the process step is avoided.
A first prime mover is operatively connected with the process
path for moving a container holding the sample along the process
path. A first pipetting system is operatively associated with
the process path for introducing the sample to the container. A
second pipetting system is operatively associated with the
process path for introducing a reagent to the container. A
device is operatively connected with the process path and is
selectively engagable with the container for mixing the sample
and the reagent in the container. A second prime mover is
operatively connected with the process path for selectively
positioning the container in a selected one of the process step
performance lane and the process step avoidance lane. A reader
is operatively connected with the process path for determining
the item of interest in the sample based upon a reaction between
the sample and the reagent.
In another embodiment, the structure comprises a process
path. The process path comprises a process lane accepting a
container for the sample. The process lane includes a process
step performance lane where a process step is performed, and a
process step avoidance lane where the process step is avoided.
In an additional embodiment, a structure comprises a
process step performance lane accepting a container for the
sample where a process step is performed, and a process step
avoidance lane accepting the container where the process step is
avoided.
Another embodiment provides a structure comprising a cover,
a base connected with the cover, a disk rotatably disposed
CA 022~904~ 1999-01-13
between the cover and the base and a slot disposed on the
disk for accepting a container for the sample. The slot
has a longitudinal axis and the container is movable
along the longitudinal axis.
Yet a further embodiment provides a struct~re
comprising a process lane for accepting a container for
holding the sample. The process lane includes a bypass
region for selectively automatically performing a process
step on the sample in the container.
Other embodiments provide a structure comprising a
process lane for accepting a container for holding the
sample. The process lane includes an element for provid-
ing selective automated performance of a determination of
item of interest process step.
Embodiments described herein also provide methods of
performing a process for determining an item of interest
in a sample. In one embodiment, a container for holding
the sample is accepted in a process lane where a process
step is selectively automatically performed on the sample
in the container. The process step is selectively auto-
matically performed on the sample in the container. An
effective length of the process lane is maintained
constant while a physical length of the process lane is
selectively varied.
In another embodiment, a container for holding the
sample is accepted in a process lane where a process step
is selectively automatically performed on the sample in
the container. The process step is selectively automati-
cally performed on the sample in the container. A
physical length of the process lane is selectively
varied.
Other embodiments described herein provide methods
of performing a process for determining an item of
interest in a sample. In one embodiment, a process path
comprising a process lane including a process step
performance lane where a process step is performed, and a
. .
CA 022~904~ 1999-01-13
3a
process step avoidance lane where the process step is
avoided is provided. A container holding the sample is
moved along the process path. The sample is introduced
to the container. A reagent is introduced to the
container. The sample and the reagent are mixed in the
container. The container is selectively positioned in a
selected one of the process step performance lane and the
process step avoidance lane. The item of interest in the
sample is determined based upon a reaction between the
sample and the reagent.
In another method, a process path is provided
comprising a process lane accepting a container for the
sample. The process lane includes a process step
performance lane where a process step is performed, and a
process step avoidance lane where the process step is
avoided. The container is introduced to the process
lane. The container is selectively automatically
positioned in a selected one of the process step
performance lane and the process step avoidance lane.
In an additional method, a container for the sample
is positioned in a process step performance lane. A
process step is performed with the container in the
process step performance lane. The container is
positioned in a process step avoidance lane. The process
step is avoided with the container in the process step
avoidance lane.
In a further method, a container for holding the
sample is introduced in a process lane including a bypass
region for selectively automatically performing a process
step on the sample in the container. The process step is
performed on the sample in the container when the
container is in the bypass region.
In yet another method, a container for the sample is
inserted into a loading lane. The container is moved
from the loading lane to the process lane. The sample is
introduced to the container. The reagent is introduced
CA 022~904~ 1999-01-13
3b
to the container. Contents of the container are mixed.
The contents of the container are incubated. The
container is moved through a bypass region. A signal
generated by the contents of the container is read.
An additional embodiment provides a method where a
container for holding the sample is accepted in a process
lane where process step is selectively automatically
performed on the sample in the container. The process
step is selectively automatically performed on the sample
in the container.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a perspective view of a component of an
analyzer;
Fig. 2 shows the component of Fig. 1 with elements
thereof removed for clarity;
Fig. 3 is a perspective view of an element of the
component shown in Flg. 1;
Fig. 4 is a top view of the component of Fig. 1 with
elements thereof removed for clarity;
Figs. 5A and 5B show another element of the
component of Fig. 1 which is connected with the structure
shown in Fig. 2;
Fig. 6 is an enlarged sectional view of the
component of Fig. 1 with elements removed for clarity;
Fig. 7A is a perspective view of a container for use
with the component of Fig. 1;
Fig. 7B is a perspective view of another container
for use with the component of Fig. 1;
CA 022~904~ 1999-01-13
Fig. 8 is an enlarged sectional view of a portion of the
component of Fig. 1 showing interaction with the container of
Fig. 7B;
Fig. 9 is an enlarged sectional view, substantially similar
to that of Fig. 8, of another portion of the component of Fig.
l;
Fig. 10 is substantially similar to Fig. 9 but shows
another portion of the component of Fig. l;
Fig. 11 is substantially similar to Fig. 10 but shows
another portion of the component of Fig. li
Fig. 12 is a perspective view of an element of the
component of Fig. l;
Fig. 13 is an enlarged sectional view of a section of
another embodiment of the component shown in Fig. l;
Fig. 14 is a perspective view of an element of the
component of Fig. l;
Fig. 15 is a perspective view of an element of the
component of Fig. l;
Fig. 16 is a generic view of the component of Fig. 1
cooperating with other portions of an analyzer;
Fig. 17 is a perspective view of a frame for the structures
shown in Fig. 16;
Figs. 18A, 18B and 18C illustrate an element of the
component shown in Fig. l;
Fig. 19 is an enlarged sectional view of a section of
another embodiment substantially similar to that shown in Fig.
13;
Figs. 20A and 20B are generic views of other related
analyzers having oppositely directed components substantially
similar to the component of Fig. l;
Figs. 21A, 21B and 21C show an embodiment of a high density
data carrier which may be used with the component of Fig. l;
Fig. 22 is an isometric view of a container for use with
the process path of Fig. l;
CA 022~904~ 1999-01-13
Figs. 23A, 23B and 23C show another container for use with
the process path of Fig. l;
Figs. 24A and-24B are enlarged sectional views of a portion
of the container of Figs. 23A, 23B and 23C operatively
associated with a support;
Fig. 25 is an isometric view of a seal which may be used
with the containers of Figs. 22, 23A, 23B and 23C;
Fig. 26 is an enlarged section of another application of
the process path of Fig. l;
Fig. 27 is an enlargement of a portion of Fig. 27;
Fig. 28 is a generic view of another related analyzer
having a component substantially similar to the component of
Fig. l;
Fig. 29 is an illustration of two components of Fig. 1
joined together;
Fig. 30 is an enlarged view of a portion of Fig. 29;
Figs. 31A, 31B and 31C show another container for use with
the process path of Fig. l; and
Figs. 32A and 32B illustrate portions of another embodiment
of the process path.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
The embodiments described herein relate to methods and
structures for determining an item of interest in a sample. The
item of interest may be an antibody, an antigen, concentrations
of the former or latter or any other desired element of the
sample. In an exemplary embodiment, the item of interest is
selected from, but are not limited to, antibodies to HCV,
antibodies to HIV l/HIV 2, antibodies to hepatitis B core
antigen (HBcAb), carcinoembryonic antigen (CEA), cancer antigen
19-9 (CAl9-9), Hepatitis B Surface Antigen (HBsAg), antibodies
to Hepatitis B Surface antigen (HBsAb), alpha-fetoprotein (AFP),
Total prostate specific antigen (Total PSA), Free PSA, Thyroid
stimulating Hormone (TSH), luteinizing hormone (LH), follicle
CA 022~904~ 1999-01-13
stimulating hormone tFSH), beta human chorionic gonadotropin (B-
hCG), Free Thyroxine (Free T4), Free triiodothyronine (Free T3),
Total T4, Total T3, Progesterone, Testosterone, Estradiol,
Prolactin, vitamin B12 (B12), Folate, Glycated Hemoglobin, and
Ferritin. The structures and methods may be employed in a
number of different configurations.
For the sake of clarity of understanding, the structures
and methods will be discussed with respect to their employment
in an immunoassay analyzer which performs approximately 200
determinations of items of interest in a sample in an hour. It
is to be noted that the structures and methods can be used in
other employments, such as analyzers which perform 600, 400,
100, 50, etc. determinations in an hour. A number of analyzers
may be joined together or integrated to meet individual needs,
such as modifying the number of tests performed in a given time
period (throughput~, tailoring the items of interest to be
determined, etc. For example a number X of analyzers which
perform Y determinations in a given hour may be connected such
that the connected analyzers perform XY determinations in an
hour.
It is to be noted that all such analyzers perform all
determinations of items on interest in substantially the same
way. For instance, all determination process steps for all
items of interest are performed within the same time frame, such
as 18 seconds, irrespective of the number or type of
determinations to be performed by the given analyzer. These
analyzers may include common elements, such as reagents,
disposable articles, element, such as fluids and the like,
delivery technologies, determination step performance
mechanisms, software, etc.
In other applications, the analyzer may be joined, e.g.
with a conveyor system and the like, along with supporting
hardware and software, such that the analyzer can be used with
different analyzers, such as clinical chemistry or hematology
analyzers and the like, in the same setting. This conveyor
, ~
CA 022~904~ 1999-01-13
system may move samples among the analyzers such that different
determinations can be made with respect to one sample. Also,
while operation of-the analyzer is described herein with respect
to only one analyzer, for the sake of clarity, it is to be
remembered that multiple analyzers can operate in the same of in
different fashion, either simultaneously or at different times.
Furthermore, steps of one method of operation can be combined
with steps of another method of operation to arrive at yet more
methods of operation.
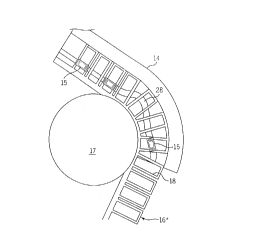
As illustrated in Fig. 1, the analyzer comprises a process
path 10. It is understood that there are other elements (not
shown), such as fluid delivery mechanisms, suppliers, and the
- like, of the analyzer that support operation of the process path
10. While the process path 10 is illustrated as being
substantially circular in configuration, the process path 10 may
take other configurations, such as linear, serpentine, etc., as
desired.
The process path 10 includes a cover 12 and a base 14. The
base 14 may be attached to a support frame (Fig. 17) and the
cover 12 is attached to the base 14. The cover 12 may be a
single piece or may comprise multiple, sometimes 6, pieces.
Various elements, some of which are described below, of the
process path 10 are connected to at least one of the cover 12
and the base 14. The cover 12 and the base 14 include
structures, such as openings and the like, for accommodating
some of the elements. In one embodiment, the base 14 has an
inner diameter of about 25.58 inches, an outer diameter of about
30.08 inches and a height of about 1.99 inches. The base 14 may
be made of any suitable material, such as a metal, a polymer and
the like. In one embodiment, the base 14 is made of anodized
aluminum, including a reduced friction coating, such as a PTFE-
impregnated anodized coating. In a particular embodiment, the
base 14 is made from 6061-T6 aluminum with a MIL-A-63576, Type I
finish. The cover 12 may be made of a material which is
substantially similar to the material of the base 14.
CA 022~904~ 1999-01-13
Fig. 2 shows the process path 10 with the cover 12 removed
from the base 14. With the cover 12 removed, a disk 16 is
visible. The disk-16 is located between the cover 12 and the
base 14 and is movable with respect to both the cover 12 and the
base 14.
In some embodiments, the disk 16 may be replaced by a belt
16~, shown in Figs. 32A and 32B, driven by a wheel 17. Use of
the belt 16S provides for orientations other than substantially
circular, i.e. serpentine and like, of the process path 10. The
belt 16t moves with respect to the cover 12 and the base 14 in
substantially the same manner as the disk 16. In other aspects,
construction of the process path 10 is substantially similar
irrespective of use of the disk 16 or the belt 16~.
The disk 16, illustrated more clearly in Fig. 3, has, in
one embodiment, an inner radius of about 25.2 inches and an
outer radius of about 29.3 inches. The disk 16 may have a
thickness of about 0.063 inches. The disk 16 may be formed from
any suitable material, such as a polymer and the like. In a
particular embodiment, the disk 16 is made from polyvinyl
chloride. The disk 16 may be machined, molded or the like. In
an exemplary embodiment, the material comprising the disk 16 is
chosen with respect to the material of the base 14 to reduce
friction between the base 14 and the disk 16.
A plurality. 112 in the illustrated embodiment, of slots 18
are disposed on the disk 16. As is discussed in greater detail
later, the slots 18 cooperate with structures on the base 14 to
move containers 15 (Figs. 7A and 7B) along the process path 10.
Each slot 18 has, with respect to the disk 16 in an exemplary
embodiment, a radial length of about 1.75 inches and a
tangential width of about 0.45 inches with a slot 18 centerline
being located at a radius of about 13.614 inches. As is
discussed further below, the slot 18 has a longitudinal axis and
the container 15 is capable of moving within the slot 18 along
the slot's 18 longitudinal axis. To facilitate movement of the
container 15 along the longitudinal axis of the slot 18, the
CA 022~904~ 1999-01-13
process path 10 may include a configuration, such as a surface,
a diverter, a prime mover engagable with the container 15, and
the like. In another embodiment, one end of the slot 18 may
include a latitudinally expanded width (Fig. 13) to facilitate
removal of a container 15 from the disk 16. In still a further
embodiment, the latitudinally expanded width may be located at
another region of the slot 18 (Fig. 19).
The disk 16 is configured to facilitate movement of the
disk 16 with respect to the cover 12 and the base 14. In one
embodiment, a plurality of teeth 20 are disposed along an outer
diameter surface of the disk 16. In an exemplary embodiment,
the teeth 20 may be about 938 in number with a diametral pitch
of about 32, a pressure angle of about 20 degrees and a pitch
diameter of about 29.3125 inches.
As shown in Fig. 6, the teeth 20 mate with a gear 22 which
is driven by a prime mover 24 attached to the base 14 by a
bracket 26. In an exemplary embodiment, the gear 22 is made
from Estane 58130 natural 92A/50D polyurethane and the motor 24
is a P21 model available from Pacific Scientific of Rockford,
Illinois. The prime mover 24, the entire process path 10 and
its supporting elements, are connected with and are operated by
a suitable controller, such as a computer (not shown) running
appropriate routlne and the like. In this manner, the disk 16
moves responsive to movement of the gear 22 by the prime mover
24. In a particular embodiment, the prime mover 24 is a stepper
motor.
Referring to Fig. 4, the base 14 includes structures to
facilitate determination of an item of interest in a sample.
The base 14 comprises at least one lane 28 for guiding movement
of a container 15 along the process path 10 responsive to
movement of the disk 16. As the disk 16 moves responsive to
activation of the prime mover 24, the container 15 moves along
the lane 28 from one processing station to another to complete
determination of the item of interest in the sample.
CA 022~904~ 1999-01-13
In the illustrated embodiment, there are a first processing
lane 28 and a loading lane 30 in the process path 10.
Complimentary portions of the lanes 28 and 30 are formed in both
the cover 12 and the base 14. Because these two lanes 28 and 30
are substantially concentric, the disk 16, which is adjacent
both lanes 28 and 30, and its slots 18 are dimensioned to accept
and to support containers 15 disposed in both the process lane
28 and the loading lane 30 at substantially the same
circumferential position, while being radially offset, on the
disk 16. In an exemplary embodiment, the lanes 28 and 30 have a
width of about 0.279 inches at the top and have a draft angle of
about 1.5 degrees.
