Note: Descriptions are shown in the official language in which they were submitted.
CA 02260930 1999-01-19
WO 98/04234 PCTtUS97/12208
LOW FOAMING THERAPEUTIC TOOTHPASTES
WITH IMPROVED CLEANING AND ABRASION PERFORMANCE
FIELD OF THE INVENTION
Low foaming thelap~ulic toothpastes cont~ining a therapeutic substance, an
abrasive, a hl-m~ct~nt, a low foam surfactant, and/or a foam control agent having
improved cle~ning and abrasion pclro~ ce wherein: packing of the abrasive into the
ch~nn~lc of a channeled bristle toothbrush, and abrasiveltooth surface contact, are
S ~Ub~ ly free from surfactant bubble il~Lclrelel~ce such that Cleaning Efficiency
Coefficient and Abrasion Efficiency Coefficient values greater than about 1.1 are
achieved along with improvements in therapeutic efficacy.
BACKGROUND OF THE INVENTION
The present invention relates to thel~cuLic toothr~etes having improved
cle~ning and abrasion pelrullllance attributed to a low foam characteristic and the
absence of substantial surfactant bubble hllelr~lence with the abrasive/tooth surface
interface during brushing. As a result of this improved cleaning and abrasion, the
15 therapeutic activity of each of these low foaming toothpastes is generally improved.
In the oral hygiene field today, too~brushing is generally carried out with a
~ toothbrush/toothpaste combination where the abrasive in the toothpaste is brought into
contact with tooth surfaces by the bristles of the toothbrush. The leading commercial
20 toothp~.ctec presently marketed are characterized by a controlled foam profile, resulting
CA 02260930 l999-01-l9
WO 98/04234 PCT/US97/12208
in foam initially filling a good part of the oral cavity, eventually dissipating at the end
of the brushing cycle, such that the residue can be conveniently expectorated.
Examples of therapeutic toothpastes include "fluoride", "anti-tartar", "anti-
S plaque", "baking soda", "anti-gingivitis", and "hypersensitivity treatment~
toothpastes, some of which are described in the following U.S. Patents: 4,254,101;
4,515,772; 4,684,518; 4,806,339; 4,806,340; 4,842,165; 4,885,155; 4,889,712;
4,891,211; 4,999,184; 5,004,597; 5,180,576; 5,374,368; and 5,424,060. These
patents are to be incorporated by reference in the present specification. The
10 toothpastes described in these patents generally use one or more abrasive substances to
abrasively clean, polish and remove stains, plaque and tartar from the surfaces of teeth
in preparation for imparting various therapeutic benefits to the oral cavity.
The current level of gum disease and tooth loss attributed to gum disease and
15 gum retraction in adults, along with high incidence of gingivitis, coronal caries and
hypersensitivity among adults, suggests the referenced toothpastes may not be cleaning
as efficiently as one would hope they would and therefore not imparting the O~)t1l11U11l
therapeutic benefits intended.
During toothbrushing, the primary function of the toothbrush bristles is to rub
abrasive particles contained in the toothpaste across the surfaces of the teeth, thereby
removing by abrasive action tooth deposits such as pellicle, stains, plaque, tartar and
the like while delivering various active ingredients such as fluoride, anti-tartar, anti-
gingivitis ingredients, etc. to the "cleaned" oral cavity.
Studies show that the most aggressive mech~nical cleansing with a
toothpaste/toothbrush combination should be directed toward the tooth surface, with
much less so toward the gingival surface and essentially none toward the base of the
CA 02260930 1999-01-19
Wo 98/04234 PCT/USg7/12208
gingival sulcus. The basis for these observations is as follows:
1. The development of gingival infl~mm~tion and dental caries is most
frequently caused by failure to remove dental plaque from the
subgingival surface of the tooth and to a much lesser extent materia alba
from the gingival surface in the subgingival space. Both dental plaque
and materia alba can form within several hours of brushing and
therefore frequent mPch~nical cleansing is essenti~l. Materia alba which
consists primarily of an acquired bacterial coating and desqu~m~ted
epithelial cells, leukocytes and a mixture of salivary proteins and lipids is
a soft sticky deposit less adherent than dental plaque. It can be flushed
away with a water spray but more completely removed from the gingiva
with a mild m.o- h~nir~l ~le~n~in~.
2. Dental plaque is formed by oral microorg~ni~m~ that synth~si7P harmful
products that are destructive to the tooth and gums when not removed
from the gingival sulcus. The toxins formed by these microorg~ni~m~
cause cellular damage to the gingiva with subsequent infl~mm~tion
(gingivitis) and eventually destruction of the supporting structures
(periodontitis). When gingivitis occurs, vascular ~ tion, capillary
proliferation, engorged vessels and sluggish venous return causes a
stretched and thinned epithPlillm that is sensitive to mPch~ni~l trauma
such as aggressive brushing.
3. Dental plaque with associated gingivitis also causes exposure of the root
surface (recession) with increased occurrence of cavities (dental caries).
Exposure of the root surfaces can also occur due to faulty brushing by
repeated direct trauma to the base of the sulcus (gingival abrasion).
CA 02260930 l999-01-l9
WO 98/04234 PC'r/US97/12208
When a pathologically deepened gingival sulcus (periodontal pocket)
occurs, the pathological condition may become exacerbated because
plaque can more readily occur. ~f dental plaque is not removed, calculus
(tartar) is formed by mineralization of the bacterial plaque. Calculus can
form within several hours of plaque formation. Calculus has a bacterial
plaque coating and exacerbates gingivitis and gingival recession by both
chemical irritation from the formed toxins and destruction from the
mPch~nical irritation of the calculus mass. Subgingival calculus usually
extends near but does not reach the base of periodontal pockets in
chronic periodontal lesions. Calculus holds the plaque against gingiva,
and
4. Since materia alba can be removed by light mPch~nical cleansing and
gingival infl~mm~tion causes thinning of the gingival epithelium the
mPrh~nical cleansing requirement of the gingival surface is much less
than the requirement for removing dental plaque from the surface of the
teeth.
Accordingly, a more efficient cle~n~ing and abrading therapeutic toothpaste that20 fulfills the foregoing requil~ll,enL~, and is more effective therapeutically, is desirable.
In order for the abrasives used in toothp~ctes today to approach O~ llUlll cleaning
abrasion perforrnance, channeled bristle toothbrushes have been developed to entrap
the abrasive and extend abrasive/toothbrush contact beyond tangential contact between
25 bristle tips/abrasive with tooth surfaces. Preferred brushes of this type are described
in U.S. Application Serial No. , Attorney Docket No. 1648/46640,
filed on even date herewith. The contents of said application are hereby incorporated
herein by reference.
