Note: Descriptions are shown in the official language in which they were submitted.
CA 022~927~ 1998-12-21
BIO 9401 1 PCT 23.05.1997
EUROFERM GmbH
Gustav-Meyer-Allee 25
13355 Berlin
Device for measuring the partial ~,~ r~e of gases
dissolved in liquids
The present invention relates to a novel device
for measuring gas partial pressure in liquid media
according to P 44 45 68.9.
There is an increasing need, primarily in the
field of fermentation technology, to measure gases by
determining their partial pressure. Special probes have
therefore been developed for determining the partial
pressure of oxygen and carbon dioxide. A common example
of these is constituted, for example, by so-called
Severinghaus electrodes. These devices operate with
membrane-covered single-rod pH electrodes
(DE-A 25 08 637, Biotechnol. Bioeng. 22 (1980), 2411-
2416, Biotechnol. Bioeng. 23 (1981), 461-466). In this
system, there is an electrolyte solution or paste between
the gas-selective membrane and the pH electrode. The
measuring principle is based on the fact that, in aqueous
solution, carbon dioxide forms carbonic acid, which
dissociates into a bicarbonate anion and a proton. This
process causes a change in the pH in the electrolyte
solution, and this change is measured using the pH probe.
A disadvantage of this measuring principle is the fact
that carbon dioxide is measured not directly, but in its
ionic form. Since the ionic form is present in a propor-
tion of less than 0.1%, this method is not sufficiently
accurate. Apart from this, other acidic or basic volatile
gases vitiate the measurement of the pH. Furthermore,
very high outlay on maintenance is needed.
PC02 optodes are also known from the prior art.
Once again, these involve a membrane-covered sensor
system (SPIE vol. 798 Fiber Optic Sensors II (1987)
pp. 249-252; Anal. Chim. Acta 160 (1984) pp. 305-309;
Proc. Int. Meeting on Chemical Sensors, Fukuoka, Japan,
Elsevier, pp. 609-619, 1983, Talanta 35 (1988) 2 pp. 109-
CA 022~927~ 1998-12-21
112, Anal. Chem. 65 (1993) pp. 331-337, Fresenius Z.
Anal. Chem. 325 (1986) pp. 387-392). In pH optodes, pH
indicators, which change their absorption or fluorescence
properties as a function of the proton concentration, are
used as indicator phase (Anal. Chem. 52 (1980) pp. 864-
869, DE-A 3 343 636 and 3 343 637, US Pat. Appl.
855 384). If a gas-permeable membrane is used to separate
the indicator from the substance to be measured, only
gases, for example carbon dioxide, can penetrate the
membrane to reach the indicator phase, where they cause
a change in the pH through hydrolysis. Carbon dioxide
optodes of this type operate in similar fashion to
Severinghaus electrodes. The disadvantages of optical pH
and therefore pC02 measurements reside in the very
limited analytical measuring range and the dep~n~ence on
ionic strengths. This, as well as the disadvantages
already mentioned with regard to the Severinghaus elec-
trodes, is a hindrance to wide application of optodes.
DE-A 2435493 discloses a differential-pressure
measuring instrument for the determination of carbonic
acid. However, it is only possible to use this instrument
in flowing media. It is therefore unsuitable, in particu-
lar, for conventional stirred or fixed-bed reactors, as
used, in particular, in the fermentation industry.
DE-A 2926138 discloses a device for continuously
measuring the dissolved carbon dioxide content in
liquids. The measuring principle is based on determining
conductivity difference. The instrument is equipped with
a membrane, one side of which has the liquid contA; n; ~
dissolved carbon dioxide flowing over it, and the other
side of which has a neutral or basic measuring liquid
flowing over it. There is a conductivity meter arranged
in the flow path of the measuring liquid both before and
after the permeable membrane. A disadvantage with the
measurement is that it is unsuitable for liquids whose
chemical and physical properties are not constant.
Furthermore, European Patent Application 0462755
discloses the determination of gases, for example C02, by
measuring infrared absorption. In this case, the infrared
CA 022~927~ 1998-12-21
light beam is passed through the fluid to be measured.
The light beam is split into two or more components.
These split light beams are then measured. A disadvantage
with this measuring arrangement is that it does not allow
partial pressures to be determined and it i8 sensitive to
scattering particles in the sample liquid.