As shown in Figs. 18A, 18B and 18C, in one embodiment, the
loading lane 30 accepts and orients containers 15 from a
container 15 supply or hopper 102. A disk 104 including a
projection 106 is moved within the hopper 102 by a prime mover
108. In some embodiments, structures may be included with the
hopper 102, such as a baffle for directing container 15 movement
within the hopper 102 responsive to disk 104 movement, an
~inherent flat springn actuated by a cam driven mechanism
associated with the disk 104 to move containers lS within the
hopper 102, and the like, to facilitate movement of the
containers 15. As the disk 104 moves within the hopper 102, the
projection 106 is inserted through the top surface 42 of a
container 15 in the hopper 102. The projection 106 carries the
container 15 toward a loading mechanism 110, which may include a
mover 111, such as a barrel cam and the like, for moving a
container 15 from the hopper 102 toward the loading lane 30. As
the container 15 approaches the loading lane 30, in one
embodiment, another mover 112, such as a solenoid-driven rod and
the like, moves the container 15 into a slot 18 in the disk 16
at the loading lane 30. Alternatively, the container 15 may
move from an end of the mover 111 into a slot 18 in the disk 16
at the loading lane 30 under the influence of gravity.
CA 022~904~ l999-0l-l3
In an exemplary embodiment, the hooper 102 is made from
Lexan WR2210 (GE Plastics of Pittsfield, Massachusetts) with a
black SPI Bl finish and has a volume substantially within the
range of about 396 to about 540 cubic inches, thereby allowing
the hopper 102 to hold approximately 1000 containers 15. The
disk 104 is made from Lexan 500 with a finish of gray SPI Bl and
the projection 106 is made from Lexan WR2210 with a finish of
black SPI Bl. The disk 104 includes four projection 106 mounts
spaced equidistantly along a circumference of the disk 104, i . e.
every 90 degrees, at a radius of about 4.5 inches from a center
of the disk 102. To assist movement of containers 15 within the
hopper 102, the disk 102 includes a plurality, such as four, of
nubs having a spherical radius of about 0.165 inches spaced
equidistantly along a circumference of the disk 104, i . e. every
90 degrees, at a radius of about 3.312 inches from a center of
the disk 102. The projection 106 has a nominal thickness of
about 0.1 inches and a length of about 0.9 inches. The
projection 106 is aligned substantially tangentially to a 4.5
inch radius of the disk 102. The mover 108 may be No. 78431-101
20 from Pacific Scientific of Elgin, Illinois. The mover 111
includes a screw made from Delrin 500 having a black SPI Bl
finish. The screw is about 7.126 inches long and has 18 threads
of a diameter measuring about 0. 706 inches and of a pitch of
about 0. 394 inches. The screw is connected to a drive gear made
25 from Celcon M90 having a finish of black SPI Bl. The drive gear
is an involute gear having 24 teeth with a diametral pitch of
about 32, a pressure angle of about 20 degrees and a pitch
diameter of about 0. 75 inches. The mover 112 may be No . 78851-
102 available from Haydon Switch & Instrument of Waterbury,
30 Connecticut. In other embodiments, No. 78425-101 available from
SPM/Portland of Hillsboro, Oregon may be used for some of the
components.
As shown in Figs. 7A and 7B, the container 15 includes a
sample receiving chamber 32 and a pair of support surfaces 34A
35 and 34B connected with the sample receiving chamber 32. As
CA 022~904~ 1999-01-13
shown in Fig. 8, the support surfaces 34A and 34B rest on
portions of the disk 16 which bound the slot 18. The chamber 32
is formed by two sets of side walls 36A, 36B, 38A and 38B and a
bottom wall 40. In an exemplary embodiment, the largest
external distance between the side walls 36A and 36B, which have
a rib width of about 0.020 inches, is about 0.26 inches, the
largest external distance between the side walls 38A and 38B is
about 0.44 inches, the support surfaces 34A and 34B extend a
distance measuring about 0.085 inches from the side walls 38A
and 38B, respectively, the maximum length of the container 15 is
about 1.445 inches, an open end of the sample receiving chamber
32 measures about 0.391 inches by about 0.219 inches, a nominal
thickness of the walls 36A, 36B, 38A and 38B is about 0.030
inches, an inside depth of the sample receiving chamber 32 is
about 1.34 inches having a volume of about 1.4 ml and a volume
of the sample receiving chamber 32 at a location, from which
determination measurements are made, measuring about 0.699
inches from a bottom of the container 15 is about 0.45 ml. A
top surface 42 of the container 15 is located a distance
measuring about 0.18 inches from the support surfaces 34A and
34s. The container 15 may be made from Escorene 3345-E5 (Exxon,
Houston, Texas) or Montell PD701N (Wilmington, Delaware) with an
internal finish of polished SPE/SPE 1 B-2.
Returning to Figs. 4 and 8, cooperation among the container
15, the slots 18 in the disk 16 and the lanes 28 and 30
facilitate movement of the container 15 along the process path
10. Specifically, the dimensions of the container 15, the slots
18 and the lanes 28 and 30 are predetermined such that the
support surfaces 34A and 34B of the container 15 radially
slidingly engage the disk 16 adjacent to the slot 18 in which
the container 15 is disposed while the container 15 itself is
restrained from rotation within the slot 18. In one embodiment,
the process lane 28 has a radius of about 27.6 inches and a
width of about 0.28 inches while the loading lane 30 has a
smaller radius but a similar width. The container 15 is
CA 022~904~ 1999-01-13
disposed such that axes of the side walls 36A and 36B are
positioned substantially radially with respect to the process
path 10 and the support surfaces 34A and 34B are aligned
substantially circumferentially with respect to the process path
10. In this manner, as the disk 16 moves responsive to
activation of the prime mover 24, the container 15 within the
slot 18 moves substantially tangentially to the process path 10
within the lanes 28 and 30.
As the process path 10 may be used with biological samples,
it is desirable to maintain the process path 10, or portions
thereof, at a suitable temperature, such as 37 degrees Celsius,
to facilitate determination of the item of interest. Thus, a
heater (not shown), such as an electrlc heater and the like, may
be thermally associated with the process path 10. In an
exemplary embodiment, a plurality of electric resistive flexible
strip heaters may be applied, such as by a suitable adhesive and
the like, to the cover 12 and/or the base 14 of the process path
10. These heaters apply sufficient thermal energy to the
process path 10 such that the contents of the container 15 is
maintained at the desired temperature. Also, because the
loading lane 30 is part of the process path 10, it is possible
to bring the container 15 to the desired temperature prior to
addition of anything to the container 15. For example, if
determination of an item of interest in a sample is performed
optimally at a given temperature, the container 15 in the
loading lane 30 can be brought to that given temperature at a
certain time period after introduction of the container 15 from
the hopper to the loading lane 30 but before the container 15 is
needed to perform the desired determination. Suitable
temperature control devices, such as thermistors and the like,
are also provided along the process path 10. Additionally, in
some embodiments, materials, such as reagents and the like, to
be added to the container 15 may be heated prior to addition to
the container 15. In some cases, the material delivery
CA 022~904~ 1999-01-13
apparatus, such as a fluid conduit and the like, may be
associated with appropriate heaters and heat sensors.
When a container 15 is needed to perform a given item of
interest determination, the container 15 is moved from the
loading lane 30 to the process lane 28. This function is
performed at location 48 shown at Fig. 4 To move the container
15 from the loading lane 30 toward the process lane 28, as shown
in Fig. 10, a prime mover 44, mounted on the process path 10, is
operated. A container 15 engaging member 46 operatively
connected with the prime mover 44 bears against the side wall
36A of the container 15 and moves the container 15 radially
outward with respect to the disk 16 within the slot 18 from the
loading lane 30 towards the process lane 28 responsive to
activation of the prime mover 44. In an exemplary embodiment,
the member 46 is made from 6061-T6 aluminum with a MIL-A-63576,
Type I finish. The member 46 may include structures, such as a
slot, which mate with complimentary structures, such as a pin,
on the prime mover 44 to provide desired alignment of the mover
44 and the arm 46 and to limit undesired movement, such as
rotation, of the member 46. Operation of the prime mover 44
causes the member 46 to move a distance of about 0.5 inches with
a minimum starting force of about 7.08/.25 gm/oz and a mi~iml1m
ending force of about 56.7/2.0 gm/oz.
To accommodate movement of the container 15, a passageway
50 is formed on the cover 12 and the base 14 connecting the
process lane 28 with the loading lane 30. Once the container 15
is in the process lane 28, the prime mover 44 moves the
container 15 engaging member 46 away from the container 15 just
moved to a waiting position to move another container 15 from
the loading lane 30 toward the process lane 28. In an exemplary
embodiment, the prime mover 44 is a solenoid, a pneumatically
actuated motor, a linear positioner or the like. In a
particular embodiment, the prime mover 44 is an electric
solenoid with its windings modified such that the solenoid
CA 022~904~ 1999-01-13
travel occurs without splashing or spilling of container 15
contents.
Now that the container 15 has been moved from the loading
lane 30 to the process lane 28, movement of the disk 16 causes
the container 15 to move along the process lane 28 for
performance of determination of an item of interest in a sample.
In some cases, the sample, such as blood or other bodily fluids,
added to the container 15 is in liquid form. Also, in some
cases, other substances, such as reagents and the like, are
added to the sample in the container 15 during determination of
an item of interest in the sample. These other substances may
also be in liquid form.
As these liquids are added to the container 15 it is
possible that some of the liquids may not end up within the
container 15 but may be disposed on the disk 16 or other
portions of the process path 10. To substantially remove these
liquids, drain ducts 52 are provided on the base 14 of the
process path 10. These drain ducts 52 are recessed from a
groove 54 on the base 14 in which the disk 16 is disposed. In
an exemplary embodiment, the drain ducts 52, about 112 in
number, are equidistantly spaced along a circumference of the
base 14, recess a distance of about 0.125 inches from the groove
54, have an internal angle of about 90 degrees and are about
0.05 inches deep and about 0.1875 inches wide. In some
embodiments, the drain ducts 52 may be inclined toward the
process lane 28 such that liquid within the drain ducts 52 will
move under the influence of gravity toward and into the process
lane 28. In the illustrated embodiment, the drain ducts 52 are
oriented in an expected direction of disk 16 rotation. In this
embodiment, liquid movement within the drain ducts 52 is
encouraged by movement of the disk 16. Similar drain ducts 52
may be formed on the cover 12. To facilitate substantial
removal of the liquids from the process lane 28, drain holes 56
are provided in the base 14 at various locations along bottom
portions of the process lane 28.
CA 022~904~ l999-0l-l3
16
The process of determining an item of interest in a sample
comprises a number of steps. However, given the specific item
of interest to be determined, different steps are to be
performed. For instance, for determination of a first item of
interest, three process steps are to be performed, whereas for a
second item of interest, only two process steps are to be
performed. These process steps may include, for example,
solid/liquid phase (for example, magnetic) separation,
aspiration of container 15 contents, container 15 contents
washing, etc. To offer determination of both the first and
second items of interest, the process path 10 includes
structures for selective automated performance of process steps.
However, it is to be noted that the process path 10 includes all
structures necessary to perform all process steps for
determining a predetermined set of items of interest.
At at least one location along the process lane 28,
structures or elements for providing selective automated
performance of a determination of item of interest process step
are disposed. As shown in Fig. 4, in one embodiment, these
structures or elements are located in a bypass region of the
process path 10. In the illustrated embodiment, the process
path 10 includes three bypass regions 58A, 58B and 58C. At the
bypass regions 58A, 58B and 58C, the process lane 28 is radially
expanded with respect to other portions of the process lane 28.
In an exemplary embodiment, the process lane 28 at the bypass
regions 58A, 58B and 58C is about 0.65 inches wide radially.
The radial expansion of the process lane 28 at the bypass
regions 58A, 58B and 58C allows the container 15 to be
positioned at multiple places longitudinally along the slot 18
and radially with respect to the disk 16 at the bypass regions
58A, 58s and 58C. Depending on the position of the container 15
within the slot 18 in the disk 16, the container 15 may or may
not participate in the item of interest determination process
step performed at the bypass regions 58A, 58s and 58C.
CA 022s904~ l999-0l-l3
In an alternate embodiment, the structures or elements for
providing selective automated performance of a determination of
item of interest process step may include routines, such as
those embodied in software, hardware and the like, for
S selectively activating or deactivating certain process path 10
elements, such as a wash zone and the like, selectively moving
process path 10 elements into and out of a process step
performance position with respect to the process path 10, such
as moving a magnet and the like, or any appropriate combination
of the methods discussed herein.
The cover 12 also includes structures forming the bypass
regions 58A, 58B and 58C on the process path 10. As shown in
Figs. 5A and 5B, a wall 60 on the cover 12 separates the process
lane 28 on the cover 12 at the bypass regions 58A, 58B and 58C
15 into a process step performance lane 62 and a process step
avoidance lane 64 offset radially on the cover 12. The wall 60
engages a portion of the side walls 36A and 36B adjacent of top
surface 42 of the container 15 to guide the container 15 through
either the process step performance lane 62 or the process path
20 avoidance lane 64.
To encourage a desired container 15 into the desired one of
the process step performance lane 62 or the process step
avoidance lane 64, a prime mover 44 connected with a container
engaging member 46 is provided attached to the process path 10,
25 as shown in Fig. 9. The structure illustrated in Fig. 9 is
substantially similar to the construction illustrated in Fig.
10, hence the like reference numbers. Activation of the prime
mover 44 enables selective radial positioning of the container
15 at either an inner 66 or outer radial edge 68 (Figs. 5A and
30 5B) of the process lane 28. Once so positioned, advancement of
the disk 16 with respect to the base 14 moves the container 15
into the preselected one of the process step performance lane 62
or the process step avoidance lane 64.
In some embodiments, the prime mover 44, and/or the wall 60
35 may be constructed to take advantage of natural movement of the
CA 022~904~ l999-0l-l3
18
container 15 in the process lane 16. For instance, the
container 15 may tend to move radially outwardly along the
process lane 28. In this case, the prime mover 44 and/or the
wall 60 may be constructed such that a container 15 moved toward
5 the process step avoidance lane 64 moves toward that lane 64
under centrifugal force without any assistance from the prime
mover 44. In this case, the prime mover 44 would only act on a
container 15 to be moved into the process step performance lane
62.
In the illustrated embodiment, the bypass regions 58A, 58B
and 58C are positioned along the process lane 28 dependent upon
the anticipated frequency of performance and avoidance of a
particular process step. This frequency is, in turn, dependent
upon a particular step of determinations of items of interest to
15 be performed with the process path 10. Also, depending upon the
determinations to be performed, there may be more or less bypass
regions 58A, 58B and 58C provided.
Illustrating further by example, the process lane 28
diverges radially prior to entering the bypass region 58A (Figs.
5A and 5B). The process lane 28 enters the bypass region 58A
along its outer radial edge. Since performance of the process
step occurs at the inboard process step performance lane 62 of
the bypass region 58A, the prime mover 44 associated with the
bypass region 58A moves the container 15 radially inward toward
25 the process step performance lane 62 only if performance of this
process step were desired. If performance of this process step
were not desired, then the prime mover 44 would not be activated
and the container 15 would remain on the outer radius surface of
the process lane 28 and move into the process step avoidance
lane 64 upon movement of the disk 16. This construction favors
performance of a set of determinations where performance of the
relevant process step is required for a minority of the
determinations to be performed.