CA 02260930 l999-01-l9
WO 98/04234 PCT/US97/12208
In addition to the e,llldplllent of toothpaste abrasive in the channeled bristles,
improved toothpaste cleiqning efficiency and improved toothpaste abrasion efficiency
requires that the abrasive particles entrapped in these bristle channels be brought into
S direct contact without bubble illlelr~lel~ce with those tooth surfaces requiring cleaning,
polishing, stain removal, etc. This contact is most effective when the bubbles
produced by surfactants are minimiql and preferably excluded from the abrasive/tooth
surface interference.
The use of high foaming surfactants in toothractes as taught in the referenced
patents of market leading toothpa~tes such as Crest$, Colgate$, Arm & ~iqmrnPr~....
although creating the Con~ù~ "impression of cleiqning" in fact interferes with
abrasive packing in bristle ch~qnn~lc and with the abrasive/tooth surface contacts
required, for OPL1111U11l cleiqning and abrasion p~lrolll~nce, similar to the way high
15 foarning detelgelll~ interfere with soil removal. Eventually, high foaming detergents
gave way to the more efficient low foarning detergenls and today have been totally
replaced in the laundry market by low foaming (low sudsing) detergents.
The advent of abrasive ellll~ppillg toothbrush bristles calls for the use of low20 foarning surfactants in tool1,pqiles in order to optimize abrasive Up~lfing'' in the
toothbrush bristles and to Ol~Lill~ize abrasive/tooth surface contact during
toothbrushing, thereby o~ ;..g toothpaste cleaning and abrasion efficiencies.
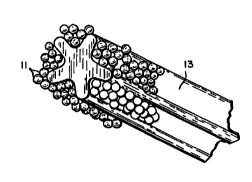
Surfactant ~bubble" ~lelr~l~nce with entrapped abrasive/tooth surface contact is25 illustrated in Figure 1 of the drawings and is contrasted with substantial bubble free
enLrapped abrasive/tooth surface contact as is illustrated in Figure 3. Bubble
intelr~rence with abrasive packing illustrated in Figure 2 is contrasted with Figure 4,
which illustrates bubble free abrasive packing in the bristle rhiqnnpl.
. . .
CA 02260930 l999-01-l9
W O 98/04234 PCT~US97/12208
OBJECTIVES
The present invention has for its prirnary objective the enhancement in therapeutic
toothpastes of tooth cleaning and polishing through improved toothpaste cleaning and
abrasion efficiency wherein irnproved contact between toothpaste abrasives and tooth
surfaces is achieved with a ~ of surfactant bubble interference and with a
corresponding improvement in therapeutic results. This improvement in cleaning
efficiency is measured by a Cleaning Efficiency Coefficient (CEC). The improvement
in abrasion efficiency is measured by an Abrasion Efficiency Coefficient (AEC). Both
of these terms are discussed in detail below as are the improvements in various
therapeutic effects.
A further objective of the present invention is to enhance the cleaning of thosetooth surfaces contiguous to the gingival margin and to the illle~ploximal surfaces
while avoiding d~m~ging the soft tissue by using generally lower RDA abrasives,
which abrasives are presented to tooth surfaces substantially surfactant bubble free
resulting in enh~nred CEC and AEC scores.
A further objective of the present invention is to improve the abrasive/tooth surface
contact of various cornrnercial therapeutic toothpastes by reducing subst~nti~lly the
sudsing and bubble content of thel~l,eulic toothpastes resulting in improved therapeutic
pelrollnance. These improved cornrnercial therapeutic toothpastes include low
foaming versions of the various toothracte~ described in: U.S. 4,254,101; 4,515,772;
4,684,518; 4,806,339; 4,806,340; 4,842,165; 4,885,155; 4,889,712; 4,891,211;
4,999,184; 5,004,597; 5,180,576; 5,374,368; and 5,424,060.
Yet another objective of the invention is to provide an improved method of caring
for teeth and gums using a low foaming thel~peulic toothpaste with improved CEC and
CA 02260930 1999-01-19
WO 98/04234 PCT/US97112208
AEC values.
SUMMARY OF THE INVENTION
The foregoing and other objectives, advantages and features are achieved with the
present invention through the use of low foaming therapeutic toothpastes whereby the
abrasive/tooth surface contact is subst~nti~lly free from toothpaste surfactant bubbles
as shown in Figure 3 and the Cleaning Efficiency Coefficient (CEC) and Abrasion
Efficiency Coefficient (AEC) values of the toothpaste are at least 1.1, with
corresponding improvement in therapeutic efficacy.
Improved p~kin~ of abrasives into the channels of ch~nnPled bristle toothbrushes(which are described in Copending Application Serial No. 08/ , , supra) is
obtained with the low foaming thel~cuLic toothpactçs of the present invention asillustrated in Figure 4, along with improved efficiency of various thclapeulic
~ubsl~lces contained in the low foaming toothp~ct~Ps of the present invention.
Specific embo~limPnt~ of low foaming toothpastes of the present invention will now
be described with Lerclcnce to the accompanying drawings. In the description that
follows, specific low foaming therapeulic toothpaste formulations are described for
purposes of clarity, but these are not intPn-lecl to define or limit the scope of the
invention, which is defirled in the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 illustrates schem~tir~lly the interference of surfactant bubbles with
abrasive/tooth surface contact in a ch~nnPled bristle toothbrush.
.
CA 02260930 l999-01-l9
WO 98/04234 PCT/US97/12208
Figure 2 illustrates schem~tically the interference of surfactant bubbles with
abrasive packing in a channeled bristle toothbrush.
Figure 2(a) is a cross-sectional view of channeled bristle, 13.
Figure 3 illustrates schçm~tic~lly the improved abrasive/tooth surface contact
achieved with low foaming therapeutic toothpastes of the present invention in a
channeled bristle toothbrush.
Figure 4 illustrates schem~tically the irnproved abrasive packing in the bristlechannels achieved with low foaming therapeutic toothpastes of the present invention.
Figure 4(a) is a cross-sectional view of charmeled bristle, 13.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
For the purposes of the present invention, a low foarning toothpaste is defined as a
toothpaste formulation cont~ining an abrasive, a hllm~ct~nt, a surfactant and a foam
controlling agent wherein the abrasive/tooth surface interface and abrasive packing in
20 channeled bristle toothbrushes is minim~l resulting in CEC and AEC values of at least
about 1.1.