Splitting into two beam paths has already been
disclosed by GB 2194333. In this method, only one of the
light beams is guided through the substance to be
measured. The rest of the radiation is used as reference
light, 80 as likewise to increase the accuracy.
A further publication discloses a so-called
chopped gas analyzer, which likewise operates using
light-emitting diodes (~aser und Optoelektronik 17 (1985)
3, p. 308-310, Wiegleb, G.: Einsatz von LED-Strahlungs-
quellen in Analysengeraten [Use of LED Radiation Sources
in Analyzers]).
These instruments and methods have in common the
fact that they only determine concentrations. The sub-
stance to be measured is placed and measured directly inthe beam path. This is po6sible for gases and liquids
which do not contain scattering particles and have
constant physical composition, in which noise can be
quantified using a blank measurement. However, partial
pressures cannot be deter~;n~d using the described
optical methods. Neither is it possible to use them for
varying physical composition and liquids cont~;n;ng
particles giving rise to turbidity.
The ATR (Attenuated Total Reflectance) analysis
method is known and has already been described in the
prior art.
The measurement makes use of the phenomenon of
the formation of evanescent wave~ or surface waves at the
interface between two media with different optical
densities. In a medium with high refractive index, a
light beam is reflected back into the optically denser
medium, at the interface with an optically th;nne~
medium, if the angle between the incident light beam and
the normal to the interface exceeds the critical angle
CA 022~927~ 1998-12-21
for total reflection. However, a fraction of the light
waves penetrates a few wavelengths into the ad;oining
th;nner medium and is only from there reflected back into
the optically denser medium. If there are substances
which absorb light in the region of this short optical
path, then a lower fraction of the light will be
reflected. This attenuation can be detected and
correlated with the amount of absorbing substance.
A large number of configurations for the use of
thi~ light-absorption phenomenon are now state of the
art. Most ATR devices contain crystals, usually trapezoi-
dally sectioned prisms. DE-A 42 27 813 describes the
simplest geometrical shapes for the ATR element. Simple
commercially available planoconvex microlenses made of
glass and plastic, which have the shape of hemispheres,
are employed.
DE-A 44 18 180 uses a cube corner reflector in
the form of a triple prism. The advantage with this
arrangement is its compact design. The emitted light is
thereby deviated through 180~. This makes it possible to
have an arrangement in a thin rod. The supply of the
incident light and the removal of the residual light is
achieved in the design by the use of optical waveguides.
DE-A 40 38 354 describes an ATR probe completely
avoiding the use of prisms, lenses and similar
components. The light is once again transported via light
guides. In the invention, the supplying and removing
light guides and the actual ATR sensor consist of a
common optical fibre. In the region of the sample to be
investigated, the cl~;ng of the light guide is removed.
The optical waveguide is mechanically supported and
arranged in a measuring space in a probe body, so that
this measuring space is in contact with the medium to be
examined.
It is also known to determine the C02 concentra-
tion in liquids using attenuated total reflection (The
Chemical Engineer 498 (1991) p. 18). In a continuous
measuring cell for fluid substances, for example beer, a
sapphire ATR (Attenuated Total Reflectance) crystal is
., .. ,, ~ .. .. _ .....
CA 022~927~ 1998-12-21
arranged perpendicularly to the flow direction. The
infrared light fed to one side of the crystal passes
through the crystal and undergoes repeated total reflec-
tion. On each reflection, the radiation travels several
~m into the ~ample liquid and is attenuated by the carbon
dioxide which i8 present. The residual light intensity at
the other end of the crystal is measured. A disadvantage
with this method is that it i8 not possible to measure
partial pressures. Furthermore, in the case of fluids
which undergo changes, variations in the reflection
properties can lead to errors in the results.
One point which all the described arrangements
have in common i8 that they are directly in contact with
the medium cont~;ning the substance to be determined.
Because of this arrangement, however, only concentrations
can be determined. It is not possible to determine the
partial pressure of gases dissolved in liquids. Neither
can they be used for media with changing composition, in
particular with changing partial concentrations which
interfere with the measurement.