If the set of determinations were to change such that
35 performance of the relevant process step is required for a
18
CA 022~904~ l999-0l-l3
- 19
majority of the determinations to be performed, then it may be
desirable to construct the bypass region 58A substantially
similarly to the bypass regions 58B and 58C. At the bypass
regions 58B and 53C, the process lane 28 enters the bypass
5 regions 58B and 58C at its inner radial edge. Thus, if the
prime mover 44 is not activated, then the container 15 would
move under the influence of movement of the disk 16 into the
process step performance lane 62 and the process step would be
performed. The prime mover 44 would be activated only to move
those containers 15 that did not require performance of this
process step. Of course, this would represent a minority of the
determinations to be performed with the process path 10.
Once a container 15 is in one of the bypass regions 58A,
58B or 58C, movement of the container 15 through the bypass
15 region 58A, 58B or 58C is controlled by cooperation among the
disk 16, edges of the process step performance and avoidance
lanes 62 and 64 and the wall 60. The container 15 moves
substantially tangentially through the process path 10 under the
influence of rotation of the disk 16. The position of the
20 container 15 radially within the radially inner-most one of the
process step performance lane 62 (e.g. bypass region 58A) or the
process step avoidance lane 64 (e.g. bypass region 58B) is
maintained by an inner radial edge of the wall 60. A radius
defining this inner radial edge of the wall 60 gradually
25 increases along the wall 60 from a first end 70 to a second end
72 thereof. The container 15 is moved radially outward as the
container 15 moves through the bypass region 58A, 58B or 58C. A
radius defining an inner edge of the process step performance
lane 62 (e.g. bypass region 58A) and the process step avoidance
lane 64 (e.g. bypass region 58B) also increases from one end of
the bypass region 58A, 58B or 58C adjacent the first end 70 of
the wall 60 to an opposite end of the bypass region 58A, 58B or
58C adjacent the second end 72 of the wall 60. Thus, a portion
of the container 15 adjacent its top surface 42 is maintained
19
CA 022~904~ l999-0l-l3
adjacent the wall 60, thereby maintaining intended positioning
of the container 15 within the bypass regions 58A, 58B and 58C.
Once the determination of an item of interest is complete,
the relevant container 15 is removed from the process lane 28
5 and the process path 10 altogether. As shown in Fig. 11, a
prime mover 74 is connected with the process path 10. The prime
mover 74 drives a container 15 engaging surface 76 which acts on
the container 15 adjacent the top surface 42 of the container
15. The prime mover 74, which may be a stepper motor and the
like, drives the container engaging surface 76 to rotate the
container 15 about 90 degrees with respect to the disk 16. This
occurs at location 78 shown in Fig. 4. The process path 10 at
the location 78 is configured to allow axial rotation of the
container 15 and includes an aperture 80 having dimensions
15 larger than corresponding dimensions of the container 15.
In an exemplary embodiment, the prime mover 74 may be a
solenoid such as P/N 197855-001 BTA 2 DV 90- available from
Lucas Control Systems Products of Vandalia, Ohio. The surface
76 may be made from 6061-T6 aluminum with a MIL-A-63576, Type I
20 finish and moves approximately 90 degrees responsive to
operation of the prime mover 74.
Once the container 15 has been rotated, the support
surfaces 34A and 34B of the container 15 are no longer in
engagement with the disk 16. Under the influence of gravity,
25 the container 15 falls through the aperture 80 in the process
path 10 into a waste receptacle (not shown). In some
constructions, a chute may be provided to guide the container 15
from the process path 10 toward the waste container. In other
constructions, liquid present in the container 15 may be removed
30 from the container 15 prior to encountering the prime mover 74.
With the container 15 being removed from the process lane
28, another container 15 within the same slot 18 on the disk 16
can be moved from the loading lane 30 to the process lane 28 as
soon as the relevant slot 18 reaches the location 48. In some
3 5 instances, it may not be desirable to remove a container 15 from
CA 022~904~ 1999-01-13
the process lane 28 once that container 15 reaches location 78.
In this case, the prime mover 74 will not be activated. Also, a
container 15 disposed within the same slot 18 on the disk 16 but
in the loading lane 30 will not be moved from the loading lane
30 to the process lane 28 when the relevant slot 18 reaches
location 48.
In an alternative embodiment shown in Figs. 13 and 19, the
disk 16 is constructed to facilitate removal of a container 15
from the process path 10. In this embodiment, the slots 18 on
the disk 16 include an enlarged container 15 removal area 82.
Also, a diverter 84 is disposed in the process lane 28 adjacent
to the location 78. The diverter 84, along with movement of the
disk 16, urges the container 15 radially outward with respect to
the disk 16 toward the container removal area 82 of the slot 18.
The container removal area 82 is wider than the remainder of the
slot 18 such that, when the container 15 reaches the container
removal area 82 of the slot 18, gravity causes the container 15
to fall from the disk 16 and the process path 10 through the
aperture 80 and into the waste receptacle. However, this
embodiment does not allow a container 15 to pass the location 78
and still remain with the disk 16. But, if it were desirable to
do allow the container 15 to remain with the disk 16 in this
embodiment, then the diverter 84 may be replaced with a prime
mover, similar to the prime mover 44, to move the container 15
within the slot 18 toward the container removal area 82.
Another construction of the disk 16, the slot 18 and the
container removal area 82 is shown in Fig. 19. This
construction functions in a manner substantially similar to that
of Fig. 13. It is to be noted that, in the embodiment
illustrated in Fig. 19, an end of the process lane 28 is defined
by the aperture 80.
Additional features may be incorporated into the process
path 10 as desired. For example, liquid level sensing devices,
such as radio frequency liquid level sense devices and the like,
may be incorporated at positions along the process path 10 where
CA 022~904~ 1999-01-13
liquid movement may occur. Also, any suitable structures, such
as any of those disclosed in U.S. Patent No's. 5,358,691,
5,536,471 and 5,482,861 may be added, sometimes with appropriate
modifications. Those patents are assigned to the assignee of
the present case and the disclosures thereof are incorporated
herein in their entirety by this reference.
It may also be desirable to construct the process lane 28
to reduce light in portions of the process lane 28. In one
embodiment, the process lane 28 is constructed such that there
is a radial divergence of that lane 28 prior to and following
any position on the process path 10 where light, such as
chemiluminescently generated light, measurements are performed.
Such a radial divergence of the process lane 28 may increase the
sensitivity of the light measurer by reducing introduction of
stray or ambient light into the light measuring position of the
process lane 28.
The process path 10 described above allows sequential
automated performance of multiple determination of item of
interest process steps. The motion of a container 15 along the
process lane 28 may be executed in discrete steps, that is
discrete with respect to time and with respect to position along
the process lane 28. At regular time intervals, such as about
18 seconds, the disk 16 rotates a distance substantially equal
to the angular distance between two adjacent slots 18. This
rotation causes each container 15 to move to the position along
the process path 10 previously occupied by the container 15 in
the adjacent slot 18. The disk 16 and the container 15 remain
stationary for a remainder of the regular time period prior to
the next rotation or indexing of the disk 16. The process lane
28 may be considered as having a fixed number of process
positions, positions at which a process step comprising the
determination of an item of interest in a sample occur, equal to
the number of slots 18 in the disk 16.
In the examples described here, there are 112 slots 18 in
the disk 16, and consequently the process lane 28 may be
... . _
CA 022~904~ 1999-01-13
considered as having 112 process positions. The total
processing time of a container 15 and its contents may be
thought of as integral multiples of the index period. For
example, if the index period is 18 seconds, a container 15 in
the 10th process position has undergone a total of 180 seconds
of processing. Similarly, a process step that is performed over
20 process positions takes a total of 360 seconds of process
time on an individual container 15.
An example of process steps that may be performed during
determination of an item of interest in a sample may be
described by specifying the process position at which each
process step occurs, as is provided in the following examples.
This example may be more easily understood with reference to
Fig. 16. The dotted line 129 indicates a boundary of a support
on which the process path 10 is mounted.
A reagent carousel 131 is located substantially
concentrically with the process path 10 and is rotatable. The
reagent carousel 131 may include one or more carousels and may
provide for axial rotation of individual containers, i.e.
magnetic microparticle containers, disposed thereon. In one
embodiment, the reagent carousel 131 may include multiple
substantially concentric carousels to provide simultaneous
and/or shared access of multiple containers by multiple pipette
assemblies, such as assemblies 128 and 134. Such an arrangement
may facilitate performance of the Formats discussed later. The
reagent carousel 131 may be constructed substantially similarly
to the structure disclosed in GB 2,081,118B issued on September
7, 1983, with appropriate, well known bearings and gear trains
being provided as and where needed (See Fig. 24B), as disclosed
on Page 3, lines 86-91 of that patent. In an exemplary
embodiment, the carousel 131 may be No. 77829-101 available from
SPM/Portland of Hillsboro, Oregon, with appropriate motors
available from Pacific Scientific, gears from Turnamatic of
Richardson, Texas and SPM/Portland and sensors from Aromat of
Rolling Meadows, Illinois.
CA 022~904~ 1999-01-13
24
The reagent carousel 131 may be maintained within a
thermostaticly controlled environment. The thermostaticly
controlled environment may be provided by an air cooling unit
which provides forced cooled air to a housing 133 (Figs. 29 and
30) containing the reagent carousel 131. In an exemplary
embodiment, the housing 133 may be similar to No. 76848
available from General Pattern of Blaine, Minnesota. This may
reduce evaporation of fluid from the containers held on the
reagent carousel 131. To further reduce evaporation, open
mouths of the containers may be fitted with a seal 184 as shown
in Fig. 25. The seal 184 may be made of a polymeric material,
such as an elastomer and the like, and may include a slit 186
for allowing a pipettor access to the interior of the container.
In one embodiment, the reagent carousel 131 supports a
plurality of reagent containers. These containers may be of at
least four types, such as microparticle, conjugate,
determination specific diluent and pretreatment, dependent upon
the type of reagent contained therein. Figures 22, 23A, 23B and
23C give two exemplary configurations of the containers. A
bottom portion 174 of the containers 176 (Fig. 22) and 177
- (Figs. 23A, 23B and 23C) is constructed to fit with mating
portions of the reagent carousel 131.
As shown more clearly in Figs. 24A and 24B, the bottom
portion 174 of the container 177 bears a projection 178 which
engages a complementary portion 188 of the reagent carousel 131.
The engagement between the projection 178 and the portion 188 of
the reagent carousel 131 provides a user who is placing the
container 177 on the reagent carousel 131 with positive
feedback, i.e. tactile feel, indicative of proper positioning of
the container 177 with respect to the carousel 131.
As shown in Fig. 24B, the portion 188 of the carousel 131
is operatively connected by a shaft 191 with a drive gear 190
which drivingly engages a gear 202 which is connected with a
prime mover (not shown). The gear 202 engages all drive gears
190 associated with the carousel 131. Operation of the prime
24
CA 022S904~ 1999-01-13
mover moves the gear 202 which, in turn, moves the gear 190.
Movement of the gear 190 causes axial rotation, which may be bi-
directional, of the portion 188 and the containe~ 177. The
shaft 191 also electrically contacts a plate 204 which is
electrically connected with a conductor 206. In this manner,
the plate 204 and the conductor 206, and possibly the portion
188 of the carousel 131, if it is electrically conductive,
comprise a portion of a radio frequency liquid level sense
mechanism with determines a fluid level inside the container
177.
To further facilitate manipulation of the container 177, a
substantially annular rib 180 (Figs. 23A, 23B and 23C) may be
provided on an outer surface of the container 177. Also, if it
were desirable to maintain the container contents in a
substantially homogeneous state, i.e. magnetic particles
substantially uniformly dispersed in a liquid medium, then at
least one fin 182 (Figs. 24A and 24B) may be provided on an
interior, fluid facing surface of the container 177 to agitate
container contents upon axial rotation, as discussed above, of
the container 177.
Illustrating constructions of the containers and seals with
specific examples, the containers may be made from DOW 30460M
HDPE or Chevron 90512 HDPE with a finish of SPI A3. The fins
182 may have a finish of SPI Cl. The seals may be made from
Lexington Medical 3401005 EPDM. The containers may have a neck
inner diameter measuring about 1.069 inches. The rib may have a
thickness of about 0.025 inches, a width, from an inner wall of
the container, measuring about 0.31 inches, a top geometry
measuring about 45 degrees, and a bottom geometry tapering to a
center at an angle of about 48 degrees. The seal may have a
diameter of about 1.094 inches when installed with a container,
a maximum thickness of about 0.070 inches at a centerline of the
seal, and a reinforced hinge section measuring about 0.025
inches thick by about 0.062 inches deep from an underside of a
pipettor contact area on the seal. The slit on the seal may
CA 022~904~ 1999-01-13
comprise two slits having a length of about 0.5 inches through a
center of the seal and offset about 90 degrees from each other.
To facilitate identification of the containers, at least
some of the containers may bear a label 133A, 133B, or 133C,
substantially similar to those shown in Figs. 21A, 21B and 21C.
The labels 133A, 133B and 133C include a high density data
carrier 135A, 135B and 135C, respectively, which includes
information to facilitate performance of the determinations.
In a specific embodiment, the high density data carrier
135A, 135s and 135C is a two dimensional bar code utilizing PDF
417 technology to provide desired data capacity. This
technology allows for inclusion of more information than a
common one dimensional bar code. Usage of such a high density
data carrier 135A, 135B and 135C provides structural
flexibility, i.e. individual containers for a given
determination do not have to be physically joined together. The
data carrier 135A, 135B and 135C contains information desirable
for performance of a given determination. This information may
include master lot number, container lot number, container
contents, i.e. reagent, lot number and expiration date,
calibration curve data, container contents type, etc. The
information may also contain a serial number specific to the
particular container to facilitate tracking of process path 10
resources.
In the illustrated embodiment, the data carrier 135A is
used with magnetic microparticle containers and holds
approximately 185 characters of information. The data carrier
135A is approximately 1.5 inches tall and about 0.75 inches
wide, as viewed by the bar code reader. Because the
microparticle container is rotated as discussed above, this
rotation may be utilized while reading the data carrier 135A.
In this case, the orientation of the data carrier 135A with
respect to the bar code reader may not be important.
The data carriers 135B and 135C of the illustrated
embodiment comprise two dimensional bar codes containing about
26
CA 022~904~ 1999-01-13
15 characters of information each. The data density of the
carrier 135B and 135C is adjusted to allow the carrier 135s and
135C to be about 0.7 inches high. Eurthermore, the data carrier
135B and 135C is printed with error correction, x bar, Y bar and
a column count that allows the carrier 135B and 135C to be about
3.125 inches wide. In this manner, the data carrier 135B and
135C can be disposed along an outer circumference of a container
such that the carrier 135B and 135C is accessible to the bar
code reader through approximately 220 to approximately 270
10 degrees of visibility, depending on container size.
Alternatively, instead of the carrier 135B which includes only
one bar code, the data carrier 135C includes a plurality of
repetitions of a similar, but narrower in form bar code with
gaps between adjacent code repetitions. Additionally, various
15 modifications of the data carriers 135A, 135B and 135C are also
possible. For instance, one dimensional bar codes could be
used, but the surface area of the one dimensional bar code would
have to be sufficient for the amount of data contained in the
two dimensional bar code.
Exam~ le -- DETERMINING AN ITEM OF INTEREST IN A SAMPLE
.