For the purposes of the present invention, a therapeutic toothpaste is defined as a
toothpaste formulation cont~ining one or more active ingredients for the tre~tm~nt of
25 oral conditions ranging from chronic plaque and tartar buildup to gingivitis, caries,
hypersensitivity, etc.
Referring to Figures 1 to 4 of the drawings, the il-lelrelellce of surfactant bubbles
CA 02260930 l999-01-l9
WO 98~'0~'~?1 PCT/US97/12208
(10) with abrasive (ll)/tooth surface (12) contact and with abrasive p~c~ing in
- channeled bristle toothbrushes (13) is sc'nem~tic~lly illustrated. Improved
abrasive/tooth surface contact and abrasive packing in the absence of surfactantbubbles is also sçh~m~ti~lly illustrated in Figures 3 and 4.
For the purposes of the present invention, the Cleaning Efficiency Coefficient
(CEC) is the cleaning improvement obtained with the low foaming toothr~ctçc of the
present invention as measured against a standard foaming toothpaste, with both
toothr~ctes using the same channeled bristle toothbrush.
Specifically, the CEC, is a number that relates the cleaning efficiency of the novel
low foaming therapculic toothractçs of the present invention to a current standard
collll.lclcial foaming toothpaste, where both toothpactçs are tested using idçnti~l
ch~.nn~led toothbrushes.
The CEC is a ratio of the efficiency of the low foaming toothpaste to the efficiency
of a standard foaming toothpaste under standardized brushing conditions. The ratio is
expressed as the reduction in the parameter measured, plaque for example, by the low
foaming toothpaste, divided by the reduction in plaque produced by the standard
20 toothpaste under i~entir~l toot_brushing test conditions.
This relationship is expressed as:
CEC = Baseline Test - Final Test
Baseline Standard - Final Standard
Low foa~ g thelapculic toothpactes with CEC values above about 1.1,
.. . ..
CA 02260930 l999-01-l9
W O 98/04234 PCT~US97/12208
- 10-
particularly above 1.5, are l,refelled.
For the purposes of the present invention, Abrasion Efficiency Coefficient (AEC)is defined as the ratio of the results of a standard RDA, Stain Index or Polishing Index
5 procedure of low surfactant toothpaste to the results of an identical procedure using a
standard foaming toothpaste with the same bristled toothbrush used in both instances.
This relationship is expresses as
AECRDA Baseline RDATEST - Final RDATEST
Baseline RDAs,al,dard - Final RDAs~rd
or
Baseline Stn IndTEsT - Final Stn IndTEsT
AECs~ nd =
Baseline Stn ~n-l~. - Final Stn Inds~ dard
Ra~Pline Polish IndTEsT - Final Polish IndTEsT
AECpolish Ind
Baseline Polish Inds~ dard - Final Polish Inds,, ~,dard
For the purposes of the present invention, AEC values for RDA, Stain Index and
Polish Index above about 1.1 are pLe~ d with values about 1.5 particularly
35 preferred.
Relative Dental Abrasion (RDA) has long been the standard measurement for
predicting the pelrolmal~ce of a given toothpaste formulation, and/or the functionality
CA 02260930 l999-01-l9
WO 98/04234 PCT/US97/12208
of a series of abrasives having varying particle sizes, compositions of rnatter, crystal
structures, fracture edges, etc. Typically a measured number of strokes with a
standard toothbrush with a fixed applied p~ssu-e against a piece of dental enamel
fixed in a holding plate is the basis of the test. So~ s a plate of soft metal, such
5 as copper, is substituted for the dental enamel as an inexpensive approximation
method. The dental enamel is measured for loss of surface enamel (or metal) by avariety of methods, including weight loss, optical comparison and radioactive
techniques.
lO A similar measurement using artificially stained enamel measures the abrasiveremoval of stain. In a similar fashion, one can evaluate the polishing of tooth
surfaces, a process that increases the reflectance propelLies of the enamel without a
high level of enamel removal or "sc,atching".
In the present invention, the rh~ngjng of the toothpaste to a low foaming toothpaste
impacts abrasivity whether using RDA, Stain or Polishing measurements. It is
suggested that because the delivery of the abrasive to the tooth surface is substantially
bubble free and therefore more efficient, certain abrasives will have a higher RDA
when used in the low foaming toothpastes of the present invention.
Conversely, if non-scratching abrasives are more efficiently delivered to the tooth
surface by the low foaming toothp~te~ of the present invention, improved cleaning
and abrasion results can be produced without resorting to high RDA abrasives and the
inherent potential damage they could cause to tooth surfaces, dentin and soft tissue.
The advantage of these low foaming de,.liflices is that the teeth are more efficiently
cleaned without risking enamel or soft tissue damage that may occur with higher RDA
abrasives.
. . . . . . . .
CA 02260930 l999-01-l9
WO 98/04234 PCT/US97/12208
The improved CEC and AEC values obtained with the low foaming therapeutic
toothpastes of the present invention result in an improvement in various therapeutic
effects ranging from plaque and tartar control to anti-gingivitis and anti-caries effects
5 as well as improved hypersensitivity tre~tmPnt Surprisingly, the rate of "tubule"
closure is improved with low foaming hypersensitivity treating toothpastes of the
present invention.
The low foarning therapeutic toothpaste compositions of the present invention
10 comprise an abrasive, a hllmPct~nt, a surfactant, a foam control substance, water and
an active therapeutic ingredient. Each of these components as well as optional
ingredients such as binding agents, flavoring and sweet~ning substances are described
in detail as follows:
1 5 ABRASIVE
The therapeutic toothpaste compositions of the present invention contain from
between about 1% and about 90%, preferably from between about 105'c and 50% by
weight, of an abrasive material described in detail below. These abrasives in the low
20 foarning dentifrices of the present invention provide the unique abrasion benefits of
exceptionally efficient cleaning, i.e. CEC values above about 1.1 along with
exceptional polishing, stain removal and abrasion as indicated by AEC values of at
least about 1.1. The exceptional AEC values are obtained without unduly abradingtooth enamel or dentin.