The object of the application P 44 45 68.9 on
which this application is based is to provide a device
for measuring the partial pressure of gases dissolved in
liquids using optical methods which does not have the
abovementioned disadvantages of the devices known from
the prior art, and which, in particular, allows the
partial pressures of gases to be measured accurately,
with extended long-term stability for the device, and in
media whose physico-chemical composition may change, as
well as in clear or turbid media or media whose turbidity
varies.
This object is achieved in that the device
consists of
a) a measuring chamber which i~ separated, by means of
a gas-permeable membrane which is permeable to the
gas to be determined, from a sample space which
contains the liquid and, dissolved therein, the gas
to be determined,
b) a light-emission source for generating a light beam
CA 022~927~ 1998-12-21
which passes through the measuring chamber and has
a wavelength which is absorbed by the gas to be
deter~;ne~, and
c) a measuring arrangement for determining the light
beam emerging from the measuring chamber.
The measuring chamber, the light-emission source
and the measuring arrangement are here arranged in a rod-
shaped probe. When it is intended to be used in the field
of biotechnology, for example for fermentation, the
production of drinks or waste-water purification, it i8
designed as a sterilizable device. Since, in the field of
fermentation technology, sterilization is predominantly
carried out using steam, the materials of the probe
should be tailored to such conditions. For this reason,
membrane materials which are tried and tested in this
field are also primarily to be used here. In particular,
these include polytetrafluoroethylene (silicone and other
fluoride polymers). Gas-selective membranes which have
proved successful are solubility membranes. When they are
introduced into the sample space, they can establish
equilibrium between the sample liquid and the internal
mixture.
The measuring chamber is preferably filled with
a chemically and biologically inert fluid. This fluid is
selected in such a way that it absorbs the gas to be
determined, which diffuses through the membrane into the
measuring chamber. To this end, suitable liquids or gases
may equally well be used. The nature of the said fluids
is dictated by the gases which are to be measured.
Light-emitting diodes are preferably used as the
light source. Using these devices has the following
advantages:
The emission has a relatively narrow band, which
means that it is not absolutely necessary to use inter-
ference filters in order to determine the correspo~;ng
gas selectively. By virtue of the relatively low power
consumption, it is in principle possible to design the
measuring structure as portable with battery operation.
A decisive advantage over conventional infrared sources
CA 022~927~ 1998-12-21
is that the power iB extremely constant. It may therefore
be possible to make do without comparison paths or to
construct compensation circuits without moving parts. A
system of this type is merh~n;cally robust. At the same
time, the fact that the power is very constant ensures
extended operation without recalibration. The light-
emitting diodes are small enough for the injection of
light into optical waveguides to be straightforward. The
sensitive parts can thus be located externally, and are
not subjected to the thermal and mechanical stresses of
steam sterilization.
In the method according to P 44 45 68.9, it is
also possible to operate with different wavelengths, for
example two different wavelengths, in order to increase
the accuracy. The methods for increasing the accuracy of
the measurement and for compensating for fluctuations in
the electronic components are widely known and published
(Meas. Sci. Technol. 3 (1992) 2 191-195, Sean F.
Johnston: Gas Monitors Employing Infrared LEDs).
Furthermore, the detectors which are compatible
with the light-emitting diodes are used. Suitable
examples are, in particular, photodiodes, photoresistors
and lead selenide photo-detectors (PbSe detectors). The
latter operate pre~ in~ntly in the infrared range and
are suitable, above all, for the determination of carbon
dioxide.
Optical waveguides are used to guide the light
waves from the light-emission source to the measuring
chamber. The same is true for guiding the light from the
measuring chamber to the measuring arrangement for
determ;n;ng the unabsorbed light intensity. The measuring
arrangement is preferably connected to a special circuit
for evaluating, storing and diQplaying the signals.
Because of this, the device is suitable, in particular,
for the automation of systems. When an integrated evalua-
tion unit is used, all the data can be acquired
automatically and a control process can be carried out.
A further advantage is the possibility of the
device having a pressure-proof design. It is merely
. ~ .... . . _
CA 022~927~ 1998-12-21
-- 8
necessary to tailor the design of the housing of the
probe accordingly. In this way, the device according to
the invention can be used at pressures of 200 bar.
Preferably, the probe is used at pressures of up to
20 bar. In the case of use for fermentation processes, it
is merely necessary to ensure that the probe withstands
the elevated pressures which occur under sterilization
conditions.