The process path 10 illustrated in Fig. 1 is utilized to
25 perform a sequence of process steps, executed with a index
period of about 18 seconds. Each index step comprises about 1
second of rotation of the disk 16 (and consequent motion of the
containers 15 disposed within the disk 16) and about 17 seconds
during which the containers 15 are stationary at their
30 respective process positions. The process step performed at
each process position is as follows:
Process Process Ste~ Descril~tion
Position
CA 022~904~ l999-0l-l3
28
1 Container 15 load Container 15 moved from loading
lane 30 to process lane 28 as
required
1 Sample Pipettor Sample deposited into container
15 by pipetting system 128. The
sample may be obtained from
position 130A or 130B which are
located on appropriate conveyors,
sample handlers or structures
associated with a laboratory
automation system
2 Reagent Pipettor 1 Reagent obtained from reagent
carousel 131 deposited into
container 15 by pipetting system
132. Liquid present in the
pipetting system 132 may also be
added to the container 15.
3 Mixer Contents of container 15 are
mixed by a device 86 imparting
motion to the container 15
4 - 23 Incubation Contents of container 15 are
incubated at a controlled
temperature, about 3 7 degrees
Celsius
2 4 Sample Pipettor Sample may be aspirated from
container 15 by pipetting system
128 for deposition into a second
container 15 at position 1
2 5 - 3 9 Incubation Contents of container 15 are
incubated at a controlled
temperature
CA 022~904~ l999-0l-l3
29
Bypass region 58A Container 15 iS selectively
start positioned at entry to
performance lane 62 or avoidance
lane 64 of bypass region 58A
41 Wash zone 1 Container 15 in performance lane
62 undergoes magnetic separation
and fluid addition
42 Wash zone 1 Container 15 in performance lane
62 undergoes magnetic separation,
container 15 contents aspiration
and fluid addition
43 Wash zone 1 Container 15 in performance lane
62 undergoes magnetic separation,
container 15 contents aspiration
and fluid addition
44 Wash zone 1 Container 15 in performance lane
62 undergoes magnetic separation
and container 15 contents
aspiration
45.5 Bypass region 58A Performance lane 62 and avoidance
end lane 64 of bypass region 58A
merge (midway between positions
45 and 46)
46 Container 15 load New containers 15 are loaded into
into loading lane loading lane 30
30 - ~
48 Reagent Pipettor 2 Reagent selectively deposited
into container 15 by pipetting
system 134
49 Mixer Contents of container 15 are
mixed by a device 86 imparting
motion to the container 15
29
CA 022~904~ 1999-01-13
50 - 62 Incubation Contents of container 15 are
incubated at a controlled temperature
63 Bypass region 58B Container 15 is selectively
positioned at entry to
performance lane 62 or avoidance
lane 64 of bypass region 58B
64 Wash zone 2 Container 15 in performance lane
62 undergoes magnetic separation
and fluid addition
Wash zone 2 Container 15 in performance lane
62 undergoes magnetic separation,
container 15 contents aspiration
and fluid addition
66 Wash zone 2 Container 15 in performance lane
62 undergoes magnetic separation,
container 15 contents aspiration
and fluid addition
67 wash zone 2 Container 15 in performance lane
62 undergoes magnetic separation
and container 15 contents
aspiration
68 Bypass region 58B Performance and avoidance lanes
end 62 and 64 of bypass region 58B
merge
69 - 70 Incubation Contents of container 15 are
incubated at a controlled
temperature
71 Reagent Pipettor 2 Reagent selectively deposited
into container 15 by pipetting
system 134
CA 022~904~ 1999-01-13
72 Mixer Contents of container 15 are
selectively mixed by a device 86
- imparting motion to the container
73 - 86 Incubation Contents of the container 15 are
incubated at a controlled
temperature
Motor/Encoder Gear 22 on prime mover 24 engages
teeth 20 on disk 16 at this
position
81.5 Home Sensor Electrical, magnetic, optical, or
other sensor 136 is present to
generate signal corresponding to
the position of the disk 16
86 Bypass region 58C Container 15 is selectively
positioned at entry to
performance lane 62 or avoidance
lane 64 of bypass region 58C
87 Wash zone 3 Container 15 in performance lane
62 undergoes magnetic separation
and fluid addition
88 Wash zone 3 Container 15 in performance lane
62 undergoes magnetic separation,
container 15 contents aspiration
and fluid addition
89 Wash zone 3 Container 15 in performance lane
62 undergoes magnetic separation,
container 15 contents aspiration
and fluid addition
CA 022~904~ 1999-01-13
Wash zone 3 Container 15 in performance lane
62 undergoes magnetic separation,
and container 15 contents aspiration
91 Bypass region 58C Performance and avoidance lanes
end 62 and 64 of bypass region 58C
merge
91 - 93 Incubation Contents of container 15 are
incubated at controlled
temperature
94 Pre-Trigger and Reagent added to container 15 and
Mixer mechanically mixed
95 - 97 Incubation Contents of container 15 are
incubated at controlled
temperature
98 Shutter, reader, Indicator reaction (such as
and trigger chemiluminescent reaction)
triggered and read with magnetic
particles pulled out of solution
with magnet. Shutter blocks
ambient light.
99 Magnet Magnetic particles are held at a
wall of the container 15
100 Liquid Waste Magnetic particles are held at a
Aspirate wall of the container 15 and all
liquid in container 15 is
aspirated and discarded
110 Container 15 unload Container 15 selectively removed
from process lane 28
111 Container 15 unload System optically verifies that
sensor slot 18 in process lane 28 is
- vacant prior to loading of second
container 15
CA 022~904~ 1999-01-13
Adding more specificity to the example, in a particular
embodiment, the determination of an item of interest in a sample
is an immunoassay. When the process path 10 is used to perform
an immunoassay, the container 15 is moved into the process lane
28 at position 1. Also at position 1, a known quantity of
sample (for example, 50 ~l of blood) is deposited into the
container 15 by a pipetting system 128. The pipetting system
128 comprises a pipettor, which may be substantially similar to
the pipettors 116A, 116B and 116C, mounted on an arm for
movement up and down and angularly, as shown in Fig. 16.
After indexing the container 15 to position 2, a known
quantity of a first reagent, possibly along with an amount of
fluid present in the pipetting system 132, is deposited into the
container 15 by a second pipetting system 132. The first
reagent may contain magnetically responsive microparticles
coated with antibodies or other binding substances that
specifically bind to the item of interest in the sample. The
first reagent may be added with an assay specific diluent. In
some cases, the first reagent and a conjugate, possibly along
with an amount of fluid present in the pipetting system 132, may
be added at position 2.
At position 3, a mechanical device 86 (illustrated in Fig.
12) is provided to mechanically move the container 15 and cause
mixing of the contents of the container 15. The mechanical
mixing device 86 includes a bore 88 formed within a body 89,
which is eccentrically formed in the illustrated embodiment,
that moves axially and rotatably under the influence of a prime
mover 90 connected with the body 89. When the prime mover 90 is
activated, the body 89 rotates clockwise, and a protrusion 92
connected with the prime mover 90 moves within a slot 94 in a
second body 96. The second body 96 rotates freely about a drive
shaft of the prime mover 90.
As the protrusion 92 moves within the slot 94, the body 89
and the bore 88 move toward the bottom 40 of the receptacle 15
CA 022~904~ 1999-01-13
34
as the body 89 rotates. When the body 89 and the bore 88 move
toward the container 15, the bore 88 engages the bottom 40 of
the container 15 and imparts an orbital (but not rotational)
motion to the bottom 40 of the container 15. The portions of
the container 15 adjacent the top surface 42 remain relatively
stationary within the slot 18 in the disk 16.
The mechanical motion imparted to the container 15 mixes
the sample with the first reagent. After the contents of the
container 15 have been mixed for a predetermined time period,
the prime mover 90 rotates its drive shaft counterclockwise,
causing the protrusion 92 to move in an opposite direction
within the slot 94, thereby moving the first body 89, the bore
88 and the second body 96 away from the bottom 40 of the
container 15.
Illustrating further with a specific example, in one
embodiment, the body 89 is made of PEEK with a black finish, the
protrusion 92 is made of AISI 301 stainless steel with a #10
passivated finish, the second body 96 is made of Acetron GP with
a white finish and the slot 94 has a #32 finish. The bore 88 in
the body 89 is offset from an axis of the body 89 and has a
radius of about 0.020 inches. An interface between the body 89
and the container 15 provides a minimum of about 0.05 inches of
eccentric rotation of the container 15. The slot 94 provides a
rise of about 0.315 inches over a rotation of the second body 96
of about 226.8 degrees. The prime mover 90 is a 3 phase, 8
pole, Y connected DC brushless motor P/N DR538-504 available
from Shinano Kenshi of California. The prime mover 90 is
supplied with a 36 Volt potential and operates substantially
within the range of about 500 to about 5500 rpm~s with a torque
constant of about 620 g~cm/A.
The container 15 is freed from the bore 88 and processing
of the container 15 contents continues. Subsequently, the
container 15 contents is incubated for a predetermined time
period.
CA 022~904~ 1999-01-13
At position 24, depending upon the particular item of
interest in the sample to be determined, the first pipetting
system 128 may withdraw a portion of the contents of the
container 15 for deposition into another container 15 located at
position 1. This may be appropriate when a particular
determination requires pretreatment, such as pre-heating, heated
incubation with a first reagent prior to second reagent
introduction, and the like, prior to introduction of
magnetically responsive microparticles comprising the first
reagent.
At position 37, the process path 10 selectively positions
the container 15 for performing or avoiding a series of magnetic
separation and wash steps. Structures for performing the wash
and separation comprise a wash station 114, shown in Figs. 14
and 15.
Each wash station 114 includes a plurality, 3 in the
illustrated embodiment, of movable pipettors 116A, 116B and 116C
and at least one stationary nozzle (not shown) for moving fluids
at least one of into and out of the container 15. In some
embodiments, the movable pipettors 116A, 116B and 116C may be
used to move fluids out of the container 15 while the at least
one stationary nozzle moves fluid into the container 15.
Sensors, such as thermistors and the like, may be operatively
associated with the pipettors 116A, 116B and 116C to verify
fluid movements.
The pipettors 116A, 116B and 116C move liquids both into
and out of the container 15 whereas the nozzle only moves liquid
into the container 15. The movable pipettors 116A, 116B and
116C are connected to a common base plate 118 which moves with
respect to the cover 12 under the influence of a prime mover
120, such as a stepper motor and the like. Responsive to the
prime mover 120, the pipettors 116A, 116B and 116C move into and
out of the container 15. Suitable fluid delivery conduits, not
shown, are connected with the pipettors 116A, 116B and 116C and
the nozzle. The pipettors 116A, 116B and 116C are spring-loaded
CA 022~904~ 1999-01-13
36
to facilitate their replacement and to cushion any contact
between the pipettors 116A, 116B and 116C and another surface,
such as the bottom-40 of the container 15 and the like.
The pipettors 116A, 116B and 116C are movable to remove
fluid from the container 15. Because the item of interest is
connected with the magnetic particles, a magnet assembly 122 is
also included at the wash station 114. The magnet assembly 122
is disposed in a receptacle 124 in the base 14. The magnet
assembly 122 includes a portion of the performance lane 62 and
holds a plurality of permanent magnets 126. In an exemplary
embodiment, the assembly 122 is made from 6061 T6 aluminum with
a finish of MIL-A-63576 Type I and the magnets 126 are neodymium
Iron Boron (NdFeB) magnets with a residual flux density (Br)
substantially within the range of about 12.1 to about 13.2 KG, a
coercive force (Hc) substantially within the range of about 11.0
to about 12.0 KOe, an intrinsic coercive force (Hci)
substantially within the range of about 17.0 to about 19.0 KOe
and a total energy product (BHmax) substantially within the
range of about 34.0 to about 41.0 MGOe. The field intensity of
the magnets 126 at a distance of about 0.030 inches from the
container 15 is about 4470 Gauss and at a distance of about
0.176 inches from the container 15 is about 1570 Gauss.
At the wash station 126, the magnets 126 hold the magnetic
particles, and thereby the item of interest, against a side wall
36A or 36B of the container 15. This allows removal of contents
of the container 15 other than the magnetic particles and the
item of interest bound to the magnetic particles. In some
constructions, the pipettors 116A, 116B and 116C may be
positioned such that the pipettors 116A, 116B and 116C move
substantially along a central axis of elongation of the
container 15, may be biased away from a side wall 36A or 36B
against with the magnetic particles are held, or otherwise
constructed to reduce the chances of the pipettors 116A, 116B
and 116C removing magnetic particles and the item of interest
bound to the magnetic particles from the container 15.
CA022~904~l999-0l-l3
In an exemplary embodiment, the pipettors 116A, 116B and
116C are made from Inccnel. The pipettors 116A, 116B and 116C
are disposed such that longitudinal center lines of the
pipettors 116A, 116B and 116C are offset a distance measuring
about 0. 029 inches from a center line of the containers 15 into
which the pipettors 116A, 116B and 116C are inserted. This
offset distances the pipettors 116A, 116B and 116C from magnetic
particles within the containers 15. When the pipettors 116A,
116B and 116C dispense fluid into the containers 15, the
10 pipettors 116A, 116B and 116C are located a distance measuring
about 0. 342 inches from a side wall of the containers 15
adjacent the magnets 126. The pipettors 116A, 116B and 116C are
mounted with springs so as to absorb up to 0.1 inches of
overdrive. The pipettors 116A, 116B and 116C are fluidly
connected with a valve which allows for bubble flushing without
use of a container 15. The stationary nozzle is made of 0. 031
inch inner diameter PEEK tubing. The base plate 118 is a two
piece, thermally bonded assembly of acrylic with a clear Iridite
finish on top and an opaque finish on the bottom to allow fluid
visibility and light protection for a chemiluminescence reader.
If, for a particular determination, magnetic separation and
washing is required at position 37, the container 15 is moved to
the performance lane 62. Containers 15 in the performance lane
62 undergo, at each processing position between 41 and 44,
magnetic separation (effected by permanent magnets 126 at fixed
locations adjacent to the performance lane 62), fluid
aspiration, and fluid dispensing, performed by fluid handling
devices introduced through an opening 98 (Fig. 1) in the cover
12. In one embodiment, one of these wash stations (position 41)
includes only a magnetic separation and fluid dispensing step
that introduces a wash buffer to the container 15. In some
cases, wash buffer or other fluid is added such that the amount
of fluid present within the container 15 facilitates separation
(magnetic) of the particles from the fluid in the container 15.
At positions 42 and 43, separation, fluid aspiration, and fluid
CA 022~904~ 1999-01-13
dispensing occur. In position 44, the magnetic particles are
separated from the fluid in the container 15 by magnets 126 and
fluid is aspirated. In this example, these steps would remove
substantially all substances within the container 15 that have
not bound to binding conjugate elements on the magnetic
particles deposited as the first reagent. Containers 15 within
the avoidance lane 64 are undisturbed and continue incubation.
The performance and avoidance lanes 62 and 64 merge between
positions 45 and 46.
A second reagent may be deposited into the container 15 at
location 48 (Fig. 4) by a third pipetting system 134, again
followed by a mechanical device 86 at position 49 to mix the
container 15 con~ents. The second reagent may include an
indicator substance, such as a chemiluminescent substance,
linked to binding elements that also bind to the item of
interest (remaining occurrences of which are bound to the
magnetic particles of the first reagent). The contents of the
container 15 are incubated at positions 50-59.
The second bypass region 58B begins at position 60, where
the container 15 may selectively automatically undergo a set of
magnetic separations, fluid aspirations, and fluid dispensing
steps.
The third pipetting system 134 may deposit a third reagent
into the container 15 at position 71, with subsequent mixing at
position 72 and incubation between positions 73 and 86.