Suitable abrasive materials for the low foaming therapeutic toothpastes of the
present invention include: talc, calcium pyrophosphate, calcium hydrogen phosphate
dihydrates, anhydrous dicalcium phosphate, calcium carbonate, ~ min~, tin dioxide,
CA 02260930 1999-01-19
WO 98/04234 PCT/US97/12208
silica, zirconiurn silicate, sodium bicarbonate, sodium percarbonate, etc., and mixtures
thereof. Particularly prerelled are abrasive mixtures where the secondary abrasive is
the type used in translucent dentifrice gels at levels up to about 20 % . Some of these
are described in U.S. 3,927,200; 3,906,090, 3,937,321; 3,911,102; 4,036,949;
4,891,211; 4,547,362; 5,374,368; 5,424,060; 5,180,576; 4,943,429; 4,160022;
4,623,536; 4,663,153; and 4,721,614.
Other useful abrasives include: sodium metaphosphate, potassium metaphosphate,
m~gnPsil-m orthophosphate, trim~gn~sium phosphate, ~lnmin~ silicate and hetonite as
described in U.S. 4,806,340 incorporated herein by refe~ence. See also Thorpe's
DictionarY of Applied Chemistry, Volurne 9, 4th Edition, pp . 510-511.
Particularly ~ Ç~lled abrasives that are comr~tihle with sources of soluble fluoride
include those precipitated silica or silica gels such as the silica xerogels described in
U.S. 3,538,230 incorporated herein by reference. Preferred are the silica/xerogels
m~rk~ted under the tr~-lçn~m~ Syloid by W.R. Erecex Co., Davison Chemical
Division. Especially pLerelled are the precipitated silica materials such as those
marketed by the J.M. Huber Corporation under the tradçn~m~ Zeodent, particularlythe silica carrying the design~tion Zeodent 119. Other silica dental abrasives useful in
the toothp~tçs of the present invention are disclosed in U.S. 3,862,307 and 4,340,583
incorporated herein by refelellce.
Other abrasives useful in the low roalll,llg therapeutic clçntifri~e compositions of
the present invention include calcium pyrophosphate including the B-phase calcium
pyrophosphate prepared in accordance with the te~hing of U.S. 3,112,247
incorporated herein by lefe~ellce. Another class of abrasives suitable for use with the
low foarning toothpastes of the present invention include particulate therrnosetting
polym~ri7.ed resins as described in U.S. 3,0750,510 including m~l~minPs, phenolics,
.. . .. .... . .. .... .
CA 02260930 l999-Ol-l9
WO 98/04234 PCT/US97/12208
- 14-
ureas, mel~mine-ureas, melamineformaldehydes, urea-formaldehydes, melamine-urea-formaldehydes, cross-linked epoxides and cross linked polyesters. See also U.S.
4,070,510 incorporated herein by reference.
The size of the abrasive particles are most cornmonly expressed in "mean
diameter", i.e. the arithm~tic~l average of the diameters of particles in a representative
sample. The mean di~m~ter value of abrasive particles is usually described in
rnicrons. Abrasives having particle sizes between about 3 and 25 microns and
preferably between about 6 and about 20 microns are particularly preferred for the
channel designs of the toothbrush bristles of the present invention.
The preparation of suitable particle size abrasives can be accomplished by
conventional techniques well known to the art. Basically, these techniques involve
milling various abrasive materials, followed by standard screen sieving (or air
separation) to segregate the desired particle si_e range. Other techniques employ
cryst~lli7~tion or related techniques to control si_e and crystal variants.
SURFACTANT
Organic surface active substances are used in the low foaming therapeutic
toothpastes of the present invention to achieve increased cleaning action, assist in
complete dispersion of various active ingredients throughout the oral cavity, optimize
therapeutic activity, etc. Organic synthetic surfactants which may be so utilized can be
non-soap, anionic, nonionic, cationic, zwitteronic or amphoteric in nature.
Low foarning nonionic surfactants are preferred for the low foaming therapeutic
toothpastes of the present invention. Where high foaming surfactants are used, an
~piol)iiate level of a foam control substance along with a nonionic surfactant is added
CA 02260930 1999-01-19
WO 98t04234 PCT/US97/12208
to the formulation to achieve the low foam i~lle.rereilce abrasive "packing" andsubstantially bubble-free abrasive/tooth surface contact illustrated in Figures 3 and 4 of
the drawings. These foam control substances are described in detail below.
Suitable surfactants are described in U.S. 3,959,458; 3,937,807; and 4,051,234.
Anionic surfactants useful herein include the water soluble salts of alkyl sulfates
having from 10 to 18 carbon atorns in the alkyl radical and the water-soluble salts of
sulfonated monoglycerides of fatty acids having from 10 to 18 carbon atoms. Sodium
lauryl sulfate and sodium coconut monoglyceride sulfonates are examples of anionic
surfactants of this type. Mixture of anionic surfactants can also be employed.
The nonionic surfactants which can be used in the compositions of the present
invention can be broadly defined as compounds produced by the con-i~nc~tion of
alkylene oxide groups (hydrophilic in nature) with an organic hydrophobic compound
which rnay be aliph~tic or alkylaromatic in nature. Examples of suitable nonionic
sudsing agents include the Pluronics, polyethylene oxide con-lçnc~t~s of alkyl phenols,
products derived from the con~nC~tion of ethylene oxide con~lçnc~t~c of aliphatic
alcohols, long chain tertiary amine oxides, long chain tertiary phophine oxides, long
chain dialkyl sulfoxides and mixtures of such materials.
The ;~wilLelollic synthetic surfactants useful in the composition of the presentinvention can be broadly described as derivatives of aliph~tic q ~~tern~ry ammonium,
phosphonium, and sulfonium compounds, in which the aliph~tic radicals can be
straight chain or branched, and wherein one of the aliphatic substituents contains from
about 8 to 18 carbon atoms and one contains an anionic water-solubilizing group, e.g.,
carboxy, sulfonate, sulfate, phosphate, or phosphonate.
The cationic surfactants useful in the compositions of the present invention can be
CA 02260930 l999-Ol-l9
W O 98/04234 PCT~US97/12208
- 16 -
broadly defined as quatemary ammonium compounds having one long alkyl chain
cont~ining from about 8 to 18 carbon atoms such as lauryl trimethylammonium
chloride, cetyl pyridinium chloride, cetyl trimethylammonium bromide; di-
isobutylphenoxyethoxyethyl-dimethylbenzylammonium chloride; coconut-
5 alkyltrimethylammonium nitrite; cetyl pyridinium fluoride; etc.
The amphoteric surfactants useful in the present invention can be broadly described
as derivatives of aliphatic secondary and tertiary amines in which the aliphatic radical
can be straight chain or branched and wherein one of the aliphatic substituents contains
10 from about 8 to 18 carbon atoms and one contains an anionic water-solubilizing group,
e.g., carboxylate, sulfonate, sulfate, phosphate, or phosphonate.