A further subject of P 44 45 68.9 is a method for
measuring the partial pressure of gases dissolved in
liquids. In this method, the device described is ;~mersed
in the liquid present in the sample space in such a way
that the m~mbrane is fully wetted with sample liquid.
Because of this, the gas which is to be determined can
then diffuse selectively through the membrane into the
measuring chamber. Using the light-emission source, a
light beam is guided through the measuring chamber via
optical waveguides. The gas diffusing into the latter
absorbs some of the radiation. The unabsorbed part of the
light beam is fed to the measuring arrangement, via an
optical waveguide, for determining the partial pressure
of the gas. Using correspo~; ng evaluation, storage and
display devices, the measurement of the unabsorbed light
beam can be used to determine and evaluate the partial
pressure of the gas.
Use is preferably made of electromagnetic radia-
tion generated by light-emitting diodes. The infrared
range is quite particularly preferred.
The device and the method are suitable, in
particular, for use in measuring the partial pressure of
carbon dioxide. Carbon dioxide represents a considerable
production factor in the food industry, in particular in
the drinks industry. In the drinks themselves, carbon
dioxide is responsible for the shelf life and the
refreshing taste. Most determinations are currently
carried out with simultaneous pressure and temperature
monitoring.
For optimum process control of biotechnology
processes, measurement of the partial pressure of carbon
CA 022~927~ l998-l2-2l
dioxide is likewise also necessary. An important fact in
connection with this is that the supply of the micro-
organisms with gases and their inhibitory properties are
a function of the correspon~; ng partial pressures rather
than of the concentrations. In spite of this knowledge,
the partial pressure of carbon dioxide has not to date
been taken into account sufficiently. A satisfactory
601ution to its determination has not yet been found. The
main problems in choosing a suitable determination method
are the lack of available equipment and the high chemical
stability of carbon dioxide. Carbon dioxide represents
the highest oxidation state of carbon and is therefore
very unreactive at room temperature. In contrast to other
heterogeneous gases, it does not form hydrogen bonds in
its di~solved form. With a dissociation constant for
carbonic acid equal to 2 x 10-4 M, only a very small
proportion is present in the form of dissolved ions. A
measuring probe which relies on determination of the
ionic form therefore ha~ an inherent shortcoming. For an
accurate method, it is therefore necessary to determine
the dissolved carbon dioxide directly. For a measurement
at room temperatures, it is possible to measure the
absorption of carbon dioxide. Measuring absorption in the
infrared range is, with existing exhaust-gas analysis
instruments, part of the prior art. However, determina-
tion from the waste air gives concentrations and not
partial pressures. Concentrations can be converted into
partial pressures, and vice versa, using Henry' B law. In
contrast to oxygen, the conversion of concentrations into
partial pressures proves more difficult for carbon
dioxide, since Henry's constant is influenced by the pH
and the constituents of the media. Fluctuations in the pH
lead to variations in the concentration of carbon dioxide
in the outlet air over time. In particular with basic
fermentations, and in large reactors, the accumulation of
carbon dioxide in the media leads to temporal overshoots
of the measured signal when approaching a new equili-
brium. Signals of this type can be misinterpreted as
changes in metabolism.
.. .. .. . .. ... . . _
- CA 022~927~ 1998-12-21
- 10 -
When the device described according to
P 44 45 68.9 is used, the abovementioned problems in
measuring the partial pressure of carbon dioxide are
solved in particular. In this case, the measuring chamber
is filled with a carrier fluid for carbon dioxide. Carbon
dioxide must be soluble in this fluid. A further pre-
requisite is for the fluid to be chemically and biologi-
cally inert. For steam sterilization, it is furthermore
advantageous if the fluid has a higher boiling point than
the substance to be measured, in order substantially to
avoid pressure fluctuations. However, the device is not
restricted to a particular carrier liquid. Instead, the
composition and chemical nature of the latter are dic-
tated by the type of gas to be measured and the working
conditions for the probe.
One disadvantage with a system according to
P 44 45 68.9 is the relatively long response time to
changes of the partial pressure in the sample space,
which because of the required diffusion into the
measuring chamber are of the order of seconds to minutes.