The third bypass region 58C begins at position 86, where
the container 15 may selectively automatically undergo a set of
magnetic separations, fluid aspirations and fluid dispensing
steps.
In one embodiment, where it is assumed that substantially a
majority of the containers 15 will undergo magnetic separation,
fluid aspiration, and fluid dispensing at positions 87-90, no
bypass region 58C may be provided at these locations. For
example, these step would cause the removal of substantially all
indicator (chemiluminescent) substances that are not bound to
38
CA 022~904~ l999-0l-l3
39
the magnetic particles (via the analyte of interest), yielding a
container 15 holding indicator substance in an amount indicative
of the amount of the item of interest in the initial sample
deposition. However, in some determinations, it is desirable to
S avoid those process steps.
A pretrigger reagent may be deposited by a fluid dispensing
device at position 94.
A fluid dispensing device will deposit a triggering agent
at position 98, which causes the indicator reaction to occur.
For example, a chemiluminescent substance releasing reagent may
be deposited at position 94, which causes the release of the
indicator (chemiluminescent) substance from the magnetic
particles.
The contents of the container 15 are incubated between
lS positions 9 5 and 9 7, inclusive.
Position 98 may also include a magnet, which separates or
removes substantially all of the magnetic particles from the
fluid within the container 15. The magnet holds substantially
all of the magnetic particles against a side wall 36A or 36B of
20 the container 15 prior to reading of light from the
chemiluminescent substance. Preferably, all of the magnetic
particles are removed from a path of chemiluminescent photons
from the chemiluminescent substance, which remains in solution
in the fluid in the container 15, to a light detection apparatus
25 138. This read step is substantially similar to that described
in EP O 371 265 Bl issued January 1, 1994. The introduction of
the triggering reagent would initiate a chemiluminescent
reaction which would be detected and quantified by an optical
detection system (not shown) such as a photomultiplier tube or
30 photon counting system.
In an exemplary embodiment, the apparatus 138 may comprise
a reader assembly such as No. 78262 available from Thorn EMI of
Rockaway, New Jersey, a photomultiplier tube such as No. 78252-
101 available from ~mm~m~tSU of Middlesex, New Jersey and a
35 substantially light-tight shutter operable by a plunger such as
39
CA 022~904~ 1999-01-13
No. 78200-101 available from Ironwood Industries of
Libertyville, Illinois and a motor such as No. 78851-101
available from Haydon Switch & Instrument of Waterbury,
Connecticut.
The embodiment described in the following examples
demonstrates its utility in processing multiple assays of
different formats and timing requirements within a common
process path 10. In these examples, the embodiment described
enables the execution of at least the following four assay
formats, the first three of which may be executed simultaneously
with no degradation in processing capacity.
CA 022~904~ l999-0l-l3
Format A
Ste~ Position
Sample introduction
First reagent introduction and 2 - 3
mixing
First incubation (1~3 minutes) 4 - 63
Separation and wash 64 - 67
Second reagent introduction and 71 - 72
mixlng
Second incubation (4 minutes) 73 - 86
Separation and wash 87 - 90
Pretrigger introduction and 94
mixing
Third incubation (1 minute) 95-97
Trigger and read 9 8
As an example, Format A may be used to determine at least the
following items of interest: antibodies to HCV, antibodies to
HIV 1/HIV 2, antibodies to hepatitis B core antigen (HBcAb),
carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA19-9),
Hepatitis B Surface Antigen (HBsAg), antibodies to Hepatitis B
Surface antigen (HBsAb), alpha-fetoprotein (AFP), Total prostate
specific antigen (Total PSA), Free PSA, Thyroid stimulating
Hormone (TSH), luteinizing hormone (LH), follicle stimulating
hormone (FSH), beta human chorionic gonadotropin (B-hCG), Free
Thyroxine (Free T4), Free triiodothyronine (Free T3), Total T4,
Total T3, Prolactin and Ferritin. It is to be noted that almost
any item of interest discussed herein may be determined by
properly using this format. For instance, this format may also
be used to determine beta human chorionic gonadotropin (B-hCG),
prolactin and ferritin.
41
CA 022~904~ l999-0l-l3
42
Format
SteD Po~ition
Sample introduction
First reagent introduction and 2 - 3
mixing
First incubation (11 minutes) 4 - 40
Separation and wash 41 - 44
Second reagent introduction and 48 - 49
mixlng
Second incubation (11 minutes) 50 - 86
Separation and wash 87 - 90
Pretrigger introduction and 94
mixlng
Third incubation (1 minute) 95-97
Trigger and read 98
As an example, Format B may be used to determine an item of
interest in a sample where a relatively increased degree of
sensitivity, as compared with some other formats, is desired.
It is to be noted that almost any item of interest discussed
herein may be determined by properly using this format.
42
CA 022~904~ l999-0l-l3
43
Format C
Ste~ Position
Sample introduction
First reagent introduction and 2 - 3
mixing
First incubation (11 minutes) 4 - 40
Separation and wash 41 - 44
Second reagent introduction and 48 - 49
mixing
Second incubation (~ minutes) 50 - 63
Separation and wash 64 - 67
Third reagent introduction and 71 - 72
mixing
Third incubation ( 4 minutes) 73 - 86
Separation and wash 87 - 90
Pretrigger introduction and 94
mixing
Fourth incubation (1 minute) 95-97
Trigger and read 98
As an example, Format C may be used when the item of interest
relates to hepatitis, such as determinations for anti-M, HBcAb-M
and HAVAb-M.
43
CA 022~904~ l999-0l-l3
44
Format D
Ste~ Po~ition
Sample introduction
First reagent introduction and 2 - 3
mixing
First incubation (7 minutes) 4 - 23
Transfer to second container 15 24
in position 1
Second reagent introduction and 2 - 3
mixing
Second incubation (11 minutes) 4 - 40
Separation and wash 41 - 44
Third reagent introduction and 48 - 49
mixing
Third incubation ( 4 minutes) 50 - 63
Separation and wash 64 - 67
Fourth reagent introduction and 71 - 72
mixing
Fourth incubation (4 minutes) 73 - 86
Separation and wash 87 - 90
Pretrigger introduction and 94
mixing
Fifth .incubation 95-9 7
Trigger and read 9 8
CA 022~904~ l999-0l-l3
- 45
Format E
Ste~ Position
Sample introduction
First reagent 2 - 3
introducticn and mixing
First incubation (7 4 - 23
minutes)
Transfer pGrtion of 24
container 15 contents to
second container 15,
remainder of container 15
continues on process lane
28
First container 15 2 4 - 63
contents continues first
incubation (11 minutes)
Second reagent 2 - 3
introduction and mixing
with seconcl container 15
contents
First container 15 passes 64 - 67
through bypass region 58B
Second container 15 first 4 - 63
incubation ( 18 minutes)
Introduction of fourth 71 - 72
reagent into first
container 15 and mixing
(optional, enhances total
Hb chemiluminescent
signal)
CA 022~904~ l999-0l-l3
46
Second container 64 - 67
separation and wash
Fourth incubation (4 73 - 86
minutes - optional) of
first container 15
Third reagent 71 - 72
introduction into second
container 15 and mixing
First container 15 passes 87 - 90
through bypass region 58C
Third incuhation (4 73 - 86
minutes) of second
container 15
Pretrigger introduction 9 4
into first container 15
and mixing
Separation and wash of 87 - 90
second container 15
Trigger and read value 1 98
(Total Hb) from first
container 15
Pretrigger introduction 9 4
into second container 15
and mixing
Trigger and read value 2 98
(GlyHb)
Reported result = value 2 X 100
value 1
For example, in Format E, it is possible to modify the format by
disregardlng the first container 15 after the portion of the
container 15 contents has been transferred (Position 24) to the
46
CA 02259045 1999-01-13
47
second container 15. In that case, Format E may be used to
determine, for example, folate and vitamin B12.
47
CA 022~904~ l999-0l-l3
48
Format F
Ste~ Po9 it ion
Sample introduction
First reagent 2 - 3
introduction and mixing
First incubation (7 4 - 23
minutes)
Transfer portion of 24
container 15 contents to
second container 15,
remainder of container 15
continues on process lane
28
First container 15 24 - 63
contents continues first
incubation (11 minutes)
Second reagent 2 - 3
introduction and mixing
with second container 15
contents
First container 15 passes 64 - 67
through bypass region 58B
Second container 15 first 4 - 40
incubation (11 minutes)
Introduction of fourth 71 - 72
reagent into first
container 15 and mixing
(optional, enhances total
Hb chemiluminescent
signal)
48
CA 022~904~ l999-0l-l3
49
Second container 41 - 44
separation and wash
Fourth incubation (4 73 - 86
minutes - optional) of
first container 15
Third reagent 4 8 - 4 9
introduction into second
container 15 and mixing
First container 15 passes 87 - 90
through bypass region 58C
Third incubation (11 50 - 86
minutes) of second
container 15
Pretrigger introduction 9 4
into first container 15
and mixing
Separation and wash of 87 - 90
second container 15
Trigger and read value 1 98
(Total Hb) from first
container 15
Pretrigger introduction 94
into second container 15
and mixing
Trigger and read value 2 98
(GlyHb)
Reported result = value 2 X 100
value 1
This format may be used, for example, to determine at least one
of total and glycated hemoglobin. Also, this format may be
modified by disregarding the first container 15 as in Format E.
49
CA 022~904~ l999-0l-l3
Format G
Ste~ Position
Sample introduction
First reagent 2 - 3
introduction and mixing
First incubation (7 4 - 23
minutes)
Transfer portion of 24
container 15 contents to
second container 15,
remainder of container 15
continues on process lane
28
First container 15 24 - 63
contents continues first
incubation (11 minutes)
Second reagent 2 - 3
introduction and mixing
with second container 15
contents
First container 15 64 - 67
separation and wash
Second container 15 first 4 - 63
incubation (18 minutes) .
Introduction of fourth 71 - 72
reagent into first
container 15 and mixing
(optional, enhances total
Hb chemiluminescent
signal)
CA 022~904~ 1999-01-13
Second container 64 - 67
separation and wash
Fourth incubation (4 73 - 86
minutes - Gptional) of
first container 15
Third reagent 71 - 72
introductiGn into second
container 15 and mixing
First container 15 87 - 90
separation and wash
Third incubation (4 73 - 86
minutes) of second
container 15
Pretrigger introduction 94
into first container 15
and mixing
Separation and wash of 87 - 90
second container 15
Trigger and read value 1 98
(Total Hb) from first
container 15
Pretrigger introduction 94
into second container 15
and mixing
Trigger and read value 2 98
(GlyHb)
Reported result = value 2 X 100
value 1
As an example, this format may also be modified as may be done
with Format F. With that modification, this Format may be used
to determine progesterone, testosterone and estradiol.
51
CA 022~904~ 1999-01-13
52
Format H
SteD Po3ition
Sample introduction
First reagent 2 - 3
introduction and mixing
First incubation 4-86
Separation and wash 87 - 90
Pretrlgger introduction 94
and mlxlng
Second incubation 95 - 97
Trigger and read 98
As an example, this format may be used to determine, among other
things, beta human chorionic gonadotropin (B-hCG), prolactin,
progesterone, testosterone, estradiol and ferritin. It is to be
noted that almost any item of interest discussed herein may be
determined by properly using this format.
CA 022~904~ 1999-01-13
Format
Ste~ Position
Sample introduction into
first container 15,
possibly with diluent
fluid
First reagent 2
introduction into first
container 15, portion of
first container 15
contents moved into
pipettor, remainder of
container continues on
process lane 28,
bypassing all wash
stations, to Position 71
First reagent 2 - 3
introduction into second
container 15 and mixing
First incubation of 4 - 23
second container 15
Second container 15 first 24 - 63
incubation (18 minutes)
Introduction of second 71 - 72
reagent into first
container 15 and mixing
(optional, enhances total
Hb chemiluminescent
signal)
Second container. 64 - 67
separation and wash
CA 022~904~ l999-0l-l3
- 54
Fourth incubation (4 73 - 86
minutes - optional) of
first container 15
Third reagent 71 - 72
introduction into second
container 15 and mixing
First container 15 passes 87 - 90
through bypass region 58C
Third incubation (4 73 - 86
minutes) of second
container 15
Pretrigger introduction 9 4
into first container 15
and mixing
Separation and wash of 87 - 90
second container 15
Trigger and read value 1 9 8
(Total Hb) from first
container 15
Pretrigger introduction 9 4
into second container 15
and mixing
Trigger and read value 2 9 8
(GlyHb)
Reported result = value 2 X 100
value 1
As an example, in Format I, it is possible to modify the format
by disregarding the first container 15 after the portion of the
container 15 contents has been transferred (Position 24) to the
second container 15. In that case, Format I may be used to
determine, for example, folate and vitamin B12.
54
. _
CA 022~904~ 1999-01-13
Format J
Ste~ Position
Sample introduction into
container 15, possibly
with diluent fluid
First reagent 2 - 3
introduction and mixing
First incubation (27 4 - 93
minutes)
Pretrigger introduction 94
and mixing
Second incubation (1 95 - 97
minute)
Trigger and read 98
As an example, Format J may be used to determine, among other
things, total hemoglobin.
CA 022~904~ l999-0l-l3
56
The embodiments described herein also allow for sample
pretreatment which may be performed in at least two ways,
indicated as Formats K and L. During performance of sample
pretreatment, fluid present in the containers 15 indicated may
5 be processed, after they are no longer significant in the
pretreatment steps, in any appropriate manner, such as any of
the Formats discussed above. Also, as will become clear later
on, both Formats K and L are substantially similarly applicable
to the other embodiments of the process path 10 discussed below.
56
CA 022~904~ 1999-01-13
Format R
Ste~ Position
Sample introduction into
first container 15,
possibly w_th diluent
fluid
First reagent 2 - 3
introduction and mixing
First incuhation (7 4 - 23
minutes)
Transfer portion of 24
contents of first
container L5 to second
container 15 in Position
Second reagent 2 - 3
introduction to second
container 15 and mixing
(optional)
Transfer portion of 24
contents of second
container 15 to third
container 15 in Position
Third reagent 2 - 3
introduction to third
container and mixing
(optional)
As an example, the third container 15 may be processed according
to at least one of Formats A (to determine, among other things,
folate), B, C, H and J.
CA 022~904~ 1999-01-13
Format L
Ste~ Po9 it ion
Sample introduction into
first container 15,
possibly with diluent
fluid
First reagent 2 - 3
introduction and mixing
First incubation (7 4 - 23
minutes)
Transfer portion of 24
contents of first
container 15 to second
container 15 in Position
Second reagent 2 - 3
introduction to second
container 15 and mixing
(optional)
As an example, the second container 15 may be processed
according to at least one of Formats A (to determine, among
other things, folate, vitamin B12, confirm HBsAg), B, C, H and
J.
58
CA 022~904~ 1999-01-13
59
In each of the formats discussed above, it is possible to
move contents of a first container 15 at Fosition 2 into a
second container 15 at Position 1. Thereafter, the first
container 15 may or may not be ignored.
It is to be remembered, as pointed out earlier, that the
steps of one format may be mixed with steps of another format to
arrive at yet further formats. Also, it is to be remembered
that the construction of the process path 10, and its elements
and supporting components, allow for selective automated
performance (i.e. a particular step may or may not be performed,
as desired) of the above-described steps.
These examples demonstrate usefulness of the described
embodiments in controlled processing of determinations of items
of interest in a sample within a common process path 10.