HUMECTANT
Another essenti~l component of the low foaming toothpaste composition of the
present invention is a hum~ct~nt The humectant serves to keep the toothpaste
compositions from hardening upon exposure to air. Certain humectants can also
impact desirable sweetness or flavor to the toothpaste. The hum~ct~nt, on a purehumectant basis, generally comprises from between 30% and 70%, preferably from
20 between about 45 % and 65 %, by weight of the toothpaste compositions herein. (See
Examples XI, XII and XIII.)
Suitable hnm~ct~nt~ for use in this invention include edible polyhydric polyols such
as glycerin, sorbitol, xylitol, polyethylene glycol, polypropylene glycol, m~nnitol,
25 rnaltitol, etc. Sorbitol is frequently employed as a 70% aqueous solution obtained
from SPI Polyols, Inc., New Castle, Delaware. Mixtures of glycerin and sorbitol are
particularly useful in the low foaming toothpastes of the present invention.
CA 02260930 1999-01-19
Wo 98~ 31 PCT/US97/12208
In a p1ere~ied embodiment of the invention, the hllmPct~n~ is selected from liquid
oxyalkylated diols that have a molecular weight in the range between about 200 and
8000. Polyethylene glycols are commercially available under tradenames such as
Carbowax 200, 300, 400, 600, 900, 1000, 2000, 4000, 6000, and 8000 from Union
5 Carbide where the number values are approximations at average molecular weight.
Polyethylene-propylene glycols are co~ e~cially available under tr~(lçn~mPs such as
Pluracare/Pluronic L-31 and L-35 from BASF.
In the low foarning therapeutic toothpastes of the present invention, the liquid10 vehicle may comprise water and hllmPct~nt7 typically in an amount from between
about 10% and 90%, by weight of the toothpaste. In translucent low foaming gel
toothr~tes, where the retractive index is an important consideration, it is preferred to
use higher ratios of hl-mPct~nt to water than those used in opaque toothr~ctçs.
FOAM CONTROL AGENT
For these low foaming toothpaste compositions of the invention that contains foam
generating surfactants such as anionic and cationic surfactants, it may be nPces~ry to
substitute nonionic low foaming surfactants in part or in total and/or add a foam
20 control agent in order to avoid surfactant bubble ~ re.ellce with the abrasive/tooth
surface contact and in order to assure abrasive packing of the channeled bristletoothbrushes, both of which l~alules are nPcec~ry to achieve CEC and AEC values
above about 1.1.
Most toothpaste surfactants genelat~ a controlled level of foam (suds) that remains
reasonably stable, eventually breaking down near the end of the toothbrushing cycle.
It is this initial bubble formation, at the outset of toothbrushing, that poses the most
~ignifit~nt interference threat to abrasive packing in the rh~nnPled bristle toothbrushes
CA 02260930 1999-01-19
WO 98/04234 PCTNS97/12208
- 18-
and to the abrasive/tooth surface interface. In most instances, the toothbrush is not
reloaded with toothpaste once brushing starts and it is the distribution of the toothpaste
abrasive on the initial "pass" of the bristles over the tooth surfaces that determines the
CEC and AEC values of most toothpastes.
s
Examples of suitable foam control agents for the low foaming toothpastes of the
present invention include alcohols such as ethanol, low molecular weight
polydimethylsiloxanes such as Silicone, 350, and 1500 from Dow Corning
Corporation, l~ nfl7 Texas.
Low HLB (Hydrophile-Lipophile-Balance) surfactants such as ARLACEL 186
surfactants, m~mlf~c.tured by ICI Specialty Chemicals, Wilmington, Delaware, when
used at approximately 0.1%, will effectively control most foaming in commercial
toothpastes and produce abrasive packing and abrasive/tooth surface contact
15 substantially free from bubble interference. Preferred foam control agents for
commercial toothpastes include lipophilic oleates and/or laureates with an HLB range
from between about 1 and 8.
Although foam control agents can be used effectively to control the foam of
20 various commercial toothpastes, it is preferred to substitute nonionic low foarning
surfactants for the sodium lauryl sulfate-type surfactants generally used in most
commercial toothr~ctec. Such substitution is illustrated in Examples I through XIII
below.
WATER
Water is another essential element of the toothractec of this invention. Water
employed in the preparation of commercially suitable low foaming, therapeutic
CA 02260930 1999-01-19
WO 98/04234 PCT/US97/12208
- 19-
toothr~te~ should preferably be deionized and free of organic hllpulilies. Watercomprises from about 10% to 45%, preferably from about 20% to 35%, by weight of
the toothpaste compositions herein. These amounts of water include the free water
that is added plus that which is introduced with other materials.
ACTIVE THERAPEUTIC INGREDIENTS
Therapeutic ingredients for the tre~tmPnt of hypersensitivity that can be included in
the low foaming toothr~ctes of the present invention include potassium nitrate,
10 stannous fluoride, zinc chloride and various abrasives that demol~llate a propensity
for "tubule" closure during brushing with the low foaming toothpastes of the
invention.