There are also problems with use at very high partial
pressures, since in this case too much light is absorbed
and the measurement signal consequently becomes too weak.
The object of the present invention i~ to provide
a device for measuring the partial pressure of gases
dissolved in liquids, by means of optical methods, which
no longer presents the aforementioned disadvantages of
the devices known from the prior art and which, in
particular, permits measurement of gas partial pressures,
with greater long-term stability of the device, precisely
and in media with changing chemical/physical composition
and in clear, turbid and varyingly turbid media. In
comparison with P 44 45 68.9, the device is intended to
have considerably shorter response times and, in particu-
lar, to be usable even when the partial pressures of thegas are high.
This object is achieved by a device which con-
tains
a) a measuring location which is partially separated by
- CA 022~927~ 1998-12-21
means of a gas-permeable membrane, which is
permeable to the gas to be determined,
b) a light-emission source for generating a light beam
which interacts with the liquid in the measuring
location and has a wavelength which is absorbed by
the gas to be determined, and
c) a measuring arrangement for determining the light
leaving the measuring location,
and which is characterized in that
d) the measuring location is in contact with the
interface of a light-guiding element, and
e) the light is channelled through this element in such
a way that attenuated total reflection is brought
about at the interface.
The element exhibiting attenuated total reflec-
tion will be referred to below as the "ATR elementn.
According to the invention, the ATR element, the
light-emission source and the measuring arrangement are
arranged in a rod-shaped probe. If the latter is to be
employed in the field of biotechnology, it will be
designed as a sterilizable device. Since, in the field of
fermentation technology, sterilization is predominantly
carried out using steam, the materials of the probe
should be tailored to these conditions.
The type of light-emission source, the way in
which it is arranged and the way in which it is used, the
type of detectors, the way in which they are arranged and
the way in which they are used, the type of optical
waveguides and the pressure-proof configuration corre-
spond as described above to P 44 45 68.9.
Any design may be chosen for the ATR element.
This includes the use of prisms, lenses or optical
waveguides. For use under steam-sterilization conditions,
they must be able to withstand high temperatures. For the
W to NIR range, quartz glass is in particular available,
and sapphire, in particular, for longer-wave light. If an
optical waveguide is used, quartz-glass fibres are
suitable for the W to NIR range, and chalcogenide,
fluoride or sil~er halide fibres, in particular, are
.
~ CA 022~927~ 1998-12-21
- 12 -
suitable for the longer-wave range.
In terms of design and principle, the membrane
may be arranged in two different ways with respect to the
ATR element. If the membrane material exhibits no
absorption, or constant absorption, for the wavelength
range the membrane may be applied directly to the ATR
unit. If this is not the case, a gap measuring of the
order of a few wavelengths of the light may be left
between the membrane and the ATR element. This gap is
then, according to the invention, filled with a
chemically and biologically inert fluid. This fluid is
chosen in such a way that it absorbs the gas which is to
be determined and diffuses through the membrane into the
gap. For this purpose, suitable liguids or gases can be
used in the same way. The type of the said fluids depends
on the gases to be measured.
While, in the first case, the measuring location
is thus still inside the membrane (on the same side as
the ATR element), in the second case there is an individ-
ual liquid-filled space/gap between the ATR element and
the membrane. In any case, the gas to be determined can
diffuse out from the sample into the measuring location
in the shortest time because of the very small thickness
of the latter, measuring only a few micrometres (=
penetration depth of the light which undergoes total
reflection) and the immediate proximity with the mem-
brane. Partial pressure changes in the sample are there-
fore registered with an extremely short response time, in
the range of milliseconds to seconds. In contrast,
diffusion for a device according to P 44 45 68.9 requires
a time in the region of minutes.
Furthermore, because of the thin measuring
location, the device according to the invention is
especially suitable for measuring high partial pressures,
at which excessive absorption of the measurement signal
takes place in customary systems. In particular by the
arrangement of a fluid which absorbs the gas between the
ATR element and the membrane, it is however possible to
measure even very low partial pressures, since the gas
CA 022~927~ 1998-12-21
becomes enriched in this fluid.
The membrane consists of steam-sterilizable
materials. M~brane materials tried and tested in this
field will primarily be used. These include, above all,
silicone, polytetrafluoroethylene and other fluoro-
polymers. For the application to fibres as the ATR
element, they must be liquefiable or sprayable, in
particular polytetrafluoroethylene.