As discussed earlier, multiple process paths 10 may be
connected to meet specific needs. If the process path 10 were
to perform approximately 200 determinations per hour, and if an
analyzer (Fig. 29) that performed 400 determinations per hour
were needed, then two process paths 10 could be connected. One
20 way of doing is described with reference to Figs. 17, 29 and 30.
As Fig. 17 illustrates, the process path 10 would be
located in space 140. To supply samples to the process path 10,
a load track 142 and a conveyor 146 are provided connected to a
frame 148 defining the space 140. In some embodiments, at least
25 one of the load track 142, the conveyor 146 and an unload track
192 may be provided with a cover 194 (Fig. 30). A carrier 150
supporting multiple sample tubes 152, which may be any suitable
tubes, rides along both the load track 142 and the conveyor 146.
Both the load track 142 and the conveyor 146 move the carrier
30 150 as indicated by the arrows. A transfer mechanism 154, such
as a solenoid (e.g. linear actuation) driven arm and the like,
shifts the carrier 150 from the load track 142 to the conveyor
46.
The carrier 150 moves along the conveyor 146 until the
35 carrier 150 iS stopped by a retention member 156, which is, in
59
CA 022~904~ 1999-01-13
the illustrated embodiment, a stepper motor driving a star wheel
which mates with the carrier 150. The pipetting system 128
accesses sample at the position 130B and supplies that sample to
a container 15 on the process path 10. Of course, suitable
identification structures, such as bar codes on the sample tubes
152 and a bar code reader, can be provided. When the pipetting
system 128, or any of the pipetting systems 128, 132 or 134
access a fluid, pipetting system pressure can be monitored as
described in commonly owned U.S. Patent application, Serial No.
08/572,835 filed on December 14, 1995. The disclosure of that
application is incorporated herein in its entirety. Appropriate
liquid level sense devices, such as radio frequency based
devices and the like, may also be located in suitable positions.
In an exemplary embodiment, the load track 142 may be No.
77325-101 and the conveyor 146 may be No. 77425-101 both
available from Dorner Manufacturing of Hartland, Wisconsin. The
unload track 192 may be No. 77525-101 available from
SPM/Portland of Hillsboro, Oregon. The retention member 156 may
be No. 77476-101 available from Pacific Scientific of Elgin,
Illinois. The transfer mechanism 154 may comprise a solenoid
such as No. 77952 available from Lucas/Ledex of Vandalia, Ohio,
a belt such as No. 6R25-M225090 and a pulley such as No. A 6725-
020DF0908 both available from Stock Drive Parts of New Hyde
Park, New York, and a stepper motor such as No. P21NSXS-LSS-NS-
07 available from Pacific Scientific of Elgin, Illinois.
In some cases, a level of sample in a sample tube 152 maybe insufficient for access by the pipetting system 128. In
these cases, the sample within-the sample tube 152 may be moved
by an operator into another container 208 shown in Figs. 31A,
31B and 31C. The container 208 comprises a barrel 210 and a
flange 212. The barrel 210 is dimensioned to fit within the
sample tube 152 as shown in Fig. 31C. The flange 212 is offset
from an outer diameter surface of the barrel 210 by a distance
sufficient to accommodate any suitable sample tubes 152. In
this way, sample can be moved from the sample tube 152 into the
CA 022~904~ 1999-01-13
61
container 208 and the container 208 can be placed within the
sample tube 152. The sample tube 152 bearing the container 208
may then be placed into the carrier 150. Because the sample is
now in the container 208, the level of the sample is elevated
with respect to the level of the sample in the sample tube 152,
thereby facilitating sample access by the pipetting system 128.
In an exemplary embodiment, the container 208 may be made
from DOW 666 polystyrene and is dimensioned to fit within sample
tubes having outer diameters substantially within the range of
about 0.4 inches through about 0.7 inches. The barrel 210 has
an outer diameter measuring about 0.4 inches and a length of
about 1.964 inches. The flange 212 has an outer diameter
measuring about 0.776 inches, depends from an open end of the
container 208 by a distance of about 0.216 inches and is offset
from the outer diameter surface of the barrel 210 by a distance
of about 0.258 inches.
In some embodiments, the load track 142 is removed and
replaced by a sample supply conveyor having a similar retention
member 156. If this were done, then the pipetting system 128
would access sample at position 130A. If this case, then, in
additional embodiments, a carousel 189 may be operatively
connected with the frame 148 by a connection member 193 as shown
in Eigs. 26 and 27. The connection member 193 locates the
carousel 189 with respect to the process path 10 such that the
pipetting system 128 can also access containers on the carousel
189 at position 130B. The carousel 189 may be used, for
instance, to house determination calibrators and controls and
certain samples, such as emergency samples that need to be
processed immediately. In an exemplary embodiment, the carousel
189 may be a 2 or 3 part injection molded polymeric (Ass~ GE-
Cycolac or the like) article constructed substantially similar
to a TDx~ unit dose carousel, an IMx Select~ carousel (Abbott
Laboratories, Abbott Park, Illinois) and the like.
In some instances, the retention member 156 may not retain
the carrier 150 for sample access, but may allow the carrier 150
~1
CA 022~904~ l999-0l-l3
62
to move toward an end 158 of the conveyor 146 towards another
process path 10. In this case, the frame 148 includes a
connecting structure 160 for operatively coupling one process
path 10 to another, or more specifically, one frame 148 holding
5 one process path 10 to another frame 148 holding another process
path 10. In an exemplary embodiment, the connecting structure
160 may be constructed such that two adjacent frames 148 are
offset by a distance substantially within the range of about
0.25 inches to about 1. 5 inches.
The connecting structure 160 comprises a first bracket 162
and a second bracket 164. The first bracket 162 iS connected
with one frame 148 and the second bracket 164 iS connected with
the another frame 148. To connect the frames 148, a fastener,
such as a bolt and the like, is placed between aligned apertures
166 in the first and second brackets 162 and 164. Another
fastener is inserted into slots 168 disposed on opposite ends of
the brackets 162 and 164. The conveyors 146 supported by both
frames 148 have sufficient tolerance such that more precise
alignment of the frames 148 iS not required. As a carrier 150
leaves an end 158 of one conveyor 146, the carrier 150 iS
supported by an opposing end 196 of an the adjacent conveyor
146. Once the carrier 150 reaches the end 158 of the last
conveyor 146, the carrier 150 iS moved to an unload track 192
(Fig. 29), constructed and operated substantially similarly to
the load track 142, by another transfer mechanism 197, which may
be substantially similar to the transfer mechanism 154.
The construction of the process path 10 is also adaptable
in other ways to meet other requirements. For example, it may
be desirable to provide a process path 10 that performs 100, 50
or any desired number of determination per hour. Viewing this
requirement in another way, it may be desirable to provide a
process path 10 that fits within a certain physical space, such
as a table surface. To meet such requirements, the process path
10 may be scaled, i.e. altered in size or determinations per
35 hour while still including elements discussed above, such as a
CA 022~904~ 1999-01-13
63
bypass region, a mixing device, a pipetting system, a wash
station and a reader.
Another embodiment is a process path 10-, constructed to
perform 100 determinations per hour, and is illustrated in Figs.
20A and 20B. This embodiment utilizes elements substantially
similar to those described above, hence the like reference
characters. The same index period and assay formats are used,
thereby allowing the same reagents to be used. Because of the
reduced number of determinations per hour, it is possible to
reduce correspondingly the physical dimensions of the
embodiment. For instance, whereas the process path 10 of the
previous Figures comprises 112 positions, the process path 10-
comprises about 55 positions. In another embodiment, which
performs 50 determinations per hour, the corresponding process
path comprises approximately 32 positions.
Whereas the determinations performed with the process path
10 are completed without a container 15 passing the same
location along the process lane 28 more than once, the
containers 15 used with the process path 10- may pass the same
location along the process lane 28 more than once. Depending
upon the particular needs to be addressed, the process path may
be modified such that a container 15 passes the same location
along the process path any appropriate number of times. Of
course, depending upon the particular employment, a given
container 15 may be positioned in a different one of a
performance lane 62 and an avoidance lane 64 of a given bypass
region 58 at different times passing through the same bypass
region 58 during ~ given determination.
Illustrating further by example, the following describes
the procedures performed at each location along the process path
10-- which performs 100 determinations in an hour. As noted
above, a particular container 15 may pass a given location along
the process path 10- more than once. Therefore, Process
Position 1 indicates the first time the container 15 encounters
Process Position 1, while Process Position 1' indicates the
63
. .. _
CA 022~904~ l999-0l-l3
64
second time the container 15 encounters Process Position 1.
Also, in similar fashion, Process Position 1" indicates the
third time the container 15 encounters Process Position 1.
Furthermore, the process path 10- is constructed such that once
a container 15 reaches Process Position 46 a first time, the
next Process Position reached by the container 15 may be Process
Position 1', i.e. the container 15 moves from one end of the
process path 10- to an opposite end of the process path 10-.
In the illustrated embodiment of the process path 10-, a
second processing lane 170 iS included. The second processing
lane 170 may be located in any suitable position with respect to
the processing lane 28 SO that a container 15 can move between
the process lane 28 and the process lane 170. In some
embodiments, the position of the process lane 170 may be chosen
to maintain the process path 10- within specified physical
dimensions.
A prime mover, which may be substantially similar to the
prime movers discussed earlier, is located, in an exemplary
embodiment, adjacent position 46 along the process lane 28.
20 This prime mover is operable to move a container 15 from the
process lane 28 to the process lane 170 when desired, viz. for
reading a determination reaction, removal of a container 15 from
the process path 10-, etc. The process lane 28 may be joined to
the process lane 170 by suitable connection structures 172, such
25 as those associa~ed with a bypass region. In this manner, a
container 15 may be selectively automatically moved between the
process lane 28 and the process lane 170. Thus, upon reaching
Process Position 46, a container 15 may move to Process Position
1 of the process lane 28, or, alternatively, may move from
Process Position 46 of the process lane 28 to Process Positions
47 through 55 of the process lane 170. Once in the process lane
170, process steps detailed in the example below are performed.
Of course, structures, similar to those discussed above, that
perform those process steps are disposed along the process lane
64
CA 022~904~ 1999-01-13
- 65
170 which has sufficient dimensions to accommodate those structures.
Process Process Step Description
Position
1 Container 15 load Container 15 moved from loading
lane 30, if present, to process
lane 28 as required
1 Sample Pipettor Sample deposited into container
15 by pipetting system 128. The
sample may be obtained from
position 130A or 130B which are
located on appropriate conveyors
2 Reagent Pipettor 1 Reagent obtained from reagent
carousel 131 deposited into
container 15 by pipetting system
132
3 Mixer Contents of container 15 are
mixed by a device 86 imparting
motion to the container 15
4 - 16 Incubation Contents of container 15 are
incubated at a controlled
temperature, about 37 degrees
Celsius
17 Bypass region start Container 15 is selectively
positioned at entry to
performance lane 62 or avoidance
lane 64 of bypass region
18 Wash zone 1 Container 15 in performance lane
62 undergoes magnetic separation
and fluid addition
, ................ . . _ .
CA 022~904~ l999-0l-l3
66
19 Wash zone 1 Container 15 in performance lane
62 undergoes magnetic separation,
container 15 contents aspiration
and fluid addition
Wash zone 1 Container 15 in performance lane
62 undergoes magnetic separation,
container 15 contents aspiration
and fluid addition
21 Wash zone 1 Container 15 in performance lane
62 undergoes magnetic separation,
and container 15 contents
aspiration
22 Bypass region end Performance and avoidance lanes
62 and 64 of bypass region merge
23 - 24 Incubation Contents of container 15 are
incubated at a controlled
temperature
24 Sample Pipettor Sample may be aspirated from
container 15 by pipetting system
128 for deposition into a second
container 15 at position 1
2 5 Reagent Pipettor 2 Reagent obtained from reagent
carousel 131 may be deposited
into container by pipetting
system 132
26 Mixer Contents of container 15 are
mixed by a device 8 6 imparting
motion to the container 15
27 - 39 Incubation Contents of container 15 are
incubated at a controlled
temperature
66
- T
CA 022~904~ l999-0l-l3
67
Bypass region start Container 15 iS selectively
positioned at entry to
performance lane 62 or avoidance
lane 64 of bypass region
41 Wash zone 2 Container 15 in performance lane
62 undergoes magnetic separation
and fluid addition
42 Wash zone 2 Container 15 in performance lane
62 undergoes magnetic separation,
container 15 contents aspiration
and fluid addition
43 Wash zone 2 ~ontainer 15 in performance lane
62 undergoes magnetic separation,
container 15 contents aspiration
and fluid addition
44 Wash zone 2 Container 15 in performance lane
62 undergoes magnetic separation
and container 15 contents
aspiration
45.5 Bypass region end Performance lane 62 and avoidance
lane 64 of bypass region merge
(midway between positions 45 and
46)
46 Process lane Container moves from Process
transfer Position 46' of process lane 28
to Process Position 46 of process
lane 170
46 - 47 Incubation Contents of container 15 are
incubated at a controlled
temperature
48 Pre-Trigger and Reagent added to container 15 and
Mixer mechanically mixed
67
~ ..
CA 022~904~ 1999-01-13
68
49 - 51 Incubation Contents of container 15 are
incubated at controlled
temperature
52 Shutter, reader, Indicator reaction (such as
and trigger chemiluminescent reaction)
triggered and read with magnetic
particles pulled out of solution
with magnet. Shutter blocks
ambient light.
54 Liquid Waste Magnetic particles are held at a
Aspirate wall of the container 15 and all
liquid in container 15 is
aspirated and discarded
Container 15 unload Container 15 removed from process
lane 2 8
Given these modifications, it is possible to utilize
determination formats that are substantially similar to those
discussed previously. For the sake of clarity, those formats,
as performed by the process path 10- are listed below.
68
CA 022~904~ l999-0l-l3
69
Format A
Ste~ POs ition
Sample introduction
First reagent introduction and 2 - 3
mixing
First incubation ( 18 minutes) 4 - 17
Container 15 passes through 18 - 21
bypass region, first incubation
continues
First incubation continues 22 - 40
Container 15 passes through 41 - 44
bypass region, first incubation
continues
First incubation continues 45 - 17 '
Separation and wash 18' - 21'
Second reagent introduction and 25' - 26'
mixing
Second incubation ( 4 minutes) 27 ' - 40 '
Separation and wash 41' - 44'
Container 15 transferred from 46' of 28
process lane 28 to process lane to 46 of
170 170
Pretrigger introduction and 48
mixing
Third incubation (1 minute) 49 - 51
Trigger and read 52
Container 15 evacuate 54
Container 15 removal 55
59
CA 022~904~ 1999-01-13
As an example, Format A may be used to determine at least the
following items of interest: antibodies to HCV, antibodies to
HIV 1/HIV 2, antibodies to hepatitis B core antigen (HBcAb),
carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA19-9),
Hepatitis B Surface Antigen (HBsAg), antibodies to Hepatitis B
Surface antigen (HBsAb), alpha-fetoprotein (AFP), Total prostate
specific antigen (Total PSA), Free PSA, Thyroid stimulating
Hormone (TSH), luteinizing hormone (LH), follicle stimulating
hormone (FSH), beta human chorionic gonadotropin (B-hCG), Free
Thyroxine (Free T4), Free triiodothyronine (Free T3), Total T4,
Total T3, Prolactin and Ferritin. It is to be noted that almost
any item of inter-est discussed herein may be determined by
properly using this format. For instance, this format may also
be used to determine beta human chorionic gonadotropin (B-hCG),
prolactin and fer-ritin.