The ll~ of caries requires a thel~eulic ~ub~ ce that functions as a source
15 of fluoride ion. The number of such sources is great and includes those disclosed in
U.S. 3,535,421, incorporated herein by reference. Typical materials include:
stannous fluoride, potassium fluoride, lithium fluoride, cesium fluoride, ammonium
fluoride, ~hl",i..l...~ fluoride, capric fluoride, indium fluoride, stannous
fluorozirconate, lead fluoride, ferric fluoride, nickel fluoride, p~ m fluoride, silver
20 fluoride, zinc fluoride, zirconium fluoride, hexylamine hydrofluoride, laurylamine
hydrofluoride, lllyli~Lylarnine hydroflnori-le, decanolamine hydrofluoride,
oct~-lec~nylamine hydrofluoride, myristoxyamine hydrofluoride, diethylamino-
ethyloleylamide hydrofluoride, ~ieth~nolamino-ethyloleylamide hydrofluoride,
~ieth~nf~laminopropyl-N'-oct~ec~nylamine dihydrofluoride, l-ethanol-2-
25 h~r~lPcylimitl~7.oline dihydrofluoride, octoylethanolamine hydrofluoride,o~;Lyllli,lleLllylammonium fluoride, dodecyliethyldimethylammonium fluoride,
tetraethylammonium fluoride, diaryidmethylamonium fluoride diazoryl-
dimethylammoniurn fluoride, ~8 9-oct~(lec~nylbenzyldimethylammonium fluoride,
, ~
CA 02260930 l999-Ol-l9
WO 98/04234 PCT/US97/12208
- 20 -
dioctyldiethyla~mmonium fluoride, cyclohexylcetyldimethylammonium fluoride,
furfuryllauryldmethylammonium fluoride, phenoxyethylcetyldimethylammonium
fluoride, N,N'-tetramethyl-N,N'-dilaurylethylen~ mmonium difluoride, N-
cetylpyriclini-lm fluoride, N,N-dilaurylmorpholinium fluoride, N-myristyl-N~
5 ethylmorpholinium fluoride, N-(octylaminocarbonylethyl)-N-benzyl-
dimethylammonium fluoride, N-(,B-hydroxydodecyl)trimethylarnmonium fluoride, N-
phenyl-N-hexadecyldiethylammonium fluoride, N-cyclohexyl-N-octadecyl-
dimethylammonium fluoride, N-(2-carbomethoxyethyl)-N-benzyldimethylammonium
fluoride, N-(2-carbocyclohexoxyethyl)-N-myristyldimethylammonium fluoride, N-~2-
10 carbobenzyloxyethyl)-N-dodecyldimethyammonium fluoride, N-~2-(N,N' -dimethyl-aminocarbonyl)-ethyl)-N-dodecyldiethylammonium fluoride, N-carboxymethyl-N-
cicoxyldimethylarnmonium fluoride, betaine hydrofluoride, sarcosine stannous
fluoride, alanine stannous fluoride, glycine potassium fluoride, sarcosine potassium
fluoride, glycine hydrofluoride, lysine hydrofluoride, alanine hydrofluoride, betaine
15 zircor~ium fluoride, sodium monofluorophosphete and mixtures thereof. Sodium
fluoride is the preferred fluoride source. The amount of the fluoride ion source should
be sufficient to provide from about 50 ppm to 3500 ppm, preferably from about 500
ppm to 3000 ppm of fluoride ions.
Anticalculus active mgredients include various pyrophosphate substances. The
pyrophosphate salts useful in the present composition include dialkali metal
pyrophosphates and mixtures of the dialkali metal and tetraalkali metal pyrophosphate
salts. Na2H2P2O" Na2P2O7 and K~P2O, in their unhydrated as well as hydrated forms
are tne preferred species. The levels of each of these species that preferably are used
in the compositions are as follows (all are in the unhydrated forrn):
CA 02260930 l999-Ol-l9
W098/04234 PCTAUS97/12208
Na2H2P2O,. 0-5% 13.8%
Na2P2O, 0 6.0%
K,P2O, ~ 4.0 %
Preferred P2O3~ in the present compositions is 1.5 % which can be provided solely
S by Na2H2P2O, or mixtures of Na2H2P2O7 with either or both of the tetra alkali metal
salts. Preferred are binary rnixtures of the sodium salts and ternary ~ Lures of those
with the tetra potassium salt. The upper limits on the sodium species are determined
by solubility considerations while the tetra potassium level is established for taste
reasons.
The pyrophosphate salts are described in more detail in Kirk & Othmer,
Encyclopedia of Chernical Technolo~y, Second Edition, Volume 15, Interscience
Publishers (1968) incorporated herein by re~rence.
This reference discloses sodium salts including tetrasodium pyrophosphate,
disodium dihydrogen pyrophosph~te, tri~o~ lm hydrogen phosphate and sodium
trihydrogen pyrophosphate on the bottom of page 243 and the top of page 244;
potassium pyrophosphate on page 249; and ~i~mn~onium dihydrogen pyrophosphate,
triammonium hydrogen phosphate and tetraammonium pyrophosphate on page 249.
The leference further discloses condensed phosphoric acids exemplified by
pyrophosphoric acid on page 214. The solubilities of sodium pyrophosph~t~s are
presented in a diagram at the top of page 243. The reference in total not only
discloses a wide range of soluble pyrophosphate sources but also their properties.
.. ....
CA 02260930 l999-Ol-l9
W O 98/04234 PCTrUS97/12208
- 22 -
Bis-biguanide antiplaque agents can also be added to the composition of this
invention. Such agents include chlorhexidine (1,6bis [N5-p-chlorophenyl-N'-
biguanido]hexane), the soluble and insoluble salts thereof and related materials such as
1,2-bis(n5-p-tri-fluoromethylphenyl-NI-biguanido)ethane are described more fully in
Haefels, U.S. Pal. No. 3,923,002, U.S. Pat. No. 3,937,807, Belgian Pat. No.
843,244, and Belgian Pat. No. 844,764. These patents are incorporated herein by
reference.
If present, these antiplaque agents generally comprise from about 0% to about 5%10 by weight of the compositions herein.
Poloxamer polydimethylsiloxane emulsions which function as antiplaque active
ingredients available under ~e trademarks MICRODENT~ and ULTRAMULSION~
from WhiteHill Manufacturing, Stafford, Texas, are also suitable therapeutic
15 ingredients for the low foarning toothpastes or tne present invention.
It is well accepted that hydrogen peroxide and other peroxygen-cont~ining agentsare effective in curative and prophylactic tre~tm~nts with respect to dental plaque,
calculus, gingivitis, mouth odor, tooth stains, mucosal infections, and the like.
Many oral care products have been form-l~t~d which include a peroxy compound,
and more recently oral care products have been developed which include a peroxy
compound having improved stability. References that describe peroxy-cont~ining
toothpastes include: U.S. Pat. Nos. 2,275,979; 3,577,521; 3,657,413; 3,885,028;
25 3,886,265; 4,226,851; 4,302,441; 4,405,599; 4,426,108; 4,431,631; 4,521,403;
4,522,805; 4,528,180; 4,567,036; 4,592,487; 4,592,488; 4,592,489; 4,687,663;
4,812,308; 4,837,008; 4,839,152; 4,849,213; 4,867,988; 4,891,211; 4,897,258;
4,925,655; 4,971,782; 4,980,152; 4,988,450; 5,000,941; 5,041,280; 5,085,853;
CA 02260930 1999-01-19
PCT/US97/12208
WO 98/04234
5,256,402; and the like, incorporated herein by reference.