The invention furthermore relates to a method for
measuring the partial pressure of gases dissolved in
liquids. In this method, the device according to the
invention is dipped in the liquid present in the ~ample
space in such a way that the m~hrane is fully wetted
with the sample liquid. As a consequence of this, the gas
to be determined can then diffuse into the mDmbrane, in
the case when the membrane is applied directly to the ATR
element, and through the membrane selectively into the
gap, in the case when a fluid-cont~;n;ng gap is arranged
between the ATR element and the membrane. The gas which
diffuses there forms a fraction of the radiation. The
unabsorbed fraction is guided via an optical waveguide to
the measuring arrangement for determining the gas partial
pressure. By means of correspon~; ng evaluation, storage
and display instruments, the gas partial pressure can be
determined and evaluated with the aid of the measurement
of the unabsorbed light beam.
The device according to the invention and the
method according to the invention are suitable, in par-
ticular, for use in the measurement of the partial
pressure of carbon dioxide. In this case, in particular,
the aforementioned specific problems of measuring the
partial pressure of carbon dioxide are solved. Dep~n~;ng
on the measurement range, a gap may be provided which is
filled with a carrier fluid. Particular advantages are,
in this case, the short response time and the suitability
for deter~;n;ng high partial pressures.
The invention will be described in more detail
below with reference to Figures 1 to 4.
Fig. 1 shows the probe overall.
CA 022~927~ 1998-12-21
- 14 -
Fig. 2 shows the probe tip with a gap.
Fig. 3 shows the probe tip with an optical
waveguide as an ATR element without a gap.
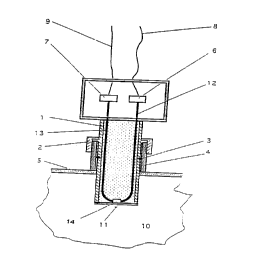
Fig. 1 shows the device according to the inven-
tion in the form of a probe 1. In the example accordingto the invention, the body of the probe is made of
stainless steel. It is, however, possible to make it from
any other desired material, but in general the material
should not corrode.
The probe 1 has a connector 2 which makes it
possible for the probe 1 to be fitted in pressure-proof
fashion into the pipe or the wall 5 of a vessel. The
connector 2 and the 0-ring arrangement 3 allow the probe
1 to be fastened in leaktight fashion in an access tube
4 on the wall 5. The access tube 4 has the corresp~n~ ng
connector to the connector 2.
This structure affords the possibility of sub-
jecting the probe head to steam sterilization and using
it in sterile operation.
A light source 6 and a measuring arrangement 7
are present inside the probe 1. In the example according
to the invention, the light source 6 is a light-emitting
diode and the measuring arrangement 7 is a photodetector.
Both instrument parts are provided with electrical leads
8 and 9. The light-emitting diode 6 is supplied with
electricity via the lead 8. The photodetector 7 transmits
a signal pulse, via the lead 9, to a means for amplifying
and recording the signal.
The light-emitting diode 6 and the photodetector
7 are arranged outside the liquid space 10. They are used
via the extrinsic optical waveguides 12 and 13, which
serve to transmit the light 12 from the light-emitting
diode 6 and the unabsorbed light to the photodetector 7.
The optical waveguides may be made of any materials
suitable for the transmission of light. In the example
according to the invention, operation is carried out in
the infrared range. Light guides made of transparent
material, for example silver halides and chalcogenides,
are therefore preferable. These optical waveguides can
CA 022~927~ 1998-12-21
withstand thermal stresses and are therefore suitable for
use in a steam-sterilizable environment.
The ATR element 14 is located at the tip of the
head of the probe 1. In the example according to the
invention, this element is a sapphire crystal.
The ATR element 14 is separated from the sample
space 10 by the gas-permeable membrane 11. In the example
according to the invention, the membrane 11 is a
thermally stable mP~hrane which is made of steam-
sterilizable material. According to the invention, poly-
tetrafluoroethylene and/or teflon are preferred for this.