CA 022~904~ 1999-01-13
Format s
Ste~ Position
Sample introduction
First reagent introduction and 2 - 3
mixing
First incubation (11 minutes) 4 - 40
Separa~ion and wash 41 - 44
Second reagent introduction and 2~ - 3
mixing
Second incubation (11 minutes) 4~ - 40~
Separation and wash 41' - 44'
Container 15 transferred from 46' of 28
process lane 28 to process lane to 46 of
170 170
Pretrigger introduction and 48
mixing
Third incubation (1 minute) 49 - 51
Trigger and read 52
Container 15 evacuate 54
Container 15 removal 55
As an example, Format B may be used to determine an item of
interest in a sample where a relatively increased degree of
sensitivity, as compared with some other formats, is desired.
It is to be noted that almost any item of interest discussed
herein may be de-ermined by properly using this format.
CA 022~904~ 1999-01-13
Format C
Ste~ Position
Sample introduction
First reagent introduction and 2 - 3
mixing
First -ncubation (11 minutes) 4 - 40
Separation and wash 41 - 44
Second reagent introduction and 2' - 3
mixing
Second incubation (4 minutes) 4' - 17'
Separation and wash 18' - 21'
Third reagent introduction and 25' - 26'
mixing
Third incubation (4 minutes) 27' - 40'
Separation and wash 41~ - 44~
Container 15 transferred from 46' of 28
process lane 28 to process lane to 46 of
170 170
Pretrigger introduction and 48
mixing
Fourth incubation (1 minute) 49 - 51
Trigger and read 52
Container 15 evacuate 54
Container 15 removal 55
As an example, Format C may be used when the item of interest
relates to hepatltis, such as determinations for anti-M, HBcAb-M
and HAVAb-M.
CA 022~904~ 1999-01-13
Format D
Ste~ Position
Sample introduction
First :reagent introduction and 2 - 3
mixing
First incubation (7 minutes) 4 - 17
Transfer to second container 15 25
in position 1
Second reagent introduction and 2 - 3
mixing
Second incubation (11 minutes) 4 - 40
Separation and wash 41 - 44
Third reagent introduction and 2' - 3
mixing
Third incubation (4 minutes) 4' - 17'
Separation and wash 18' - 21'
Fourth reagent introduction and 25' - 26'
mixing
Fourth incubation (4 minutes) 27' - 40'
Separation and wash 41' - 44'
Container transferred from 46' of 28
process lane 28 to process lane to 46 of
170 170
Pretrigger introduction and 48
mixing
Fifth lncubation (1 minute) 49 - 51
Trigger and read 52
Container i5 evacuate 54
Container 15 removal 55
CA 022~904~ l999-0l-l3
74
Format E
Ste~ Pocition
Sample introduction
First reagent 2 - 3
introducticn and mixing
First incubation (7 4 - 24
minutes)
Transfer portion of 25
container 15 contents to
second container 15,
remainder of container 15
continues on process lane
28
First container 15 25 - 17 '
contents continues first
incubation (11 minutes)
Second reagent 2 - 3
introduction and mixing
with second container 15
contents
First container 15 passes 18 ' - 21 '
through bypass region 58B
Second container 15 first 4 - 17
incubation ( 18 minutes)
Introduction of fourth 25' - 26'
reagent into first
container 15 and mixing
(optional, enhances total
Hb chemiluminescent
signal)
74
. . .
CA 022~904~ 1999-01-13
Second container 18~ - 21'
separation and wash
Fourth incubation (4 27' - 40'
minutes - cptional) of
first container 15
Third reagent 25' - 26
introduction into second
container 15 and mixing
First container 15 passes 41~ - 44
through bypass region
Third incubation (4 27' - 40'
minutes) of second
container 15
First container 15 46' of 28 to 46 of 170
transferred from process
lane 28 to process lane
170
Pretrigger introduction 48
into first container 15
and mixing
Separation and wash of 41' - 44
second container 15
Second container 15 46' of 28 to 46 of 170
transferred from process
lane 28 to process lane
170
Trigger and read value 1 52
(Total Hb) from first
container 15
CA 022~904~ 1999-01-13
76
Pretrigger introduction 48
into second container 15
and mixing
Trigger and read value 2 52
(GlyHb)
Reported result = value 2 X 100
value 1
For example, in Format E, it is possible to modify the format by
disregarding the first container 15 after the portion of the
container 15 contents has been transferred (Position 24) to the
second container 15. In that case, Format E may be used to
determine, for example, folate and vitamin B12.
76
CA 022~904~ 1999-01-13
Format F
Ste~ Po~ition
Sample introduction
First reagent 2 - 3
introduction and mixing
First incubation (7 4 - 24
minutes)
Transfer portion of 25
container 15 contents to
second container 15,
remainder of container 15
continues on process lane
28
First container 15 25 - 17'
contents continues first
incubation (11 minutes)
Second reagent 2 - 3
introduction and mixing
with second container 15
contents
First container 15 passes 18' - 21'
through bypass region 58B
Second container 15 first 4 - 40
incubation (11 minutes)
Introduction of fourth 25' - 26'
reagent into first
container 15 and mixing
(optional, enhances total
Hb chemiluminesc.ent
signal)
CA 022~904~ 1999-01-13
Second container 41 - 44
separation and wash
Fourth incubation (4 27' - 40
minutes - cptional) of
first container 15
Third reagent 2~ - 3
introduction into second
container 15 and mixing
First container 15 passes 41' - 44
through bypass region
Third incubation (11 4~ - 40
minutes) of second
container 15
First container 15 46' of 28 to 46 of 170
transferred from process
lane 28 to process lane
170
Pretrigger introduction 48
into first container 15
and mixing
Separation and wash of 41' - 44'
second container 15
Trigger and read value 1 52
(Total Hb) from first
container 15
Second container 15 46' of 28 to 46 of 170
transferred from process
lane 28 to process lane
170
CA 022~904~ 1999-01-13
79
Pretrigger introduction 48
into second container 15
and mixing
Trigger and read value 2 52
(GlyHb)
Reported result = value 2 X 100
value 1
This format may be used, for example, to determine at least one
of total and glycated hemoglobin. Also, this format may be
modified by disregarding the first container 15 as in Format E.
79
CA 022~904~ 1999-01-13
Format G
Ste~ Position
Sample introduction
First reagent 2 - 3
introduction and mixing
First incubation (7 4 - 24
minutes)
Transfer portion of 25
container 15 contents to
second container 15,
remainder of container 15
continues cn process lane
28
First container 15 25 - 17
contents continues first
incubation (11 minutes)
Second reagent 2 - 3
introduction and mixing
with second container 15
contents
First container 15 18' - 21
separation and wash
Second container 15 first 4 - 17'
incubation (18 minutes) .
Introduction of fourth 25~ - 26'
reagent into first
container 15 and mixing
(optional, enhances total
Hb chemiluminescent
signal)
CA 022~904~ 1999-01-13
Second container 18' - 21'
separation and wash
Fourth incubation (4 27' - 40
minutes - cptional) of
first container 15
Third reagent 25' - 26'
introducticn into second
container 15 and mixing
First container 15 41' - 44
separation and wash
Third incubation (4 27' - 40'
minutes) of second
container 15
First container 15 46' of 28 to 46 of 170
transferred from process
lane 28 to process lane
170
Pretrigger introduction 48
into first container 15
and mixing
Separation and wash of 41' - 44'
second container 15
Second container 15 46' of 28 to 46 of 170
transferred from process
lane 28 to ~process lane
170
Trigger and read value 1 52
(Total Hb) from first
container 15
CA 022~904~ 1999-01-13
82
Pretrigger introduction 48
into second container 15
and mixing
Trigger and read value 2 52
(GlyHb)
Reported result = value 2 X 100
value 1
As an example, this format may also be modified as may be done
with Format F. ~-ith that modification, this Format may be used
to determine progesterone, testosterone and estradiol.
82
CA 022~904~ l999-0l-l3
83
Format H
Ste~ Position
Sample introduction
First reagent 2 - 3
introduction and mixing
First incubation 4-41'
Separation and wash 42' - 44'
Container 15 transfer 46' of 28 to 46 of 170
from process lane 28 to
process lane 170
Pretrigger introduction 48
and mixing
Second incubation 49 - 51
Trigger and read 52
As an example, this format may be used to determine, among other
things, beta human chorionic gonadotropin (B-hCG), prolactin,
progesterone, testosterone, estradiol and ferritin. It is to be
noted that almost any item of interest discussed herein may be
determined by properly using this format.
83
CA 022~904~ 1999-01-13
84
Format
Ste~ Po~ition
.
Sample introduction into
first container 15,
possibly with diluent
fluid
First reagent 2
introduction into first
container 15, portion of
first container 15
contents moved into
pipettor, remainder of
container continues on
process lane 28,
bypassing all wash
stations, to Position 25'
First reagent 2 - 3
introduction into second
container 15 and mixing
Second container 15 first 4 - 17'
incubation (18 minutes)
Introducticn of second 25' - 26'
reagent into first
container 15 and mixing
(optional, enhances total
Hb chemiluminescent
signal)
Second container 18' - 21
separation and wash
84
CA 022~904~ l999-0l-l3
Fourth incubation (4 27' - 40'
minutes - optional) of
first container 15
Third reagent 25 ' - 26 '
introduction into second
container 15 and mixing
First container 15 passes 41 ' - 44 '
through bypass region 58
Third incubation (4 27' - 40'
minutes) of second
container 15
Pretrigger introduction 48
into first container 15
and mixing
Separation and wash of 41' - 44'
second container 15
Trigger and read value 1 52
(Total Hb) from first
container 15
Pretrigger introduction 48
into second container 15
and mixing
Trigger and read value 2 52
(GlyHb)
Reported result = value 2 X 100
value 1
5 AS an example, in Format I, it is possible to modify the format
by disregarding the first container 15 after the portion of the
container 15 contents has been transferred (Position 24) to the
second container 15. In that case, Format I may be used to
determine, for example, folate and vitamin B12.
CA 022~904~ 1999-01-13
Format J
Ste~ Position
Sample introduction into
container 15, possibly
with diluent fluid
First reagent 2 - 3
introduction and mixing
First incubation (27 4 - 47
minutes - two times along
process lane 28)
Pretrigger introduction 48
and mixlng
Second incubation (1 49 - 51
minute)
Trigger and read 52
AS an example, Format J may be used to determine, among other
things, total hemoglobin.
CA 022~904~ 1999-01-13
The embodiments described herein also allow for sample
pretreatment which may be performed in at least two ways,
indicated as Formats K and L. During performance of sample
pretreatment, fluid present in the containers 15 indicated may
be processed, after they are no longer significant in the
pretreatment steps, in any appropriate manner, such as any of
the Formats discussed above. Also, as will become clear later
on, both Formats K and L are substantially similarly applicable
to the other embodiment of the process path 10 discussed below.
87
CA 022~904~ 1999-01-13
88
Format K
Ste~ PoRition
Sample introduction into
first container 15,
possibly with diluent
fluid
First reagent 2 - 3
introduction and mixing
First incubation (7 4 - 23
minutes)
Transfer portion of 24
contents of first
container 15 to second
container 15 in Position
Second reagent 2 - 3
introduction to second
container 15 and mixing
(optional)
Transfer portion of 24
contents of second
container 15 to third
container 15 in Position
Third reagent 2 - 3
introduction to third
container and mixing
(optional)
As an example, the third container 15 may be processed according
to at least one of Formats A (to determine, among other things,
folate), B, C, H and J.
88
., _ ......................................................... ...
CA 022~904~ 1999-01-13
89
Format L
Ste~ Po~ition
Sample introduction into
first container 15,
possibly with diluent
fluid
First reagent 2 - 3
introduction and mixing
First incubation (7 4 - 23
minutes)
Transfer portion of 24
contents of first
container 15 to second
container 15 in Position
Second reagent 2 - 3
introduction to second
container 15 and mixing
(optional)
As an example, the second container 15 may be processed
according to at least one of Formats A (to determine, among
other things, folate, vitamin B12, confirm HBsAg), B, C, H and
J.
~9
CA 022~904~ 1999-01-13
Another embodiment is a process path 10 , substantially
similar to the previous embodiment, of the process path 10 is
constructed to perform 50 determinations per hour. Elements
similar to those described earlier, along with the same index
period and assay formats are used, thereby allowing use of the
same reagents albeit in an embodiment having relatively smaller
physical dimensions. Following the examples discussed above,
the following examples relate to this process path 10 . In
these examples, it is assumed that only one pipettor is
utilized. Also, whereas the previous examples performed
determinations while moving a container 15 along the process
lane 28 twice, the process path 10~ performs determinations
while moving a container 15 along the process lane 28 four
times. Thus, the second time Process Position 1 is encounter is
indicated as 1', the third time as 1~ and the four time as '".
However, it is to be noted that more or less movements along the
process lane 28 may be employed. Also, the process lane 28 of
this embodiment includes 23 Process Positions with Process
Position 23 being located adjacent to Process Position 1.
Process Process Step Descri~tion
Position
1 Container 15 load Container 15 moved from loading
lane 30, if present, to process
lane 28 as required
1 Pipettor - Sample deposited into container
15 by pipetting system
2 Pipettor Reagent obtained from reagent
carousel 131 deposited into
container 15
CA 022~904~ 1999-01-13
91
3 Mixer Contents of container 15 are
mixed by a device 86 imparting
motion to the container 15
4 - 16 Incubation Contents of container 15 are
incubated at a controlled
temperature, about 37 degrees
Celsius
17 Bypass region start Container 15 is selectively
positioned at entry to
performance lane 62 or avoidance
lane 64 of bypass region
18 Wash zone 1 Container 15 in performance lane
62 undergoes magnetic separation
and fluid addition
19 Wash zone 1 Container 15 in performance lane
62 undergoes magnetic separation,
container 15 contents aspiration
and fluid addition
Wash zone 1 Container 15 in performance lane
62 undergoes magnetic separation,
container 15 contents aspiration
and fluid addition
21 wash zone 1 Container 15 in performance lane
62 undergoes magnetic separation,
and container 15 contents
aspiration
22 sypass region end Performance and avoidance lanes
62 and 64 of bypass region merge
23 Process lane Container 15 selectively
transfer transferred from process lane 28
to process lane 170
CA 022~904~ 1999-01-13
92
24 Pre-Trigger and Reagent added to container 15 and
Mixer mechanically mixed
2 6 - 2 8 Incubation Contents of container 15 are
incubated at controlled
temperature
29 Shutter, reader, Indicator reaction (such as
and trigger chemiluminescent reaction)
triggered and read with magnetic
particles pulled out of solution
with magnet. Shutter blocks
ambient light.
31 Liquid Waste Magnetic particles are held at a
Aspirate wall of the container 15 and all
liquid in container 15 is
aspirated and discarded
32 Container 15 unload Container 15 removed from process
lane 2 8
Given these modifications, it is possible to utilize
determination formats that are substantially similar to those
discussed previously. For the sake of clarity, those formats,
as performed by a process path that performs 50 determinations
per hour, are listed below.