OPTIONAL INGREDIENTS
S Low foaming therapeutic toothpastes, creams, gels and powders of the invention
typically also contain a natural or synthetic thickener or gelling agent in proportions of
about 0.1 % to about 10%, preferably about 0.5 % to about 5 %, by weight. Suitable
organic thickeners include sodium carboxymethyl cellulose, gum traga~anth, starch,
carrageenan, polyvinylpyrrolidone, hydroxy~lhylpn~pyl cellulose, hydroxybutylmethyl
cellulose, hydro~ypru~ylmethyl cellulose or hydroxyethyl cellulose, and are usually
used in collce~llrations of 0.1% to 2.0%. Inorganic thirk~on~rs such as hydrated silicas
rnay also be used at levels of about 0.5~o to 10%.
Suitable flavoring and sweetening agents may also be employed in the dentifricesof the invention. Examples of suitable flavorants include the flavoring oils, for
example, oils of spearn~int, peppermint, wintergreen, sassafras, clove, sage,
eucalyptus, marjoram, cinnamon, lemon and orange, as well as methylsalicylate.
Suitable sweeteners include sodium cyclamate, perillartine, saccharin, sodium
saccharin and arnmoniated glycyrrhizin (e.g. its mono~mm-)nium salt), and the like.
Suitably, the flavoring and sweetenin~ agent together comprise from about 0.01% to
5 % or more by weight of the dentifrice. Preferably, the amount of flavoring oil is
above 0.3%, e.g. 0% to 1.2%.
The pH of ~e compositions herein is in the range of 6.0 to 10.0, preferably from7.3 to 9Ø The pH is preferably achieved through a proper balancing of the
pyrophosphate salts or by the addition of an ~lk~lin~ or acidic agent.
, .. ,~.. ,_ ....
CA 02260930 1999-01-19
WO 98/04234 PCT/US97112208
- 24 -
EXAMPLES
The present invention will be further illustrated with reference to the following
examples which aid in the understanding of the present invention, but which are not to
5 be construed as limitations thereof. All percentages reported herein, unless otherwise
specified, are percent by weight. All temperatures are expressed in degrees Celsius.
EXAMPLE 1
The following is a low foarning therapeutic toothpaste representative of the present
invention.
C(lmprmPnt %
Distilled Water 16.484
Sorbitol ('70% Aqueous Solution) 49.565
Sodium Saccharin 0.300
Dye SolutioD 0.350
Plc~i~Jildt~d Silica 20.000
Sodi~ Fluoride 0.243
Flavor 1.330
Low foaming nonionic polo~mP~ Sulr~ 2.000
foarn control Arlacel 186 (at 0.1 %) rnixture
Carbopol 940* 0.180
Xanthan Gum 0.600
Na4P,O, 2.400
Na2H2P2O7 1.190
K4P2O7 (6L5 Aqueous Solution) 3.360
100.000
*Carboxy vinyl polymer offered by H. F. Goodrich Company.
CA 02260930 1999-01-19
W O 98/04234 PCTAUS97tl2208
The above composition is made by combining the water and part of the sorbitol inan ~git~ted mixture and heating this mixture to 140~F. The Na2H2P2O7, Na4P,O"
saccharin, sodium fluoride and preci~ ted silica are then added in order and the total
mixture was mixed for from S to 10 miml~ec The flavor, dye and the poloxamer
S surfactant are then added. In a separate vessel the rem~in-ler of the sorbitol, the
Carbopol and the Xanthan gum are slurried together and then added to the main mix
tank. The complete batch is mixed for about one-half hour and subsequently milled
and deaerated.
EXAMPLE 2
The following is another representative toothpaste of the present invention.
Cnmr n.~n~ %
Sorbitol (70% Aqueous Solution)50.723
Distilled Water 16.484
Sodiurn Saccharin 0.300
Dye Solution 0,350
P~ Silica 20.000
Sodium Fluoride 0.243
Flavor 1.330
Low foarning ~nionic Pluronic surfactant/ 5.000
Arlacel 186 (at 0.1 %) rnixture
Carbopol 940S 0.180
Xanthan Gum 0.600
Na4P,O, 3.400
Na2HzP2o7 100.000
Both the . , - of Example I and that of Example II are
effective in reducing calculus and possess --~rt~ cosmetic
properties.
.. ..... .. . ..
CA 02260930 1999-01-19
WO 98/04234 PCT/US97/12208
- 26 -
EXAMPLES 3-6
The following dentifrice compositions are representative of the present invention.
Weigh~ %
C~lrnr-)nPnt Ex. 3 Ex. 4 Ex. S Ex. 6
Water 12.500 12.500 12.500 12.500
Sorbitol (70% Solution) 47.891 45.727 43.437 41.328
Glycerin 10.198 10.198 10.198 10.000
PEG-12 ~~ ~~
Titaniurn Dioxide 2.525 0.525 0.525 0.525
Silica 20.000 20.000 20.000 20.000
Na Carboxymethyl Cellulose 1.010 1.050 1.050 1.000
Na ~'-- . A~
~ '~gm~ci~lm Alurnina Silicate0.408 0.408 0.408 --
Hyd~ yl Cellulose -- -- -- --
Nonionic low foaming p~nS~ n~r with 0.1 % 4.000 4.000 4.000 4.000
Arlacel 186
Na Gluconate 0.632 2.395 4.750 3.314
Stannous Eluoride 0.454 0.454 0.454 0.454
Star~ous Chloride Dihydrate -- 1.141 1.141 2.198
Stannous P)~lu~h~, '- I .040 -- --
Na .S~ hArin O 700 0.200 0.200 0.230
Flavor 0.831 0.851 0.851 1.000
FD&C Blue #1 (1% Solution) 0.051 0.051 0.051 0.051
Na Hydroxide (50% Solution) 0.200 0.300 0.385 0.850
pH 4.5 4.5 4.5 4.5
CA 02260930 l999-01-l9
W O 98/04234 PCTAUS97/12208
EXAMPLES 7-10
The following dentifrice compositions are representative of the present invention.