The dissolved gas diffuses into the membrane 11
until an equilibrium is established. Since the diffusion
of gases into a membrane is controlled by partial pres-
sure, the probe 1 determines the partial pressure. The
probe therefore measures a biologically meAn;ngful
parameter, since the supply to the microorganisms is,
like all transport processes out of or into cells,
controlled by partial pressure rather than concentration.
The light-emitting diode 6 emits narrow-band
light which is absorbed selectively by the gaR to be
determined. In accordance with the gas to be examined,
the wavelength may be either in the W/VIS or in the
infrared range. For carbon dioxide, this wavelength is
preferably 4.3 ~m. The emitted wavelength range can be
restricted by a heat radiator with interference filter,
or preferably by a narrow-band light-emitting diode. The
particular advantage in using light-emitting diodes is
that the radiation can be modulated, which ~nhAnces the
detection and minimizes effects such as DC drift.
The emitted radiation is fed, via the optical
waveguide 12, to the ATR element 14. The gas which is
present Apecifically attenuates the emitted radiation.
Some of the attenuated light is picked up by the optical
waveguide 13 and fed to the photodetector 7. The latter
mea~ures the attenuated light and produces an electrical
signal proportional to the attenuated light. If modulated
light is used, the electrical signal may likewise be
modulated.
..... . ... ~
CA 022~927~ 1998-12-21
A device according to P 44 45 68.9 could be
obt~;ne~ from the arrangement according to Fig. 1 by
removing the ATR element 14. In this case, a fluid-filled
chamber, through which the measurement light can be
guided, is left behind the membrane 11.
Figure 2 shows the tip of the probe 1 for the case
when the membrane 11 absorbs light at the correspo~i ng
wavelength. The ATR element 14 i8 arranged not flush with
the probe head (as in Figure 1), but somewhat set back,
80 as to create a gap 15. The gas in the sample space 10
then diffuses through the membrane 11 into the gap, until
equilibrium is set up, and it can be determined without
additional absorption by the membrane 11. The same
arrangement is chosen for the case when there are low
partial pressures. For this case, the gap i8 filled with
a carrier fluid which has a high physical absorption
capacity for the gas. This configuration may also be
chosen if the ATR element 14 consists of an unclad fibre
and is operated with a gap.
Figure 3 show~ the configuration of the probe tip
for the case when an optical fibre i8 used as the ATR
element and is operated without a gap. The optical fibres
12 and 13 supplying and removing the light, as well as
the ATR element 14, consist of a fibre. The actual ATR
element 14 is an optical fibre unclad in this portion. A
membrane 11 is applied to this fibre. In order to protect
the exposed fibre against mechanical stresses resulting
from the medium, a cage 16 is fastened on the probe tip.
The advantages achieved with the invention
consist, in particular, in that, primarily in the case of
measuring the partial pressure of carbon dioxide,
separating the measuring space from the sample space
avoids effects due to the presence of particles which
give rise to turbidity and have a concentration which
varies. Furthermore, implementation of the membrane
ensures that the partial pressure is measured. Although
it is in principle possible to use Henry's law to convert
concentration into partial pressures, this requires
simultaneous knowledge of temperature and pressure, as
CA 022~927~ l998-l2-2l
- 17 -
well as the properties of the media. The latter is
difficult, in particular when using fermentation media.
Furthermore, the long-term stability, accuracy and
measuring range are increased in comparison with pH-
sensitive partial pressure probes.
A quite particular advantage with the system
according to the present invention is the extremely short
response time and the suitability for determining high
partial pressures. At the same time, the probe structure
is simplified since it is not necessary to provide
separate light emitters and light detectors, for which
problems may occur with alignment and sterilization.
The probe according to the invention can be used
particularly well both in the drinks industry and in
biotechnology. Probes for measuring rangee of up to
10 bar can be made for use in food technology.
For use in measuring the partial pressure of
carbon dioxide in the field of fermentation technology,
it is advantageous for precalibration to be possible.
This is because, in view of the inhibiting effect of
carbon dioxide on most organisms, calibration can no
longer be carried out subsequently. A further advantage
in this field of application is that, during steriliza-
tion, the probe withstands thermal stresses and is
readily stable at temperatures of 150~C. Lastly, it is
advantageous that, in contrast to the prior methods
involving the measurement of absorption, interference by
materials which likewise absorb in the infrared range is
ruled out.