92
CA 022~904~ 1999-01-13
93
Format A
Ste~ Position
Sample introduction
First reagent introduction and 2 - 3
mixing
First incubation (18 minutes) 4 - 17
Container 15 passes through 18 - 21
bypass region, first incubation
continues
First incubation continues 22 - 17"
Separation and wash 18" - 21"
Second reagent introduction and 2'N - 3
mixing
Second incubation (4 minutes) 4'" - 17' N
Separation and wash 18'" -
21'"
Container 15 transferred from 23'" of 28
process lane 28 to process lane to 23 of
170 170
Pretrigger introduction and 25
mixing
Third incubation (1 minute) 26 - 28
Trigger and read 29
Container 15 evacuate 31
Container 15 removal 32
As an example, Format A may be used to determine at least the
following items of interest: antibodies to HCV, antibodies to
HIV 1/HIV 2, antibodies to hepatitis B core antigen (HscAb),
carcinoembryonic antigen (CEA), cancer antigen 19-9 (CA19-9),
93
CA 022~904~ 1999-01-13
94
Hepatitis B Surface Antigen (HBsAg), antibodies to Hepatitis B
Surface antigen ~HBsAb), alpha-fetoprotein (AFP), Total prostate
specific antigen (Total PSA), Free PSA, Thyroid stimulating
Hormone (TSH), luteinizing hormone (LH), follicle stimulating
hormone (FSH), beta human chorionic gonadotropin (B-hCG), Free
Thyroxine (Free T4), Free triiodothyronine (Free T3), Total T4,
Total T3, Prolactin and Ferritin. It is to be noted that almost
any item of interest discussed herein may be determined by
properly using this format. For instance, this format may also
be used to determine beta human chorionic gonadotropin (B-hCG),
prolactin and ferritin
94
CA 022~904~ 1999-01-13
Format B
Ste~ Position
Sample introduction
First reagent introduction and 2 - 3
mixing
First incubation (11 minutes) 4 - 17'
Separation and wash 18' ~ 21'
Second reagent introduction and 2/~ ~ 3
mlxing
Second incubation (11 minutes) 4" - 17'"
Separation and wash 18~N ~
21 ~ ~
Container 15 transferred from 23~ of 28
process lane 28 to process lane to 23 of
170 170
Pretrigger introduction and 25
mixing
Third incubation (1 minute) 26 ~ 28
Trigger and read 29
Container 15 evacuate 31
Container 15 removal 32
As an example, Format B may be used to determine an item of
interest in a sample where a relatively increased degree of
sensitivity, as compared with some other formats, is desired.
It is to be noted that almost any item of interest discussed
herein may be determined by properly using this format.
CA 022~904~ 1999-01-13
96
Format C
Ste~ Position
Sample introduction
Flrst reagent introduction and 2 - 3
mlxlng
First incubation (11 minutes) 4 - 17'
Separation and wash 18' - 21
Second reagent introduction and 2" - 3"
mixing
Second incubation (4 minutes) 4" - 17"
Separation and wash 18" - 21"
Third reagent introduction and 2'a - 3'"
mixing
Third incubation (4 minutes) 4'~ - 17'"
Separation and wash 18'" -
21' N
Container 15 transferred from 23'" of 28
process lane 28 to process lane to 23 of
170 170
Pretrigger introduction and 25
mixing
Fourth incubation (1 minute) 26 - 28
Trigger and read 29
Container 15 evacuate 31
Container 15 removal 32
As an example, Format C may be used when the item of interest
relates to hepatltis, such as determinations for anti-M, HBcAb-M
and H.-VAb-M.
96
CA 022~904~ 1999-01-13
97
Format D
Ste~ Position
Sample introduction
First reagent introduction and 2 - 3
mixing
First incubation ( 7 minutes) 4 - 17
Transfer to second container 15 23
in position 1
Second reagent introduction and 2 - 3
mixing
Second incubation (11 minutes) 4 - 17 '
Separation and wash 18' - 21'
Third reagent introduction and 2~ - 3
mixing
Third incubation (4 minutes) 4~ - 17 "
Separation and wash 18" - 21"
Fourth reagent introduction and 2'" - 3'N
mixing
Fourth incubation (4 minutes) 4~" - 17 ' "
Separation and wash 18'" -
21'"
Container transferred from 23~ of 28
process lane 28 to process lane to 23 of
170 170
Pretrigger introduction and 25
mixing
Fifth incubation (1 minute) 26 - 28
Trigger and.read 29
Container 15 evacuate 31
97
CA 02259045 1999-01-13
9 8
Container 15 removal 32
9~
CA 022~904~ 1999-01-13
99
Format E
Ste~ Position
Sample introduction
First reagent 2 - 3
introduction and mixing
First incubation (7 4 - 17
minutes)
Transfer portion of 23
container 15 contents to
second container 15,
remainder of container 15
continues on process lane
28
First container 15 23 - 17'
contents continues first
incubation (11 minutes)
Second reagent 2 - 3
introduction and mixing
with second container 15
contents
~irst container 15 passes 18" - 21"
through bypass region 58B
Second container 15 first 4 - 17"
incubation (18 minutes)
Introduction of fourth 2'" - 3~ N
reagent into first
container 15 and mixing
(optional, enhances total
Hb chemiluminescent
signal)
99
CA 022~904~ 1999-01-13
100
Second container 18' - 21'
separation and wash
Fourth incubation (4 4'" - 17'"
minutes - optional) of
first container 15
Third reagent 25' - 26'
introduction into second
container 15 and mixing
First container 15 passes 18'" - 21'"
through bypass region
Third incubation (4 4'" - 17'"
minutes) of second
container 15
First container 15 23'" of 28 to 23 of 170
transferred from process
lane 28 to process lane
170
Pretrigger introduction 25
into first container 15
and mixing
Separation and wash of 18'" - 21'"
second container 15
Second container 15 23'" of 28 to 23 of 170
transferred from process
lane 28 to process lane
170
Trigger and read value 1 29
(Total Hb) from first
container 15
100
CA 022~904~ 1999-01-13
101
Pretrigger introduction 25
into second container 15
and mixing
Trigger and read value 2 29
(GlyHb)
Reported result = value 2 X 100
value 1
For example, in Format E, it is possible to modify the format by
disregarding the first container 15 after the portion of the
container 15 contents has been transferred (Position 24) to the
second container 15. In that case, Format E may be used to
determine, for example, folate and vitamin B12.
101
CA 022~904~ l999-0l-l3
102
Format F
Ste~ Position
Sample introduction
First reagent 2 - 3
introduction and mixing
First incubation (7 4 - 17
minutes)
Transfer portion of 23
container 15,contents to
second container 15,
remainder of container 15
continues on process lane
28
First container 15 23 - 17"
contents continues first
incubation (11 minutes)
Second reagent 2 - 3
introduction and mixing
with second container 15
contents
First container 15 passes 18" - 21"
through bypass region 58B
Second container 15 first 4 - 17'
incubation (11 minutes)
Introduction of fourth 2'~' - 3'"
reagent into first
container 15 and mixing
(optional, enhances total
Hb chemiluminescent
signal)
102
CA 022~904~ l999-0l-l3
103
Second container 18' - 21'
separation and wash
Fourth incubation (4 4'" - 17'"
minutes - optional) of
first container 15
Third reagent 2'" - 3'"
introduction into second
container 15 and mixing
First container 15 passes 18 ' " - 21' "
through bypass region
Third incubation (11 4'~ - 17'"
minutes) of second
container 15
First container 15 23 ' " of 28 to 23 of 170
transferred from process
lane 28 to process lane
170
Pretrigger introduction 2 5
into first container 15
and mlxlng
Separation and wash of 18'" - 21'"
second container 15
Trigger and read value 1 29
(Total Hb)-from first
container 15
Second container 15 28~N cf 28 to 23 of 170
transferred from process
lane 28 to process lane
170
103
CA 022~904~ l999-0l-l3
104
Pretrigger introduction 25
into second container 15
and mixing
Trigger and read value 2 29
(GlyHb)
Reported result = value 2 X 100
value 1
This format may he used, for example, to determine at least one
of total and glycated hemoglobin. Also, this format may be
modified by disregarding the first container 15 as in Format E.
104
CA 022~904~ 1999-01-13
105
Format G
Ste~ Position
Sample introduction
First reagent 2 - 3
introduction and mixing
First incubation (7 ~ - 23
minutes)
Transfer portion of 23
container 15 contents to
second container 15,
remainder of container 15
continues on process lane
28
First container 15 24 - 17"
contents continues first
incubation (11 minutes)
Second reagent 2 - 3
introduction and mixing
with second container 15
contents
First container 15 18" - 21a
separation and wash
Second container 15 first ~ - 17"
incubation (18 minutes)
Introduction of fourth 2'" - 3'"
reagent into first
container 15 and mixing
(optional, enhances total
Hb chemiluminescent
signal)
105
CA 022~904~ l999-0l-l3
106
Second container 18~ - 21
separation and wash
Fourth incubation (4 4'" - 17'"
minutes - optional) of
first container 15
Third reagent 2'" - 3
introduction into second
container 15 and mixing
First container 15 18'" - 21'"
separation and wash
Third incubation (4 4'" - 17
minutes) of second
container L5
First container 15 23'" of 28 to 23 of 170
transferred from process
lane 28 to process lane
170
Pretrigger introduction 25
into first container 15
and mixing
Separation and wash of 18'" - 21
second container 15
Second container 15 23~ of 28 to 23'" of 170
transferred from process
lane 28 to process lane
170
Trigger and read value 1 29
(Total Hb) from first
container L5
106
CA 022~904~ l999-0l-l3
107
Pretrigger introduction 25 .
into second container 15
and mixing
Trigger and read value 2 29
(GlyHb)
Reported result = value 2 X 100
value 1
As an example, this format may also be modified as may be done
with Format F. With that modification, this Format may be used
to determine progesterone, testosterone and estradiol.
107
CA 022~904~ l999-0l-l3
108
Format H
Ste~ Position
Sample introduction
First reagent 2 - 3
introduction and mixing
First incubation 4-17'"
Separation and wash 18'" - 21'"
Container 15 transfer 23'" of 28 to 23 of 170
from process lane 28 to
process lane 170
Pretrigger introduction 2 5
and mixing
Second incubation 26 - 28
Trigger and read 2 9
As an example, this format may be used to determine, among other
things, beta human chorionic gonadotropin (B-hCG), prolactin,
progesterone, testosterone, estradiol and ferritin. It is to be
noted that almost any item of interest discussed herein may be
determined by properly using this format.
108
CA 022~904~ l999-0l-l3
109
Format
S t e ~ P o s i t i on
Sample introduction into
first container 15,
possibly with diluent
fluid
First reagent 2
introduction into first
container 15, portion of
first container 15
contents moved into
pipettor, remainder of
container continues on
process lane 28,
bypassing all wash
stations, to Position 25'
First reagent 2 - 3
introduction into second
container 15 and mixing
Second container 15 first 4 - 17"
incubation (18 minutes)
Introduction of second 2'" - 3'"
reagent into first
container 15 and mixing
(optional, enhances total
Hb chemiluminescent
signal)
Second container 18'" - 21
separation and wash
109
CA 022~904~ 1999-01-13
110
Fourth incubation (4 4~ 17'"
minutes - optional) of
first container 15
Third reagent 2~ - 3
introduction into second
container 15 and mixing
First container 15 passes 18'" - 21'"
through bypass region 58
Third incubation (4 4'" - 17 ~ a
minutes) of second
container 15
Pretrigger introduction 25
into first container 15
and mixing
Separation and wash of 18'N - 21'"
second container 15
Trigger and read value 1 29
(Total Hb) from first
container 15
Pretrigger introduction 25
into second container 15
and mixing
Trigger and read value 2 29
(GlyHb)
Reported result = value 2 X 100
value 1
As an example, in Format I, it is possible to modify the format
by disregarding the first container 15 after the portion of the
container 15 contents has been transferred (Position 24) to the
second container 5. In that case, Format I may be used to
determine, for example, folate and vitamin B12.
110
CA 022~904~ 1999-01-13
111
Format J
Ste~ Po~ition
Sample introduction into
container 15, possibly
with diluent fluid
First reagent 2 - 3
introduction and mixing
First incubation (27 4 - 47
minutes - four times
along process lane 28)
Pretrigger introduction 25
and mixing
Second incubation (1 26 - 28
minute)
Trigger and read 29
AS an example, Format J may be used to determine, among other
things, total hemoglobin.
111
CA 022~904~ l999-0l-l3
112
The embodiments described herein also allow for sample
pretreatment which may be performed in at least two ways,
indicated as Formats K and L. During performance of sample
pretreatment, fluid present in the containers 15 indicated may
be processed, after they are no longer significant in the
pretreatment steps, in any appropriate manner, such as any of
the Formats discussed above. Also, as will become clear later
on, both Formats K and L are substantially similarly applicable
to the other embodiment of the process path 10 discussed below.
112
CA 022~904~ l999-0l-l3
113
Format R
Ste~ Po~ition
Sample int~oduction into
first container 15,
possibly with diluent
fluid
First reagent 2 - 3
introduction and mixing
First incubation (7 4 - 23
minutes)
Transfer portion of 2 4
contents of first
container 15 to second
container :L5 in Position
Second reagent 2 - 3
introduction to second
container 15 and mixing
(optional)
Transfer portion of 2 4
contents of second
container 15 to third
container ~5 in Position
Third reagent 2 - 3
introductic~n to third
container and mixing
(optional)
As an example, the third container 15 may be processed according
to at least one of Formats A (to determine, among other things,
folate), B, C, H and J.
113
CA 022~904~ l999-0l-l3
114
Format L
Ste~ Po~ition
Sample introduction into
first container 15,
possibly w th diluent
fluid
First reagent 2 - 3
introduction and mixing
First incubation (7 4 - 23
minutes)
Transfer portion of 24
contents of first
container 15 to second
container 15 in Position
Second reagent 2 - 3
introduction to second
container 15 and mixing
(optional)
As an example, the second container 15 may be processed
according to at least one of Formats A (to determine, among
other things, folate, vitamin B12, confirm HBsAg), B, C, H and
J.
114
CA 022~904~ l999-0l-l3
115
Given commonality among the various embodiments of the
process path discussed and exemplified above, it is to be
appreciated that assay formats performed on each of the various
embodiments are essentially the same. The time frames are
5 identical. Reagents for a particular assay used on one of the
embodiments may also be used on other embodiments.
Upon consideration of all of these examples and their
common features, it is to be understood that the process path
10, or in other words the process lane 28, has a variable
physical length. However, the effective length of the process
path 10 is constant in all embodiments. This effective length
represents the total distance traveled by the container 15 along
the process path 10 during performance of a certain
determination. The physical length, i.e. the physical
dimensions of the process path 10, is variable, for instance, to
make the process path 10 fit within a given space. The
effective length of the process path 10 is maintained constant
by moving the container 15 multiple times along the same process
path 10 (4 times in the last set of examples). Maintenance of
20 the effective length is achieved with appropriate combination of
selective automatic performance of a given determination process
step. In all instances, the effective length remains constant
even though the physical length of a given process path 10 may
be longer or shorter than other process paths 10.
It is to be noted that all of the above discussed
embodiments of the process path 10 include and utilize certain
common elements, such as reagents, a sample/reagent pipettor, a
mixer, a wash zone and a reader. The structural elements are
arranged along each embodiment of the process path 10 such that
each embodiment is able to perform the same determinations in
substantially the same manner by keeping the effective length of
the process path 10 constant. Each of the embodiments of the
process path executes determinations with approximately the same
number, such as 98 in the above examples, "steps" of the
3 5 container 15 along the process path 10 between sample
115
CA 022~904~ l999-0l-l3
116
introduction and reading. Determination of a given item of
interest by one c,f the embodiments of the process path 10 takes
substantially the same amount of time as a determination of the
same item of interest by another embodiment of the process path
10. Thus, it is possible to construct a structure for
performing item c,f interest determinations which conforms to
desired physical dimensions, throughput requirements, etc.,
while using the common elements discussed herein by maintaining
the effective length of the process path constant.
11~