Weight %
~r ~I Ex. 7 Ex. 8 Ex. 9 Ex. 10
Water 12.500 16.500 12.500 12.500
Sorbitol (70% Solution) 45.712 42.135 43.730 48.708
Glycerin 10.000 10.000 10.000 10.000
PEG- 12 -- 3 ooo
Titanium Dio~ide 0.525 0.525 0.515 0.325
Silica 20.000 20.000 20.000 20.000
Na C~l"~.... ~l Cellulose 1.000 -- 1.000 0.900
Na r---,,~ 0.330 0.450 0.310 0.350
~' . Alumina Silicate - -- -
II~u,.~ Cellulose - 0.400 --
Low Foarning Pluronic F127/ 4.000 4.000 4.000 4.000
Arlacel 186 mixture
Na Gluconate 2.082 2.395 2.395 2.082
Stannous Fluoride 0.454 0.454 0.454 0.908
Stannous Chloride Dihydrate 1.500 1.141 1.141 0.346
Stannous I~.. ,~ .' -- --
Na Saccharin 0.230 0.230 0.230 0.230
Flavor 1.000 1.000 1.000 1.000
Fl)&C Blue #1 (1% Solution) 0.051 0.050 0.051 0.051
NaHydroxide (50% Solutiûn)0.6000.600 0.600 0.700
pH 4.5 4.5 4.5 4.5
CA 02260930 l999-01-l9
WO 98/04234 PCTIUS97/12208
- 28 -
PROCEDURE FOR MAKING LOW FOAMING THERAPEUTIC DENTIFRICE
In preparing the dentifrice formulations for Examples 3 to 10, sorbitol and one half
of the water are added to the mix tank and heating to 77~C initi~ted. Saccharin,titanium dioxide, and silica may be added to the mixture during this heating period.
Sufficient agitation is m~int~inPd to prevent the settling of the insoluble components.
The glycerin is added to a separate vessel and is also heated to 77~C. When both the
solutions have ~ in~d the required temperature, the carboxymethyl cellulose (CMC)
10 and carrageenan are blended together and slowly added to the glycerin under vigorous
agitation. When the CMC and carrageenan are sufficiently dispersed in the glycerin,
this mixture is added to the sorbitol/water mixture. The resulting rnixture is then
blended for a sufficient period of time to allow complete hydration of the binders
(about 15 minllres). When the paste is of acceptable texture, the flavor, Pluronic
15 F127/Arlacel 186 mixture and color are added. One half of the rem~ining water is
then added to a separate mix tank and allowed to heat to 77~C. After the water attains
the necessary temperature, the sodium gluconate is added under medium agitation and
allowed to dissolve completely. The stannous chloride dihydrate is then added to the
gluconate solution and also allowed to dissolve. This mixture is added to the main
20 rnix. The stannous fluoride is added to the rem~ining water (also at 77~C) and the
resulting solution is added to the main mix and allowed to blend thoroughly before
final pH adjustment with sodium hydroxide. The completed paste is ~git~ted for
approximately 20 minutes before being milled and deaerated.
It is particularly preferred to incorporate the following ingredients: sodiurn/alkali
metal pyrophosphate-cont~ining, calculus inhibiting ingredients in the low foaming
toothpastes or dental creams of the invention.
CA 02260930 l999-01-l9
WO 98/04234 PCT/US97/12208
- 29 -
EXAMPLE 1 1
Toothr~t~s or Dental Creams
Amounts, Percent by Weight
(Unless Otherwise Indicated)
Ingredient Broad Range Preferred Range
SodiumBic~ut 20.00 to 65.00 30.00 to 60.00
~ rl~ Salt 2.S0 to 13.00 2.50 to 3.00
ll - 3.00 to 60.00 10.00 to 35.00
Organic Thickener 1.00 to 2.00 0.30 to 1.50
Inorganic Thickener 9.00 to 10.00 0.00 to 5.00
Nonionic Low Foam Surfactant 0.05 to 5.00 0.10 to 1.00
Water Insoluble Abrasive 0.00 to 50.00 0.00 to 20.00
Sweetener 0.00 to 10.00 0.30 to 2.00
r ~ gAgentasfluorideion 23.00 to 3000ppm 850 to 1500ppm
Flavoring Agent 0.01 to 5.00 0.30 to 2.00
Water 3.00 to 60.00 5.00 to 35.00
CA 02260930 1999-01-19
WO 98/04234 PCT/US97/12208
- 30 -
In another particularly preferred embodiment, the following ingredients are
incorporated in sodium bicarbonate/alkali metal pyrophosphate-cont~ining, calculus
inhibiting low foaming dental gels.
EXAMPLE 12
Dental Gels
Arnounts, Percent by Weight
(Unless Otherwise Indicated)
Ingredient Broad Range Preferred Range
Sodium Bic~; ~- 20.00 to 60.00 20.00 to 40.00
Pyl~h ,~' Salt 2.50 to 15.00 2.50 to 5.00
Tl.. ,.r. ~ 10.00 to 60.00 10.00 to 50.00
Organic Thickener 0.10 to 2.00 0.30 to 1.50
Inorganic Thickener 0.00 to 10.00 3.00 to 8.00
Nonionic Low Foarn Surfactant 0.00 to 10.00 0.30 to 1.00
Water Insoluble Abrasive 0.00 to 50.00 0.00 to 20.00
Sweetener 0.00 to 10.00 0.30 to 2.00
~Iu~)li~tiug Agent as fluoride ion 15.00 to 5000 ppm 850 to 1500 ppm
Flavoring Agent 0.01 to 5.00 0.30 to 2.00
Water 3.00 to 30.00 5.00 to 20.00
CA 02260930 1999-01-19
WO 98/04234 PCT/US97/12208
- 31 -
In another preferred embodiment of the invention, anticalculus low foaming toothpowders contain the following ingredients.
EXAMPLE 13
Toothpaste Powders
Amounts, Percent by Weight
(Unless Otherwise Indicated)
Ingredient Broad Range Preferred Range
Sodium Bical; 20.00 to 95.00 50.00 to 95.00
~r~ Salt 2.50 to 13.00 2.50 to 5.00
Nonionic Low Foam S r _ ' 0~OO tO 10~00 0~00 tO 2.00
Water Insoluble Abrasive 0.00 to 95.00 0.00 to 50.00
Sweetener 0.00 to 10.00 0.30 to 2.00
~ ~; ' e Agent as fluoride ion 25.00 to 3000 ppm 850 to 1500 ppm
Flavoring Agent 0.01 to 5.00 0.30 to 2.00
Anti-ca~ng Agent 0.00 to 5.00 0.05 to 0.20
10 In addition to the levels and combin~tion~ of ingredients shown in these examples,
others can be used which are consislelll with the invention disclosed and cl~im~d
herein.
The present invention has been described in detail, inrlllcling the prefellcd
l 5 embc~lim~nt~ thereof. However, it will be appreciated that those skilled in the art,
upon consideration of the present disclosure, may rnake modifications and/or
improvements on this invention and still be within the scope and spirit of this invention
as set forth in the following clairns.